Abstract
Despite repeated findings of abnormal corpus callosum structure in autism, the developmental trajectories of corpus callosum growth in the disorder have not yet been reported. In this study, we examined corpus callosum size from a developmental perspective across a 30-year age range in a large cross-sectional sample of individuals with autism compared to a typically developing sample. Midsagittal corpus callosum area and the 7 Witelson subregions were examined in 68 males with autism (mean age 14.1 years; range 3–36 years) and 47 males with typical development (mean age 15.3 years; range 4–29 years). Controlling for total brain volume, increased variability in total corpus callosum area was found in autism. In autism, increased midsagittal areas were associated with reduced severity of autism behaviors, higher intelligence, and faster speed of processing (p=0.003, p=0.011, p=0.013, respectively). A trend toward group differences in isthmus development was found (p=0.029, uncorrected). These results suggest that individuals with autism benefit functionally from increased corpus callosum area. Our cross-sectional examination also shows potential maturational abnormalities in autism, a finding that should be examined further with longitudinal datasets.
Keywords: autism, corpus callosum area, development, MRI
1. Introduction
A central long-range goal of in vivo magnetic resonance brain imaging research in autism is to help identify and describe neuropathological changes during development that are linked to the disorder, further understand inter-individual differences in severity, course, and outcome, and discover biological targets for the development of specific treatments (Lainhart and Lange, 2010). Multiple lines of evidence suggest that one of the brain structures most commonly affected in autism is the corpus callosum (CC), the major white matter fiber system connecting the cerebral hemispheres. It remains unclear if CC pathology in autism is primary or secondary, or how risk genes, environmental factors and daily life experience may affect CC development. For autism imaging research to move closer to its goal, several basic uncertainties about pathological development of CC size in autism need to be addressed.
Many studies have suggested that structural pathology contributes to cortical underconnectivity in autism and that there is reduced CC area, volume, and white matter (WM) density in autism compared to typical development (Alexander et al., 2007; Casanova et al., 2011, 2009; Chung et al., 2004; Frazier and Hardan, 2009; Freitag et al., 2009; Hardan et al., 2009; He et al., 2008; Hong et al., 2011; Keary et al., 2009; Kilian et al., 2008; Waiter et al., 2005) with a few exceptions (Elia et al., 2000; Kilian et al., 2008; McAlonan et al., 2002; Rice et al., 2005; Tepest et al., 2010). Genetic research aimed to benefit individuals with autism depends more strongly on individual phenotypes than it does on clinical group means. Although decreased mean CC size is one of the most replicated findings in autism neuroimaging research, the CC size distribution has not been examined in detail and the proportion of affected individuals with abnormally small CC not yet reported. Previous reports show that not all individuals with autism present with larger head circumferences, increased numbers of prefrontal neurons (Courchesne et al., 2011; Lainhart and Lange, 2011), and abnormalities in CC microstructure (Alexander et al., 2007); such is likely the case for small CC size. Decreased CC size appears most evident when it is considered relative to total brain volume (TBV; BogerPage Megiddo et al., 2006; Just et al., 2007), though an exception has been found in a high-functioning adult sample (Tepest et al., 2010). However, scaling CC size to TBV in autism and the age-invariance of such scaling have not yet been examined. Similarly, although studies report atypically decreased mean CC size in individual samples of young children, older children, adolescents, and adults with autism, information about age-related changes in CC area is limited (Chung et al., 2004). Other investigators have shown the danger of assuming a typical relationship between age and changes in WM tracts in individuals with developmental neuropsychiatric disorders (Jones et al., 2006).
Clinico-pathological studies using structural MRI have suggested a relationship between CC size and clinical features of autism (Hardan et al., 2009; Keary et al., 2009). Functional magnetic resonance imaging (fMRI) studies have shown correlations between the size of CC subregions and functional connectivity measured during tasks that tap cognitive skills frequently impaired or relatively preserved in autism (Damarla et al., 2010; Just et al., 2007, 2004; Kana et al., 2009, 2006; Keary et al., 2009; Mason et al., 2008; Schipul et al., 2011). Nonetheless, the relation between severity of core diagnostic features of autism, intelligence quotient and CC size from childhood into adulthood is not yet known.
In this study we examined CC size from a developmental perspective across a 30-year age range in a large cross-sectional sample of individuals with autism. Consistent with the underconnectivity theory of CC involvement in autism, we hypothesized 1) smaller mean CC size in autism, especially when considered relative to TBV, and 2) association of small CC size with more severe core features of autism, lower IQ, and slower processing speed. In addition, we predicted that there are 3) atypical age-related changes in CC size, and 4) differences in the distribution of CC area in the autism group.
2. Materials and Methods
2.1 Participants
Sixty-eight (68) individuals with autism spectrum disorder (lifetime diagnosis autistic disorder in 62, PDD-NOS in 6) and 47 typically developing controls were selected from a large, ongoing neuroimaging study. Participants were selected if they met the following inclusion criteria: male; age between 3 and 36 years; performance IQ ≥70; quantitative handedness score, which ranges from completely left-handed (-100) to completely right-handed (+100), ≥ 0; and very good quality of scan at the 1st wave of data collection. The inclusion criteria were chosen to decrease heterogeneity other than age in the autism sample and potential associated neuroanatomic heterogeneity, and, as a result, increase statistical power. Handedness in particular may be associated with CC morphology in both typical and atypical development (Gilliam et al., 2011; Witelson, 1985).
Diagnoses of autism were based on the Autism Diagnostic Interview-Revised [ADI-R; (Lord et al., 1994)], Autism Diagnostic Observation Schedule-Generic [ADOS-G; (Lord et al., 2000)], and DSM-IV (American Psychiatric Association, 1994). Participants were excluded if history, Fragile-X gene testing, karyotype, or examination identified medical causes of autism or other medical conditions that could affect brain morphometry, such as history of severe head injury, hypoxia-ischemia, seizures, and other neurologic disorders. Forty-one percent of the autism participants were taking psychotropic medication (28% serotonin reuptake inhibitor, 13% stimulant, 9% neuroleptics, 1.5% atypical agents, 16% multiple medications). Possible effect of psychotropic use on CC size was explored in the data analysis.
Control participants underwent neuropsychological testing, standardized psychiatric assessments (Leyfer et al., 2006), and were assessed with the ADOS-G (Lord et al., 2000) to confirm typical development. Controls with any history of developmental, learning, cognitive, neurological, or neuropsychiatric conditions were excluded. All autism and control participants were recruited, assessed, and scanned at the University of Utah.
2.2 Assessments
Severity of Core Features of Autism
ADOS-G Algorithm scores were used as estimates of autism severity (Lord et al., 2000). Because all participants were cognitively high-functioning and standardized methods are not yet available to calculate severity scores across all 4 modules (Gotham et al., 2009), raw Social scores were used and Communication and Total algorithm scores across modules were equated by prorating.
IQ
Verbal (VIQ) and performance IQ/non-verbal ability (PIQ) were ascertained with the Differential Abilities Scale (DAS) or WISC-III in children, and the WAIS-III in adults (Elliott, 1990; Wechsler, 1997, 1991). For the DAS, VIQ was estimated from the Verbal Cluster, and PIQ estimated from the Nonverbal Cluster (preschool) and Special Nonverbal Composite (school-age) Standard Scores.
Processing Speed
A difference score from the Trail Making Test (Trails B – Trails A) was used as a measure of processing speed (Lezak et al., 2004). This score removes the common motor component, providing an estimate in processing time to complete attention set shifting, working memory, and executive functioning required by the more complex Trails B (Sanchez-Cubillo et al., 2009). Both the child (age <15 years) and adult (age ≥15 years) Trail Making Test versions were administered and results examined separately.
Head circumference
It was measured as maximal occipital frontal head circumference.
Handedness
The Edinburgh Handedness Inventory (Oldfield, 1971) was used.
2.3 Magnetic Resonance Imaging
Magnetic resonance images were acquired on a single Siemens Trio 3.0 Tesla Scanner. An 8-channel, receive-only RF head coil was used to acquire 3D T1-weighted image volumes with 1mm isotropic resolution using an MP-RAGE sequence (TI=1100msec, TR=1800msec, TE=2.93msec, flip angle=12degrees, sagittal, field of view=25.6cm, matrix=256×256×160).
2.3.1 Corpus Callosum Measurement
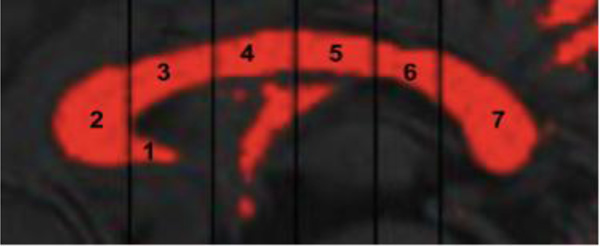
DICOM images were converted to ANALYZE® format (Robb, 2001, 1995) and re-sampled to a 1mm3 volume. The midsagittal slice was selected in MRIcro (Rorden and Brett, 2000). Images were imported into ImageJ (Rasband, 1997–2011) and the midsagittal image was rotated (pitch-adjusted) such that a horizontal line was fit from the most anterior to the most posterior CC. Manual thresholding classified white matter voxels. The CC was subdivided into 7 Witelson regions using an automated macro (see Figure 1; Witelson, 1989). The CC was measured 4 times per individual. Two independent raters (MBDP, TLM) blind to diagnosis and age measured each CC twice and intra-rater CC areas were averaged. Final CC areas were calculated by averaging inter-rater areas. Intra-rater reliability was ICC > 0.95 and inter-rater reliability ICC > 0.90.
