Abstract
Design of an efficient site-specific drug delivery system based on degradable functional polymers still remains challenging. In this work, we synthesized and characterized three degradable functional polyesters belonging to the poly(malic acid) family: the poly(benzyl malate) (PMLABe), the poly(ethylene glycol)-b-poly(benzyl malate) (PEG42-b-PMLABe), the biotin-poly(ethylene glycol)-bpoly( benzyl malate) (Biot-PEG62-PMLABe). Starting from these building blocks, we were able to prepare the corresponding well-defined degradable functional nanoparticles whose toxicity was evaluated in vitro on normal and cancer cell lines. Results have evidenced that the prepared nanoparticles did not show any significant cytotoxicity even at high concentrations. A model anti-cancer drug (doxorubicin, Dox) or a fluorescent probe (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, DiD oil) has been encapsulated into PMLABe, PEG42-PMLABe or Biot-PEG62-PMLABe based nanoparticles in order to evaluate, respectively, the in vitro cytotoxicity of Dox-loaded nanoparticles on normal and cancer cell lines and the ligand (biotin) effect on cellular uptake in vitro using mmt 060562 cell line. Dox-loaded PMLABe, PEG42-PMLABe or Biot-PEG62-PMLABe nanoparticles showed an in vitro cytotoxicity similar to that of free Dox. Moreover, the DiD oil loaded Biot-PEG62-PMLABe based nanoparticles showed a better in vitro cellular uptake than ligand-free DiD oil loaded nanoparticles. Both results evidence the great potential of such degradable functional poly(malic acid) derivatives for the design of highly efficient site-specific anti-cancer nanovectors.
Keywords: Degradable polyesters, Poly(malic acid) derivatives, Degradable functional nanoparticles, In vitro cytotoxicity, In vitro cellular uptake
1. Introduction
Cancer, characterized by an abnormal and anarchical cell proliferation within a normal tissue of the body, is a very complex disease and a major cause of mortality (Misra et al., 2010). Over the past decade, the better understanding of the molecular basis of cancer unveiled new therapeutic targets especially proteins involved in cell signalling pathways regulating cell division and/or programmed cell death. In the meantime, considerable efforts to prevent and treat this disease have lead to the identification of new drugs with protective effects or anti-cancer properties through the inhibition of cell division and/or induction of apoptosis. Administration of these new potent anti-cancer therapies is however often associated with severe side effects such as hair loss, damages to liver, kidney, bone narrow and heart (Misra et al., 2010; Jaracz et al., 2005; Torchilin, 2006), which limit their use for clinical applications. In this context, numerous research works are focused on the search of optimal cancer therapeutic strategies decreasing significantly the dramatic side effects of the actual chemotherapies (Misra et al., 2010; Jaracz et al., 2005; Torchilin, 2006; Hoffman, 2008; Malam et al., 2009). Therefore, an objective of research in the pharmaceutical and biomedical fields is concerned with the development of drug delivery systems able to protect effectively the drug from non-specific degradation and/or biodistribution, and to target the drug to its site of action (cells, tissues, organs) (Hoffman, 2008; Malam et al., 2009). This concept should allow decreasing not only the administrated doses of drugs but also the non-specific drug distribution. Several kinds of drug delivery systems have been reported, among which the most frequent ones are liposomes formed by the association of lipids (Misra et al., 2010; Malam et al., 2009) and nanoparticles consisting of polymers which may be (bio)degradable or not (Misra et al., 2010; Torchilin, 2006;Hoffman, 2008; Malam et al., 2009; Liu et al., 2009).
While certain PEGylated liposomes containing anti-cancer drugs have reached the market, most of the polymeric nanoparticles are still in the preclinical phase of development (Misra et al., 2010; Torchilin, 2006; Hoffman, 2008; Malam et al., 2009; Liu et al., 2009). However, it is noteworthy that two polymeric nanoparticles formulations are in phase II of clinical trials: the Doxorubicin Trandrug® produced by the society BioAlliance Pharma (http://www.bioalliancepharma.com) and the Mitoxantrone-poly(alkylcyanocrylate) nanoparticles (Zhou et al., 2009). Improvement of several properties of these polymeric nanoparticles needs to be reconsidered, such as (i) the drug loading efficiency, (ii) the targeting of the site of action, (iii) the particle in vivo stability and the control of drug release, (iv) the improvement of nanovector biocompatibility and its elimination from the organism after tumor treatment.
Within this context, we have focused our attention on the following objectives: (i) synthesis of polymers combining biologically active molecules to a biocompatible and degradable macromolecular backbone, (ii) formulation and characterization of the corresponding biodegradable nanoparticles, (iii) in vitro and in vivo toxicity and a favourable biodistribution, (iv) anti-cancer drug encapsulation for site-specific delivery to liver.
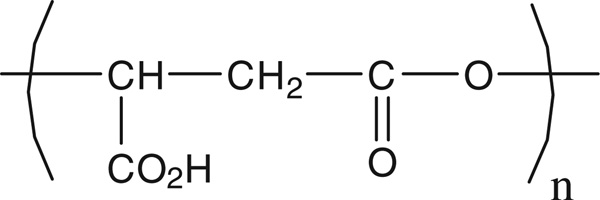
Towards this goal, we have first synthesized a degradable and biocompatible nanoparticle that is derived from poly(malic acid), PMLA shown in Fig. 1.
Fig. 1.
Structure of PMLA.
This polymer is synthesized by certain fungi and slime molds (Rathberger et al., 1999) and has been shown to be non-toxic and biodegradable into malic acid under physiological conditions (Vert and Lenz, 1979). PMLA was originally synthesized for applications in the biomedical field. Moreover, PMLA has been successfully used as platform in the synthesis of nanovectors (Abdellaoui et al., 1998;Osanai and Nakamura, 2000; Cammas et al., 2000; Cammas-Marion and Guérin, 2000; Martinez Barbosa et al., 2004) or as a constituent in macromolecular conjugates bearing several functionalities to treat human brain and breast tumors in mouse models (Fujita et al., 2006, 2007; Ljubimova et al., 2008; Ding et al., 2010). In all of these investigations it has been concluded that PMLA was a promising building block for the design of efficient drug delivery systems.
PMLA and its derivatives having well-defined structures are accessible either from natural PMLA extracted from fungi or myxomycetes, for example, from the slime mold Physarum polycephalum (Ljubimova et al., 2008) or by anionic ring opening polymerization (ROP) of β-substituted β-lactones (MLAR) synthesized from aspartic acid (Cammas et al., 1996) or malic acid (Cammas et al., 1993). In the frame of the present work, we have chosen to prepare the selected poly(malic acid) derivatives by anionic ROP of MLAR synthesized from aspartic acid. Besides the bio-compatibility, current research in drug delivery aims at designing new nanoparticles that exhibit stealth properties, i.e. a prolonged circulation time in the bloodstream without being recognized by the reticulo-endothelial system, and that preferentially target a specific cell type. For stealth properties, we selected the poly(ethylene glycol), PEG, which is the most widely used material for achieving such steric stabilization (Romberg et al., 2008). We have also selected the biotin (Biot) as a targeting moiety because this vitamin (vitamin B7 or H) is a promoter of cell growth in many cell types and expression of its receptors is increased in several tumoral cells (Yang et al., 2009).
