Abstract
We have previously shown that laminin-8, a vascular basement membrane component, was over-expressed in human glioblastomas multiforme and their adjacent tissues compared to normal brain. Increased laminin-8 correlated with shorter glioblastoma recurrence time and poor patient survival making it a potential marker for glioblastoma diagnostics and prediction of disease outcome. However, laminin-8 therapeutic potential was unknown because the technology of blocking the expression of multi-chain complex proteins was not yet developed. To inhibit the expression of laminin-8 constituents in glioblastoma in vitro and in vivo, we used Polycefin, a bioconjugate drug delivery system based on slime-mold Physarum polycephalum-derived poly(malic acid). It carries an attached transferrin receptor antibody to target tumor cells and to deliver two conjugated morpholino antisense oligonucleotides against laminin-8 α4 and β1 chains. Polycefin efficiently inhibited the expression of both laminin-8 chains by cultured glioblastoma cells. Intracranial Polycefin treatment of human U87MG glioblastoma-bearing nude rats reduced incorporation of both tumor-derived laminin-8 chains into vascular basement membranes. Polycefin was thus able to simultaneously inhibit the expression of two different chains of a complex protein. The treatment also significantly reduced tumor microvessel density (p < 0.001) and area (p < 0.001) and increased animal survival (p < 0.0004). These data suggest that laminin-8 may be important for glioblastoma angiogenesis. Polycefin, a versatile nanoscale drug delivery system, was suitable for in vivo delivery of two antisense oligonucleotides to brain tumor cells causing a reduction of glioblastoma angiogenesis and an increase of animal survival. This system may hold promise for future clinical applications.
Keywords: Tumor angiogenesis, Glioma, Laminin-8, Multiple drug targeting, Poly(malic acid)
Introduction
Laminins are major basement membrane components important for cell differentiation, migration, and proliferation. They participate in tumor invasion as barriers for tumor cell penetration of surrounding tissues. At the same time, some laminins produced by tumor cells facilitate their migration via integrin receptors [1, 2]. Laminin-8 (α4β1γ1), a vascular basement membrane component, plays important roles in angiogenesis (capillary maturation) and cell migration [1, 2]. We have also documented previously laminin-8 overexpression in grade IV human glioma (glioblastoma multiforme, GBM) and ductal breast carcinoma [3–5]. Antisense oligonucleotide (AON) inhibition of two laminin-8 chains (α4 + β1) was able to block glioma invasion in vitro [6]. Laminin-8 involvement in vessel formation and its overexpression in tumors suggested that its inhibition could reduce tumor neo-vascularization in vivo.
To test this hypothesis, AON to laminin-8 needed to be delivered specifically to tumor cells. Targeted drug delivery is crucial for treating tumors and reducing side effects for normal cells. High-molecular-mass compounds as drug delivery vehicles recently attracted special attention because tumors can selectively accumulate these molecules by the enhanced permeability and retention (EPR) effect. It was observed in tumor tissue for macromolecules [7–9] and is used for cancer drug delivery. Tumor vasculature can be targeted with a synthetic polymer, N-(2-hydroxypropyl)methacrylamide [8], conjugated with O-(chloracetyl-carbamoyl) fumagillol (TNP-470) [10]. Another promising drug carrier used here is β-poly(L-malic acid) (PMLA), a natural product of Physarum polycephalum [11–13]. Compared to other chemically functional polymers, PMLA has such advantages as lack of toxicity in vitro and in vivo, non-immunogenicity, biodegradability, stability in the blood stream, and easy cellular uptake. Antisense inhibitors of multiple molecular targets can be attached to a single PMLA molecule [14].
Combined blocking of several tumor markers with simultaneous delivery of respective inhibitors is a promising approach to cancer therapy but previously had considerable technical problems. In this report, we demonstrate simultaneous delivery into the tumor cells of two different AONs that simultaneously block in vivo expression of two laminin-8 chains using a novel nanoscale PMLA-based drug, Polycefin. Polycefin inhibited laminin-8 synthesis in cultured human glioma cells U87MG and T98G [14]. It significantly increased survival of human GBM-bearing nude rats after intracranial administration by interfering with tumor angiogenesis and reducing GBM vessel density and area.
Materials and methods
Cell lines and culture conditions
Invasive human GBM U87MG and rat glioma RG2 cell lines were from American Type Culture Collection (Rockville, MD). Mouse glioma GL26 cells were from the Division of Cancer Treatment Tumor Repository (National Cancer Institute, Frederick, MD). Normal human brain microvascular endothelial cells (HBMVEC) were from Dr. Ken Samoto (Kyushu University, Fukuoka, Japan). Cells were cultured in Eagle’s MEM or a mixture of DMEM-Ham’s F-12 (1:1) with 10% fetal calf serum (FCS), L-glutamine, antibiotics, and sodium pyruvate.