Figure 1.
Sample image of midsagittal corpus callosum area and Witelson regions. Subregions are labeled as follows: (1) rostrum, (2) genu, (3) rostral body, (4) anterior midbody, (5) posterior midbody, (6) isthmus, (7) splenium
2.3.2 Brain and Total Intracranial Size Measurement
Total brain volume (TBV) and total intracranial volume (TICV) were generated using Freesurfer version 4.3.1. TBV included GM and WM of the forebrain and hindbrain (cerebellum and brainstem). Although only the forebrain is directly connected by the CC, 95% of the variance in TBV as measured is explained by forebrain volume (Jancke et al., 1997).
2.4 Statistical Analyses
Linear regression models were used to assess group differences in demographics and brain measures. Models examining group differences in total CC area and the seven subregions included the following:
CC area = Intercept + β1*group + β2*age + β3*age2 + β4*group by age + ε
Age was centered and quadratic age was included to allow for nonlinear CC growth with age (Giedd et al., 1999). The inclusion of a group by age2 interaction term was based on model fit according to the Akaike Information Criterion (AIC; Akaike, 1974). The inclusion of handedness did not improve the model fit. Analyses were repeated including TBV as a covariate because evidence suggests the CC is smaller than expected for TBV in autism (Boger-Megiddo et al., 2006). IQ, and group by IQ interaction effects were also examined. The relationships between CC area and autism severity and processing speed measures were examined using linear regressions controlling for age and TBV. A Bonferroni correction of p=0.05/7=0.007 was employed to calculate a significance value that controlled for multiple comparisons of the seven CC subregions.
Within group linear regressions were also used to examine the relationship between CC area and TBV. Total CC area and a ratio of total CC area/TBV were examined. Centered age, age2, TBV, TBV2 and TBV by age interactions were included as potential covariates. In accordance with the study byJancke et al. (1999), our descriptive correlations between TBV and CC area and ratio of CC/TBV were performed in our groups split into child (age ≤14) and adult (age ≥18) samples.
To estimate the distributions of CC size in the autism group relative to our control sample, we calculated age-normalized z-scores for total CC area and the ratio of total CC area/TBV, using our control data as the reference data in four age-bins (3–5yr, 6–11yr, 12–18yr, 19+yr). Levene’s Test of Homogeneity of Variance was used to examine group differences in the variances of the z-score distributions. Participants were also classified as having an abnormally large or small CC (“macro-CC” and ”micro-CC”, respectively) using standard clinical criteria for abnormality of size of a structure: z-scores > 1.88 standard deviations from the control mean (Lainhart et al., 2006). Group differences in rates of macro-CC and micro-CC were tested with independent samples t-tests. Analyses were performed in PASW Statistics 18.0.
3. Results
As indicated in Table 1, the sample groups do not significantly differ in age or handedness, and IQ is decreased in the autism group. After controlling for age, no group differences are found in mean head circumference, TBV, or total intracranial volume (TICV).
Table 1.
Demographic Information
| Autism n=68 |
Control n=47 |
Group Difference |
||||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | t | p | |
| Age (years) | 14.1 (7.5) | 3–36 | 15.3 (6.5) | 4–29 | 0.9 | nsg |
| Handednessa | 79.8 (22) | 0–100 | 76.7 (26) | 0–100 | 0.6 | ns |
| VIQb | 99.8 (23) | 51–145 | 116.7 (14) | 91–151 | 5.1 | <0.001 |
| PIQc | 101.2 (16) | 70–135 | 117.8 (15) | 90–155 | 5.2 | <0.001 |
| HCd | 55.2 (2.7) | 49–60.7 | 55.4 (2.2) | 51.6–59.3 | 0.1 | ns |
| TBVe | 1442 (143) | 1049–1735 | 1469 (122) | 1242–1889 | 1.1 | ns |
| TICVf | 1669 (149) | 1311–1991 | 1713 (168) | 1399–2171 | 1.2 | ns |
Edinburgh Handedness Inventory (Oldfield 1971): ranges from -100 (left handed) to +100 (right handed);
Verbal IQ; control N=47, ASD N=67;
Performance IQ/Non-verbal Ability;
Head Circumference in cm;
Total Brain Volume in cm3;
Total Intracranial Volume in cm3;
ns indicates p-values > 0.2
3.1 Typically Developing Sample
Because the generalization of the results of our case-control comparisons depends on the representativeness of our control group, we compared our control sample to published findings in other typically developing samples. Age-related changes in CC size in our control group are similar to changes in two very large mixed cross-sectional and longitudinal typically developing samples from other studies (Giedd et al., 1999; Gilliam et al., 2011). Similar to these larger samples, our control group shows an anterior-posterior gradient of age-related increase in CC area from early childhood to the early twenties (Section 3.2.2). Compared to other typically developing samples, our control group shows similar scaling of CC area to TBV (Jancke et al., 1999, 1997; Section 3.2.4), a roughly symmetric distribution of age-standardized CC area particularly when adjusted for TBV (Section 3.2.5), and negative correlations between CC area and intellgence quotient (IQ) (Ganjavi et al., 2011; Luders et al., 2011) and positive correlations with processing speed (Jancke and Steinmetz, 1994; Section 3.2.6).
3.2 Autism Sample
3.2.1 Mean Corpus Callosum Areas
Table 2 provides CC group means and group effects from linear models controlling for linear and nonlinear age and group by age interactions. In the total CC, rostrum, genu, rostral body, anterior midbody, and isthmus, the inclusion of the group by age2 interaction term improved model fit. Total CC, isthmus, and splenium areas are smaller in the autism group than the control group. After adjusting for TBV, the total CC and isthmus remains smaller, suggesting that case-control differences are not due to global TBV effects. None of these results survive our correction for multiple comparisons (p<0.007). Neither PIQ nor VIQ were significant predictors of CC area but the inclusion of PIQ improved model fit for the rostrum only, where a group by IQ interaction emerged. This finding is discussed in Section 3.2.6.
Table 2.
