Abstract
BACKGROUND
Low platelet count is a marker of portal hypertension but is not routinely included in the standard preoperative evaluation of patients with hepatocellular carcinoma (HCC) because it pertains to liver function (Child/model for end-stage liver disease [MELD] score) and tumor burden (Milan criteria). We hypothesized that low platelet count would be independently associated with increased perioperative morbidity and mortality after resection.
STUDY DESIGN
Patients treated with liver resection for HCC between January 2000 and January 2010 at 3 institutions were eligible. Preoperative platelet count, Child/MELD score, and tumor extent were recorded. Low preoperative platelet count (LPPC) was defined as <150 × 103/μL. Postoperative liver insufficiency (PLI) was defined as peak bilirubin >7 mg/dL or development of ascites. Univariate and multivariate regression was performed for predictors of major complications, PLI, and 60-day mortality.
RESULTS
A total of 231 patients underwent resection, of whom 196 (85%) were classified as Child A and 35 (15%) as Child B; median MELD score was 8. Overall, 168 (71%) had tumors that exceeded Milan criteria and 134 (58%) had major hepatectomy (≥3 Couinaud segments). Overall and major complication rates were 55% and 17%, respectively. PLI occurred in 25 patients (11%), and 21 (9%) died within 60 days of surgery. Patients with LPPC (n = 50) had a significantly increased number of major complications (28% versus 14%, p = 0.031), PLI (30% versus 6%, p = 0.001), and 60-day mortality (22% versus 6%, p = 0.001). When adjusted for Child/MELD score and tumor burden, LPPC remained independently associated with increased number of major complications (odds ratio [OR] 2.8, 95% confidence intervals [CI] 1.1 to 6.8, p = 0.026), PLI (OR 4.0, 95% CI 1.4 to 11.1, p = 0.008), and 60-day mortality (OR 4.6, 95% CI 1.5 to 14.6, p = 0.009).
CONCLUSIONS
LPPC is independently associated with increased major complications, PLI, and mortality after resection of HCC, even when accounting for standard criteria, such as Child/MELD score and tumor extent, used to select patients for resection. Patients with LPPC may be better served with transplantation or liver-directed therapy.
Hepatocellular carcinoma (HCC) represents the third leading cause of cancer mortality worldwide.1 The rising incidence in the US mirrors the increasing prevalence of viral hepatitis C infection.2,3 There are a variety of treatment options for HCC, of which resection remains at the forefront, especially for patients with normal livers or well-compensated cirrhosis.4 In some areas, resection is the mainstay of surgical treatment because of limited organ availability for transplantation. Within the last 2 decades, outcomes after liver resection have substantially improved owing to refinements in surgical technique, perioperative care, and improvements in patient selection.5,6 However, postoperative outcomes after HCC resection still vary substantially among series. A recent nationwide in-patient sample study reported an overall in-hospitality mortality rate of 6.5% after resection of HCC.7
When patients are selected for resection of HCC, it is essential to consider both the extent of tumor burden and liver function to optimize outcomes. Common tumor factors include size and number of lesions and whether there is radiographic evidence of major vascular invasion. These variables, which comprise the Milan criteria for transplantation, help determine appropriateness for resection primarily from an oncologic standpoint.8–10 The status of liver function is assessed by evaluating various parameters. Abnormal serum values of bilirubin, albumin, and international normalized ratio (INR) are common indicators of liver dysfunction. The Child-Turcotte-Pugh and unadjusted model for end-stage liver disease (MELD) scores are assessment tools frequently used to determine liver function. Other measures include indocyanine green (ICG) clearance, which is more frequently used in Asia.11 The presence of portal hypertension is also indicative of poor liver function and may be associated with postoperative liver insufficiency (PLI) and increased morbidity.12 Radiographic evidence of portal hypertension includes the presence of varices and splenomegaly. Additionally, a low platelet count serves as a noninvasive indicator of portal hypertension. However, the value of a low preoperative platelet count to predict perioperative outcomes is not well defined in patients undergoing resection for HCC.
The aim of this study was to assess the value of a low preoperative platelet count in predicting perioperative outcomes after liver resection for HCC in the current era of liver surgery. We hypothesized that a low platelet count would be independently associated with increased postoperative morbidity and mortality, even when accounting for standard criteria, such as Child/MELD score and extent of tumor burden, used to select patients for resection.
METHODS
After individual IRB approval was obtained at each institution, a retrospective analysis of prospectively maintained databases from 3 hepatobiliary centers was performed for patients who underwent liver resection for HCC between January 2000 and January 2010. Anatomic and nonanatomic resections of at least 1 segment performed with curative intent were included. Preoperative portal vein embolization was performed selectively per individual surgeon preference based on the estimated future liver remnant. The target future liver remnant ranged from 25% to 40% depending on the degree of liver dysfunction. Operative liver biopsies, solely ablative procedures, and transplant procedures were excluded. All institutions provided preoperative, operative, and postoperative data as specified through a common menu-driven database file.
Assessment of preoperative liver function
Preoperative Child score was calculated for each patient based on preoperative data according to the method described by Pugh and colleagues13 in 1973. Scores were generated by assigning points for serum levels of total bilirubin, albumin, and INR, and the presence of ascites or hepatic encephalopathy. Unadjusted MELD score was calculated from preoperative levels of total bilirubin, INR, and serum creatinine.14 The most recent preoperative platelet count was recorded for each patient.
Operative and pathologic details
Operative factors including estimated blood loss, type of resection (anatomic versus nonanatomic), extent of resection (major versus minor hepatectomy), and use of intraoperative RBC transfusion were recorded. Major hepatectomy was defined as any resection of 3 or more Couinaud segments.