In this paper, we report the synthesis and characterization of three copolymers of PMLA: (i) a lipophilic polymer, poly(benzyl malate) (PMLABe), (ii) a amphiphilic block copolymer, poly(ethylene glycol)-b-poly(benzyl malate) (PEG42-b-PMLABe), and (iii) a amphiphilic block copolymer possessing a targeting moiety (biotin) at the hydroxyl terminal end of PEG (the hydrophilic block) (Biot-PEG62-b-PMLABe). All these copolymers self-assembled into nanoparticles. These have been characterized and their in vitro cytotoxicity has been evaluated on healthy and cancer cell lines. Moreover, an anti-cancer model drug, doxorubicin (Dox) or a fluorescent probe, 1,1′-dioctadecyl-3,3,3′,3′- tetramethylindodicarbocyanine (DiD oil), have been encapsulated into PMLABe, PEG42-b-PMLABe and Biot-PEG62-b-PMLABe nanoparticles. Results obtained for their in vitro cytotoxicity on healthy and cancer cells as well as their in vitro cellular uptake using the mouse breast cancer mmt 060562 cells overexpressing biotin receptor are reported.
2. Materials and methods
2.1. Materials
The racemic benzyl malolactonate (MLABe) was synthesized from dl-aspartic acid according to the previously reported synthesis (Cammas et al., 1996). All chemicals were used as received. Anhydrous ethanol was prepared just before use by distillation over natrium under N2 atmosphere. Anhydrous THF was obtained by distillation over natrium/benzophenone under N2 atmosphere.
Nuclear magnetic resonance spectra (1H NMR) were recorded on a Brucker ARX 400 instrument (1H at 400 MHz). Data are reported as follows: chemical shift (multiplicity, number of hydrogen). The chemical shifts (δ) are reported as parts per million (ppm) referenced to the appropriate residual solvent peak. Abbreviations are as follows: s (singlet), d (doublet), t (triplet), q (quartet), dd (doublet of doublet), m (multiplet).
Weight average molecular weight (M̄w) and molecular weight distribution (M̄w/M̄n = Ip) values were determined by size exclusion chromatography (SEC) in THF at 30 °C (flow rate = 1.0 mL min−1) on a GPC2502 Viscoteck apparatus equipped with a refractive index detector, a LT5000L mixed medium org 300 × 7.8 mm gel column and a GPC/SEC OmniSEC software. The polymer samples were dissolved in THF (2 mg mL−1). All elution curves were calibrated with poly(styrene) standards.
The size (average diameter obtained by the cumulant result method), polydispersity and zeta potential of the formulations were measured by Dynamic Light Scattering using a Delsa™ Nano Beckman Coulter apparatus at 25 °C.
UV spectra were recorded on a Secoman apparatus at 485 nm. Seven cancer cell lines have been selected within the frame if this project: PC3, a human prostatic adenoma derived cell lines (Kaighn et al., 1979); NCI, a human lung cancer derived cell line (Gazdar et al., 1980); Caco, a human colorectal cancer derived cell line (Jumarie and Mao, 1991); HCT116, a human colorectal cancer derived cell line (Brattein et al., 1981); MDA, a human breast cancer derived cell line (Gazdar et al., 1998); HuH7, a human hepatocarcinoma derived cell line (Nakabayashi et al., 1982), all these cells were provided by the ImPACcell platform (IFR 140) and mmt 060562, a mice mammary tumor derived cell lines which over expressed the biotin receptors (Akatsu et al., 1998).
One healthy cell line has also been selected used for in vitro cytotoxicity assays: the immortalized human foreskin fibroblasts, primary cells immortalized by transfection of human Telomer reverse transcriptase Tert (Loyer, unpublished data).
2.2. Synthesis and characterization of PMLABe, PEG42-b-PMLABe and Bio-PEG62-b-PMLABe
2.2.1. PMLABe
The poly(benzyl malate), with a theoretical molecular weight of 15,000 Da, was synthesized as described previously (Cammas et al., 1996). Briefly, 840 µL of an ethanol solution of tetraethylammonium benzoate (6.65 × 10−5 mol) were placed in the polymerization flask, the alcohol was eliminated under vacuum at room temperature. The tetraethylammonium benzoate was dried under vacuum at room temperature for 2 h. One gram of MLABe was transferred into the polymerization flask containing the initiator under N2. The polymerization was conducted under nitrogen at 37 °C for 8 h (disappearance of the lactone peak at 1850 cm−1 from the IR spectrum). The polymer was dissolved in acetone, precipitated into ethanol and dried under vacuum for 48 h.
1H NMR (400.1 MHz; (CD3)2CO), δ (ppm) = 2.83–3.03 (m, 2nH, CHCH2CO2), 5.05–5.21 (m, 2nH, CO2CH2C6H5), 5.48–5.52 (m, 1 nH, CHCH2CO2), 7.27–7.38 (m, 5nH, CO2CH2C6H5)
SEC (THF, polystyrene standards, 1 mL/min): M̄w = 14, 000 Da, Ip = M̄ w/M̄ n = 1.33.
2.2.2. PEG42-b-PMLABe
The potassium naphtalide solution was prepared as reported elsewhere (Cammas et al., 1995). A THF solution of 18-crown-6 ether was previously prepared (50 mg of 18-crown-6 ether dissolved in 1 mL of anhydrous THF under N2 atmosphere). THF (2 mL), α-methyl-ω-carboxyl-PEG42 (6.65 × 10−5 mol), potassium naphtalide (6.65 × 10−5 mol) and 18-crown-6 ether (6.65 × 10−5 mol) were introduced in a round-bottom flask under N2 atmosphere. MLABe (with a monomer/initiator ratio of 73 to obtain a PMLABe with a theoretical molecular weight of 15,000 Da), dissolved in anhydrous THF, was added under N2 to the initiator solution. The polymerization reaction was followed by IR and stopped after disappearing of the characteristic lactone peak (νC=O of the lactonic ring at 1850 cm−1). Acetic acid was added and the polymer was purified by precipitation in a large excess of ethanol. The resulting polymer was characterized by 1H NMR, to verify the polymer structure and determine the molecular weight (MNMR) of the polyester, and by SEC, giving access to the weight average molecular weight (M̄w) and polydispersity index (Ip = M̄w/M̄n).
1H NMR (400.1 MHz; (CD3)2CO), δ (ppm): 2.77–2.83 (m, 2nH, CO2CH2C6H5), 3.56–3.59 (m, 4mH (m = 42), CH3O(CH2CH2O)42), 4.97–5.06 (m, 2nH, CHCH2CO2), 5.39–5.44 (m, 1nH, CHCH2CO2), 7.19–7.27 (m, 5nH, CO2CH2C6H5).
MNMR = 14,000 Da for the PMLABe block.
SEC (THF, polystyrene standards, 1 mL/min): M̄w = 9600 Da, Ip = 1.30.
2.2.3. Biot-PEG62-b-PMLABe
The preparation of the Biot-PEG62-b-PMLABe was similar to that described for the PEG42-b-PMALBe using the α-biotin ω-carboxyl- PEG62 (6.65 × 10−5 mol) as initiator in the presence of 1 equiv. of 18-crown-6 ether and 1 equiv. of potassium naphtalide.
