Abstract
Aim
Although weight loss usually decreases very-low-density lipoprotein–triglyceride (VLDL-TG) secretion rate, the change in VLDL-TG kinetics is not directly related to the change in body weight. Circulating leptin also declines with weight loss and can affect hepatic lipid metabolism. The aim of this study was to determine whether circulating leptin is associated with weight loss-induced changes in VLDL-TG secretion.
Methods
Ten extremely obese subjects were studied. VLDL-TG secretion rate and the contribution of systemic (derived from lipolysis of subcutaneous adipose tissue TG) and nonsystemic fatty acids (derived primarily from lipolysis of intrahepatic and intraperitoneal TG, and de novo lipogenesis) to VLDL-TG production were determined by using stable isotopically-labeled tracer methods before and 1 y after gastric bypass surgery.
Results
Subjects lost 33±12% of body weight, and VLDL-TG secretion rate decreased by 46±23% (p=0.001), primarily due to a decrease in the secretion of VLDL-TG from nonsystemic fatty acids (p=0.002). Changes in VLDL-TG secretion rates were not significantly related to reductions in body weight, body mass index, plasma palmitate flux, free fatty acid or insulin concentrations. The change in VLDL-TG secretion was inversely correlated with the change in plasma leptin concentration (r=−0.72, p=0.013), due to a negative association between changes in leptin and VLDL-TG secretion from nonsystemic fatty acids (r=−0.95, p<0.001).
Conclusions
Weight loss-induced changes in plasma leptin concentration are inversely associated with changes in VLDL-TG secretion rate. Additional studies are needed to determine whether the correlation between circulating leptin and VLDL-TG secretion represents a cause-and-effect relationship.
Keywords: tracers, VLDL, lipoprotein, liver, adipokines
INTRODUCTION
An increase in plasma triglyceride (TG) concentration is associated with obesity and is an important risk factor for cardiovascular disease [1]. Very-low-density lipoprotein (VLDL) is the major carrier of TG in plasma during postabsorptive conditions, and hypertriglyceridemia associated with obesity is primarily due to an increase in hepatic VLDL-TG secretion rate [2–4]. In general, weight loss decreases VLDL-TG secretion and plasma TG concentrations [5–9]. However, a direct relationship between the amount of weight lost and the decline in VLDL-TG secretion rate has not been detected. A moderate 10–15% diet-induced decrease in body weight is associated with considerable variability in the change in VLDL-TG kinetics, from a slight increase to a decline of up to 80% in VLDL-TG secretion rate [5,6,9,10]. In addition, a similar 40–50% reduction in VLDL-TG secretion rate has been reported after both moderate (~10%) [7] and marked (~25%) [8] weight loss. These observations suggest that weight loss-induced alterations in hepatic VLDL-TG secretion rate are regulated by other factors than simply weight loss alone.
Leptin, the protein product of the ob/ob gene, has pleotropic neuroendocrine effects and is likely involved in the regulation of hepatic TG metabolism [11]. Data from studies conducted in animal models demonstrate that leptin administration lowers plasma TG and VLDL-TG concentrations [12–15], reduces intrahepatic TG content [13–17] and suppresses VLDL-TG synthesis and secretion [13,15,16], independently of its effects on food intake and body weight. Moreover, leptin replacement in leptin-deficient, lipodystrophic animals [18] and human subjects [19,20] markedly reduces hepatic TG content and plasma TG concentrations. In contrast, many obese persons have increased intrahepatic TG content, plasma TG concentrations and VLDL-TG secretion rates [2], despite increased plasma leptin concentrations [21,22]. The dissociation between leptin concentration and its purported action in obese persons has been attributed to “leptin resistance” and attenuated hepatic leptin signaling [23–25]. For instance, leptin reduces intrahepatic TG content in lean but not in diet-induced obese animals [25]. Weight loss reduces plasma leptin concentrations [21,22] and presumably restores hepatic leptin sensitivity [11,13,14,17,23].
The purpose of the present study was to evaluate the relationship between weight loss-induced changes in hepatic VLDL-TG secretion rate and changes in circulating leptin. We hypothesized that the decline in VLDL-TG secretion rate would correlate directly with the decline in circulating leptin. VLDL-TG secretion was determined in vivo in morbidly obese subjects before and 1 y after Roux-en-Y gastric bypass (RYGBP) surgery, because these subjects usually experience a large range in treatment-induced body weight loss and VLDL kinetics [8].