Pathology reports were reviewed to assess the extent of tumor burden, including number and size of lesions, margin status, and presence of microvascular and macrovascular invasion. The tumor burden was classified according to whether or not it fulfilled Milan criteria.15
Perioperative outcome
Length of hospital stay and postoperative complications were recorded. Severity of complications was graded using a validated classification scheme as described by Dindo and colleagues.16 Accordingly, major complications were defined as any complications requiring surgical or radiologic intervention, life-threatening organ failure, or death.
Postoperative liver function was mainly assessed by peak levels of serum total bilirubin and INR and development of ascites (drain output exceeding 500 mL per 24 hours or clinical evidence of development of ascites). PLI was defined as a peak total bilirubin level greater than 7 mg/dL and/or development of ascites.17 Coagulation tests were not used in our definition because of high rates of perioperative plasma transfusions, which made interpretation of the INR value difficult. Postoperative mortality was defined as death within 60 days of surgery.
Statistical analysis
Receiver–operating characteristic analysis for the outcome of PLI was used to determine the most appropriate cut-off point for preoperative platelet count to define a low platelet count. The distributions of clinicopathologic characteristics and perioperative outcomes between patients with high and low preoperative platelet counts were compared using chi-square or Fisher exact test for categoric variables and Student’s t-test for continuous variables. Univariate and multivariate binary logistic regression analyses were performed with preoperative variables for 3 specific end points: major complications, PLI, and 60-day mortality. Variables demonstrating association with each end point to the level of p ≤ 0.1 on univariate analysis were entered into a multivariate model. A modified Child score that incorporated preoperative platelet count (<100/nL, 100 to 149/nL, and ≥ 150/nL) in place of encephalopathy was calculated and tested in a multivariate regression model for the same 3 end points of major complications, PLI, and 60-day mortality. Statistical significance was defined as p < 0.05. Data were analyzed using SPSS (version 17.0 for Windows, SPSS, Inc).
RESULTS
Patient characteristics and assessment of preoperative liver function
A summary of clinical and pathology data for 231 patients who underwent hepatic resection of HCC at 3 institutions is shown in Table 1. Median age at operation was 64 years (range 19 to 89 years), and there was a male predominance (65%). The median Child score was 5 (range 5 to 9), and 85% of patients (n = 196) were classified as Child A and 15% (n = 35) as Child B. The median unadjusted MELD score was 8 (range 6 to 32). The etiology of liver disease was documented as alcohol abuse in 44 patients (20%), chronic hepatitis B infection in 26 (11%), and hepatitis C infection in 53 patients (23%). Three patients were infected with both hepatitis B and C viruses.
Table 1.
Clinicopathologic Data for Patients Undergoing Liver Resection of Hepatocellular Carcinoma (N = 231)
| Clinical variable | n | % | Median (range) |
|---|---|---|---|
| Age, y | 64 (19–89) | ||
| Sex | |||
| Female | 80 | 35 | |
| Male | 151 | 65 | |
| Diabetes mellitus | 63 | 27 | |
| ASA* | |||
| 2 | 70 | 30 | |
| 3 | 147 | 64 | |
| 4 | 13 | 6 | |
| Preoperative platelet count, per nL | 228 (30–747) | ||
| ≥150 | 181 | 78 | |
| 100–149 | 29 | 13 | |
| <100 | 21 | 9 | |
| Splenomegaly | 18 | 8 | |
| Hepatic encephalopathy | 3 | 1 | |
| Preoperative ascites | |||
| Mild | 22 | 10 | |
| Severe | 5 | 2 | |
| Preoperative bilirubin, mg/dL | 0.7 (0.1–8.4) | ||
| Preoperative albumin, g/dL | 3.7 (1.6–5.6) | ||
| Preoperative INR | 1.5 (0.8–2.3) | ||
| Child-Turcotte-Pugh score, overall | 5 (5–9) | ||
| Class A | 196 | 85 | |
| Class B | 35 | 15 | |
| Modified Child score | 5 (5–11) | ||
| Class A | 178 | 77 | |
| Class B | 47 | 20 | |
| Class C | 6 | 3 | |
| MELD | 8 (6–32) | ||
| Viral hepatitis | |||
| Hepatitis B | 26 | 11 | |
| Hepatitis C | 53 | 23 | |
| Major resection | 134 | 58 | |
| Pathology variables | |||
| Positive margin | 15 | 7 | |
| Distance to margin, cm | 0.7 (0–5.5) | ||
| Tumor size, cm | 6.6 (0.8–32.7) | ||
| Multiple lesions | 45 | 20 | |
| Satellites | 35 | 15 | |
| Tumors beyond Milan criteria | 163 | 71 | |
| Grade/differentiation | |||
| Well | 57 | 25 | |
| Moderate | 140 | 61 | |
| Poor | 29 | 13 | |
| Unknown | 7 | 3 | |
| Fibrolamellar type | 7 | 3 | |
| Microvascular invasion | 80 | 35 | |
| Macrovascular invasion | 20 | 9 | |
| Direct invasion (nonvascular) | 11 | 5 | |
| Positive lymph nodes | 6 | 3 | |
One value not available.
ASA, American Society of Anesthesiologists; INR, international normalized ratio; MELD, model for end-stage liver disease.
The median preoperative platelet count was 228/nL (range 30 to 747/nL). With receiver–operating characteristic analysis for the end point of PLI, the optimal cut-off for a high and low preoperative platelet count was 150/nL (area under the curve 0.794). Fifty patients (22%) had a platelet count of <150/nL, 21 patients (9%) had a platelet count of <100/nL, and 181 (78%) had a platelet count of ≥150/nL. Splenomegaly was detected in 18 patients (8%), which was associated with a significantly lower platelet count as compared with patients with normal-sized spleens (94/nL versus 247/nL, p = 0.001).