The Biot-PEG62-b-PMLABe was purified by precipitation in a large excess of ethanol. The resulting polymer was characterized by 1H NMR, to verify the polymer structure and determine the molecular weight (MNMR) of the polyester, and by SEC, giving access to the weight average molecular weight (M̄w) and polydispersity index (Ip = M̄w/M̄n).
1H NMR (400.1 MHz; (CD3)2CO), δ (ppm): 2.83 (s, 2nH, CO2CH2C6H5), 3.56–3.59 (m, 4mH (m = 62), Biot (CH2CH2O)62), 5.00–5.05 (m, 2nH, CHCH2CO2), 5.41–5.44 (m, 1nH, CHCH2CO2), 7.19–7.23 (m, 5nH, CO2CH2C6H5).
MNMR = 15,500 Da for the PMLABe block.
SEC (THF, polystyrene standards, 1 mL/min): M̄w = 8200 Da, Ip = 1.20.
2.3. Nanoparticles preparation and characterization
2.3.1. Nanoparticles preparation and characterization
Nanoparticles were prepared by the nanoprecipitation method as described by Fessi et al. (Thioune et al., 1997). Twenty-five mg of the selected polymer, PMLABe, PEG42-b-PMLABe or Biot-PEG62- b-PMLABe, were dissolved in 5 mL of acetone. This solution was rapidly added into 10 mL of water under vigorous stirring. The suspension was left under stirring during 15 min and the acetone was evaporated under vacuum at 40 °C. The final polymer concentration was 2.5 g/L. The nanoparticles were characterized by Dynamic Light Scattering (DLS) and zetametry in water at room temperature with a concentration in polymer of 2.5 mg/mL.
PMLABe based nanoparticles: 130 nm, Ip = 0.10, ζ = −50 mV.
PEG42-b-PMLABe based nanoparticles: 70 nm, Ip = 0.10, ζ = −8 mV.
Biot-PEG62-b-PMLABe based nanoparticles: 100 nm, Ip = 0.20, ζ =−6 mV.
2.3.2. In vitro cytotoxicity assays
We used six human cancer cell lines (PC3, NCI, Caco, HCT116, MDA and HuH7) and one normal human cell line (immortalized human foreskin fibroblasts). The same protocol was used for all the cytotoxicity assays and performed using the ImPACcell platform (IFR 140, University of Rennes 1). The cells were seeded in 96-well plates with 4000 cells in 60 µL of DMEM culture medium per well and cultured for 24 h at 37 °C under 5% CO2 atmosphere. Then, after cell adhesion to the support, PMLABe, PEG42-b-PMLABe or Biot-PEG62-PMLABe based nanoparticles at different concentrations were incubated with the various cell lines. After 48 or 72 h, the culture medium was removed and cells were washed with 100 µL of PBS. Dead cells floating in culture medium were eliminated and cell viability assay consisted in measuring number of viable attached cells. The PBS was eliminated and 60 µL of a mixture alcohol/acetic acid was dropped in the wells. After 20 min at room temperature, the solvents were removed and the plates were dried 30 min at room temperature. Coloration of cell nuclei was performed with 60 µL per well of a Hoechst’s solution at 1.5 µL/mL during 20 min at room temperature. The dye was eliminated and the cells were washed with 100 µL of milliQ water. The plates were dried at room temperature and analyzed by automated image capture using an Olympus microscope and Simple PCI software. Such analysis allowed photographing 5 fields of each well of a culture plate. Each well was analyzed by the software in order to determine the number of nuclei and therefore the number of cells. The concentrations in polymers for each type of nanoparticles are the following:
PMLABe nanoparticles:
-
-
Concentration in polymer (µM): 0; 0.04; 0.13; 0.39; 1.16; 3.50; 10.40.
PEG42-b-PMLABe nanoparticles:
-
-
Concentration in polymer (µM): 0; 0.04; 0.11; 0.34; 1.03; 3.10; 9.25.
Biot-PEG62-b-PMLABe nanoparticles:
-
-
Concentration in polymer (µM): 0; 0.04; 0.11; 0.32; 0.97; 2.90; 8.70.
For each nanoparticle, the number of cells per field was plotted against the polymer concentration.
2.4. Doxorubicin and DiD oil-loaded nanoparticles
2.4.1. Doxorubicin-loaded nanoparticles
The doxorubicin hydrochloride (Dox-HCl, Sigma) was encapsulated into the three kinds of nanoparticles during the nanoprecipitation procedure. The selected polymer (5 mg) was dissolved in acetone (1 mL). Two hundred microliters of a Dox solution [1.5 mg of Dox-HCl solubilized in 0.6 mL of a mixture of chloroform (6 mL) and NEt3 (23 µL)] was added to the polymer solution. This mixture was then nanoprecipitated into 2 ml of water under vigorous stirring. After organic solvent evaporation, the unloaded doxorubicin was removed by ultracentrifugation at 15,000 × g at 15 °C for 7 min using filter with an exclusion limit 10,000 Da. The filters were returned and centrifuged for 1 min at 1000 × g at 15 °C. The volume of the recovered solutions was completed to 2 ml with distilled water in order to obtain a final concentration in nanoparticles of 2.5 g/L. The concentration of loaded Dox was evaluated by UV at 485 nm, as described elsewhere (Cammas et al., 1995). Briefly, 200 µL of Dox-loaded nanoparticles were dissolved into 800 µL of DMF and the resulting solutions were analyzed by UV at 485 nm. The absorbance of Dox encapsulated into nanoparticles was converted into a concentration using a calibration curve and the encapsulation efficiency (e.e.) was calculated using the following equation:
Cytotoxicity assays with Dox-loaded nanoparticles were conducted as described above for empty nanoparticles and compared to the cytotoxicity of the free Dox. The concentrations in Dox and in polymers used for each nanoparticle are given below:
Free Dox, concentration (nM): 0; 0.8; 2.4; 7.4; 22; 66; 200.
-
-
Dox-loaded PMLABe nanoparticles:
-
-
Concentration in Dox (nM): 0; 0.8; 2.4; 7.4; 22; 66; 200.
-
-
Concentration in polymers (nM): 0; 8; 24; 90; 260; 790; 2500.
Dox-loaded PEG42-b-PMLABe nanoparticles:
-
-
Concentration in Dox (nM): 0; 0.8; 2.4; 7.4; 22; 66; 200.
-
-
Concentration in polymers (nM): 0; 4; 12; 37; 110; 330; 1000.
Dox-loaded Biot-PEG62-b-PMLABe nanoparticles:
-
-
Concentration in Dox (nM): 0; 0.8; 2.4; 7.4; 22; 66; 200.
-
-
Concentration in polymers (nM): 0; 5.1; 12.3; 45.7; 137; 412; 1250.
For each nanoparticle, the cell viability (%) was plotted against both the Dox and the polymer concentrations.
2.4.2. DiD oil-loaded nanoparticles
The lipophilic dye, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiD oil), has been selected for the labelling of PEG42-PMLABe and Biot-PEG62- PMLABe nanoparticles in order to evaluate the ligand effect on cellular uptake using the murine mmt 060562 cell line. This dye has the following characteristics: Ex × 644 nm/Em × 665 nm, ε = 236.000. The DiD oil has been physically entrapped into PMLA derivatives based nanoparticles as followed: 5 mg of PMLABe, PEG42-PMLABe or Biot-PEG62-PMLABe were dissolved into 100 µL of DMF. Fifty µL of a DiD oil solution at a concentration of 5 mg/mL were added into the polymer solution. This mixture was nanoprecipitated into 1 mL of distilled water under vigorous stirring. After 1 h at room temperature, the non-loaded DiD oil was removed by filtration through a sephadex PD 10 column. Finally, the DiD oil-loaded nanoparticles suspensions were filtrated over a micromembrane with a porosity of 0.5 µm. DiD oil-loaded nanoparticles were analyzed by fluorescent size exclusion chromatography.