METHODS
Subjects
Ten extremely obese subjects (body mass index [BMI] > 40 kg/m2; 9 women and 1 man, aged 42 ± 11 y) who were scheduled to undergo RYGBP surgery at Barnes-Jewish Hospital, St. Louis, MO, participated in the study. All subjects completed a comprehensive medical evaluation, including a 2-hour oral glucose tolerance test (OGTT). Two subjects were newly diagnosed as having type 2 diabetes mellitus based on the results of the OGTT, but neither was being treated with diabetes medications. Subjects were excluded if they had any history or evidence of liver disease, consumed ≥ 20 g alcohol per day, had severe fasting hypertriglyceridemia (≥ 400 mg/dL), or were taking medications known to affect hepatic metabolic function or plasma lipid metabolism. All subjects gave their written informed consent before participating in this study, which was approved by the Human Studies Committee and the Center for Applied Research Sciences Advisory Committee of Washington University School of Medicine in St. Louis, MO.
Experimental protocol
Subjects were instructed to consume their normal diet for at least three days before being admitted to the Clinical Research Unit (CRU) in the afternoon before the isotope tracer infusion study, which was performed two days before RYBGP surgery. At 1900 h, they consumed a standard meal containing 12 kcal/kg adjusted body weight, which contained 55% of total energy as carbohydrate, 30% as fat, and 15% as protein. Adjusted body weight was calculated as ideal body weight (the midpoint of the medium frame of the Metropolitan Life Insurance Company Table) + 0.25 × (actual body weight – ideal body weight). Thereafter, subjects fasted until completion of the isotope infusion study the next day. At 0500 h the following morning, after subjects fasted overnight, one catheter was inserted into a forearm vein to administer stable isotopically-labeled tracers, and a second catheter was inserted into a vein in the contralateral hand, which was heated to 55°C by using a thermostatically-controlled box, to obtain arterialized blood samples. At 0600 h, a bolus of [1,1,2,3,3-2H5]glycerol (75 μmol/kg; Cambridge Isotope Laboratories, Andover, MA), dissolved in 0.9% NaCl solution, was administered, and a constant infusion of [2,2-2H2]palmitate (0.024 μmol/kg·min; Cambridge Isotope Laboratories, Andover, MA) bound to human albumin, was started and maintained for 12 h.
Blood samples were obtained immediately before starting the tracer infusion to determine plasma substrate and hormone concentrations and background substrate tracer-to-tracee ratios (TTR), and at 5, 15, 30, 60, 90, and 120 min and then every hour for 10 h to determine glycerol and palmitate TTR in plasma and in VLDL-TG. Blood was immediately placed in chilled tubes containing sodium EDTA to determine substrate concentrations and TTRs, and in chilled tubes containing EDTA and aprotinin (Trasylol) to determine plasma concentrations of leptin and insulin. All samples were immediately put in an ice bath, and plasma was separated by centrifugation within 30 min of collection. Aliquots of plasma were kept in the refrigerator to isolate VLDL, and the remaining plasma samples were stored at −80°C until additional analyses were performed. We have found that, under these conditions, ex vivo lipolysis of plasma TG is not detectable [26,27].
Two days after the tracer infusion study was performed, subjects underwent RYGBP surgery. Six subjects had open and four had laparoscopic procedures. Both procedures involved constructing a small proximal gastric pouch by stapling across the stomach. The Roux limb was formed by transecting the jejunum 30 cm distal to the ligament of Treitz and creating a jejunostomy distal to the transection; a 75-cm limb was constructed for subjects with a BMI < 50 kg/m2 and a 150-cm limb was constructed for those with a BMI ≥ 50 kg/m2. All procedures were performed by the same surgeon (JCE).
One year after RYGBP surgery, subjects were readmitted to the CRU. The same studies performed before surgery were repeated.
Sample analyses
Plasma insulin and leptin concentrations were measured by using ELISA (Diagnostic Systems Laboratories, Fremont, CA). The intra-assay and inter-assay coefficients of variation for leptin measurements were 3.8% and 4.4%, respectively. Plasma free fatty acid (FFA) concentrations were quantified by using gas chromatography (HP 5890 Series II GC, Hewlett-Packard, Palo Alto, CA) after adding heptadecanoic acid to plasma as an internal standard [28]. VLDL-TG concentrations were determined by using a colorimetric enzymatic kit (SIGMA Chemicals, St. Louis, MO) [29]. Samples obtained before and after surgery were analyzed simultaneously.