Operative and pathologic details
A total of 134 patients (58%) underwent a major hepatectomy (resection of ≥3 segments). Anatomic resections were performed in 201 cases (87%). Nineteen patients (8%) had received prior liver-directed therapy consisting of transarterial chemoembolization (16%) and radiofrequency ablation (1%). Six patients (3%) underwent preoperative portal vein embolization. Sixteen patients (7%) underwent emergency hepatectomy for ruptured lesions.
The majority of patients (71%) had tumors that exceeded Milan criteria. Pathologic review confirmed a margin-negative resection in 93% of cases, with a median distance from tumor to margin of 7 mm (range 0 to 55 mm). Median tumor size was 6.6 cm (range 0.8 to 32.7 cm), and multiple lesions were present in 45 patients (19%). Most tumors (197 [85%]) were well or moderately differentiated, and 7 (3%) were classified as fibrolamellar subtype. Eighty patients (35%) had microvascular invasion, and 20 (9%) had macrovascular invasion of main portal or hepatic veins. Direct invasion into adjacent organs was present in 11 patients (5%). Six patients (3%) had positive lymph nodes resected, although regional lymphadenectomy was not routinely performed in most cases.
Perioperative outcome
Median operative blood loss was 400 mL (range 50 to 6,000 mL). Transfusion rates of RBC, fresh frozen plasma, and platelets were 39%, 22%, and 13%, respectively. A total of 128 patients (55%) developed a postoperative complication, of which 38 (17%) were infectious in nature. A bile leak was documented in 9 cases (4%). Major complications were encountered in 39 patients (17%), including 8 patients (4%) who experienced major postoperative bleeding that required re-exploration. PLI occurred in 25 patients (11%), and 21 (9%) died within 60 days of the operation.
Outcomes were stratified by the presence of a preoperative platelet count <150/nL versus ≥150/nL (Table 2). Patients with a low preoperative platelet count (n = 50) had a significantly increased rate of major complications (28% versus 14%, p = 0.031), PLI (30% versus 6%, p = 0.001), and 60-day mortality (22% versus 6%, p = 0.001) (Fig. 1). These patients also had a higher rate of perioperative platelet transfusion (28%) and trended toward requiring re-exploration more frequently (8% versus 2%, p = 0.069). There was no difference in the rate of RBC transfusions. A subgroup analysis of patients with a preoperative platelet count of <100/nL (n = 21) compared with those with a count of 100 to 149/nL (n = 29) showed no difference in the rate of major complications (33% versus 24%, p = 0.69), PLI (33% versus 28%, p = 0.9), and 60-day mortality (24% versus 21%, p = 1.0).
Table 2.
Perioperative Outcome Stratified by Preoperative Platelet Count
| Variable | All cases (N = 231) | Platelet count <150/nL (n = 50) | Platelet count ≥150/nL (n = 181) | p Value* |
|---|---|---|---|---|
| Intraoperative blood loss, mL | 708 ± 863 | 742 ± 837 | 699 ± 872 | 0.76 |
| RBC transfusion | 89 (39) | 19 (38) | 70 (39) | 1.0 |
| Platelet transfusion | 31 (13) | 14 (28) | 17 (9) | 0.002 |
| Fresh frozen plasma transfusion | 50 (22) | 15 (30) | 35 (19) | 0.12 |
| Any complication | 128 (55) | 33 (66) | 95 (53) | 0.11 |
| Infectious complication | 38 (17) | 10 (20) | 28 (16) | 0.52 |
| Major postoperative bleeding | 9 (4) | 3 (6) | 6 (3) | 0.41 |
| Major complication | 39 (17) | 14 (28) | 25 (14) | 0.031 |
| Re-laparotomy | 8 (4) | 4 (8) | 4 (2) | 0.069 |
| Bile leak | 9 (4) | 2 (4) | 7 (4) | 1.0 |
| Postoperative peak total bilirubin, mg/dL, mean ± SD | 3.3 ± 4.4 | 5.3 ± 7.3 | 2.8 ± 3.0 | <0.001 |
| Postoperative peak international normalized ratio, mean ± SD | 1.5 ± 0.6 | 1.6 ± 0.8 | 1.5 ± 0.5 | 0.13 |
| Postoperative ascites formation | 19 (8) | 12 (24) | 7 (4) | <0.001 |
| Postoperative liver insufficiency (bilirubin >7 mg/dL and/or ascites) | 25 (11) | 15 (30) | 10 (6) | <0.001 |
| Hospital stay, d, mean ± SD | 9.0 ± 6.2 | 10.0 ± 6.5 | 8.7 ± 6.1 | 0.17 |
| 60-day mortality | 21 (9) | 11 (22) | 10 (6) | 0.001 |
Statistically significant p values are in bold.
Data are presented as n (%), unless otherwise indicated.
Figure 1.
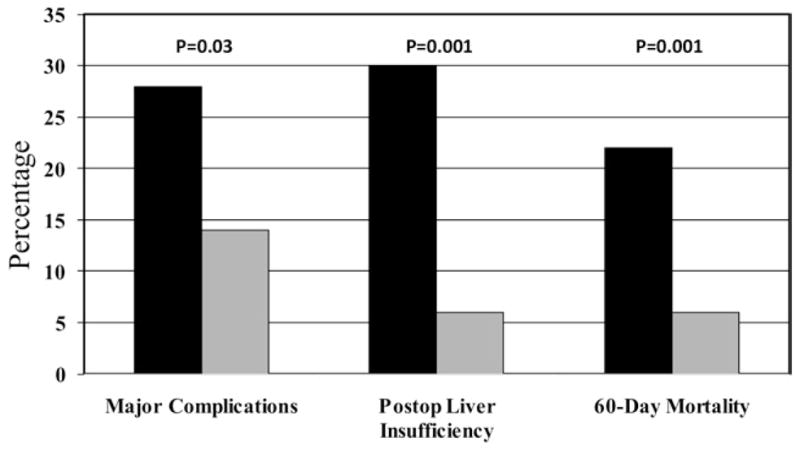
Outcomes stratified by preoperative platelet count. Black bar, <150 (n = 50); gray bar, >/=150 (n = 181).