The mouse breast cancer mmt 060562 cell line over-expressing biotin receptors have been chosen for these biological experiments.
In vitro cell viability assay with empty PEG42-b-PMLABe and Biot-PEG62-b-PMLABe nanoparticles was conducted accord ing to the following protocol. Cells were seeded in a 96-well plate (2000 cells/well in 100 µL media) and incubated for 24 h. The cells were then incubated with the selected formulations (PEG42-PMLABe or Biot-PEG62-PMLABe nanoparticles) in different concentrations in 100 µL of media for another 24 h. The viable cells were quantified using the Cell Titer 96 Aqueous One Solution Cell Proliferation Assay kit (Promega) by reading the absorbance at 490 nm with a Spectra Max Plus 384 ELISA reader. Cell viability (%) was plotted against nanoparticles concentration. Data of cell viability obtained with this method confirmed the results previously obtained by counting HuH7 cells.
In a second experiment, cellular uptake experiments were conducted in order to evidence the biotin effect. Cells (1 × 105 per chamber) were seeded on a Lab-Tek chambered cover glass (NUNC) for 24 h. The cells were washed once with serum free media followed by incubation in 150 µL media containing the samples. After 1 h incubation, the cells were washed with PBS and fixed with 2% paraformaldehyde for 20 min. After the incubation, they were washed 3 times with PBS in 5 min intervals. Then, the cell nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI), coverslipped and observed under a Leica fluorescence microscope DM 6000 B and photographed using DFC 360FX camera (Leica Microsystems, Mannheim, Germany).
3. Results and discussion
3.1. Synthesis and characterization of PMLA derivatives
In the frame of this work, we have first synthesized and characterized a β-subtituted β-lactone, benzyl malolactonate, using a well described synthetic method (Cammas et al., 1996). This lactone was used as the monomer for the design of novel degradable polyesters of the PMLA family with the aim of using them as building blocks in the preparation of degradable nanoparticles for targeted anti-cancer drug delivery.
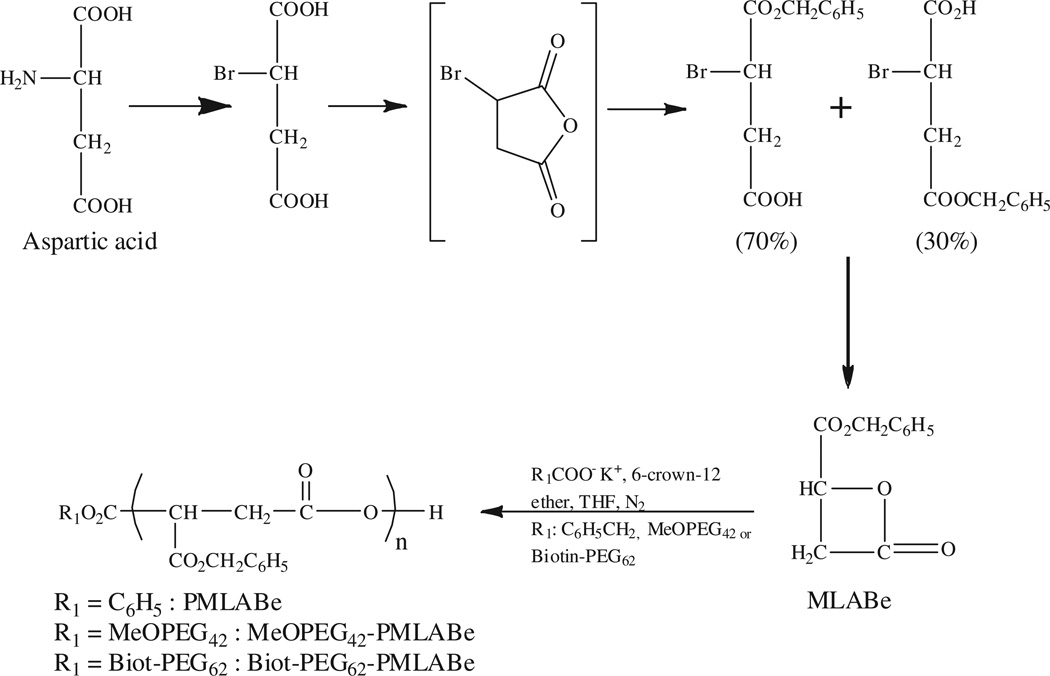
Copolymers PMLABe, PEG42-b-PMLABe and Biot-PEG62-b- PMLABe were synthesized by anionic ring opening polymerization using, respectively, tetraethylammonium benzoate, α-methoxy ω-carboxyl PEG42 and α-biotin ω-carboxy PEG62 as initiators (Scheme 1).
Scheme 1.
Synthetic route to PMLABe, PEG42-b-PMLABe and Biot-PEG62-PMLABe.
PMLABe has been prepared to evaluate the effect of the PEG chains and the biotin molecule on in vitro cytotoxicity, drug loading capacity and targeting effect on in vitro cellular uptake of the corresponding nanoparticles. The polymers have been characterized by 1H NMR and size exclusion chromatography. NMR spectra of the polymers allowed confirming the structure of the polymers and the presence of both the PEG chains and biotin molecule, and determining the molecular weight of the PMLABe block for PEG42-b-PMLABe and Biot-PEG62-b-PMLABe polymers (Table 1). Molecular weights of PMLABe block in both PEG42-b-PMLABe and Biot-PEG62-b-PMLABe polymers were calculated using the integration of the methylene protons (δ = 3.58 ppm) of the PEG block having a molecular weight of 1850 Da (n = 42) and 2730 Da (n = 62), respectively, and the integration of the methylene proton (δ = 5.01 ppm) of PMLABe block. As shown by results in Table 1, molecular weights calculated from the NMR spectra, especially with regard to the PMLABe block, were in good agreement with the theoretical ones evidencing the purity of the monomer, the completeness of the polymerization reaction and the confirmation of the type of polymer.
Table 1.
Molecular weights of PMLABe, PEG42-b-PMLABe and Biot-PEG62-b-PMLABe measured by SEC (THF, polystyrene standards, 1 mL/min) and determined by 1H NMR.
| Polymer |
M̄w (Da) measured by SEC |
Ip (SEC) |
MNMR (Da) of PMLABe block |
|---|---|---|---|
| PMLABe | 14,000 | 1.33 | – |
| PEG42-b-PMLABe | 9550 | 1.30 | 14,000 |
| Biot-PEG62-b-PMLABe | 8230 | 1.20 | 15,500 |
In addition, the three polymers were analyzed by size exclusion chromatography, which allowed measurement not only of the molecular weights of the polymers but also of the polydispersity indexes, i.e. their mass distribution. SEC results showed that the weight-averaged molecular weight of PMLABe was similar to the theoretical value, and also that the mass distribution of the polymers was quite narrow (Ip ranging from 1.20 to 1.33). However, the weight-averaged molecular weights of PEG42-b-PMLABe and Biot- PEG62-b-PMLABe were lower than the theoretical values, probably as a result of the presence of the PEG chains, which could have induced a specific conformation of the macromolecular chains in THF thus lowering the molecular weight of PEG containing polymers.