Corpus Callosum Midsagittal Area (cm2)
| Group Means | Group Differenceb | |||||||
|---|---|---|---|---|---|---|---|---|
| Autism n=68 |
Control n=47 |
Unadjusted for TBV |
Adjusted for TBV |
|||||
| Mean (SD) | Range | Mean (SD) | Range | t | p | t | p | |
| Corpus Callosum | 5.74 (0.91) | 3.71–8.49 | 6.25 (0.88) | 4.43–7.71 | 2.4 | 0.015 | 2.3 | 0.019 |
| Rostrum (1)a | 0.18 (0.08) | 0.05–0.43 | 0.19 (0.07) | 0.05–0.36 | 0.8 | nsc | 0.5 | ns |
| Genu (2) | 1.30 (0.29) | 0.66–2.27 | 1.42 (0.28) | 0.91–2.22 | 1.9 | 0.055 | 1.7 | 0.081 |
| Rostral body (3) | 0.81 (0.15) | 0.54–1.14 | 0.88 (0.15) | 0.63–1.26 | 0.4 | ns | 0.2 | ns |
| Anterior midbody (4) | 0.68 (0.13) | 0.38–1.12 | 0.74 (0.12) | 0.51–0.97 | 1.6 | 0.093 | 1.5 | 0.136 |
| Posterior midbody (5) | 0.64 (0.12) | 0.40–1.02 | 0.68 (0.12) | 0.40–0.90 | 1.1 | ns | 0.7 | ns |
| Isthmus (6) | 0.48 (0.11) | 0.26–0.70 | 0.52 (0.11) | 0.23–0.83 | 2.4 | 0.016 | 2.3 | 0.021 |
| Splenium (7) | 1.65 (0.30) | 1.07–2.50 | 1.81 (0.30) | 1.19–2.61 | 2.1 | 0.043 | 1.9 | 0.065 |
(Corresponding Witelson subdivision);
Group effect from linear regressions controlling for linear and quadratic age, group by age interaction (all regions), and group by age2 interaction (Total CC and regions 1,2,3,4,6) unadjusted and adjusted for TBV;
ns indicates p-values > 0.2
3.2.2 Age-related Changes in Corpus Callosum Area
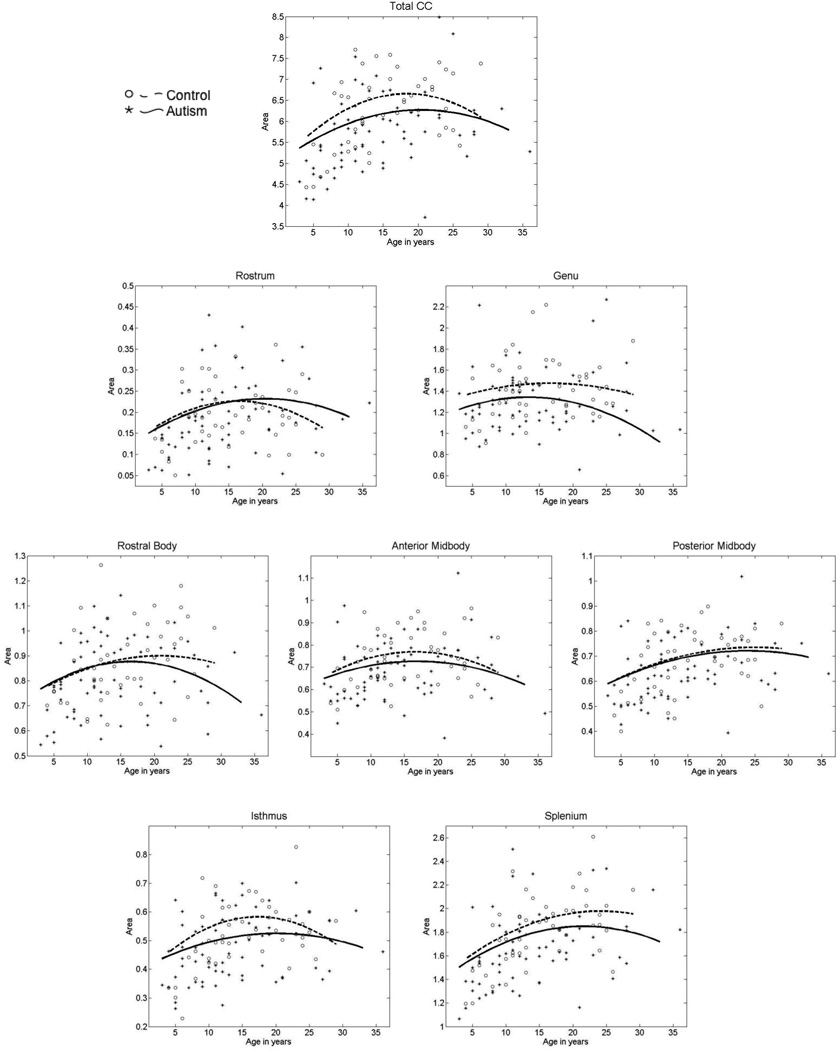
Figure 2 shows the cross-sectional age-related changes in the total CC and subregional areas for the autism and control groups. In the total CC area, similar age-related changes were found in autism. Figure 2 suggests that the trend toward decreased mean total CC area in the case-control analysis appears due to slower-than-typical age-related increase during late childhood and adolescence.
Figure 2.
Cross-sectional age-related changes in total corpus callosum area and Witelson subregions unadjusted for TBV. Smooth lines show development in autism participants (solid line and stars) versus typically developing controls (dashed line and open circles).
Anterior corpus callosum: rostrum and genu
Similar age-related changes were found in autism to typical development in the rostrum and genu. The trend toward smaller genu area in autism (p=0.08 controlling for TBV) appears to be present from early development.
Body of the corpus callosum
The rostral body, anterior midbody and posterior midbody all showed similar cross-sectional age-related changes to typical development.
Posterior corpus callosum: isthmus and splenium
The isthmus was the only subregion where cross-sectional age related changes differed in autism relative to typical development (group by age2 t=2.2, p=0.029, uncorrected). Visual inspection of Figure 2 suggests that there is a lack of expected increase in isthmus area in autism during childhood and adolescence found in typical development. Although no significant group by age interactions were found in the splenium, visual inspection of Figure 2 suggests that the trend toward decreased mean area in autism is possibly related to lack of normal growth during childhood.
3.2.3 Age-related Changes in Total Brain Volume
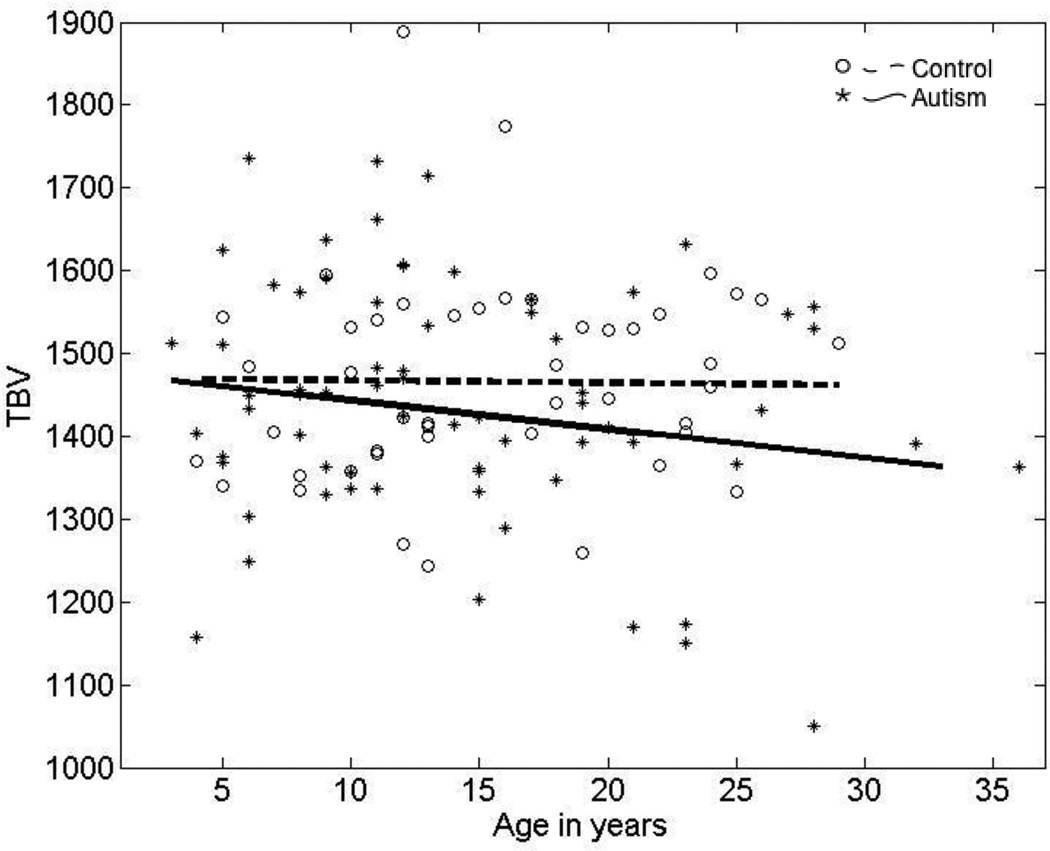
Our findings are similar to results reported previously in published cross-sectional analysis of TBV in autism: a tendency toward larger mean TBV in younger children with autism, followed by an atypically low rate of subsequent growth in later childhood and resulting in similar or decreased mean TBV compared to typically developing controls by young adulthood (Figure 3).
Figure 3.
Cross-sectional age-related changes in TBV in the autism and typically developing participants.
3.2.4 Relationship of Corpus Callosum Area to Total Brain Volume
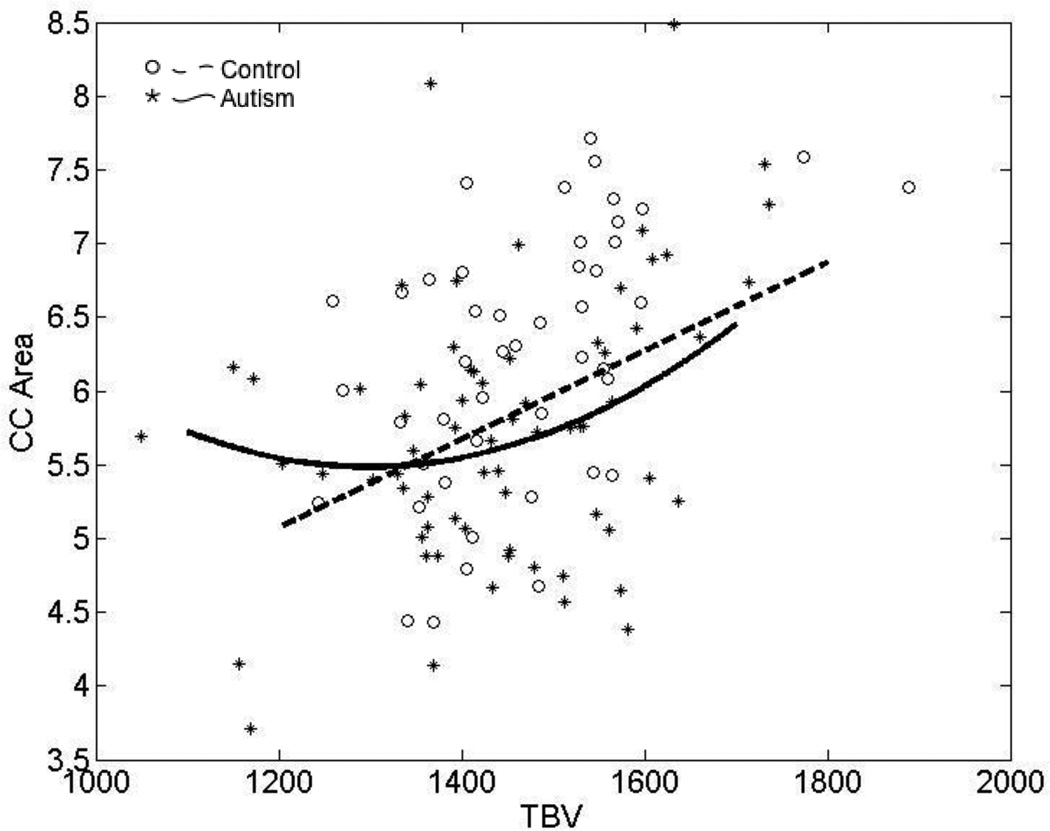
Figure 4 is a scatterplot of total CC area and TBV in the autism and control samples. CC area is related to TBV in a strongly linear manner in the typically developing control sample (t=3.8, p<0.001). In both autism and control samples, bivariate correlations between total CC area and TBV are higher during childhood, as expected when both CC area and TBV increase (typical children age <15 years, n=23, r=0.52, p=0.010; typical adults age ≥18 years, n=18, r=0.20, p=0.431; autism children, n=39, r=0.53, p<0.001; autism adults, n=20, r=0.36, p=0.113).