Predictors of major complications, PLI, and 60-day mortality
Univariate and multivariate logistic regression analyses were performed to determine predictors of postoperative major complications, PLI, and 60-day mortality. Univariate regression analysis revealed that a preoperative American Society of Anesthesiologists (ASA) score of 4, platelet count <150/nL, Child score, MELD score, and tumors that exceeded Milan criteria were associated with an increased risk of developing major complications (Table 3). When entered into a multivariate model, only ASA score of 4 (odds ratio [OR] 4.31, 95% CI 1.06 to 17.49, p = 0.041), preoperative platelet count <150/nL (OR 2.77, 95% CI 1.13 to 6.67, p = 0.026), and tumors that exceeded Milan criteria (OR 3.95, 95% CI 1.43 to 10.95, p = 0.008) were independently associated with increased postoperative major complications (Table 3).
Table 3.
Univariate and Multivariate Regression Analysis of Predictors for Major Complications
| Variable | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| Crude odds ratio (95% CI) | p Value | Adjusted odds ratio (95% CI) | p Value | |
| Age | 1.03 (0.99–1.05) | 0.066 | 1.03 (0.99–1.06) | 0.08 |
|
| ||||
| Male gender | 1.23 (0.59–2.59) | 0.58 | ||
|
| ||||
| Diabetes | 0.64 (0.28–1.49) | 0.30 | ||
|
| ||||
| ASA score | ||||
|
| ||||
| 2 | Reference | Reference | ||
|
| ||||
| 3 | 1.32 (0.58–3.02) | 0.51 | 1.27 (0.53–3.01) | 0.70 |
|
| ||||
| 4 | 5.81 (1.59–21.23) | 0.008* | 4.31 (1.06–17.49) | 0.041* |
|
| ||||
| Platelet count <150/nL | 2.43 (1.15–5.13) | 0.020* | 2.77 (1.13–6.76) | 0.026* |
|
| ||||
| Child score | 1.41 (1.02–1.96) | 0.040* | 1.09 (0.73–1.64) | 0.68 |
|
| ||||
| MELD score | 1.09 (1.00–1.18) | 0.046* | 1.08 (0.98–1.20) | 0.21 |
|
| ||||
| Ruptured lesion | 0.69 (0.15–3.15) | 0.63 | ||
|
| ||||
| Tumor >Milan criteria | 2.56 (1.02–6.44) | 0.045* | 3.95 (1.43–10.94) | 0.008* |
|
| ||||
| Major resection | 0.64 (0.32–1.27) | 0.20 | ||
Statistically significant p values.
ASA, American Society of Anesthesiologists; MELD, model for end-stage liver disease.
Preoperative factors that were associated with an increased incidence of PLI on univariate analysis were an ASA score of 4, platelet count <150/nL, Child score, and MELD score (Table 4). On multivariate analysis, only preoperative platelet count <150/nL (OR 3.98, 95% CI 1.43 to 11.08, p = 0.008) and high Child score (OR 2.4, 95% CI 1.43 to 3.44, p = 0.001) remained independent predictors of PLI (Table 4).
Table 4.
Univariate and Multivariate Regression Analyses of Predictors for Postoperative Liver Insufficiency (peak bilirubin >7 mg/dL and/or ascites)
| Variable | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| Crude odds ratio (95% CI) | p Value | Adjusted odds ratio (95% CI) | p Value | |
| Age | 0.99 (0.97–1.03) | 0.76 | ||
|
| ||||
| Male gender | 1.14 (0.47–2.77) | 0.77 | ||
|
| ||||
| Diabetes | 0.83 (0.31–2.17) | 0.70 | ||
|
| ||||
| ASA score | ||||
|
| ||||
| 2 | Reference | Reference | ||
|
| ||||
| 3 | 1.12 (0.41–3.06) | 0.82 | 1.09 (0.35–3.38) | 0.88 |
|
| ||||
| 4 | 6.67 (1.65–26.93) | 0.008* | 3.82 (0.74–19.82) | 0.11 |
|
| ||||
| Platelet count <150/nL | 7.33 (3.04–17.65) | <0.001* | 3.98 (1.43–11.08) | 0.008* |
|
| ||||
| Child score | 2.93 (1.95–4.41) | <0.001* | 2.43 (1.43–3.44) | 0.001* |
|
| ||||
| MELD score | 1.08 (0.98–1.18) | 0.10 | 0.94 (0.81–1.09) | 0.39 |
|
| ||||
| Ruptured lesion | 0.53 (0.07–4.20) | 0.55 | ||
|
| ||||
| Tumor >Milan criteria | 0.70 (0.29–1.67) | 0.42 | ||
|
| ||||
| Major resection | 0.91 (0.40–2.11) | 0.83 | ||
Statistically significant p values.
ASA, American Society of Anesthesiologists; MELD, model for end-stage liver disease.
Similarly, a preoperative platelet count <150/nL (OR 3.98, 95% CI 1.43 to 11.08, p = 0.008), Child score (OR 3.98, 95% CI 1.43 to 11.08, p = 0.001), and tumors that exceeded Milan criteria (OR 33.48, 95% CI 3.31 to 339.02, p = 0.003) persisted to be independently associated with increased 60-day mortality on both univariate and multivariate analyses (Table 5).
Table 5.