The VLDL fraction was isolated by centrifugation and tube slicing as previously described [7], and samples were stored at −80°C until final analyses were performed. Glycerol and palmitate TTRs in plasma and VLDL-TG were determined by using gas chromatography – mass spectrometry (Agilent Technologies/HP 6890 Series GC System – 5973 Mass Selective Detector, Hewlett-Packard, Palo Alto, CA), as previously described [8,29,30].
The total analytical variability of measuring plasma FFA and VLDL-TG concentrations by these methods is 3.6% and 4.9%, respectively, whereas the analytical variability of measuring the TTR of free palmitate and glycerol in plasma, and of palmitate and glycerol bound to VLDL-TG is 1.3%, 7.8%, 4.0%, and 4.0%, respectively [29].
Calculations
The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as the product of fasting plasma insulin (in mIU/L) and glucose (in mmol/L) concentrations divided by 22.5 [31].
Palmitate rate of appearance (Ra) in plasma, which provides a reliable index of total FFA Ra [32], was calculated by dividing the palmitate tracer infusion rate by the average plasma palmitate TTR value between 60 and 240 min.
The fractional turnover rate (FTR) of VLDL-TG was determined by fitting the TTR time-courses of free glycerol in plasma and glycerol in VLDL-TG to a multicompartmental model [29,30,33]. The rate of hepatic VLDL-TG secretion (in μmol per liter plasma per min), which represents the amount of VLDL-TG secreted by the liver per unit of plasma, was calculated by multiplying the FTR of VLDL-TG (in % per min) by the steady-state concentration of VLDL-TG (in μmol/L) in plasma [8,30].
The proportion of fatty acids within VLDL-TG derived from systemic plasma FFA (generated by lipolysis of subcutaneous adipose tissue TG) and nonsystemic fatty acids (generated by lipolysis of intrahepatic and intraperitoneal TG, hepatic lipolysis of circulating TG, and de novo hepatic fatty acid synthesis) were calculated by accounting for isotopic dilution between plasma and VLDL-TG palmitate by using a multicompartmental model [7,8,30,33]. The absolute secretion rates of VLDL-TG from systemic and nonsystemic fatty acids were then calculated by multiplying total VLDL-TG secretion rate by the relative contribution from each source of fatty acids [7,8,30].
Statistical analyses
All data sets were tested for normality according to the Anderson-Darling procedure; not normally distributed variables were log-transformed for analysis. Data are presented as means ± standard deviation (s.d.) for normally distributed variables and means with 95% confidence interval (c.i.) for not normally distributed variables. Results before and after surgery were compared with Student’s two-tailed t test for paired samples. Pearson’s correlation analysis was used to examine associations between variables of interest. A p-value < 0.05 was considered statistically significant.
RESULTS
At 1 y after RYGBP surgery, subjects lost an average of 33 ± 12% (52 ± 22 kg) of their initial body weight; range 16–50% (25–91 kg) (Table 1). Surgery-induced weight loss resulted in a decrease in fasting plasma glucose, insulin, leptin, FFA, and VLDL-TG concentrations (Table 1). Mean HOMA-IR value decreased from 10.8 (6.5, 17.9) at baseline to 2.0 (0.8, 5.4) after surgery (p = 0.004).
Table 1.
Body mass and metabolic profile of the study participants at baseline and 1 year after gastric bypass surgery
| Before surgery | After surgery | p-value | |
|---|---|---|---|
|
|
|||
| Body weight (kg) | 158 ± 27 | 106 ± 28 | < 0.001 |
| Body mass index (kg/m2) | 58 ± 11 | 39 ± 11 | < 0.001 |
| Glucose (mmol/L) | 6.3 ± 1.5 | 4.6 ± 0.4 | 0.013 |
| Free fatty acids (μmol/L) | 562 ± 110 | 454 ± 87 | 0.027 |
| VLDL-triglyceride (mmol/L) | 0.64 (0.38, 1.08) | 0.39 (0.21, 0.71) | 0.021 |
| Insulin (mIU/L) | 39 (24, 62) | 10 (4, 26) | 0.007 |
| Leptin (ng/mL) | 126 ± 47 | 56 ± 39 | 0.001 |
Data are means ± s.d. or means with 95% c.i.