Figure 4.
Scatterplot demonstrating the relationship between total corpus callosum area and TBV in the autism and typically participants.
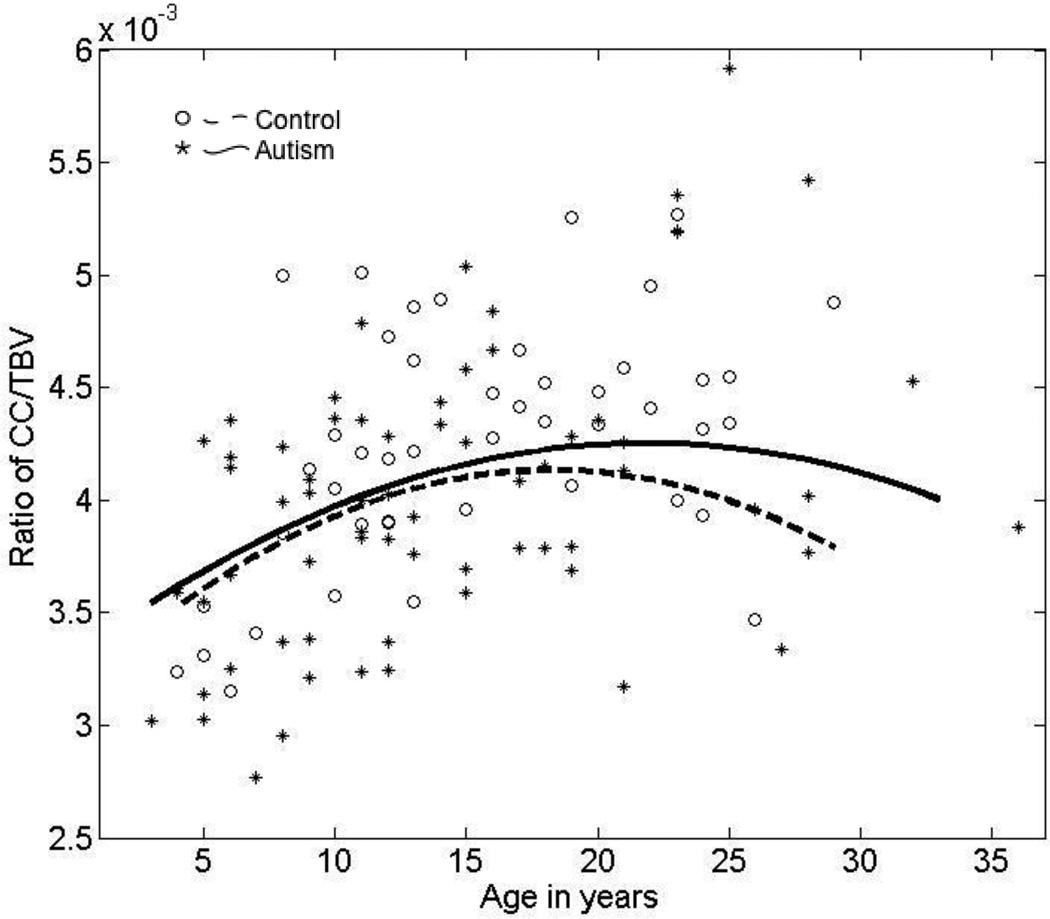
To examine scaling of CC and TBV across the broad age range, we examined the relationship between the ratio of total CC to TBV and age in the autism and control groups (Figure 5). A significant and common nonlinear quadratic age effect (t=3.0, p=0.003) is found in both groups; there is no significant group effect or group by age interaction. These findings suggest scaling of CC and TBV is similar in autism and typical development.
Figure 5.
Scatterplot of cross-sectional age-related changes in the ratio of total corpus callosum area to TBV in the autism and typically developing groups.
We then studied the relationship between CC area and TBV in small vs. large brains (Jancke et al., 1999, 1997) by first examining the relationship between total CC area/TBV and TBV. In regression analysis, TBV does not significantly predict CC/TBV in the typical controls (p=0.55) and there is no age by TBV interaction. When correlations between CC/TBV and TBV are examined in typical children and adults separately, there is a trend for a significant negative correlation in adults (≥ 18 years, r=−0.462, p=0.054) but not in children (≤ 14 years, r=-0.052, p=0.81). In the autism group, the relationship is more clear-cut with larger TBVs predicting a lower CC/TBV ratio than smaller TBVs. There are significant linear (t=2.2, p=0.020) and quadratic TBV (t=2.2, p=0.030) effects on CC area/TBV in the autism group, but the results do not survive Bonferroni correction.
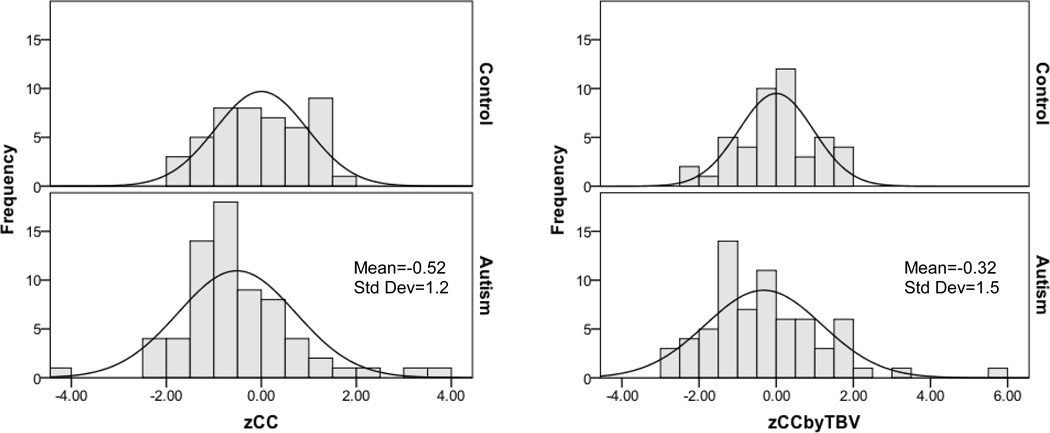
3.2.5 Distribution of Corpus Callosum Area
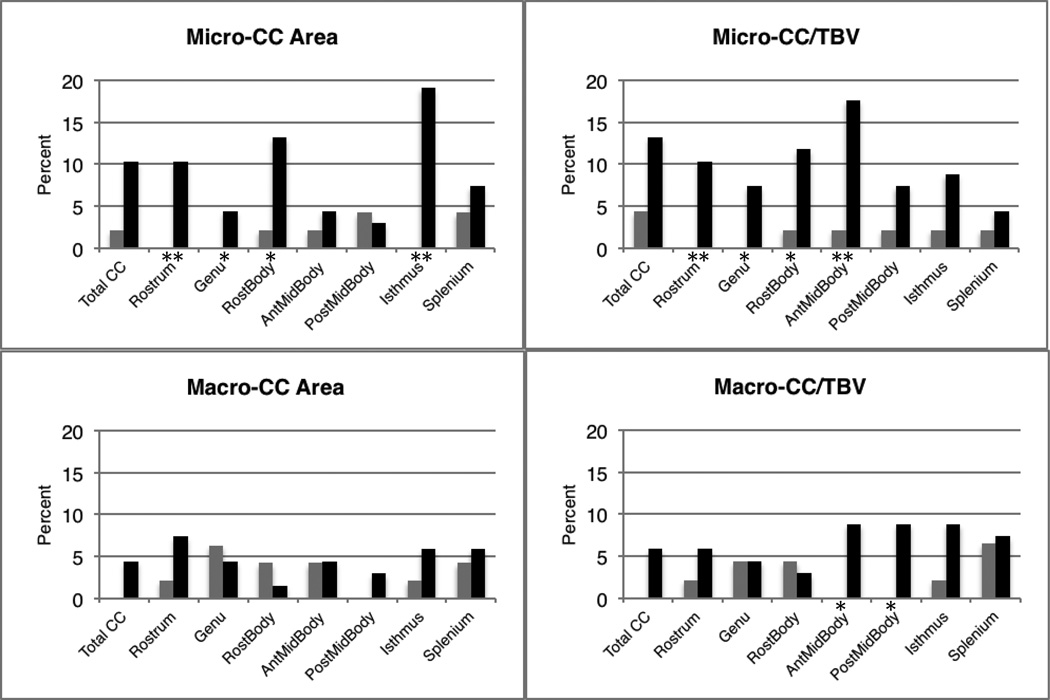
Figure 6 shows the distributions of age-standardized total CC areas (zCC, defined in Section 2.4). The estimated distributions of total standardized CC area in autism, in both the unadjusted (zCC) and adjusted for TBV (zCCbyTBV) conditions, show a mean shift to the left and increased variance relative to the typical control sample for the zCCbyTBV condition only (F=5.6, p=0.019). Figure 7 shows the percentage of autism and control participants with abnormally small and large CC total and subregional areas (defined in Section 2.4 as 1.88 SD below or above the typical group). The rates of abnormally small area are increased for several CC subregions in the autism group compared to the control group. Surprisingly, several individuals with autism show abnormally large areas of two CC subregions, the anterior and posterior midbody, relative to TBV.