3.2. Preparation and characterization of nanoparticles based on PMLA derivatives
Starting from the PMLA-based copolymers, we have set up a reproducible and reliable method to prepare well-defined nanoparticles bearing PEG chains and biotin molecules. Based on the results published in the literature concerning the preparation of nanoparticles starting from either hydrophobic or amphiphilic polymers, we have selected the nanoprecipitation technique (Thioune et al., 1997) to prepare our nanovectors. The nanoprecipitation technique consists in the rapid dilution of an organic solution of polymer (acetone or THF) into water under vigorous stirring. The nanoparticles are formed instantaneously and are recovered as an aqueous solution after solvent evaporation under vacuum. Diameters and polydispersities, as well as surface charges were measured by Dynamic Light Scattering (DLS) and zetametry, respectively.
As shown by results in Table 2, the sizes of PMLABe, PEG42-b- PMLABe and Biot-PEG62-b-PMLABe nanoparticles are ranging from 70 nm to 130 nm with polydispersity indexes varying from 0.10 to 0.20.
Table 2.
Characteristics of nanoparticles measured by DLS.
| NPs | Size (nm) | Ip | Zeta potential (mV) |
|---|---|---|---|
| PMLABe | 130 | 0.1 | −50 |
| PEG42-b-PMLABe | 70 | 0.1 | −8 |
| Biot-PEG62-b-PMLABe | 100 | 0.2 | −6 |
It is noteworthy that the diameters of PEGylated nanoparticles are significantly lower than for the nanoparticle in the absence of PEGylation. This observation is in good agreement with the literature data concerning the influence of the presence of PEG chains on the diameters of nanocarriers. Indeed, it has been shown that the presence of PEG on the surface of nanoparticles resulted in a decrease in their diameters (Thevenot et al., 2007). The low polydispersity indexes (lower than 0.20) highlighted the quite homogenous size distribution, which is a critical parameter for applications in the biomedical field, where the characteristics of the nanocarriers have to be perfectly controlled.
The negative zeta potential observed for PMLABe nanoparticles can be explained by the fact that the terminal carboxylic acid functions of PMLABe polymers can be found on the nanoparticles surfaces. It should be noted that the zeta potential of PEGy-lated nanoparticles, with or without biotin, is close to neutrality. Once again, this result is in good agreement with the properties conferred to the nanoparticles by the hydrophilic non-charged PEG chains (Thevenot et al., 2007). Such results highlighted the presence of PEG chains at the surfaces of the prepared nanoparticles. On the contrary, we did not notice a significant influence of the biotin molecules on the properties of the corresponding nanoparticles. Further studies to evidence both the presence of PEG chains and biotin molecules on the surface of nanoparticles based on PMLA derivatives are undertaken (ITC measurements, avidin–biotin interactions, activation of the complement, etc.).
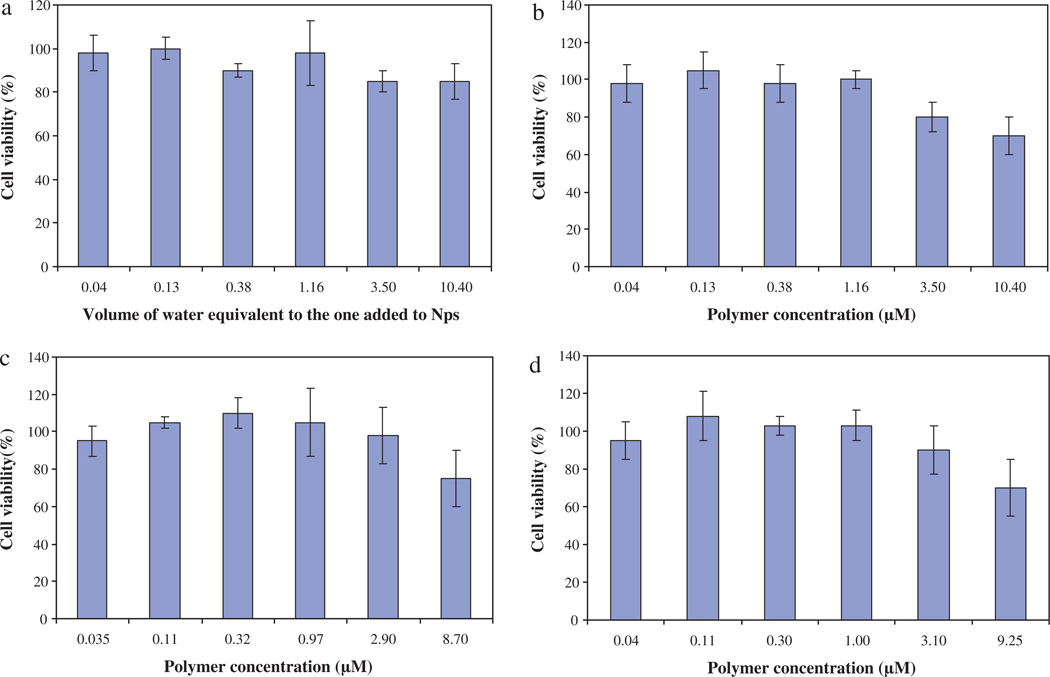
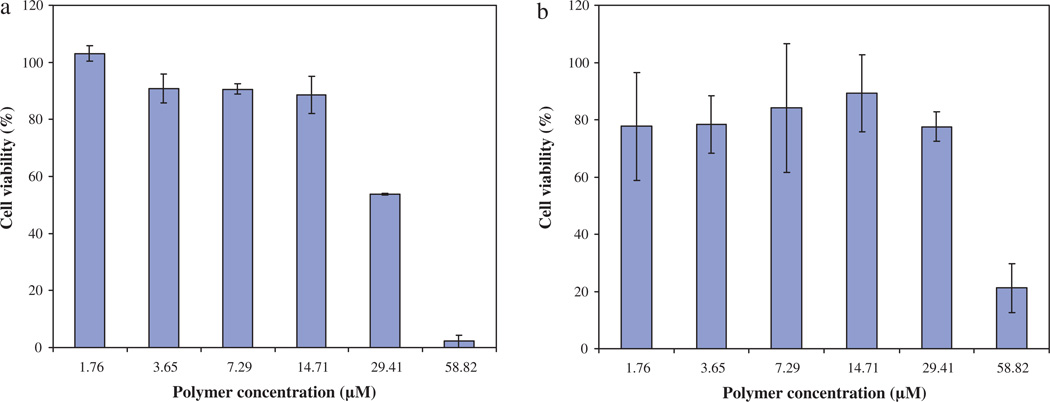
The in vitro cytotoxicity of nanoparticles has been evaluated on the seven following cancer cell lines: PC3, NCI, Caco, HCT116, MDA, HuH7 and mmt 060562. For comparison, cytotoxicity has also been evaluated on healthy human immortalized foreskin fibroblasts. Figs. 2 and 3 give the results obtained for HuH7 and mmt 060562 cell lines, respectively, treated with PMLABe nanoparticles (a), PEG42-b-PMLABe (b) or Biot-PEG62-b-PMLABe (c) in terms of cell viability as a function of polymer concentrations.