Antisense design
Custom made Morpholino™ (phosphorodiamidate morpholino oligomer) AONs (Gene Tools, Inc., St. Louis, MO) for human laminin α4 and β1 chains were as follows: α4 antisense 5′AGCTCAAAGCCATTTCTCCGCTGAC3′; β1 antisense 5′CTAGCAACTGGAGAAGCCCCATGCC3′. Standard scrambled oligonucleotide control (Gene Tools) was also used in some experiments.
Drug synthesis
Polycefin was synthesized as described previously [14].
Surgical procedures and treatment with Polycefin
A hole in a rat’s skull 3 mm laterally and 1 mm rostrally from the bregma (sagittal and transverse lines crossing point) was made using a dental drill. Nude outbred NIHRNU-M rats (Taconic Inc., Hudson, NY) underwent stereotactic intracranial injection of 105 U87MG human glioma cells to the right basal ganglia field using a 10 μl Hamilton syringe. Polycefin or saline (mock control) was injected through the same hole in the rat’s skull as tumor cells. All procedures were performed in strict accordance with an approved IACUC protocol at Cedars-Sinai Medical Center.
Groups of 12 rats each were injected intracranially with Polycefin at doses of 0.5 mg/kg AONs to laminin-8 α4 + β1 chains on days 3, 7, 10 and 14 after tumor implantation, and were euthanized after developing neurological symptoms caused by tumor progression. Preliminary experiment did not show difference between 0.5 and 2.5 mg/kg AONs, therefore, the lower dose was further used. Control groups of ten rats each were mock-injected intracranially on the same days with phosphate-buffered saline. This control has been used for the following reasons instead of sense or scrambled oligo controls: (1) abundant literature on saline as antisense control for in vivo studies [15–18], (2) abundant published data on lack of sense and scrambled oligo effects as opposed to antisense [19–21], and (3) lack of sense effects in our previous in vitro experiments [6]. Importantly, in drug development, complete drug is normally compared to its solvent/vehicle, in our case, saline, but not to a modified and inactive drug (e.g., sense). Other control groups consisted of four rats euthanized without any kind of treatment to obtain normal tissue, and of three rats without tumors injected intracranially with Polycefin as above and euthanized in 14 days to examine its effects on tissue morphology. Finally, one control group of 6 rats was injected intracranially with Polycefin without targeting mAb to transferrin receptor [Polycefin(-mAb)].
Immunohistochemistry
Cultured U87MG human glioma cells were incubated with or without drugs and at select time points were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100. Immunostaining was done with monoclonal antibodies (mAbs) to laminin α4 (mouse mAb FC10) and β1 (rat mAb LT3) chains that only reacted with human laminin, in our case synthesized by tumor astrocytes [1, 6]. In some experiments, to see all vessels in a given field, a rabbit polyclonal antibody to laminin α4 chain was used [rabbit pAb 1129 [22], a gift from Dr. Takako Sasaki, Max-Planck-Institut für Biochemie, Martinsried, Germany].
Rat brains with or without drug treatment were embedded in OCT Compound (Sakura Finetek U.S.A., Inc., Torrance, CA). 6-μm frozen sections were cut on a Leica CM1850 cryostat (McBain Instruments, Chatsworth, CA). The tumor presence was verified by hematoxylin-eosin staining. Parallel sections were stained with antibodies to human laminin α4 and β1 chains [3]. Antibodies to rat von Willebrand factor/Factor VIII and CD31 (mAb TLD-3A12), both from Chemicon International (Temecula, CA), were used to visualize blood vessels [23]. Secondary cross-species adsorbed antibodies labeled with DTAF or TRITC were from Chemicon International.
Confocal microscopy
A TCS SP spectral scanner (Leica Microsystems, Mannheim, Germany) was used for confocal microscopy. Image stacks of 160 by 160 μm in size and 7.5 μm in depth of fixed U87MG glioma cells were acquired with a Leica PlanApo 63/1.2 NA lens.
Dot blot analysis
To confirm cross-reactivity of tumor targeting mouse anti-rat transferrin receptor mAb OX-26 (Chemicon International) with human cells, we first tried Western blot but no reaction could be obtained. Therefore, dot blot was further used. Lysates of cultured U87MG human glioma cells, normal human endothelial cells (HBMVEC), rat RG2 and mouse GL26 glioma cells were prepared in 0.5% Triton X-100, 5% glycerol, protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) in 50 mM HEPES buffer pH 7.2, and applied in triplicate to nitrocellulose membrane (Invitrogen, Carlsbad, CA). Lysate volumes were 1, 4, and 8 μl (Fig. 2 shows results for 8 μl). Protein loading was normalized by β-actin content on parallel blots (Fig. 2). Membranes blocked in 5% defatted dry milk were probed with mAb OX-26 followed by chemiluminescent detection with alkaline phosphatase-conjugated secondary antibodies (Immune-Star kit, Bio-Rad, Hercules, CA). Negative controls without secondary antibodies gave background staining similar to the one with the negative control, mouse GL26 cell lysate (Fig. 2).
Fig. 2.