Total palmitate Ra decreased by 44 ± 14% at 1 y after surgery (102 ± 24 vs. 190 ± 56 μmol/min; p < 0.001). Palmitate Ra was still lower after surgery-induced weight loss after adjusting for the change in weight, by expressing the data per kg of body weight (0.97 ± 0.15 vs. 1.21 ± 0.34 μmol/kg·min, respectively; p = 0.035).
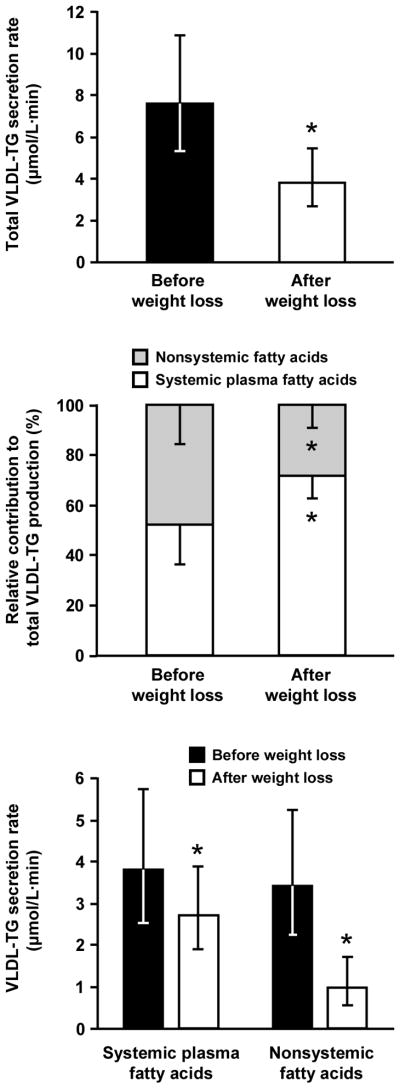
The rate of VLDL-TG secretion was ~50% lower 1 y after surgery than at baseline (p = 0.001) (Figure 1). Approximately two-thirds of the decline in VLDL-TG secretion rate was accounted for by a decrease in the contribution of nonsystemic fatty acids to VLDL-TG (3.4 [2.2–5.2] vs. 1.0 [0.5–1.7] μmol/L·min before and after weight loss, respectively; p = 0.002), whereas about one-third of the decline in VLDL-TG secretion rate was accounted for by a decrease in the contribution of systemic plasma FFA to VLDL-TG (3.8 [2.5–5.7] vs. 2.7 [1.9–3.9] μmol/L·min before and after weight loss, respectively; p = 0.043) (Figure 1). Therefore, the relative contribution of fatty acids derived from systemic and nonsystemic sources to total VLDL-TG production was different after than before surgery; nonsystemic fatty acids accounted for 48 ± 16% and 28 ± 9% of newly secreted VLDL-TG before and 1 y after surgery, respectively (p = 0.004) (Figure 1).
Figure 1.
Total very-low-density lipoprotein–triglyceride (VLDL-TG) secretion rate (top panel), relative contribution from systemic and nonsystemic fatty acids to total VLDL-TG production (middle panel), and absolute VLDL-TG secretion rates from systemic and nonsystemic fatty acids (bottom panel) before and 1 year after Roux-en-Y gastric bypass surgery. Data are means ± s.d. for the relative contribution of different fatty acid sources to total VLDL-TG production and means with 95% c.i. for absolute VLDL-TG secretion rates. * Significantly different from value before weight loss, p < 0.05.