Univariate and Multivariate Regression Analyses of Predictors for 60-day Mortality
| Variable | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| Crude odds ratio (95% CI) | p Value | Adjusted odds ratio (95% CI) | p Value | |
| Age | 1.02 (0.99–1.06) | 0.22 | ||
|
| ||||
| Male gender | 1.78 (0.63–5.05) | 0.28 | ||
|
| ||||
| Diabetes | 0.60 (0.19–1.86) | 0.38 | ||
|
| ||||
| ASA score | ||||
|
| ||||
| 2 | Reference | |||
|
| ||||
| 3 | 0.73 (0.27–1.97) | 0.53 | ||
|
| ||||
| 4 | 2.70 (0.60–12.20) | 0.20 | ||
|
| ||||
| Platelet count <150/nL | 4.82 (1.91–12.15) | 0.001* | 4.62 (1.46–14.64) | 0.009* |
|
| ||||
| Child score | 2.51 (1.68–3.74) | 0.001* | 2.42 (1.41–4.14) | 0.001* |
|
| ||||
| MELD score | 1.10 (0.99–1.20) | 0.056 | 1.03 (0.88–1.21) | 0.715 |
|
| ||||
| Ruptured lesion | 1.47 (0.31–6.98) | 0.63 | ||
|
| ||||
| Tumor >Milan criteria | 9.17 (1.20–69.75) | 0.032* | 33.48 (3.31–339.02) | 0.003* |
|
| ||||
| Major resection | 0.78 (0.32–1.91) | 0.58 | ||
Statistically significant p values.
ASA, American Society of Anesthesiologists; MELD, model for end-stage liver disease.
Modified Child score and predictors of major complications, PLI, and 60-day mortality
Only 3 patients (1.3%) had grade I encephalopathy preoperatively because most patients with hepatic encephalopathy are not considered candidates for resection. Thus, we replaced the factor of encephalopathy in the Child score with the preoperative platelet count to create a modified Child score that incorporated the preoperative platelet count. The 3 categories of preoperative platelet count were <100/nL, 100 to 149/nL, and ≥150/nL. With this strategy, the median modified Child score was 5 (range 5 to 11). Based on multivariate regression analysis, the modified Child score was found to be significantly associated with an increased risk of major postoperative complications (OR 1.36, 95% CI 1.03 to 1.79, p = 0.03; Table 6), PLI (OR 2.38, 95% CI 1.67 to 3.34, p < 0.001; Table 7), and 60-day mortality (OR 2.52, 95% CI 1.66 to 3.81, p < 0.001; Table 8). This modified Child score was the only factor significantly associated with PLI. With this revised strategy, the modified Child score moved 6 patients to class C (Table 1). In this subgroup of patients, one-third had major postoperative complications, 50% developed PLI, and the 60-day mortality was 67% (Table 9). Furthermore, the patients who were classified as Child B based on the modified Child score compared with those based on the traditional Child score had a lower incidence of PLI (30% versus 37%) and 60-day mortality (17% versus 26%).
Table 6.
Univariate and Multivariate Regression Analyses of Predictors for Major Complications Using Modified Child Score
| Variable | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| Crude odds ratio (95% CI) | p Value | Adjusted odds ratio (95% CI) | p Value | |
| Age | 1.03 (0.99–1.05) | 0.066 | 1.03 (0.99–1.06) | 0.08 |
|
| ||||
| Male gender | 1.23 (0.59–2.59) | 0.58 | ||
|
| ||||
| Diabetes | 0.64 (0.28–1.49) | 0.30 | ||
|
| ||||
| ASA score | ||||
|
| ||||
| 2 | Reference | |||
|
| ||||
| 3 | 1.32 (0.58–3.02) | 0.51 | 1.16 (0.49–2.74) | 0.73 |
|
| ||||
| 4 | 5.81 (1.59–21.23) | 0.008* | 3.74 (0.93–15.0) | 0.06 |
|
| ||||
| Modified Child score | 1.37 (1.08–1.73) | 0.008* | 1.36 (1.03–1.79) | 0.030* |
|
| ||||
| MELD score | 1.09 (1.00–1.18) | 0.046* | 1.07 (0.97–1.18) | 0.20 |
|
| ||||
| Ruptured lesion | 0.69 (0.15–3.15) | 0.63 | ||
|
| ||||
| Tumor >Milan criteria | 2.56 (1.02–6.44) | 0.045* | 3.52 (1.30–9.54) | 0.014* |
|
| ||||
| Major resection | 0.64 (0.32–1.27) | 0.20 | ||
Statistically significant p values.
ASA, American Society of Anesthesiologists; MELD, model for end-stage liver disease.
Table 7.
Univariate and Multivariate Regression Analyses of Predictors for Postoperative Liver Insufficiency (peak bilirubin >7 mg/dL and/or ascites) Using Modified Child Score
| Variable | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| Crude odds ratio (95% CI) | p Value | Adjusted odds ratio (95% CI) | p Value | |
| Age | 0.99 (0.97–1.03) | 0.76 | ||
|
| ||||
| Male gender | 1.14 (0.47–2.77) | 0.77 | ||
|
| ||||
| Diabetes | 0.83 (0.31–2.17) | 0.70 | ||
|
| ||||
| ASA score | ||||
|
| ||||
| 2 | Reference | |||
|
| ||||
| 3 | 1.12 (0.41–3.06) | 0.82 | 0.93 (0.30–2.87) | 0.90 |
|
| ||||
| 4 | 6.67 (1.65–26.93) | 0.008* | 3.42 (0.69–16.99) | 0.13 |
|
| ||||
| Modified Child score | 2.31 (1.72–3.11) | <0.001* | 2.38 (1.67–3.34) | <0.001* |
|
| ||||
| MELD score | 1.08 (0.98–1.18) | 0.10 | 0.94 (0.81–1.10) | 0.45 |
|
| ||||
| Ruptured lesion | 0.53 (0.07–4.20) | 0.55 | ||
|
| ||||
| Tumor >Milan criteria | 0.70 (0.29–1.67) | 0.42 | ||
|
| ||||
| Major resection | 0.91 (0.40–2.11) | 0.83 | ||
Statistically significant p values.
ASA, American Society of Anesthesiologists; MELD, model for end-stage liver disease.
Table 8.