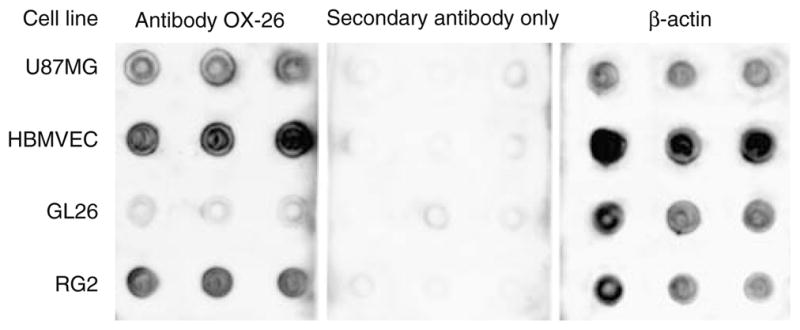
Dot blot analysis of species cross-reactivity of transferrin receptor antibody OX-26. Lysate of the rat glioma cell line RG2 (positive control) reacts well with OX-26. Mouse glioma GL26 cell lysate shows background reactivity (negative control). Lysates of human glioma U87MG and of human brain microvascular endothelial cells (HBMVEC) also react well with the antibody. Omission of primary antibody (secondary antibody; middle panel) only produced background signal. A blot for β-actin as positive loading control is shown on the right panel. Results are shown for 8 μl lysates in triplicates
Vessel density and area measurement
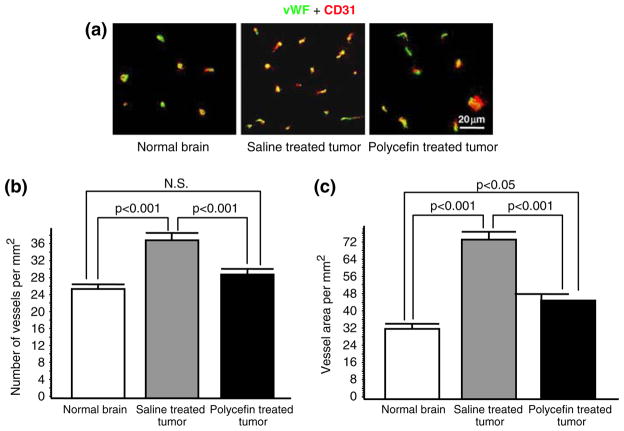
To demonstrate that Polycefin inhibition of laminin-8 expression affected tumor vessels, the number of these vessels and the vessel area (vascularity) were measured. The vessels were visualized by immunostaining for von Willebrand factor and CD31 (Fig. 5a). The number of vessels and vessel area were determined in both drug-treated and untreated animals in five microscopic fields of three serial sections (15 fields per tumor) under × 200 magnification, using a Zeiss Axioscop microscope (Thornwood, NY) with Hamamatsu (Japan) image capturing system. NIH ImageJ software was used for quantification of vessel density and area as described [24, 25]. Data are presented for control brains of three sham-operated (normal) rats (45 microscopic fields), five rats with untreated tumors (75 microscopic fields), and five rats with Polycefin-treated tumors (75 microscopic fields). Our preliminary experiments showed that brain tumors did not have “hot spots” [sites of vessel concentration, see [26]]. Therefore, the vessels were counted using global approach [26, 27].
Fig. 5.
Decreased vascular density and area in tumors treated with Polycefin. (a) Double immunohistochemical staining of rat brain vessels using antibodies to two endothelial markers, von Willebrand factor (vWF, green) and CD31 (red). The two markers were used to optimize the screening accuracy. In most vessels both markers co-distribute (yellow color). The vessel number is higher in the xenotransplanted U87MG tumor than in normal brain, and this number is decreased after four intracranial Polycefin treatments. (b) Quantitative assessment of vascular density in treated and untreated tumors compared to normal brain. Vessels were revealed by either marker (Fig. 5a) and their number was quantitated at × 200 direct magnification. Images were analyzed using NIH ImageJ software. Statistical significance was determined by ANOVA. Microvascular density in xenotransplanted U87MG human glioma mock-treated with saline is significantly increased compared to normal brain (p < 0.001). After four intracranial treatments with Polycefin, tumor vessel density was significantly decreased (p < 0.001) and became similar to normal brain tissue (NS, not significant with p > 0.05). (c) Quantitative assessment of vascular area in treated and untreated tumors compared to normal brain. Vessels were revealed by either marker (Fig. 5a) and their relative area quantitated as for vessel density. Vessel area in xenotransplanted U87MG human glioma mock-treated with saline is significantly increased compared to normal brain (p < 0.001). After four intracranial treatments with Polycefin, tumor vascular area significantly decreased (p < 0.001) but remained somewhat higher than in normal brain (p < 0.05)
Statistical analysis
Animal survival times were statistically compared using Kaplan-Meier test. Analysis of vessel density and area was performed using ANOVA. Prism4 software program (GraphPad Software, San Diego, CA) was used for all statistical analyses.