Fig. 2.
In vitro cell viability evaluation on HuH7 cell line given as 100% of cellular viability in control cultures against polymer concentration. (a) Control: cells + water. (b) Cells + PMLABe. (c) Cells + PEG42-b-PMLABe. (d) Cells + Biot-PEG62-b-PMLABe.
Fig. 3.
In vitro cell viability evaluation on mmt 060562 cell line given as 100% of cellular viability in control cultures against polymer concentration. (a) Cells + PEG42-b-PMLABe. (b) Cells + Biot-PEG62-b-PMLABe.
Within the frame of this paper, we have chosen to show results obtained for HuH7 towards future development of nanoparticles to treat liver cancer, and mmt 060562 cell line as an example to test whether cells with overexpressed biotin receptor could be targeted by Biot-PEG62-b-PMLABe. Results in Figs. 2 and 3 revealed that the three selected nanoparticles did not have significant cytotoxicity in vitro. A slightly reduced cell viability is evident, however, at elevated concentrations (from 3 to 16 µM polymer). Similar results were obtained for all the other studied cell lines. We therefore conclude that the PMLABe, PEG42-b-PMLABe and Biot-PEG62-b-PMLABe nanoparticles are only slightly cytotoxic at high concentrations (>3 µM polymer). Such results are in good agreement with other in vitro cytotoxicity studies performed on hepatocytes with poly(methylmalic acid) (Renard et al., 1996) and on J774 macrophages with poly(hexyl malate) and poly(benzyl malate) (Martinez Barbosa et al., 2004).
3.3. Dox-loaded nanoparticles: preparation, characterization and in vitro cytotoxicity
In order to evaluate the potential of our nanoparticles as drug delivery system, we have chosen to encapsulate the model anti-cancer drug doxorubicin. Dox interacts with DNA by intercalation and inhibits macromolecular biosynthesis (Di Marco et al., 1969; Momparler et al., 1976). Dox has been encapsulated during the nanoprecipitation; after removing of free drug by ultracentrifugation, the corresponding Dox-loaded nanoparticles were characterized by DLS and UV. First, we have observed that the presence of the Dox has no influence on the size and polydispersity of the resulting Dox-loaded nanoparticles (data not shown), i.e. the sizes are still ranging from 70 to 130 nm with polydispersity indexes comprised between 0.10 and 0.15. The absorbance of Dox encapsulated into nanoparticles was converted into a concentration using a calibration curve and the encapsulation efficiency was calculated using the equation given in the experimental part. Results are gathered in Table 3.
Table 3.
Concentration in polymer and in Dox into PMLABe, PEG42-b-PMLABe and Biot-PEG62-b-PMLABe nanoparticles. Encapsulation efficiency (%).
| Nps | Concentration in polymer (µM) |
Concentration in Dox (µg/mL) |
Encapsulation efficiency (%) |
|---|---|---|---|
| PMLABe | 96 | 5 | 2 |
| PEG42-b-PMLABe | 403 | 104 | 42 |
| Biot-PEG62-b-PMLABe | 294 | 80 | 32 |
The encapsulation efficiency into totally hydrophobic nanoparticles formed by PMLABe was lower than the one observed for nanoparticles composed by the amphiphilic block copolymers PEG42-b-PMLABe and Biot-PEG62-b-PMLABe. In these latter cases, a part of the encapsulated Dox is probably localized inside the hydrophilic corona of PEG. Further experiments are required to confirm this hypothesis.
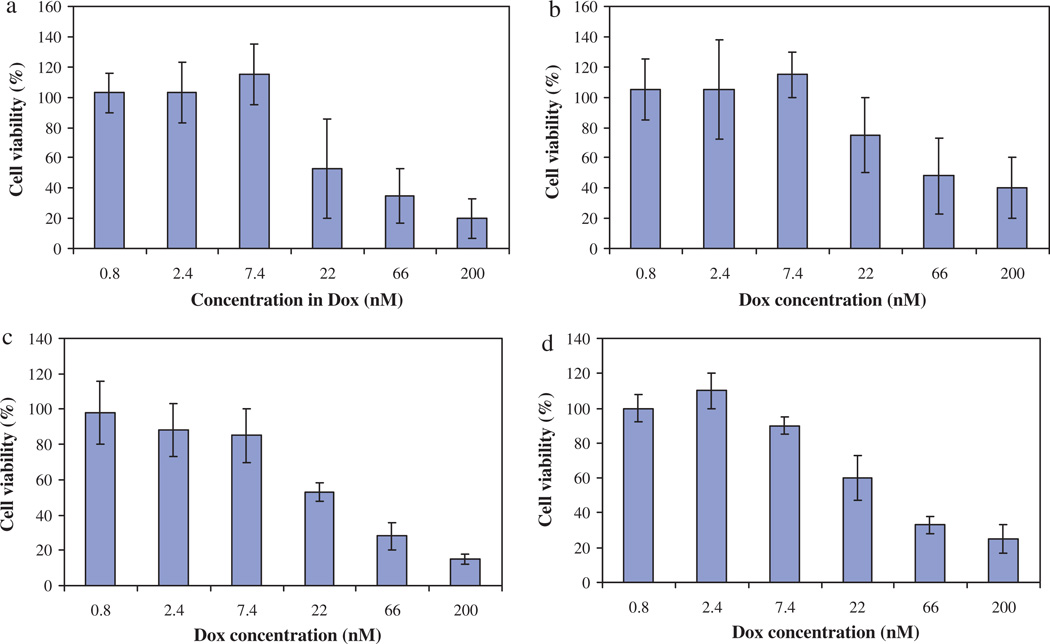
In a second time, we wished to determine whether the encapsulated Dox had the same in vitro cytotoxicity as the free drug. For that study, we have selected only one cell line, the HuH7. As shown in Fig. 4a, free Dox showed a quite high in vitro cytotoxicity with an IC50 at 22 nM.
Fig. 4.
In vitro cell viability evaluation on HuH7 cell line given as 100% cellular viability in control cultures against polymer concentration. (a) Control: cells + Dox. (b) Cells + Dox-loaded PMLABe. (c) Cells + Dox-loaded PEG42-b-PMLABe. (d) Cells + Dox-loaded Biot-PEG62-b-PMLABe.
The treatment with the Dox encapsulated into either PMLABe, PEG42-b-PMLABe or Biot-PEG62-b-PMLABe nanoparticles induced also a dose-dependent cytotoxicity with an overall response quite similar to that observed for free Dox. Such results are very encouraging because the efficacy of the drug is not affected by the encapsulation process.
3.4. DiD oil-loaded nanoparticles: preparation, characterization and ligand effect on cellular uptake in vitro
The fluorescent probe DiD oil has been loaded into PEG42- b-PMLABe and Biot-PEG62-b-PMLABe nanoparticles during the nanoprecipitation procedure using a slightly different method than the one used for Dox encapsulation. The DiD oil-loaded PEG42-b-PMLABe and Biot-PEG62-b-PMLABe nanoparticles were analyzed by fluorescent size exclusion chromatography. Both dye-loaded nanoparticles showed a retention time at around 7.9 min with no signal corresponding to the free drug. Such results confirmed the stabile encapsulation of the DiD oil into the nanoparticles.