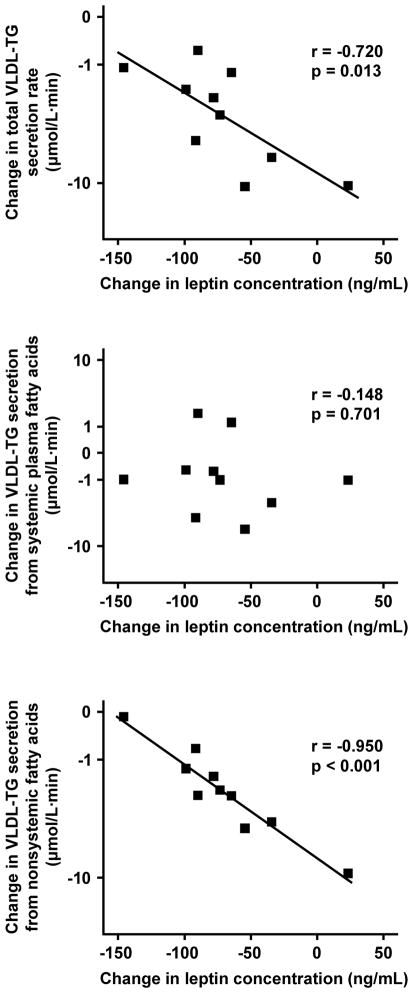
Changes in VLDL-TG secretion rates that occurred 1 y after RYGBP surgery were not significantly associated with changes in body weight, BMI, plasma FFA, glucose and insulin concentrations, HOMA-IR values, or palmitate Ra (all p-values > 0.05). However, the weight loss-induced change in total VLDL-TG secretion rate was inversely related to the change in plasma leptin concentration (r = −0.72, p = 0.013, R2 = 52%) (Figure 2), which was entirely due to a strong, inverse association between plasma leptin concentration and secreted VLDL-TG derived from nonsystemic fatty acids (r = −0.95, p < 0.001, R2 = 90%) (Figure 2). No significant relationship was detected between the change in VLDL-TG derived from systemic plasma FFA and the change in plasma leptin concentrations (r = 0.148, p = 0.701).
Figure 2.
Relationship between weight loss-induced changes in plasma leptin concentration and corresponding changes in total very-low-density lipoprotein–triglyceride (VLDL-TG) secretion rate (top panel), VLDL-TG secretion from systemic plasma fatty acids (middle panel), and VLDL-TG secretion from nonsystemic fatty acids (bottom panel). The scale for VLDL-TG secretion rates is log-transformed.
DISCUSSION
Weight loss is associated with a decline in hepatic VLDL-TG secretion rate which contributes to the decrease in plasma TG concentration. However, the decline in VLDL-TG secretion rate does not directly correlate with the decrease in body weight. In this study, we evaluated whether changes in plasma leptin concentration after RYGBP surgery-induced weight loss was associated with changes in hepatic VLDL-TG secretion rate. Our data demonstrate that the change in circulating leptin that occurs after RYGBP surgery-induced weight loss is inversely correlated with the change in VLDL-TG production; the smaller the reduction in plasma leptin, the greater the reduction in hepatic VLDL-TG secretion rate. Moreover, this relationship was entirely due to a strong inverse correlation between changes in plasma leptin and the contribution of nonsystemic fatty acids, presumably derived from lipolysis of visceral and intrahepatic TG stores and de novo hepatic lipogenesis, to VLDL-TG production. These data suggest that leptin is involved in regulating the decline in hepatic VLDL-TG secretion that occurs with weight loss, but additional mechanistic studies are needed to confirm this hypothesis.
In general, weight loss in obese subjects causes a decline in hepatic VLDL-TG secretion rate [5–8], which is almost entirely due to a decrease in the contribution of nonsystemic fatty acids to VLDL-TG production [7,8]. However, considerable variability has been reported in the response of VLDL-TG kinetics to similar reductions in body weight, and the change in VLDL-TG secretion has not been associated with corresponding changes in body weight [5,6,9,10]. We were also unable to demonstrate a relationship between changes in body weight and changes in VLDL-TG secretion rate in our group of extremely obese subjects. Although plasma insulin and FFA are important regulators of VLDL-TG metabolism in vivo [34–37], we did not detect a relationship between changes in fatty acid flux, plasma FFA concentrations, plasma insulin concentrations, or insulin sensitivity (assessed by HOMA-IR) and changes in VLDL-TG secretion rate. All our subjects exhibited an increase in insulin sensitivity, as determined by HOMA-IR, which could have contributed to the decline in VLDL-TG secretion after weight loss, even though there was not a direct correlation between the two. The effect of leptin on VLDL-TG metabolism is likely independent of insulin action. Data from studies conducted in rodents demonstrate that the reduction in intrahepatic fat content and VLDL-TG secretion rate induced by leptin are independent of hepatic insulin sensitivity [17] and the effects of insulin on the same metabolic pathways [15]. These observations and our data suggest that substrate availability and insulin sensitivity are not important regulators of VLDL-TG kinetics after weight loss. In contrast, we found a remarkably close correlation between changes in plasma leptin concentration and the hepatic secretion rate of VLDL-TG comprised of nonsystemic fatty acids. In fact, the subject who had the greatest decrease in plasma leptin concentration had a very minimal change in the rate of VLDL-TG secretion from nonsystemic fatty acids, despite losing 65 kg of weight, whereas VLDL-TG secretion from nonsystemic fatty acids was almost completely suppressed in the subject who had a small increase in plasma leptin concentration. Our findings underscore the need for additional studies to elucidate the role of leptin in VLDL metabolism in human subjects.