Results
Characteristics of a nanoscale molecular device Polycefin
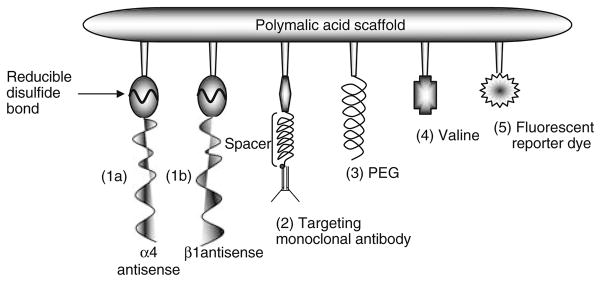
Polycefin is a nanoscale drug delivery system containing poly(β-L-malic acid) scaffold with covalently attached molecular blocks for target inhibition (AONs released by disulfide bond cleavage), pH-sensitive endosome membrane disruption (containing hydrophobic valine), protection from degradation [poly(ethylene glycol)], tumor targeting and blood brain barrier penetration [transferrin receptor mAb [14, 15]], and an optional fluorescent dye for drug imaging (Fig. 1). The device is built from biologically degradable and non-toxic molecular blocks; synthesis details have been described [14].
Fig. 1.
Schematic drawing of Polycefin. This delivery device was used for inhibiting the expression of laminin α4 and β1 chains by morpholino AONs in vitro and in vivo to prevent angiogenesis of glial tumors. The modules are (1) morpholino AON 1a (to α4 chain) and 1b (to β1 chain) conjugated to the scaffold by disulfide bonds, which are cleaved in the cytoplasm by glutathi-one to release the free drugs, (2) mAb to transferrin receptor for cancer cell targeting and receptor-mediated endocytosis, (3) PEG for protection, (4) stretches of conjugated L-valine to provide pH-dependent lipophilicity for the disruption of endosomal membranes, and (5) optional fluorescent reporter dye (Fluorescein or Alexa Fluor 680) for detection
Inhibition of laminin-8 expression by Polycefin in cultured glioma cells
Human U87MG glioblastoma cell line was treated with Polycefin (Fig. 1) to block laminin-8 expression. As shown previously [14], Polycefin without OX-26 mAb only slowly passed through the cell membrane. In contrast, Polycefin with OX-26 antibody that cross-reacted with human transferrin receptor (Fig. 2) was rapidly internalized by receptor-mediated endocytosis as also shown before [14]. Further in vitro and in vivo experiments were thus performed with complete Polycefin bearing mAb OX-26.
To confirm the inhibition of expression of laminin-8 chains in vitro, Polycefin at a concentration of 1.4 μM AON and a ratio AON:PMLA of 20:1 [14] was added to human glioma U87MG culture media on days 1 and 4. The drug with AONs to both laminin chains was used because a combination of AONs to both laminin chains decreased the expression of both chains more efficiently than AON to either chain alone [6]. In untreated cultures, distinct immunostaining was seen for laminin α4 and β1 chains in the form of perinuclear vesicles and at the cell membranes (Fig. 3, untreated). Such staining is typical to cultured cells in contrast to the in vivo situation where exclusively basement membranes stain for laminin ([28, 29]; compare with Fig. 4b). In cultures treated with Polycefin (Fig. 3) the expression of both chains was either greatly reduced (α4) or completely abolished (β1). Similar data were obtained with T98G glioma cell line using Western blot analysis [14]. Thus, AONs conjugated to the drug efficiently inhibited target protein expression in vitro.
Fig. 3.
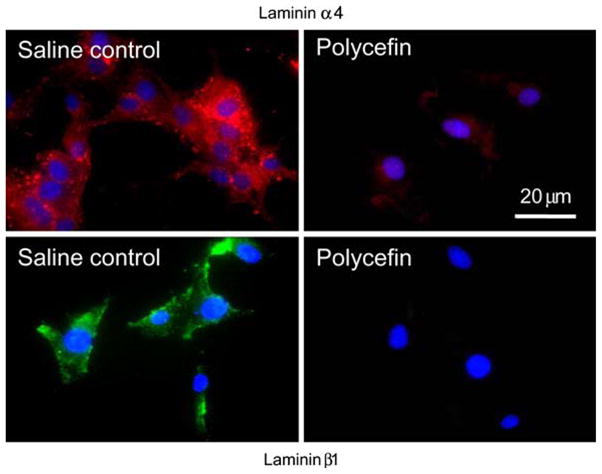
In vitro targeting and inhibition of laminin chain synthesis by Polycefin. Immunofluorescent confocal microscopy of U87MG glioma cultures stained for laminin chains. Left, untreated control cells expressing α4 and β1 laminin-8 chains. Right, Polycefin that bears AONs to α4 and β1 chains markedly inhibits the expression of both laminin chains. Nuclei are counterstained with DAPI
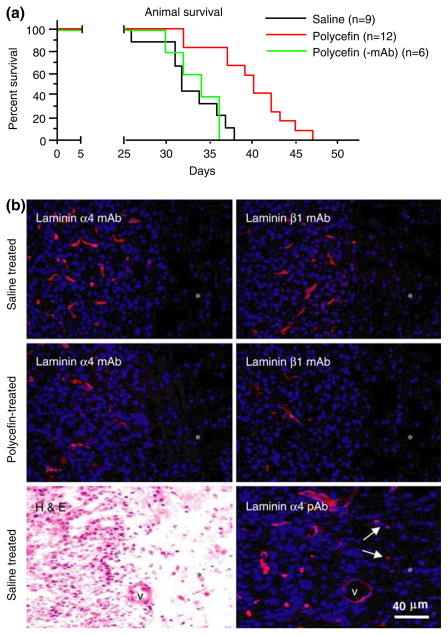
Fig. 4.