Univariate and Multivariate Regression Analyses of Predictors for 60-day Mortality Using Modified Child Score
| Variable | Univariate Analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| Crude odds ratio (95% CI) | p Value | Adjusted odds ratio (95% CI) | p Value | |
| Age | 1.02 (0.99–1.06) | 0.22 | ||
|
| ||||
| Male gender | 1.78 (0.63–5.05) | 0.28 | ||
|
| ||||
| Diabetes | 0.60 (0.19–1.86) | 0.38 | ||
|
| ||||
| ASA score | ||||
|
| ||||
| 2 | Reference | |||
|
| ||||
| 3 | 0.73 (0.27–1.97) | 0.53 | ||
|
| ||||
| 4 | 2.70 (0.60–12.20) | 0.20 | ||
|
| ||||
| Modified Child score | 1.97 (1.48–2.63) | <0.001* | 2.52 (1.66–3.81) | <0.001* |
|
| ||||
| MELD score | 1.10 (0.99–1.20) | 0.056 | 1.04 (0.88–1.21) | 0.715 |
|
| ||||
| Ruptured lesion | 1.47 (0.31–6.98) | 0.63 | ||
|
| ||||
| Tumor >Milan criteria | 9.17 (1.20–69.75) | 0.032* | 35.48 (3.40–370.85) | 0.003* |
|
| ||||
| Major resection | 0.78 (0.32–1.91) | 0.58 | ||
Statistically significant p values.
ASA, American Society of Anesthesiologists; MELD, model for end-stage liver disease.
Table 9.
Comparison of Traditional Child-Turcotte-Pugh Score and Modified Child Score Based on Major Complications, Postoperative Liver Insufficiency, and 60-day Mortality
| Class | Total, n (%)
|
Major complications, n (%)
|
PLI, n (%)
|
60-day mortality, n (%)
|
||||
|---|---|---|---|---|---|---|---|---|
| Traditional | Modified | Traditional | Modified | Traditional | Modified | Traditional | Modified | |
| A | 196 (85) | 178 (77) | 29 (15) | 23 (13) | 12 (6%) | 8 (4%) | 12 (6%) | 9 (5%) |
|
| ||||||||
| B | 35 (15) | 47 (20) | 10 (29) | 14 (30) | 13 (37%) | 14 (30%) | 9 (26%) | 8 (17%) |
|
| ||||||||
| C | –– | 6 (3) | –– | 2 (33) | –– | 3 (50%) | –– | 4 (67%) |
|
| ||||||||
| Total | 231 (100) | 39 (17) | 25 (11) | 21 (9) | ||||
PLI, postoperative liver insufficiency.
DISCUSSION
The optimal treatment for HCC is determined after careful consideration of the extent of disease as well as the overall condition of the patient, with special attention to baseline hepatic function. Primary tumor factors to consider include the size and number of lesions as well as the presence of vascular invasion,8–10 each of which is a component of the Milan criteria; adherence to these criteria has been shown to yield the best outcomes after transplantation.15 Along with assessing the oncologic appropriateness for resection, one must always consider the baseline status of hepatic function before proceeding with resection. There are a number of methods used to make a determination of the state of the liver, including the Child-Turcotte-Pugh score and the MELD score, as well as functional tests such as ICG clearance. The aim of this study was to assess the value of a low preoperative platelet count to independently predict postoperative morbidity and mortality in patients undergoing resection of HCC.
As the safety of liver surgery has improved because of better patient selection, intraoperative technique, and postoperative care, the extent and indications for resection continue to expand.5,6 However, the importance of tumor biology should not be overlooked when patients are selected for resection of HCC. Many investigators previously demonstrated poor outcomes associated with adverse tumor factors, such as large and multifocal tumors, presence of vascular invasion, and elevated α-fetoprotein levels.4,18,19 The single best preoperative assessment of tumor biology still remains whether or not the tumor burden is within Milan criteria.15 Cha and colleagues20 reported similar outcomes between resection and transplantation for tumors that met Milan criteria, although patients who underwent resection in that series predominantly had solitary lesions. However, the regional variance between wait list times for organ availability still remains a major determinant of the appropriateness of transplantation.21,22 Given these considerations, resection remains one of the mainstays of treatment for HCC.
There is no single test that has emerged as the universal best assessment of preoperative liver function.23 One common functional test that is employed mostly in Europe and Asia is the ICG clearance test; however, this is rarely performed in the US. The Child score and MELD score are the 2 most commonly used assessment tools for determining hepatic function because both of these scores are a function of multiple individual parameters. An increasing MELD score is associated with increased perioperative mortality after general surgical procedures.24 One could extrapolate that the effect of a high MELD score on perioperative outcomes after liver resection would only be compounded. An elevated Child score is also associated with increased morbidity and mortality after liver resection.25 In fact, Child B status (score 7 to 9) is a relative contraindication for liver resection, especially for major hepatectomy.26 The overall performance status of the patient, as measured by the ASA class, is also a good predictor of postoperative outcomes after liver resection.25
Despite the comprehensive attempt to assess hepatic function, the widely used scoring systems discussed previously do not incorporate an assessment of portal hypertension. The routine invasive measurement of portal pressures is not practical for patients being considered for resection, and simple radiographic evidence of varices and splenomegaly is not adequate. Thrombocytopenia, however, is a good surrogate for the presence of portal hypertension in the patient population with HCC. The etiology of low platelet count can be multifold. For example, patients who have received extensive chemotherapy for liver metastases from colorectal cancer may have thrombocytopenia as a result of bone marrow suppression. However, in patients with HCC who have underlying liver disease, the etiology of low platelet counts is nearly uniformly secondary to portal hypertension–induced hypersplenism with increased platelet sequestration.