The results from our study cannot determine whether the correlation we observed between changes in plasma leptin concentration and the secretion of VLDL-TG represents a cause-and-effect relationship or is simply an association. Data from studies conducted in both animal models and human subjects support the notion that circulating leptin is actively involved in regulating VLDL-TG production from nonsystemic fatty acids. First, leptin deficiency in both animals [38] and humans [39] is associated with an increase in intrahepatic TG content, which is likely an important source of nonsystemic fatty acids for VLDL-TG production [40]. Second, leptin administration in rodents with steatosis [13–18] and patients with lipodystrophy [19,20] decreases intrahepatic TG content, possibly by stimulating hepatic fatty acid oxidation [13,15] and decreasing de novo lipogenesis [41]. Third, leptin administration decreases VLDL-TG secretion rate and plasma TG concentration in animals [13,15,16], and leptin administration during weight loss therapy in obese men and women causes a more pronounced reduction in plasma TG concentrations than weight loss alone [42,43]. In contrast, obesity is associated with high plasma leptin concentrations, increased intrahepatic TG content and increased VLDL-TG secretion rates [2,21,22], suggestive of hepatic leptin resistance. The summation of these data and the findings from our study imply that weight loss increases hepatic leptin sensitivity, [11,13,14,17,23–25,44], which in turn increases the influence of leptin in regulating VLDL-TG metabolism in obese subjects. Therefore, the higher the plasma leptin concentration after weight loss the greater the decrease in VLDL-TG secretion rate. However, the metabolic effects of circulating leptin represent the combination of leptin concentration and sensitivity, so that accounting for differences in leptin sensitivity among our subjects could have influenced the relationship we detected between leptin concentration and VLDL-TG kinetics.
In summary, weight loss induced by RYGBP surgery causes a variable decline in hepatic VLDL-TG secretion rate that is not correlated with the amount of weight loss itself. The data from the present study demonstrate that the decrease in VLDL-TG secretion is almost entirely due to a decrease in the contribution of nonsystemic fatty acids to VLDL-TG production, which is strongly and inversely associated with changes in plasma leptin concentration. These results underscore the potential importance of circulating leptin in the regulation of weight-loss induced changes in hepatic lipoprotein metabolism; however, additional studies are needed to determine the mechanisms responsible for the link between leptin and VLDL-TG secretion after weight loss.
Acknowledgments
This study was supported by National Institutes of Health grants DK-37948, DK-56341 (Clinical Nutrition Research Unit), UL1 RR024992 (Clinical and Translational Science Award), and RR-00954 (Biomedical Mass Spectrometry Resource), and a grant from the American Heart Association (0510015Z).
The authors thank Donna Marin, Freida Custodio, and Junyoung Kwon for technical assistance, the nursing staff of the Clinical Research Unit and Barnes-Jewish Hospital Surgery Unit for their help in performing the studies, and the study subjects for their participation.
Footnotes
Author contributions: FM and SK designed research; EF, JMC, and JCE performed research; FM, EF, JMC, and BWP processed samples and analyzed data; FM and SK interpreted data and wrote the paper.
The authors have no conflicts of interest associated with the content of this manuscript.