In vivo target inhibition by Polycefin and animal survival. (a) Survival of Polycefin-treated (0.5 mg/kg body weight) and control animals. After intracranial administration of four doses of Polycefin, the animal survival time was significantly increased (p < 0.0004) compared to saline treated or Polycefin(-mAb) treated rats. (b) Immunofluorescent analysis of xenotransplanted brain tumors with anti-human mAbs to laminin α4 or β1 chains. After Polycefin treatment, the number of tumor vessels positive for either laminin chain was markedly diminished. Therefore, Polycefin inhibited the expression of both its targets and their incorporation into basement membrane by human tumor cells. Asterisks denote tumor-adjacent (normal) brain area. This area has significantly decreased cellularity (revealed by blue nuclear staining with DAPI) compared to highly cellular tumor at the left. No vascular staining is observed in tumor-adjacent area with both mAbs, because the antibodies only recognized human laminin chains. Left lower panel, a hematoxylin-eosin (H&E) stained tumor showing a sharp boundary between highly cellular tumor and surrounding brain parenchyma with significantly fewer cells. Right lower panel, staining of a serial section with a pAb to laminin α4 chain recognizing human and rat protein that reveals all vessels. Note increased vascularity and cellularity of the tumor as opposed to hypocellular surrounding tissue (asterisk) that has only scattered vessels (arrows)
Effects of Polycefin inhibition of laminin-8 in glioma-bearing rats
Four treatments with 0.5 and 2.5 mg/kg Polycefin or with saline (mock) were performed on days 3, 7, 10 and 14 after intracranial xenotransplantation of human tumor into nude rats. Drug doses gave similar results in the survival study so 0.5 mg/kg dose was further used. No effect on tissue and organ morphology was observed with either dose of Polycefin. After four intracranial Polycefin treatments, animal survival time was significantly increased compared to rats treated with saline or with Polycefin without anti-transferrin receptor mAb [Polycefin(-mAb)]. The median survival time was 32.0 days for saline-treated rats, 33.0 days for Polycefin(-mAb)-treated rats, and 40.0 days for Polycefin-treated rats, p < 0.0004 versus either control by Kaplan-Meier survival test (Fig. 4a). The tendency for prolonged survival was already observed after two Polycefin treatments in a separate experiment, but the difference was not significant. Absence of Polycefin(-mAb) effect on animal survival suggested that transferrin receptor-mediated endocytosis was responsible for AON delivery into glioma cells in vivo.
Reduction of laminin-8 deposition by glial tumor cells in vivo after Polycefin treatment
MAbs to human laminin α4 and β1 chains did not stain normal rat brain capillaries (areas marked by asterisks in Fig. 4b). However, in agreement with previous data on human GBM [3, 4], capillary basement membranes in xenotransplanted untreated human tumors showed strong staining for both laminin chains. A polyclonal antibody to laminin α4 chain that also reacted with rat laminin revealed the relative paucity of parenchymal capillaries compared to tumor tissue (Fig. 4b, lower right panel). Since mAbs only recognize human laminin α4 and β1 chains, these data confirm that glioma cells can produce laminin-8 chains in vivo and deposit them in the vascular basement membranes. This deposition apparently occurs in the outer glial leaflet that contains laminin synthesized by astrocytes [1], in our case, by tumor astrocytes. This result shows that tumor cells produce laminin-8, which is deposited in the vessels newly formed within the brain tumor. Polycefin treatment markedly decreased the number of tumor vessels positive for either α4 or β1 human laminin chain (Fig. 4b) confirming inhibition of expression of its target proteins.
Decrease of tumor vessel density and area after Polycefin treatment in vivo
To confirm the action of Polycefin in anti-angiogenesis, we compared tumor vessel density and area between Polycefin treated rats and saline treated control rats. The vessels in tumors and surrounding brain tissues were visualized by immunostaining for von Willebrand factor and CD31, with very similar results. Two panendothelial markers were used because the existing data on the accuracy of a given endothelial antigen for vessel counting are contradictory and significantly different in various tumor types [23]. Microvascular density in xenotransplanted U87MG human tumors with saline treatment was significantly higher than in normal brain (Fig. 5a, b). After four intracranial Polycefin treatments, tumor vessel density was significantly reduced compared to saline treated tumors (p < 0.001)and was similar to vessel density in normal adjacent brain tissue (Fig. 5a, b). Similar data were obtained regarding vessel area (Fig. 5c). Vessel area in xenotransplanted U87MG human gliomas significantly increased compared to normal brain (p < 0.001). After Polycefin treatments, tumor vascular area significantly decreased (p < 0.001) but remained somewhat higher than in normal brain (p < 0.05).