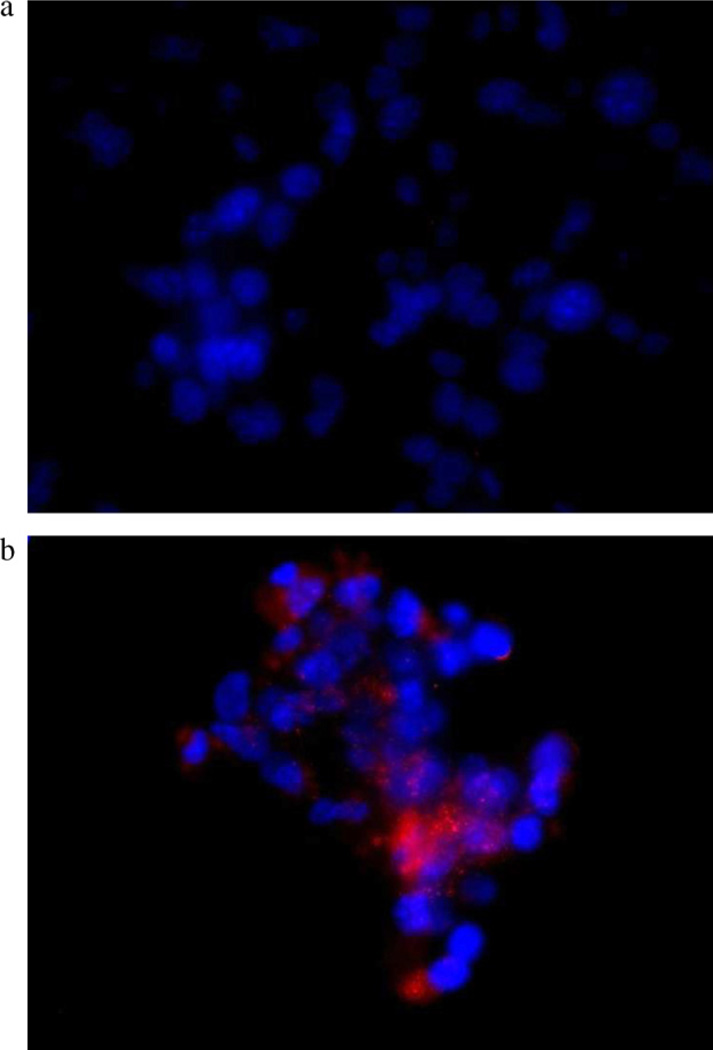
The fluorescent dye loaded nanoparticles have been prepared and characterized, to study the influence of the presence of ligand on the in vitro cellular uptake of mmt 060562, which over expressed the biotin receptor. Fig. 5 shows the results of fluorescence microscopy for (a) DiD oil loaded PEG42-b-PMLABe nanoparticles and (b) DiD oil loaded Biot-PEG62-b-PMLABe nanoparticles.
Fig. 5.
In vitro cellular uptake imaging on mmt 060562 cell line treated with (a) DiD oil-loaded PEG42-b-PMLABe; and (b) DiD oil-loaded Biot-PEG62-b-PMLABe.
Red fluorescence is seen in cells indicating uptake of DiD oil during the treatment with DiD oil loaded Biot-PEG62-b-PMLABe nanoparticles. Fluorescence was absent in mmt cells treated with DiD oil loaded PEG42-b-PMLABe nanoparticles which do not carry biotin. Therefore, in vitro uptake of DiD oil indicated targeting by biotin molecules. Although we have not confirmed targeting by the effect of free biotin competition, the results are promising for in vivo treatment of biotin overexpressing tumor animal models.
4. Conclusions
By the present study, we have demonstrated that well-defined PMLA derivatives can be synthesized bearing specific molecules such as PEG for stealth® properties and the ligand biotin for cell targeting. Starting from such building block, we were able to set up an easy and reproducible method to prepare the corresponding functional nanovectors based on the already known nanoprecipitation method. An important result of this study is that we have demonstrated the non-cytotoxicity in vitro of our formulations even at quite high concentrations. On the other hand, PMLABe, PEG42-b- PMLABe and Biot-PEG62-b-PMLABe nanoparticles can encapsulate either an anti-cancer model drug (Dox) or a hydrophobic fluorescence probe (DiD oil). If Dox encapsulation efficiency is quite low into PMLABe nanoparticles, it is much higher into PEGylated ones. However, we still have to improve this encapsulation rate by modifying the preparation method, notably by adjusting the nature of the organic solvent in which both the polymers and the Dox are solubilized.
Nevertheless, the in vitro cytotoxicity of the loaded Dox was shown to be close to the one of the free drug with IC50 values very similar. At last but not least, the preliminary results obtained for in vitro ligand effect study on cells which overexpressed the biotin receptor are very encouraging.
We are currently pursuing these studies by improving the encapsulation techniques, continuing the in vitro studies, beginning in vivo assays including biodistribution, and selecting one or two drugs more specific of liver cancer and a more adapted molecule for liver targeting.
Acknowledgements
Z.H. H. thanks the Région Bretagne and the European University of Bretagne (UEB) for a Ph.D. grant and a 4 months mobility fellowship, respectively. V.L. was a fellowship recipient from « Association de transfusion sanguine et de biogénétique Gaëtan Saleün » (EFS, Brest, France) and « Ligue Contre le Cancer ». We also thank Dr. C. Guillouzo and the ImPACcell platform (IFR140) for the cell toxicity studies. The biological data (in vitro cytotoxicity on mmt 060562 cells and cellular uptake) have been acquired in the Nanomedicine and Drug delivery laboratory of the Cedars-Sinai Medical Center, US, with the contribution of Dr. Hui Ding, Bindu Konda, Dr. Jose Portilla, Dr. Rameshwar Patil and Dr. Satoshi Inoue.