Figure 6.
Distribution of age-standardized corpus callosum area in the control and autism samples unadjusted (zCC) and adjusted for TBV (zCCbyTBV).
Figure 7.
Bar charts displaying the percentage of individuals with micro-CC (> 1.88SD below control corpus callosum areas normalized by age) or macro-CC (> 1.88SD above control corpus callosum areas normalized by age). CC area unadjusted for TBV and ratio of CC/TBV are shown. Gray bars represent control participants, black bars represent autism participants. Significant differences between group percentages are indicated: *significant difference between groups at p<0.05, **significant at p≤0.007
 Control
Control
■ Autism
3.2.6 Relationship of Corpus Callosum Area to Autism Severity, Intelligence, and Processing Speed
Autism Severity
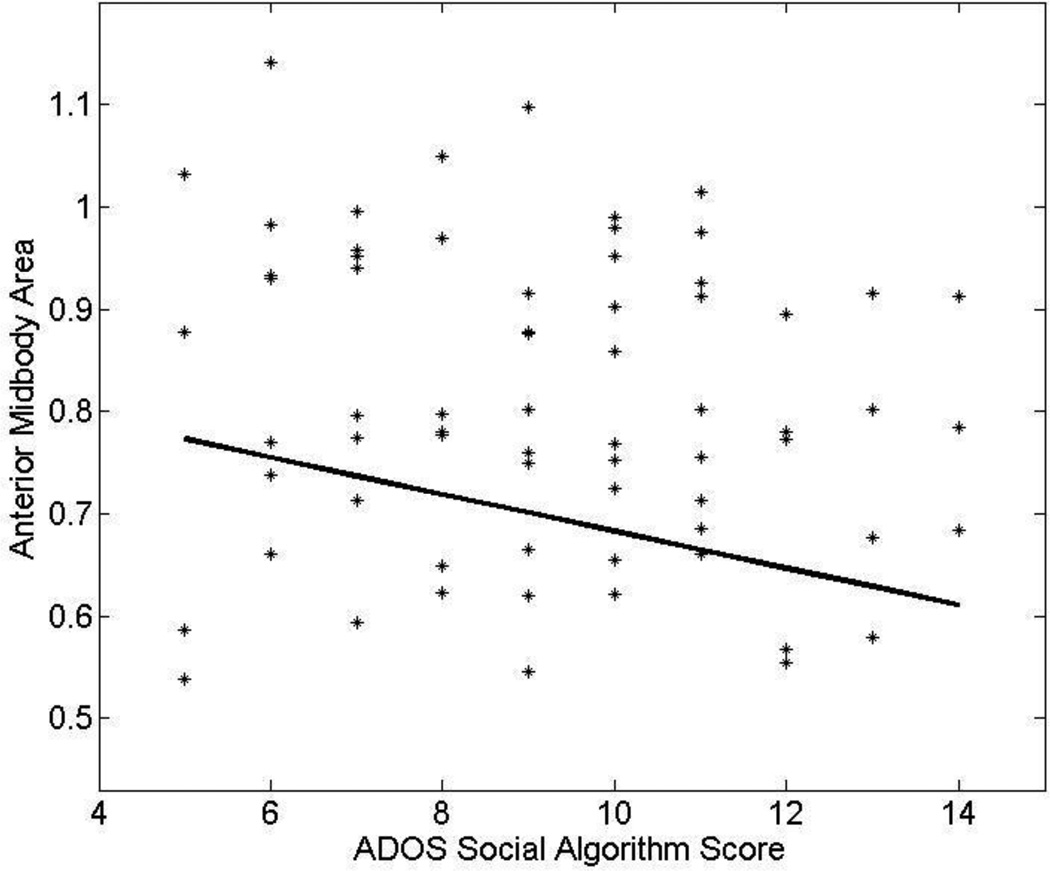
In the autism group, we examined the relationship between areas of total CC and the seven CC subregions and ADOS social and communications algorithm scores. ADOS social scores are related significantly to anterior midbody (t=-3.1, p=0.003) and genu area (t=−2.1, p=0.040); the former survived Bonferroni correction. Figure 8 provides evidence that higher (more severe) ADOS social algorithm scores are associated with smaller midsagittal anterior midbody area (correlation controlling for age r=−0.36, p=0.003). Lack of a significant interaction between age and CC area suggests the significant relationship between anterior midbody and severity of core social features is similar across the broad age range of the individuals with autism studied. No significant relationships between ADOS communication scores and CC subregions are found.
Figure 8.
Scatterplot demonstrating the relationship between anterior midbody area and ADOS Social algorithm scores in autism adjusting for age. Larger midbody areas are associated with lower Social algorithm scores.
Intelligence
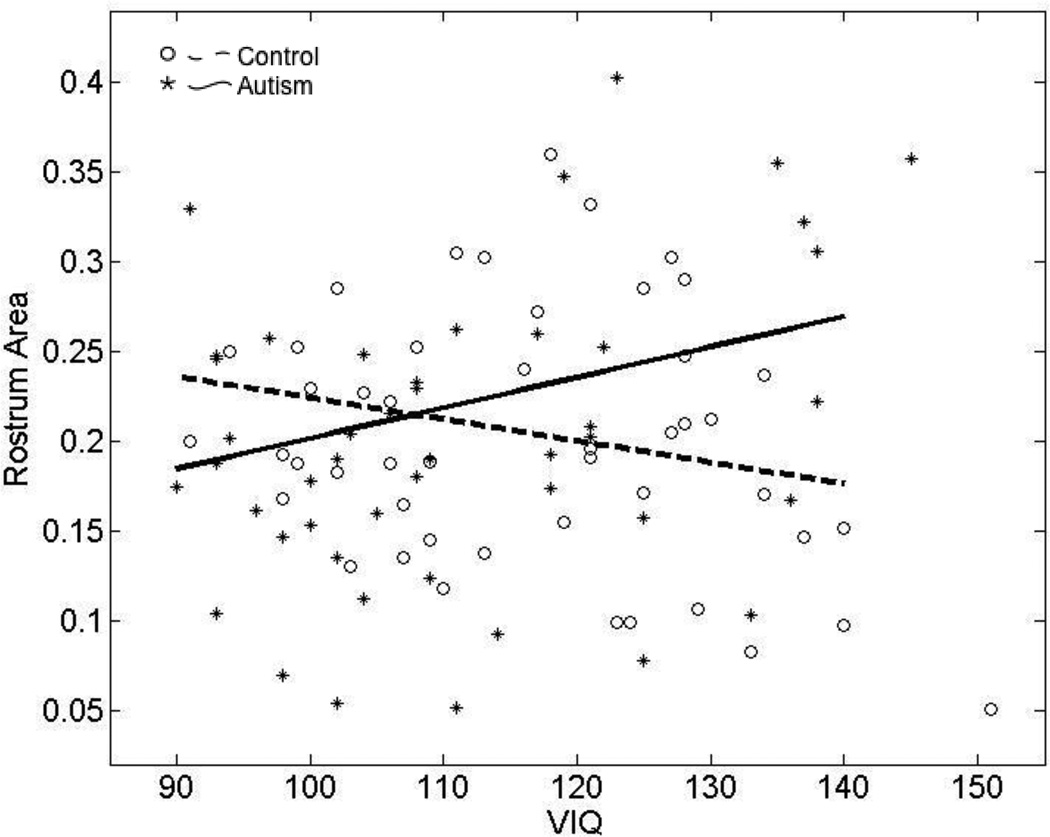
We first determined if differences in VIQ and PIQ in the autism and control groups may have affected the case-control comparison of mean CC areas. Including VIQ and PIQ separately in our regression analysis results in an improved model fit for the rostrum only, where a group effect emerges (VIQ analysis: group effect t=2.5, p=0.011; PIQ analysis: group effect t=2.1, p=0.031). VIQ or PIQ by itself does not predict CC midsagittal area but group by VIQ and group by PIQ interactions suggest the relationship between IQ and rostral area is different in the autism and control groups (VIQ analysis: group by VIQ interaction t=2.7, p=0.007; PIQ analysis: group by PIQ interaction t=2.3, p=0.022). The group by VIQ interaction survives multiple comparisons. Results are similar when only participants with autism and VIQ > 90, in the range of the typically developing group, are included in the analysis. Figure 9 shows decreased rostral area associated with increased VIQ in the typically developing group but decreased VIQ in the autism group.