Discussion
Laminin-8 is a vascular basement membrane component with very low expression in human brain capillaries [3]. It can facilitate cell migration in vitro better than several other laminin isoforms [1, 2]. Knockout of its α4 chain leads to vascular abnormalities in late embryos and neonates [1]. We have documented overexpression of laminin-8 in gliomas and breast cancer and its involvement in glioma invasion [3–6]. These data suggested that inhibition of laminin-8 expression could interfere with tumor angiogenesis and growth. This hypothesis was tested in an in vivo model using a polymer drug delivery system, Polycefin, with conjugated AONs to two laminin-8 chains.
Polymers able to deliver inhibitory agents to tumor cells increasingly gain importance because they are less immunogenic than viral vectors, and therefore, more useful for repetitive treatments [10, 30]. To prevent or inhibit tumor growth and progression, simultaneous inhibition of several molecular targets may be highly effective [31]. The novel PMLA-based polymeric drug, Polycefin, was designed to simultaneously deliver two biologically active AONs to laminin-8 α4 and β1 chains into tumor cells. AON combination was used because it was more effective in blocking the synthesis of a complex trimeric protein, laminin-8, than single AONs to either α4 or β1 chain in glioma cultures [6]. Polycefin bearing these two AONs efficiently prevented laminin-8 synthesis in vitro and in vivo.
When synthesis of laminin-8, a structural component of newly formed tumor vessels, was inhibited in tumor cells with Polycefin, this resulted in a significant reduction of tumor vessel density and area. The data suggest that tumor cell contribution to vascular basement membrane synthesis/assembly is needed for glial tumor angiogenesis. Migration-promoting laminin-8 [1, 2] may thus be a key component of tumor neovessel formation. Laminin-8 may be considered as glioma microvascular signature [32] and may be a novel specific target for future therapy to prevent tumor angiogenesis.
Drug effect on tumor vasculature may be the underlying mechanism of an increased animal survival after Polycefin treatment. Recently, treatment with drugs blocking receptors of several angiogenic growth factors was also shown to significantly increase survival of glioma-bearing rats [31]. These results hold promise for an efficient brain tumor treatment using laminin-8 as a therapeutic target, alone or in combination with other molecular markers, or with conventional chemotherapy. Because laminin-8 is also increased in breast cancer [5], Polycefin could also be efficient for inhibition of breast cancer growth and invasion.
Brain tumors are particularly difficult to treat with systemically administered drugs most of which do not pass through blood brain barrier that is also characteristic for brain tumors [33]. As a result, local intracranial delivery of drugs including antiangiogenic agents is used for brain tumor treatment [33–35]. It is often more efficient than systemic delivery because it allows one to bypass blood brain barrier and dramatically reduce effective drug doses [34]. Because of direct clinical relevance of intracranial glioma treatment, our studies with Polycefin designed to inhibit tumor vascular component, laminin-8, in glioma-bearing rats were also concentrated on this approach. Intracranially administered Polycefin inhibited laminin-8 expression, significantly increased survival of tumor-bearing animals and decreased tumor vascularity. It seems to be a promising prototype drug to treat human gliomas. Increased number of treatments and/or combination of specific anti-angiogenic drugs (such as Polycefin) with metronomic chemotherapy may further enhance the efficacy of glioma treatment [36, 37].
Acknowledgments
The authors are indebted to Dr. Takako Sasaki, Max-Planck-Institut für Biochemie, Martinsried, Germany, for a gift of polyclonal antibody to laminin α4 chain.
Contributor Information
Manabu Fujita, Maxine Dunitz Neurosurgical Institute, Cedars-Sinai Medical Center, 8631 W. Third Street, Suite 800E, Los Angeles, CA 90048, USA.
Natalya M. Khazenzon, Maxine Dunitz Neurosurgical Institute, Cedars-Sinai Medical Center, 8631 W. Third Street, Suite 800E, Los Angeles, CA 90048, USA
Alexander V. Ljubimov, Ophthalmology Research Laboratories, Cedars-Sinai Medical Center, Los Angeles, CA 90048, USA. Arrogene, Inc., Tarzana, CA 91356, USA
Bong-Seop Lee, Maxine Dunitz Neurosurgical Institute, Cedars-Sinai Medical Center, 8631 W. Third Street, Suite 800E, Los Angeles, CA 90048, USA.
Ismo Virtanen, Institute of Biomedicine/Anatomy, University of Helsinki, Helsinki, Finland.
Eggehard Holler, Arrogene, Inc., Tarzana, CA 91356, USA. Institut für Biophysik und Physikalische Biochemie der Universität Regensburg, Regensburg, Germany.