References
- Abdellaoui K, Boustta M, Vert M, Morjani H, Manfait M. Metabolite-derived artificial polymers designed for drug targeting, cell penetration and bioresorption. Eur. J. Pharm. Sci. 1998;6:61–73. doi: 10.1016/s0928-0987(97)00069-9. [DOI] [PubMed] [Google Scholar]
- Akatsu T, Ono K, Katayama Y, Tamura T, Nishikawa M, Kugai N, Yamamoto M, Nagata N. The mouse mammary tumor cell line, mmt060562, produces prostaglandin E2 and leukemia inhibitory factor and supports osteaclast formation in vitro via a stromal cell-dependent pathway. J. Bone Miner. Res. 1998;13:400–408. doi: 10.1359/jbmr.1998.13.3.400. [DOI] [PubMed] [Google Scholar]
- Brattein MG, Fine WD, Khaled FM, Thompson J, Brattein DE. Heterogeneity of malignant cells from a human colonic carcinoma. Cancer Res. 1981;41:1751–1756. [PubMed] [Google Scholar]
- Cammas S, Renard I, Boutault K, Guérin Ph. A novel synthesis of optically active 4-benzyloxy- and 4-alkyloxycarbonyl-2 oxetanones. Tetrahedron Asymmetry. 1993;4:1925–1930. [Google Scholar]
- Cammas S, Nagasaki Y, Kataoka K. Heterobifunctional Poly(ethylene oxide): synthesis of α-methoxy-ω-amino and α-hydroxy-ω-amino PEOs with the same molecular weights. Bioconjugate Chem. 1995;6:226–230. doi: 10.1021/bc00032a011. [DOI] [PubMed] [Google Scholar]
- Cammas S, Renard I, Langlois V, Guérin Ph. Poly(β-malic acid): obtaining of high molecular weights by improvement of the synthesis route. Polymer. 1996;37:4215–4220. [Google Scholar]
- Cammas S, Béar MM, Harada A, Guérin Ph, Kataoka K. New macromolecular micelles based on degradable amphiphilic block copolymers. Macromol. Chem. Phys. 2000;201:355–364. [Google Scholar]
- Cammas-Marion S, Guérin Ph. 4-Alkyloxycarbonyl-2-oxetanones and 3-alkyloxycarbonyl-2-oxetanones as versatile chiral precursors in the design of functionalized polyesters with controlled architecture. Des. Monomers Polym. (DMP) 2000;3:77–93. [Google Scholar]
- Di Marco A, Gaetani M, Scarpinato B. Adriamycin (NSC-123,127): a new antibiotic with antitumor activity. Cancer Chemother. Rep. 1969;53:33–37. [PubMed] [Google Scholar]
- Ding H, Inoue S, Ljubimov LV, Patil R, Portilla-Arias J, Hu J, Konda B, Wawrowsky KA, Fujita M, Karabalin N, Sasaki T, Black KB, Holler E, Ljubimova JY. Inhibition of brain tumor growth by intravenous poly(β-L-malic acid) nanobioconjugate with pH-dependent drug release. Proc. Natl. Acad. Sci. U.S.A. 2010;107:18143–18148. doi: 10.1073/pnas.1003919107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Khazenzon NM, Ljubimov AV, Lee BS, Virtanen I, Holler E, Black KL, Ljubimova JY. Inhibition of laminin-8 in vivo using a novel poly(malic acid)-based carrier reduces glioma angiogenesis. Angiogenesis. 2006;9:183–191. doi: 10.1007/s10456-006-9046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Lee BS, Khazenzon NM, Penichet ML, Wawrowsky KA, Patil R, Ding H, Holler E, Black KL, Ljubimova JY. Brain tumor tandem targeting using a combination of monoclonal antibodies attached to biopoly(β-l-malic acid) J. Control. Release. 2007;122:356–363. doi: 10.1016/j.jconrel.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar AF, Carney DN, Rusell EK, Sims HL, Baylin SB, Bunn PA, Guccion JG, Minna JD. Establishment of continuous clonable cultures of small-cell carcinoma of the lung which have amine precursor uptake and decarboxylation cell properties. Cancer Res. 1980;40:3502–3507. [PubMed] [Google Scholar]
- Gazdar AF, Kurvari V, Virmani A, Gollahon L, Sakaguchi M, Westerfield M, Kodagoda D, Stasnv V, Cunnihgahm HT, Wistuba II, Tomlinson G, Tonk V, Ashfag R, Leitch AM, Minna JD, Shav JW. Characterization of paired tumor and non-tumor cell lines established from patients with breast cancer. Int. J. Cancer. 1998;78:766–774. doi: 10.1002/(sici)1097-0215(19981209)78:6<766::aid-ijc15>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Hoffman AS. The origins and evolution of “controlled” drug delivery systems. J. Control. Release. 2008;132:153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Jaracz S, Chen J, Kuznetsova LV, Ojima I. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg. Med. Chem. 2005;13:5043–5054. doi: 10.1016/j.bmc.2005.04.084. [DOI] [PubMed] [Google Scholar]
- Jumarie C, Mao C. Caco-2 cells cultured in serum-free medium as a model for the study of enterocytic differentiation in vitro. J. Cell. Physiol. 1991;149:24–33. doi: 10.1002/jcp.1041490105. [DOI] [PubMed] [Google Scholar]
- Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest. Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- Liu S, Maheshwari R, Kiick KL. Polymer-based therapeutics. Macromolecules. 2009;42:3–13. doi: 10.1021/ma801782q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubimova JY, Fujita M, Khazenzon NM, Lee BS, Wachsmann-Hogiu S, Farkas DL, Black KL, Holler E. Nanoconjugate based on polymalic acid for tumor targeting. Chem. Biol. Interact. 2008;171:195–203. doi: 10.1016/j.cbi.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nano-sized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009;30:592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Martinez Barbosa ME, Cammas S, Appel M, Ponchel G. Investigation of the degradation mechanisms of poly(malic acid) esters in vitro and their related cytotoxicities on J774 macrophages. Biomacromolecules. 2004;5:137–143. doi: 10.1021/bm0300608. [DOI] [PubMed] [Google Scholar]
- Misra R, Acharya S, Sahoo SK. Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Discov. Today. 2010;15:842–850. doi: 10.1016/j.drudis.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Momparler RL, Karon M, Siegel SE, Avila F. Effect of adriamycin on DNA, RNA and protein synthesis in cell-free systems and intact cells. Cancer Res. 1976;36:2891–2895. [PubMed] [Google Scholar]
- Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cell lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- Osanai S, Nakamura K. Effects of complexation between liposome and poly(malic acid) on aggregation and leakage behaviour. Biomaterials. 2000;21:867–876. doi: 10.1016/s0142-9612(99)00210-0. [DOI] [PubMed] [Google Scholar]
- Rathberger K, Reisner H, Willibald B, Molitoris HP, Holler E. Comparative synthesis and hydrolytic degradation of β-poly(l-malate) by myxomycetes and fungi. Mycol. Res. 1999;103:513–520. [Google Scholar]
- Renard I, Cammas S, Langlois V, Bourbouze R, Guérin Ph. Poly(β-3-methyl malic acid), an hydrolyzable polyester: access to tailor-made derivatives with defined configurational structures and study of in vitro compatibility. Polym. Preprints. 1996;37:137–138. [Google Scholar]
- Romberg B, Hennink WE, Strom G. Sheddable coatings for long-circulating nanoparticles. Pharm. Res. 2008;25:55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenot J, Troutier AL, David L, Delair T, Ladavière C. Steric stabilization of lipid/polymer particle assemblies by poly(ethylene glycol)-lipids. Biomacromolecules. 2007;8:3651–3660. doi: 10.1021/bm700753q. [DOI] [PubMed] [Google Scholar]
- Thioune O, Fessi H, Devissaguet JP, Puisieux F. Preparation of pseudolatex by nanoprecipitation: influence of the solvent nature on intrinsic viscosity and interaction constant. Int. J. Pharm. 1997;146:233–238. [Google Scholar]
- Torchilin VP. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Vert M, Lenz RW. Preparation and properties of poly-beta-malic acid: a functional polyester of potential biomedical importance. Am. Chem. Soc. Div. Polym. Chem. Prepr. 1979;20:608–611. [Google Scholar]
- Yang W, Cheng Y, Xu T, Wang X, Wen LP. Targeting cancer cells with biotin–dendrimer conjugates. Eur. J. Med. Chem. 2009;44:862–868. doi: 10.1016/j.ejmech.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Sun X, Zeng L, Liu J, Zhang Z. A randomized multicenter phase II clinical trial of mitoxantrone-loaded nanoparticles in the treatment of 108 patients with unresected hepatocellular carcinoma. Nanomed. Nanotechnol. Biol. Med. 2009;5:419–423. doi: 10.1016/j.nano.2009.01.009. [DOI] [PubMed] [Google Scholar]