Figure 9.
Scatterplot showing the relationship between rostrum area and VIQ in autism and typical development. Larger rostrum area is associated with higher VIQ in autism and lower VIQ in controls.
Processing Speed
Increased isthmus area predicts faster processing speed (t=2.6, p=0.013) in the autism sample only (correlations: autism r=−0.40, p=0.041; control r=−0.33, p=0.147). Processing speed is significantly correlated with total CC area and posterior midbody area in controls but not the autism group (total CC: controls r=-0.57 p=0.007, autism r=−0.03 p=0.86; posterior midbody: controls r=−0.50 p=0.021, autism r=0.06, p=0.78).
There are case-control differences in participants tested with the adult version of the Trail Making Test (age ≥15 years; autism n=27, control n=22) but not the child version (autism n=18, control n=17). Significantly slower processing speed is found in autism (t=3.7, p=0.001). Processing speed improves with age from mid-adolescence into adulthood in the control group but not in the autism group (control r=−0.48, p=0.025; autism r=−0.18, p=0.37).
4. Discussion
This study has presented a collection of new findings regarding atypical and typical age-related changes in corpus callosum (CC) areas, increased rates of abnormally small subregions even in the absence of case-control differences in mean area, an association between anterior midbody area and severity of impairment in the social domain, and atypical relationships between area and verbal IQ and processing speed in individuals with autism. Overall, we found evidence of pathology involving CC area in autism to be more complex than previously described. Most importantly, we found evidence of multiple functional implications of atypical changes in CC area in autism.
4.1 Age-related changes
Similar to longitudinal MRI studies of typical development (Giedd, 2004; Giedd et al., 1999; Gilliam et al., 2011), our cross-sectional sample of typically developing individuals had an anterior-to-posterior gradient of age-related increase in midsagittal CC area. During later childhood, adolescence, and young adulthood, area increased mainly in posterior regions. Although our autism sample showed typical age-related change the CC as a whole and most subregions, these changes were atypical in the isthmus. The tendency toward decreased area in total CC, the genu, isthmus and splenium, present by late childhood and persisting into adulthood in our autism sample, is consistent with results of a previous 2-year follow-up study of CC volume in adolescence in autism (Frazier et al., 2012).
Atypical age-related isthmus changes in the autism sample were complex. In the autism sample, the isthmus appeared to increase at a much slower rate than the typically developing group during later childhood and adolescence. Fibers traversing the isthmus connect superior temporal and parietal regions in the two hemispheres (Pannek et al., 2010; Park et al., 2008; Witelson, 1989; Zarei et al., 2006), regions strongly implicated in idiopathic autism (Bigler et al., 2007, 2003; Boddaert et al., 2004; Hoeft et al., 2011; Lange et al., 2010; Lee et al., 2009, 2007).
4.2 Mean Area and the Distribution of Corpus Callosum in Autism
Smaller mean area in autism was found in the total CC, isthmus, and splenium, although case-control differences were modest and did not survive correction for multiple comparisons. Although a meta-analysis of CC size in autism found case-control mean difference effect sizes largest in the anterior part of the CC (Frazier and Harden, 2009), studies using voxel based morphometry (VBM) have shown strong effects both anteriorly and in the splenium (Chung et al., 2004; Vidal et al., 2006).
Examination of the distribution of CC areas provided additional information. Increased variance was observed in the autism distribution while controlling for TBV and increased rates of abnormally small CC subregional areas were found even in subregions not showing group differences in mean area. The proportions of individuals with abnormally small CC areas in the anterior third of the CC and the isthmus were increased in the autism group. Surprisingly, increased variation in the autism sample was not only due to a tendency toward small CC areas. The middle third of the CC areas were larger than expected given TBV. Similar to the distribution of head circumference in autism (Lainhart et al., 2006), the distribution of CC area in autism is abnormally wide.
A number of reasons for discrepant results in past studies of CC area in autism were apparent, in addition to known effects of gender and handedness. Our data support past reports suggesting clinical heterogeneity in IQ and severity of autism may affect results (Boger-Megiddo et al., 2006; Elia et al., 2000; McAlonan et al., 2002), and strongly implicate the effect of age and stage of development (Frazier and Hardan, 2009; Tepest et al., 2010). The wide distribution of CC area in autism suggests studies using small samples will, by chance alone, differ in the proportion of subjects with small CC area. Given the known variability of CC area in normative samples (Giedd et al., 1999), representativeness of control groups can also affect the results of case-control studies particularly when sample sizes are small. Our data provide additional evidence of a complex interaction between CC area and TBV. Scaling of CC area to TBV differs in individuals with autism who have larger versus smaller brains (Kilian et al., 2008; Rice et al., 2005), a finding consistent with those ofJancke et al. (1997), who found that CC area increased proportionately less in typical individuals with larger total brain volumes.
4.3 Clinical Correlations
CC pathology, manifested by atypical changes in cross-sectional area, was related to clinically important types of inter-individual variation in the autism sample. Variations in social impairment, IQ, and processing speed were related to variations in the area of CC subregions. Age-invariance of the relationships between CC area and social impairment and IQ suggests that a biological link between CC area and these clinical features is established early in childhood and persists into adulthood.
4.3.1 Smaller Corpus Callosum Area and Greater Social Impairment in Autism
Inter-individual variation in severity of social impairment, as measured by the ADOS social algorithm score, was associated with variation in area of the anterior midbody. Smaller anterior midbody area was associated with more severe social impairment in autism. Despite this association, only a trend toward smaller anterior midbody area in autism was found in the group comparison. This trend may be due to a number of different factors, one of which may be the high level of functioning in the autism group. These results are in agreement with other studies suggesting a relationship between smaller CC volume and more severe impairment of core diagnostic features of autism (Hardan et al., 2009), worse performance on related neuropsychological tasks (Keary et al., 2009), lower fMRI functional connectivity while performing tasks (Mason et al., 2008; Schipul et al., 2011), and decreased interhemispheric transfer of information (Hardan et al., 2009).
The anterior midbody contains fibers connecting the premotor and supplementary motor cortices and part of the pars opercularis of the inferior frontal gyrus (Hofer and Frahm, 2006; Pannek et al., 2010; Witelson, 1989; Zarei et al., 2006). Anterior midbody connections are thus involved in guidance, planning, coordination of motor movements, and in language. Reduced gray matter in the pars opercularis in individuals with autism or Asperger’s disorder has been associated with more impairment in a social communication factor that included imitation and verbal and nonverbal communication (Yamasaki et al., 2010).
4.3.2 Smaller Corpus Callosum Area and Lower Intelligence in Autism
Rostrum area was associated with verbal IQ in our typically developing and autism samples but in different directions: smaller rostrum area was associated with higher verbal IQ in controls but with lower verbal IQ in autism. The rostrum contains interhemispheric fibers connecting homotopic orbitofrontal cortex and the subgenual part of the ventromedial prefrontal cortex (Hofer and Frahm, 2006; Huang et al., 2005; Pannek et al., 2010; Peltier et al., 2010a; Witelson, 1989; Zarei et al., 2006). Volumetric abnormalities have been found in the orbitofrontal cortex in autism (Girgis et al., 2007; Hardan et al., 2006). The rostrum also contains heterotopic fibers connecting orbitofrontal cortex with contralateral temporal pole and running through the temporal stem, the “callosal radiations of Peltier” (Peltier et al., 2010a, 2010b). Temporal pole and orbitofrontal cortex are both important in social cognition, particularly inferring the mental state of others (Kringelbach, 2005; Vollm et al., 2006), a cognitive process involved in Theory of Mind and empathy, impaired in many children and adults with autism (Baron-Cohen and Belmonte, 2005).
This opposing structure-function relationship between the groups suggests that CC subregions may be similar in size but have very different functional correlates and neurodevelopmental mechanisms. Similar group differences between structure and function has been found in cortical thickness and IQ in individuals with autism and typically developing controls, interestingly in the orbitofrontal cortex (Hofer and Frahm, 2006; Huang et al., 2005; Pannek et al., 2010; Witelson, 1989; Zarei et al., 2006). Thinning of the cortex with age was previously associated with increased IQ in typical controls but not autism spectrum disorder subjects (Misaki et al., 2012). Our data suggest that smaller rostrum area may be functionally adaptive in typically developing controls and functionally maladaptive in autism.