Keith L. Black, Maxine Dunitz Neurosurgical Institute, Cedars-Sinai Medical Center, 8631 W. Third Street, Suite 800E, Los Angeles, CA 90048, USA. Arrogene, Inc., Tarzana, CA 91356, USA
Julia Y. Ljubimova, Email: ljubimovaj@cshs.org, Maxine Dunitz Neurosurgical Institute, Cedars-Sinai Medical Center, 8631 W. Third Street, Suite 800E, Los Angeles, CA 90048, USA. Arrogene, Inc., Tarzana, CA 91356, USA.
References
- 1.Hallmann R, Horn N, Selg M, et al. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 2.Fujiwara H, Gu J, Sekiguchi K. Rac regulates integrin-mediated endothelial cell adhesion and migration on laminin- 8. Exp Cell Res. 2004;292:67–77. doi: 10.1016/j.yexcr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Ljubimova JY, Lakhter AJ, Loksh A, et al. Overexpression of α4 chain-containing laminins in human glial tumors identified by gene microarray analysis. Cancer Res. 2001;61:5601–5610. [PubMed] [Google Scholar]
- 4.Ljubimova JY, Fugita M, Khazenzon NM, et al. Association between laminin-8 and glial tumor grade, recurrence, and patient survival. Cancer. 2004;101:604–612. doi: 10.1002/cncr.20397. [DOI] [PubMed] [Google Scholar]
- 5.Fujita M, Khazenzon NM, Bose S, et al. Overexpression of β1 chain-containing laminins in capillary basement membranes of human breast cancer and its metastases. Breast Cancer Res. 2005;7:411–421. doi: 10.1186/bcr1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khazenzon NM, Ljubimov AV, Lakhter AJ, et al. Antisense inhibition of laminin-8 expression reduces invasion of human gliomas in vitro. Mol Cancer Ther. 2003;2:985–994. [PubMed] [Google Scholar]
- 7.Torchilin VP, Lukyanov AN. Peptide and protein drug delivery to and into tumors: challenges and solutions. Drug Discov Today. 2003;8:259–266. doi: 10.1016/s1359-6446(03)02623-0. [DOI] [PubMed] [Google Scholar]
- 8.Peterson CM, Shiah J, Sun Y, et al. HPMA copolymer delivery of chemotherapy and photodynamic therapy in ovarian cancer. Adv Exp Med Biol. 2003;519:101–123. doi: 10.1007/0-306-47932-X_7. [DOI] [PubMed] [Google Scholar]
- 9.Maeda H, Fang J, Inutsuka T, et al. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol. 2003;3:319–328. doi: 10.1016/S1567-5769(02)00271-0. [DOI] [PubMed] [Google Scholar]
- 10.Satchi-Fainaro R, Puder M, Davies JW, et al. Targeting angiogenesis with a conjugate of HPMA copolymer and TNP-470. Nat Med. 2004;10:255–261. doi: 10.1038/nm1002. [DOI] [PubMed] [Google Scholar]
- 11.Lee BS, Vert M, Holler E. Water-soluble aliphatic polyesters: poly(malic acid)s. In: Doi Y, Steinbüchel A, editors. Biopolymers. 3a. Wiley-VCH; New York: 2002. pp. 75–103. [Google Scholar]
- 12.Gasslmaier B, Holler E. Specificity and direction of depolymerization of β-poly(L-malate) catalysed by polymalatase from Physarum polycephalum. Fluorescence labeling at the carboxy-terminus of β-poly(L-malate) Eur J Biochem. 1997;250:308–314. doi: 10.1111/j.1432-1033.1997.0308a.x. [DOI] [PubMed] [Google Scholar]
- 13.Domurado D, Fournié P, Braud C, et al. In vivo fates of degradable poly(β-malic acid), and of its precursor, malic acid. J Bioact Compat Pol. 2003;18:23–32. [Google Scholar]
- 14.Lee BS, Fujita M, Khazenzon NM, et al. Polycefin, a new prototype of a multifunctional nanoconjugate based on poly(β-L-malic acid) for drug delivery. Bioconjug Chem. 2006;17:317–326. doi: 10.1021/bc0502457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang YF, Bryant J, et al. Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clin Cancer Res. 2004;10:3667–3677. doi: 10.1158/1078-0432.CCR-03-0740. [DOI] [PubMed] [Google Scholar]
- 16.Troncoso P, Ortiz AM, Dominguez J, et al. Use of FTY 720 and ICAM-1 antisense oligonucleotides for attenuating chronic renal damage secondary to ischemia-reperfusion injury. Transplant Proc. 2005;37:4284–4288. doi: 10.1016/j.transproceed.2005.10.093. [DOI] [PubMed] [Google Scholar]
- 17.Kutryk MJ, Foley DP, van den Brand M, et al. Local intracoronary administration of antisense oligonucleotide against c-myc for the prevention of in-stent restenosis: results of the randomized investigation by the Thoraxcenter of antisense DNA using local delivery and IVUS after coronary stenting (ITALICS) trial. J Am Coll Cardiol. 2002;39:281–287. doi: 10.1016/s0735-1097(01)01741-7. [DOI] [PubMed] [Google Scholar]