References
- 1.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–219. [PubMed] [Google Scholar]
- 2.Grundy SM. Metabolic complications of obesity. Endocrine. 2000;13:155–165. doi: 10.1385/ENDO:13:2:155. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Mok HY, Zech L, Steinberg D, Berman M. Transport of very low density lipoprotein triglycerides in varying degrees of obesity and hypertriglyceridemia. J Clin Invest. 1979;63:1274–1283. doi: 10.1172/JCI109422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittendorfer B, Patterson BW, Klein S. Effect of sex and obesity on basal VLDL-triacylglycerol kinetics. Am J Clin Nutr. 2003;77:573–579. doi: 10.1093/ajcn/77.3.573. [DOI] [PubMed] [Google Scholar]
- 5.Olefsky J, Reaven GM, Farquhar JW. Effects of weight reduction on obesity. Studies of lipid and carbohydrate metabolism in normal and hyperlipoproteinemic subjects. J Clin Invest. 1974;53:64–76. doi: 10.1172/JCI107560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginsberg HN, Le NA, Gibson JC. Regulation of the production and catabolism of plasma low density lipoproteins in hypertriglyceridemic subjects. Effect of weight loss. J Clin Invest. 1985;75:614–623. doi: 10.1172/JCI111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittendorfer B, Patterson BW, Klein S. Effect of weight loss on VLDL-triglyceride and apoB-100 kinetics in women with abdominal obesity. Am J Physiol Endocrinol Metab. 2003;284:E549–556. doi: 10.1152/ajpendo.00379.2002. [DOI] [PubMed] [Google Scholar]
- 8.Klein S, Mittendorfer B, Eagon JC, et al. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1564–1572. doi: 10.1053/j.gastro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 9.Streja DA, Marliss EB, Steiner G. The effects of prolonged fasting on plasma triglyceride kinetics in man. Metabolism. 1977;26:505–516. doi: 10.1016/0026-0495(77)90094-4. [DOI] [PubMed] [Google Scholar]
- 10.Jourdan M, Margen S, Bradfield RB. The turnover rate of serum glycerides in the lipoproteins of fasting obese women during weight loss. Am J Clin Nutr. 1974;27:850–858. doi: 10.1093/ajcn/27.8.850. [DOI] [PubMed] [Google Scholar]
- 11.Cohen P, Friedman JM. Leptin and the control of metabolism: role for stearoyl-CoA desaturase-1 (SCD-1) J Nutr. 2004;134:2455S–2463S. doi: 10.1093/jn/134.9.2455S. [DOI] [PubMed] [Google Scholar]
- 12.Chen G, Koyama K, Yuan X, et al. Disappearance of body fat in normal rats induced by adenovirus-mediated leptin gene therapy. Proc Natl Acad Sci U S A. 1996;93:14795–14799. doi: 10.1073/pnas.93.25.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang W, Dedousis N, Bandi A, Lopaschuk GD, O’Doherty RM. Liver triglyceride secretion and lipid oxidative metabolism are rapidly altered by leptin in vivo. Endocrinology. 2006;147:1480–1487. doi: 10.1210/en.2005-0731. [DOI] [PubMed] [Google Scholar]
- 14.Huang W, Dedousis N, O’Doherty RM. Hepatic steatosis and plasma dyslipidemia induced by a high sucrose diet are corrected by an acute leptin infusion. J Appl Physiol. 2007;102:2260–2265. doi: 10.1152/japplphysiol.01449.2006. [DOI] [PubMed] [Google Scholar]
- 15.Huang W, Metlakunta A, Dedousis N, Ortmeyer HK, Stefanovic-Racic M, O’Doherty RM. Leptin augments the acute suppressive effects of insulin on hepatic very low density lipoprotein (VLDL) production in rats. Endocrinology. 2009;150:2169–2174. doi: 10.1210/en.2008-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen P, Miyazaki M, Socci ND, et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 17.Fishman S, Muzumdar RH, Atzmon G, et al. Resistance to leptin action is the major determinant of hepatic triglyceride accumulation in vivo. FASEB J. 2007;21:53–60. doi: 10.1096/fj.06-6557com. [DOI] [PubMed] [Google Scholar]
- 18.Nagao K, Inoue N, Ujino Y, et al. Effect of leptin infusion on insulin sensitivity and lipid metabolism in diet-induced lipodystrophy model mice. Lipids Health Dis. 2008;7:8. doi: 10.1186/1476-511X-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebihara K, Kusakabe T, Hirata M, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2007;92:532–541. doi: 10.1210/jc.2006-1546. [DOI] [PubMed] [Google Scholar]