Although the presence of portal hypertension is not an absolute contraindication to liver resection and some have reported on the feasibility of resection in patients with severe thrombocytopenia (<50/nL),27,28 most investigators who have assessed this issue report increased morbidity and mortality in patients with a low preoperative platelet count who undergo resection. The mechanism for this association is likely secondary to worsening portal hypertension after resection, although alternate mechanisms have been proposed. Some have suggested that thrombocytopenia may greatly compromise the critical role that platelets have in the initiation of liver regeneration, as has been demonstrated in experimental rodent models.29
Two of the larger reported series of patients who underwent liver resection in which preoperative thrombocytopenia was found to be associated with poor perioperative outcomes included a heterogeneous population for whom resections were performed for a variety of different tumor types.5,6 As previously mentioned, the etiology of thrombocytopenia can vary, and thus its effect on outcome should ideally be assessed in a homogeneous patient population. Accordingly, Kaneko and coworkers30 reported in a retrospective analysis of 198 patients who underwent resection of HCC that preoperative thrombocytopenia was associated with increased postoperative mortality. This study, however, included patients over a 14-year period starting as early as 1990. Because hepatic resection has evolved tremendously since the 1990s in terms of operative technique and perioperative care, the relevance of their findings to current practice of liver resection may be questioned. Furthermore, the primary selection criteria used to assess adequacy for liver resection was ICG clearance, thus making their findings less applicable to practice in the US. Although ICG clearance was still used as the main selection criteria for extent of resection, other investigators from Japan have reported a more modern series of 213 patients who underwent resection of HCC between 1997 and 2002 in which a preoperative platelet count <100/nL was associated with an increased rate of postoperative complications (OR 4.6, 95% CI 1.5 to 14.3, p = 0.007).31 Similarly, Ishizawa and colleagues32 reported an increased incidence of large-volume ascites formation after resection in patients with preoperative platelet count <100/nL.
In the present series of 231 patients who underwent resection of HCC in the current era of liver surgery, a preoperative platelet count <150/nL was the only preoperative factor to be significantly associated with all 3 postoperative outcomes assessed, specifically major complications, PLI, and 60-day mortality. In this study, we sought to determine the independent value of a low preoperative platelet count when placed in the context of other common preoperative factors used to select patients for resection of HCC, namely Child score, MELD score, and tumor burden. Although an elevated ASA class and Child score as well as tumor burden beyond Milan criteria had varying predictive value, a low preoperative platelet count was the only factor that persisted in all 3 multivariate models (Tables 3–5). The cut-off point of 150/nL was determined based on receiver–operating characteristic analysis for the end point of PLI. This is clinically applicable given that 150/nL is the lowest value that still qualifies as a normal platelet count in most hospital laboratories. Furthermore, there was no significant difference in the poor outcomes of patients with a preoperative platelet count of 100 to 149/nL compared with those with a count of <100/nL.
There is no standard definition for what constitutes PLI. In an effort to create such a standard, Mullen and colleagues17 analyzed 669 patients who underwent hepatic resection for a variety of pathologies. They reported that a peak postoperative bilirubin level >7.0 mg/dL was the most powerful predictor of major complications and 90-day mortality. Given that the formation of ascites is also a clinical indicator of liver insufficiency, we incorporated this factor along with a peak bilirubin level >7.0 mg/dL into our definition of PLI. The postoperative INR value is often confounded by the transfusion of fresh frozen plasma, which was 22% in the current series, and thus this factor was not included in our definition of PLI.
Similarly, there is no standard for what defines postoperative mortality. Traditionally, 30-day mortality has been most commonly used, however, some suggest that 90-day mortality may be more appropriate for patients undergoing liver resection because death in the first few months may still be related to postoperative liver dysfunction. Mullen and colleagues17 found that the 90-day mortality was 4.7% compared with 3.2% for 30-day mortality, thus leading them to advocate for the longer time interval as a measure of postoperative mortality after hepatic resection. In the current series, we assessed 30-, 60-, and 90-day mortality and found that only 2 patients died within the 60- and 90-day intervals, whereas indeed there was a 50% increase in the number of patients who died between 30 and 60 days. Thus, for this study, we employed 60-day mortality as the standard for postoperative mortality because it may also potentially avoid any confounding 90-day deaths that occurred secondary to early uncontrolled recurrence.
When patients are selected for resection, the presence of hepatic encephalopathy is usually a contraindication to resection. In fact, in this series, only 3 patients (1.3%) had mild encephalopathy preoperatively. Thus, this factor most often does not contribute any value to the preoperative Child score assessment because nearly all patients being considered for resection have no encephalopathy; therefore, all got a score of 1 for this factor. In an attempt to modify and improve the utility of the Child score as a comprehensive assessment of the candidacy for resection, we replaced the factor of encephalopathy with the preoperative platelet count (1 = <100/nL, 2 = 100 to 149/nL, and 3 = ≥150/nL). Indeed, this modified Child score was significantly associated with major complications, PLI, and 60-day mortality on multivariate regression (Tables 6–8), and was the only factor that was significantly associated with PLI. The modified Child score was able to further stratify and identify those patients who had a prohibitive risk of morbidity and mortality after resection. Six patients were moved to a Child class C (score of 10 or 11); 50% of these patients developed PLI, and they had a 60-day mortality rate of 67%. Furthermore, the modified Child score seemed to better stratify the Child B patients. There was a decrease in the incidence of PLI and 60-day mortality in the patients classified as Child B based on the modified Child score compared with the traditional scoring system (Table 9). Thus, if the preoperative platelet count is preserved, it seems that the modified Child scoring system may better identify those select patients who may be offered resection with acceptable morbidity and mortality who otherwise would have been denied.
In summary, low preoperative platelet count is independently associated with increased major complications, PLI, and mortality after resection for HCC, even when accounting for standard criteria, such as Child/MELD score and tumor extent, used to select patients for resection. A modified Child score that incorporates the preoperative platelet count in place of encephalopathy may be a more appropriate preoperative assessment tool for selecting patients for resection of HCC. Patients with a low preoperative platelet count may be better served with either transplantation or liver-directed therapy.