- 18.Lambert DL, Malik N, Shepherd L, et al. Localization of c-Myb and induction of apoptosis by antisense oligonucleotide c-Myb after angioplasty of porcine coronary arteries. Arterioscler Thromb Vasc Biol. 2001;21:1727–1732. doi: 10.1161/hq1101.098552. [DOI] [PubMed] [Google Scholar]
- 19.Yoshizumi T, Yonemitsu Y, Ikeda Y, et al. Tumor necrosis factor-a antisense transfer remarkably improves hepatic graft viability. Liver Int. 2006;26:451–456. doi: 10.1111/j.1478-3231.2006.01252.x. [DOI] [PubMed] [Google Scholar]
- 20.Countryman RA, Kaban NL, Colombo PJ. Hippocampal c-fos is necessary for long-term memory of a socially transmitted food preference. Neurobiol Learn Mem. 2005;84:175–183. doi: 10.1016/j.nlm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Lai SK, Ng TK, Lau WK, et al. Selective knockdown of gene expression of N-methyl-D-aspartate receptor one ameliorates parkinsonian motor symptom in 6-hydroxydopamine-lesioned rats. Neurochem Int. 2004;45:11–22. doi: 10.1016/j.neuint.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki T, Mann K, Timpl R. Modification of the laminin α4 chain by chondroitin sulfate attachment to its N-terminal domain. FEBS Lett. 2001;505:173–178. doi: 10.1016/s0014-5793(01)02812-5. [DOI] [PubMed] [Google Scholar]
- 23.Rubio L, Burgos JS, Morera C, et al. Morphometric study of tumor angiogenesis as a new prognostic factor in nasopharyngeal carcinoma patients. Pathol Oncol Res. 2000;6:210–216. doi: 10.1007/BF03032375. [DOI] [PubMed] [Google Scholar]
- 24.Chen B, Pogue BW, Zhou X, et al. Effect of tumor host microenvironment on photodynamic therapy in a rat prostate tumor model. Clin Cancer Res. 2005;11:720–727. [PubMed] [Google Scholar]
- 25.Ozawa MG, Yao VJ, Chanthery YH, et al. Angiogenesis with pericyte abnormalities in a transgenic model of prostate carcinoma. Cancer. 2005;104:2104–2115. doi: 10.1002/cncr.21436. [DOI] [PubMed] [Google Scholar]
- 26.de Jong JS, van Diest PJ, Baak JP. Heterogeneity and reproducibility of microvessel counts in breast cancer. Lab Invest. 1995;73:922–926. [PubMed] [Google Scholar]
- 27.Gasinska A, Urbanski K, Adamczyk A, et al. Prognostic significance of intratumour microvessel density and haemoglobin level in carcinoma of the uterine cervix. Acta Oncol. 2002;41:437–443. doi: 10.1080/028418602320405023. [DOI] [PubMed] [Google Scholar]
- 28.Sirica AE, Gainey TW. A new rat bile ductular epithelial cell culture model characterized by the appearance of polarized bile ducts in vitro. Hepatology. 1997;26:537–549. doi: 10.1002/hep.510260302. [DOI] [PubMed] [Google Scholar]
- 29.Kadoya Y, Yamashina S. Intracellular accumulation of basement membrane components during morphogenesis of rat submandibular gland. J Histochem Cytochem. 1989;37:1387–1392. doi: 10.1177/37.9.2768808. [DOI] [PubMed] [Google Scholar]
- 30.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 31.Jeffes EW, Zhang JG, Hoa N, et al. Antiangiogenic drugs synergize with a membrane macrophage colony-stimulating factor-based tumor vaccine to therapeutically treat rats with an established malignant intracranial glioma. J Immunol. 2005;174:2533–2543. doi: 10.4049/jimmunol.174.5.2533. [DOI] [PubMed] [Google Scholar]
- 32.Ruoslahti E. Vascular zip codes in angiogenesis and metastasis. Biochem Soc Trans. 2004;32:397–402. doi: 10.1042/BST0320397. [DOI] [PubMed] [Google Scholar]
- 33.Ningaraj NS, Rao MK, Black KL. Adenosine 5′-triphosphate- sensitive potassium channel-mediated blood-brain tumor barrier permeability increase in a rat brain tumor model. Cancer Res. 2003;63:8899–8911. [PubMed] [Google Scholar]
- 34.Fulda S, Wick W, Weller M, et al. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 35.Storm PB, Renard VM, Moriarity JL, et al. Systemic BCNU enhances the efficacy of local delivery of a topo-isomerase I inhibitor against malignant glioma. Cancer Chemother Pharmacol. 2004;54:361–367. doi: 10.1007/s00280-004-0800-7. [DOI] [PubMed] [Google Scholar]
- 36.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 37.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]