- 21.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 22.Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab. 1996;81:4406–4413. doi: 10.1210/jcem.81.12.8954050. [DOI] [PubMed] [Google Scholar]
- 23.Brabant G, Muller G, Horn R, Anderwald C, Roden M, Nave H. Hepatic leptin signaling in obesity. FASEB J. 2005;19:1048–1050. doi: 10.1096/fj.04-2846fje. [DOI] [PubMed] [Google Scholar]
- 24.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Dedousis N, Bhatt BA, O’Doherty RM. Impaired activation of phosphatidylinositol 3-kinase by leptin is a novel mechanism of hepatic leptin resistance in diet-induced obesity. J Biol Chem. 2004;279:21695–21700. doi: 10.1074/jbc.M401546200. [DOI] [PubMed] [Google Scholar]
- 26.Krebs M, Stingl H, Nowotny P, et al. Prevention of in vitro lipolysis by tetrahydrolipstatin. Clin Chem. 2000;46:950–954. [PubMed] [Google Scholar]
- 27.Zambon A, Hashimoto SI, Brunzell JD. Analysis of techniques to obtain plasma for measurement of levels of free fatty acids. J Lipid Res. 1993;34:1021–1028. [PubMed] [Google Scholar]
- 28.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- 29.Magkos F, Patterson BW, Mittendorfer B. Reproducibility of stable isotope labeled tracer measures of VLDL-triglyceride and VLDL-apolipoprotein B-100 kinetics. J Lipid Res. 2007;48:1204–1211. doi: 10.1194/jlr.D600048-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. J Clin Endocrinol Metab. 2007;92:1311–1318. doi: 10.1210/jc.2006-2215. [DOI] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes. 2003;52:1641–1648. doi: 10.2337/diabetes.52.7.1641. [DOI] [PubMed] [Google Scholar]
- 33.Patterson BW, Mittendorfer B, Elias N, Satyanarayana R, Klein S. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J Lipid Res. 2002;43:223–233. [PubMed] [Google Scholar]
- 34.Lewis GF. Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol. 1997;8:146–153. doi: 10.1097/00041433-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Gibbons GF, Brown AM, Wiggins D, Pease R. The roles of insulin and fatty acids in the regulation of hepatic very-low-density lipoprotein assembly. J R Soc Med. 2002;95 (Suppl 42):23–32. [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis GF, Uffelman KD, Szeto LW, Steiner G. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes. 1993;42:833–842. doi: 10.2337/diab.42.6.833. [DOI] [PubMed] [Google Scholar]
- 37.Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95:158–166. doi: 10.1172/JCI117633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasty AH, Shimano H, Osuga J, et al. Severe hypercholesterolemia, hypertriglyceridemia, and atherosclerosis in mice lacking both leptin and the low density lipoprotein receptor. J Biol Chem. 2001;276:37402–37408. doi: 10.1074/jbc.M010176200. [DOI] [PubMed] [Google Scholar]
- 39.Reeds DN, Mittendorfer B, Patterson BW, Powderly WG, Yarasheski KE, Klein S. Alterations in lipid kinetics in men with HIV-dyslipidemia. Am J Physiol Endocrinol Metab. 2003;285:E490–497. doi: 10.1152/ajpendo.00118.2003. [DOI] [PubMed] [Google Scholar]
- 40.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen SM, Werrmann JG, Tota MR. 13C NMR study of the effects of leptin treatment on kinetics of hepatic intermediary metabolism. Proc Natl Acad Sci U S A. 1998;95:7385–7390. doi: 10.1073/pnas.95.13.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hukshorn CJ, Saris WH, Westerterp-Plantenga MS, Farid AR, Smith FJ, Campfield LA. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab. 2000;85:4003–4009. doi: 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- 43.Hukshorn CJ, Westerterp-Plantenga MS, Saris WH. Pegylated human recombinant leptin (PEG-OB) causes additional weight loss in severely energy-restricted, overweight men. Am J Clin Nutr. 2003;77:771–776. doi: 10.1093/ajcn/77.4.771. [DOI] [PubMed] [Google Scholar]
- 44.Unger RH. The physiology of cellular liporegulation. Annu Rev Physiol. 2003;65:333–347. doi: 10.1146/annurev.physiol.65.092101.142622. [DOI] [PubMed] [Google Scholar]