4.3.3 Smaller Corpus Callosum Area and Slower Processing Speed in Autism
We found slower processing speed in autism during adolescence and adulthood, as in similar previous studies (Kleinhans et al., 2005; Minshew et al., 2002; Rumsey and Hamburger, 1988). Case-control differences in processing speed, as measured by the Trail Making Test, were not evident in young individuals in our study. Processing speed improved between mid-adolescence and adulthood in the typically developing group but not in the autism group. In the typical group, processing speed had a strong linear relationship with total CC area, apparently driven by posterior midbody area. In adolescents and adults with autism, smaller isthmus area was associated with slower processing speed. A previous study by our group found a strong relationship between processing speed and CC diffusion tensor imaging (DTI) measures (radial diffusivity in particular; Alexander et al., 2007). Although microstructural properties of the CC cannot be identified by area alone, future studies linking multi-modal imaging will help identify properties of the CC that may be developing abnormally in autism.
4.4 Limitations
The most important limitation of the study is the cross-sectional nature of the dataset, the results require verification by longitudinal data. In addition, only males with autism with general cognitive normality (PIQ>70) were studied; thus, findings may not apply to females, individuals with low-functioning autism, or very young or old individuals with the disorder. Future research will benefit from the use multimodal imaging measures of CC growth, development, pathology, and function beyond CC area. Postmortem studies of the corpus callosum are needed to determine the microscopic histological basis of abnormalities identified using in vivo neuroimaging. Finally, demonstrating the specificity of our findings to autism will require comparison to groups of individuals with other neurodevelopmental disorders.
4.5 Implications for Treatment and Intervention
Assuming CC area is an index of structural connectivity in autism, our findings imply that young people with the disorder may benefit from interventions that normalize interhemispheric communication, and CC growth and development. In some regions of the CC, the greatest opportunity for change may continue into the late twenties (Pujol et al., 1993). Given complex excitatory and inhibitory effects of CC fibers on cortical neurons (Innocenti, 2009) and the interaction of interhemispheric connectivity and cerebral lateralization, it is not known if increasing or decreasing interhemispheric connectivity in autism will provide the most benefit.
Highlights.
We examine developmental trajectories of midsagittal corpus callosum area in autism.
Case-control differences in isthmus development are suggested.
Corpus callosum area is more variable in autism compared to typical development.
Increased midsagittal areas are associated with improved functioning in autism.
Acknowledgements
We sincerely thank the participants and families for their time and participation. The project was supported by an Autism Speaks Predoctoral Fellowship (JEL for MBDP), NRSA Predoctoral Fellowship NIH NIDCD F31 DC010143 (MBDP), NIH NIDCD T32DC008553, NIH RO1 MH080826, and NIH RO1 MH084795. Past data collection was supported in part by NICHD/NIDCD U19 HD035476, part of the CPEA. We thank Kathryn Maasberg and Heidi Paine for their assistance and acknowledge contributions of William McMahon, Judith Miller, Michael Johnson, Jubel Morgan and Jeffrey Lu in early stages of this work. The content of this project is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Mental Health, NICHD, NIDCD, or National Institutes of Health.
Abbreviations
- CC
corpus callosum
- TBV
total brain volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19(6):716–723. [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, Miller JN, Lu J, Jeong EK, McMahon WM, Bigler ED, Lainhart JE. Diffusion tensor imaging of the corpus callosum in Autism. NeuroImage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV, 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Baron-Cohen S, Belmonte MK. Autism: a window onto the development of the social and the analytic brain. Annu Rev Neurosci. 2005;28:109–126. doi: 10.1146/annurev.neuro.27.070203.144137. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, Lu J, Provencal SL, McMahon W, Lainhart JE. Superior temporal gyrus, language function, and autism. Developmental Neuropsychology. 2007;31:217–238. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Tate DF, Neeley ES, Wolfson LJ, Miller MJ, Rice SA, Cleavinger H, Anderson C, Coon H, Ozonoff S, Johnson M, Dinh E, Lu J, Mc Mahon W, Lainhart JE. Temporal lobe, autism, and macrocephaly. AJNR American Journal of Neuroradiology. 2003;24:2066–2076. [PMC free article] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthelemy C, Mouren MC, Artiges E, Samson Y, Brunelle F, Frackowiak RS, Zilbovicius M. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. NeuroImage. 2004;23:364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Boger-Megiddo I, Shaw DW, Friedman SD, Sparks BF, Artru AA, Giedd JN, Dawson G, Dager SR. Corpus callosum morphometrics in young children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2006;36:733–739. doi: 10.1007/s10803-006-0121-2. [DOI] [PubMed] [Google Scholar]
- Casanova MF, El-Baz A, Elnakib A, Switala AE, Williams EL, Williams DL, Minshew NJ, Conturo TE. Quantitative analysis of the shape of the corpus callosum in patients with autism and comparison individuals. Autism. 2011;15:223–238. doi: 10.1177/1362361310386506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, El-Baz A, Mott M, Mannheim G, Hassan H, Fahmi R, Giedd J, Rumsey JM, Switala AE, Farag A. Reduced gyral window and corpus callosum size in autism: possible macroscopic correlates of a minicolumnopathy. Journal of Autism and Developmental Disorders. 2009;39:751–764. doi: 10.1007/s10803-008-0681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Dalton KM, Alexander AL, Davidson RJ. Less white matter concentration in autism: 2D voxel-based morphometry. NeuroImage. 2004;23:242–251. doi: 10.1016/j.neuroimage.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Barnes CC, Pierce K. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- Damarla SR, Keller TA, Kana RK, Cherkassky VL, Williams DL, Minshew NJ, Just MA. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism Res. 2010;3:273–279. doi: 10.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia M, Ferri R, Musumeci SA, Panerai S, Bottitta M, Scuderi C. Clinical correlates of brain morphometric features of subjects with low-functioning autistic disorder. Journal of Child Neurology. 2000;15:504–508. doi: 10.1177/088307380001500802. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential Ability Scales: Introductory and Technical Handbook. New York: The Psychological Corporation; 1990. [Google Scholar]
- Frazier TW, Hardan AY. A meta-analysis of the corpus callosum in autism. Biological Psychiatry. 2009;66:935–941. doi: 10.1016/j.biopsych.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Keshavan MS, Minshew NJ, Hardan AY. A Two-Year Longitudinal MRI Study of the Corpus Callosum in Autism. J Autism Dev Disord. 2012 doi: 10.1007/s10803-012-1478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM, Luders E, Hulst HE, Narr KL, Thompson PM, Toga AW, Krick C, Konrad C. Total brain volume and corpus callosum size in medication-naive adolescents and young adults with autism spectrum disorder. Biological Psychiatry. 2009;66:316–319. doi: 10.1016/j.biopsych.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganjavi H, Lewis JD, Bellec P, MacDonald PA, Waber DP, Evans AC, Karama S. Negative associations between corpus callosum midsagittal area and IQ in a representative sample of healthy children and adolescents. PLoS One. 2011;6:e19698. doi: 10.1371/journal.pone.0019698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Gilliam M, Stockman M, Malek M, Sharp W, Greenstein D, Lalonde F, Clasen L, Giedd J, Rapoport J, Shaw P. Developmental trajectories of the corpus callosum in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:839–846. doi: 10.1016/j.biopsych.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis RR, Minshew NJ, Melhem NM, Nutche JJ, Keshavan MS, Hardan AY. Volumetric alterations of the orbitofrontal cortex in autism. Progress in Neuro- Psychopharmacology & Biological Psychiatry. 2007;31:41–45. doi: 10.1016/j.pnpbp.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Girgis RR, Lacerda AL, Yorbik O, Kilpatrick M, Keshavan MS, Minshew NJ. Magnetic resonance imaging study of the orbitofrontal cortex in autism. Journal of Child Neurology. 2006;21:866–871. doi: 10.1177/08830738060210100701. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Pabalan M, Gupta N, Bansal R, Melhem NM, Fedorov S, Keshavan MS, Minshew NJ. Corpus callosum volume in children with autism. Psychiatry Research. 2009;174:57–61. doi: 10.1016/j.pscychresns.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Karsch K, Duan Y. Abnormalities in MRI traits of corpus callosum in autism subtype. Engineering in Medicine and Biology Society, 2008. EMBS 2008; 30th Annual International Conference of the IEEE, 2008; 2008. pp. 3900–3903. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Walter E, Lightbody AA, Hazlett HC, Chang C, Piven J, Reiss AL. Neuroanatomical differences in toddler boys with fragile x syndrome and idiopathic autism. Arch Gen Psychiatry. 2011;68:295–305. doi: 10.1001/archgenpsychiatry.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited-- comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hong S, Ke X, Tang T, Hang Y, Chu K, Huang H, Ruan Z, Lu Z, Tao G, Liu Y. Detecting abnormalities of corpus callosum connectivity in autism using magnetic resonance imaging and diffusion tensor tractography. Psychiatry Res. 2011;194:333–339. doi: 10.1016/j.pscychresns.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, van Zijl PC, Hillis AE, Wytik R, Mori S. DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. NeuroImage. 2005;26:195–205. doi: 10.1016/j.neuroimage.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Innocenti GM. Dynamic interactions between the cerebral hemispheres. Exp Brain Res. 2009;192:417–423. doi: 10.1007/s00221-008-1484-8. [DOI] [PubMed] [Google Scholar]