Abbreviations and Acronyms
- HCC
hepatocellular carcinoma
- ICG
indocyanine green
- MELD
model for end-stage liver disease
- PLI
postoperative liver insufficiency
Footnotes
Disclosure information: Nothing to disclose.
Presented at Southern Surgical Association 122nd Annual Meeting, Palm Beach, FL, December 2010.
Author contributions
Study conception and design: Maithel, Kneuertz, Kooby, Scoggins, Weber, Staley
Acquisition of data: Maithel, Kneuertz, Kooby, Scoggins, Weber, Martin, McMasters, Cho, Winslow, Staley
Analysis and interpretation of data: Maithel, Kneuertz, Kooby, Scoggins, Weber, Martin, McMasters, Cho, Winslow, Wood, Staley
Drafting of manuscript: Maithel, Kneuertz
Critical revision: Maithel, Kneuertz, Kooby, Scoggins, Weber, Martin, McMasters, Cho, Winslow, Wood, Staley
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 4.Schiffman SC, Woodall CE, Kooby DA, et al. Factors associated with recurrence and survival following hepatectomy for large hepatocellular carcinoma: a multicenter analysis. J Surg Oncol. 2010;101:105–110. doi: 10.1002/jso.21461. [DOI] [PubMed] [Google Scholar]
- 5.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708. doi: 10.1097/01.sla.0000141195.66155.0c. discussion 708–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simons JP, Ng SC, Hill JS, et al. In-hospital mortality from liver resection for hepatocellular carcinoma: a simple risk score. Cancer. 2010;116:1733–1738. doi: 10.1002/cncr.24904. [DOI] [PubMed] [Google Scholar]
- 8.Nathan H, Schulick RD, Choti MA, et al. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249:799–805. doi: 10.1097/SLA.0b013e3181a38eb5. [DOI] [PubMed] [Google Scholar]
- 9.Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 10.Pawlik TM, Poon RT, Abdalla EK, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery. 2005;137:403–410. doi: 10.1016/j.surg.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Lam CM, Fan ST, Lo CM, et al. Major hepatectomy for hepatocellular carcinoma in patients with an unsatisfactory indocyanine green clearance test. Br J Surg. 1999;86:1012–1017. doi: 10.1046/j.1365-2168.1999.01204.x. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 13.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 14.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 15.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. discussion 862–864. [DOI] [PubMed] [Google Scholar]
- 18.Fong Y, Sun RL, Jarnagin W, et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–799. doi: 10.1097/00000658-199906000-00005. discussion 799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vauthey JN, Klimstra D, Franceschi D, et al. Factors affecting long-term outcome after hepatic resection for hepatocellular carcinoma. Am J Surg. 1995;169:28–34. doi: 10.1016/s0002-9610(99)80106-8. discussion 34–35. [DOI] [PubMed] [Google Scholar]
- 20.Cha CH, Ruo L, Fong Y, et al. Resection of hepatocellular carcinoma in patients otherwise eligible for transplantation. Ann Surg. 2003;238:315–321. doi: 10.1097/01.sla.0000086548.84705.ef. discussion 321–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 22.Shah SA, Cleary SP, Tan JC, et al. An analysis of resection vs transplantation for early hepatocellular carcinoma: defining the optimal therapy at a single institution. Ann Surg Oncol. 2007;14:2608–2614. doi: 10.1245/s10434-007-9443-3. [DOI] [PubMed] [Google Scholar]
- 23.Schneider PD. Preoperative assessment of liver function. Surg Clin North Am. 2004;84:355–373. doi: 10.1016/S0039-6109(03)00224-X. [DOI] [PubMed] [Google Scholar]
- 24.Teh SH, Nagorney DM, Stevens SR, et al. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology. 2007;132:1261–1269. doi: 10.1053/j.gastro.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder RA, Marroquin CE, Bute BP, et al. Predictive indices of morbidity and mortality after liver resection. Ann Surg. 2006;243:373–379. doi: 10.1097/01.sla.0000201483.95911.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manizate F, Hiotis SP, Labow D, et al. Liver functional reserve estimation: state of the art and relevance for local treatments: the Western perspective. J Hepatobiliary Pancreat Surg. 2010;17:385–388. doi: 10.1007/s00534-009-0228-x. [DOI] [PubMed] [Google Scholar]
- 27.Cucchetti A, Ercolani G, Vivarelli M, et al. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009;250:922–928. doi: 10.1097/SLA.0b013e3181b977a5. [DOI] [PubMed] [Google Scholar]
- 28.Sugimachi K, Ikeda Y, Tomikawa M, et al. Appraisal of hepatic resection in the treatment of hepatocellular carcinoma with severe thrombocytopenia. World J Surg. 2008;32:1077–1081. doi: 10.1007/s00268-007-9442-3. [DOI] [PubMed] [Google Scholar]
- 29.Alkozai EM, Nijsten MW, de Jong KP, et al. Immediate postoperative low platelet count is associated with delayed liver function recovery after partial liver resection. Ann Surg. 2010;251:300–306. doi: 10.1097/SLA.0b013e3181b76557. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko K, Shirai Y, Wakai T, et al. Low preoperative platelet counts predict a high mortality after partial hepatectomy in patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11:5888–5892. doi: 10.3748/wjg.v11.i37.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taketomi A, Kitagawa D, Itoh S, et al. Trends in morbidity and mortality after hepatic resection for hepatocellular carcinoma: an institute’s experience with 625 patients. J Am Coll Surg. 2007;204:580–587. doi: 10.1016/j.jamcollsurg.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 32.Ishizawa T, Hasegawa K, Kokudo N, et al. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg. 2009;144:46–51. doi: 10.1001/archsurg.2008.511. [DOI] [PubMed] [Google Scholar]