- Jancke L, Preis S, Steinmetz H. The relation between forebrain volume and midsagittal size of the corpus callosum in children. Neuroreport. 1999;10:2981–2985. doi: 10.1097/00001756-199909290-00020. [DOI] [PubMed] [Google Scholar]
- Jancke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H. The relationship between corpus callosum size and forebrain volume. Cereb Cortex. 1997;7:48–56. doi: 10.1093/cercor/7.1.48. [DOI] [PubMed] [Google Scholar]
- Jancke L, Steinmetz H. Interhemispheric transfer time and corpus callosum size. Neuroreport. 1994;5:2385–2388. doi: 10.1097/00001756-199411000-00043. [DOI] [PubMed] [Google Scholar]
- Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O'Sullivan M, Golesworthy P, McGuire P, Horsfield MA, Simmons A, Williams SC, Howard RJ. Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Hum Brain Mapp. 2006;27:230–238. doi: 10.1002/hbm.20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Soc Neurosci. 2009;4:135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keary CJ, Minshew NJ, Bansal R, Goradia D, Fedorov S, Keshavan MS, Hardan AY. Corpus callosum volume and neurocognition in autism. Journal of Autism and Developmental Disorders. 2009;39:834–841. doi: 10.1007/s10803-009-0689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian S, Brown WS, Hallam BJ, McMahon W, Lu J, Johnson M, Bigler ED, Lainhart J. Regional callosal morphology in autism and macrocephaly. Developmental Neuropsychology. 2008;33:74–99. doi: 10.1080/87565640701729821. [DOI] [PubMed] [Google Scholar]
- Kleinhans N, Akshoomoff N, Delis DC. Executive functions in autism and Asperger's disorder: flexibility, fluency, and inhibition. Developmental Neuropsychology. 2005;27:379–401. doi: 10.1207/s15326942dn2703_5. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Lainhart J, Lange N. The biological broader autism phenotype. In: Amaral G, Dawson G, Geschwind D, editors. Autism Spectrum Disorders. New York: Oxford University Press; 2010. [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, Deutsch CK, Dunn M, Estes A, Tager-Flusberg H, Folstein S, Hepburn S, Hyman S, McMahon W, Minshew N, Munson J, Osann K, Ozonoff S, Rodier P, Rogers S, Sigman M, Spence MA, Stodgell CJ, Volkmar F. Head circumference and height in autism: a study by the Collaborative Program of Excellence in Autism. Am J Med Genet A. 2006;140:2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainhart JE, Lange N. Increased neuron number and head size in autism. JAMA. 2011;306:2031–2032. doi: 10.1001/jama.2011.1633. [DOI] [PubMed] [Google Scholar]
- Lange N, DuBray MB, Lee JE, Froimowitz MP, Froehlich A, Adluru N, Wright B, Ravichandran C, Fletcher PT, Bigler ED, Alexander AL, Lainhart JE. Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res. 2010;3:350–358. doi: 10.1002/aur.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, Johnson M, Morgan J, Miller JN, McMahon WM, Lu J, Jeong EK, Lainhart JE. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neuroscience Letters. 2007;424:127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Lee JE, Chung MK, Lazar M, DuBray MB, Kim J, Bigler ED, Lainhart JE, Alexander AL. A study of diffusion tensor imaging by tissue-specific, smoothing-compensated voxel-based analysis. NeuroImage. 2009;44:870–883. doi: 10.1016/j.neuroimage.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, Tager-Flusberg H, Lainhart JE. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. Journal of Autism and Developmental Disorders. 2006;36:849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York: Oxford University Press; 2004. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Narr KL, Zamanyan A, Chou YY, Gutman B, Dinov ID, Toga AW. The link between callosal thickness and intelligence in healthy children and adolescents. NeuroImage. 2011;54:1823–1830. doi: 10.1016/j.neuroimage.2010.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46:269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, Simmons A, Sigmundsson T, Greenwood K, Russell A, Schmitz N, Happe F, Howlin P, Murphy DG. Brain anatomy and sensorimotor gating in Asperger's syndrome. Brain. 2002;125:1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Meyer J, Goldstein G. Abstract reasoning in autism: a dissociation between concept formation and concept identification. Neuropsychology. 2002;16:327–334. doi: 10.1037//0894-4105.16.3.327. [DOI] [PubMed] [Google Scholar]
- Misaki M, Wallace GL, Dankner N, Martin A, Bandettini PA. Characteristic cortical thickness patterns in adolescents with autism spectrum disorders: Interactions with age and intellectual ability revealed by canonical correlation analysis. NeuroImage. 2012 doi: 10.1016/j.neuroimage.2012.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pannek K, Mathias JL, Bigler ED, Brown G, Taylor JD, Rose S. An automated strategy for the delineation and parcellation of commissural pathways suitable for clinical populations utilising high angular resolution diffusion imaging tractography. NeuroImage. 2010;50:1044–1053. doi: 10.1016/j.neuroimage.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim JJ, Lee SK, Seok JH, Chun J, Kim DI, Lee JD. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Human Brain Mapping. 2008;29:503–516. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier J, Verclytte S, Delmaire C, Deramond H, Pruvo JP, Le Gars D, Godefroy O. Microsurgical anatomy of the ventral callosal radiations: new destination, correlations with diffusion tensor imaging fiber-tracking, and clinical relevance. Journal of Neurosurgery. 2010a;112:512–519. doi: 10.3171/2009.6.JNS081712. [DOI] [PubMed] [Google Scholar]
- Peltier J, Verclytte S, Delmaire C, Pruvo JP, Godefroy O, Le Gars D. Microsurgical anatomy of the temporal stem: clinical relevance and correlations with diffusion tensor imaging fiber tracking. Journal of Neurosurgery. 2010b;112:1033–1038. doi: 10.3171/2009.6.JNS08132. [DOI] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Annals of Neurology. 1993;34:71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. Bethesda, Maryland, USA: U.S. National Institutes of Health; 1997–2011. http://rsb.info.nih.gov/ij/ [Google Scholar]
- Rice SA, Bigler ED, Cleavinger HB, Tate DF, Sayer J, McMahon W, Ozonoff S, Lu J, Lainhart JE. Macrocephaly, corpus callosum morphology, and autism. Journal of Child Neurology. 2005;20:34–41. doi: 10.1177/08830738050200010601. [DOI] [PubMed] [Google Scholar]
- Robb RA. ANALYZE: Three-dimensional Biomedical Imaging. New York: VCH Publishers; 1995. [Google Scholar]
- Robb RA. The biomedical imaging resource at Mayo Clinic. IEEE Transactions on Medical Imaging. 2001;20:854–867. doi: 10.1109/42.952724. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Hamburger SD. Neuropsychological findings in high-functioning men with infantile autism, residual state. Journal of Clinical and Experimental Neuropsychology. 1988;10:201–221. doi: 10.1080/01688638808408236. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, Tirapu J, Barcelo F. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsychological Society. 2009;15:438–450. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- Schipul SE, Williams DL, Keller TA, Minshew NJ, Just MA. Distinctive Neural Processes during Learning in Autism. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepest R, Jacobi E, Gawronski A, Krug B, Moller-Hartmann W, Lehnhardt FG, Vogeley K. Corpus callosum size in adults with high-functioning autism and the relevance of gender. Psychiatry Research: Neuroimaging. 2010;183:38–43. doi: 10.1016/j.pscychresns.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Vidal CN, Nicolson R, DeVito TJ, Hayashi KM, Geaga JA, Drost DJ, Williamson PC, Rajakumar N, Sui Y, Dutton RA, Toga AW, Thompson PM. Mapping corpus callosum deficits in autism: an index of aberrant cortical connectivity. Biol Psychiatry. 2006;60:218–225. doi: 10.1016/j.biopsych.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Vollm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, Deakin JF, Elliott R. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. NeuroImage. 2006;29:90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: a voxel-based investigation. NeuroImage. 2005;24:455–461. doi: 10.1016/j.neuroimage.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children - Third Edition (WISC-III) San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Third Edition (WAIS-III) San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Witelson SF. The brain connection: the corpus callosum is larger in left-handers. Science. 1985;229:665–668. doi: 10.1126/science.4023705. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Yamasue H, Abe O, Suga M, Yamada H, Inoue H, Kuwabara H, Kawakubo Y, Yahata N, Aoki S, Kano Y, Kato N, Kasai K. Reduced gray matter volume of pars opercularis is associated with impaired social communication in high-functioning autism spectrum disorders. Biol Psychiatry. 2010;68:1141–1147. doi: 10.1016/j.biopsych.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM. Functional anatomy of interhemispheric cortical connections in the human brain. Journal of Anatomy. 2006;209:311–320. doi: 10.1111/j.1469-7580.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]