Abstract
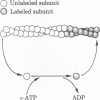
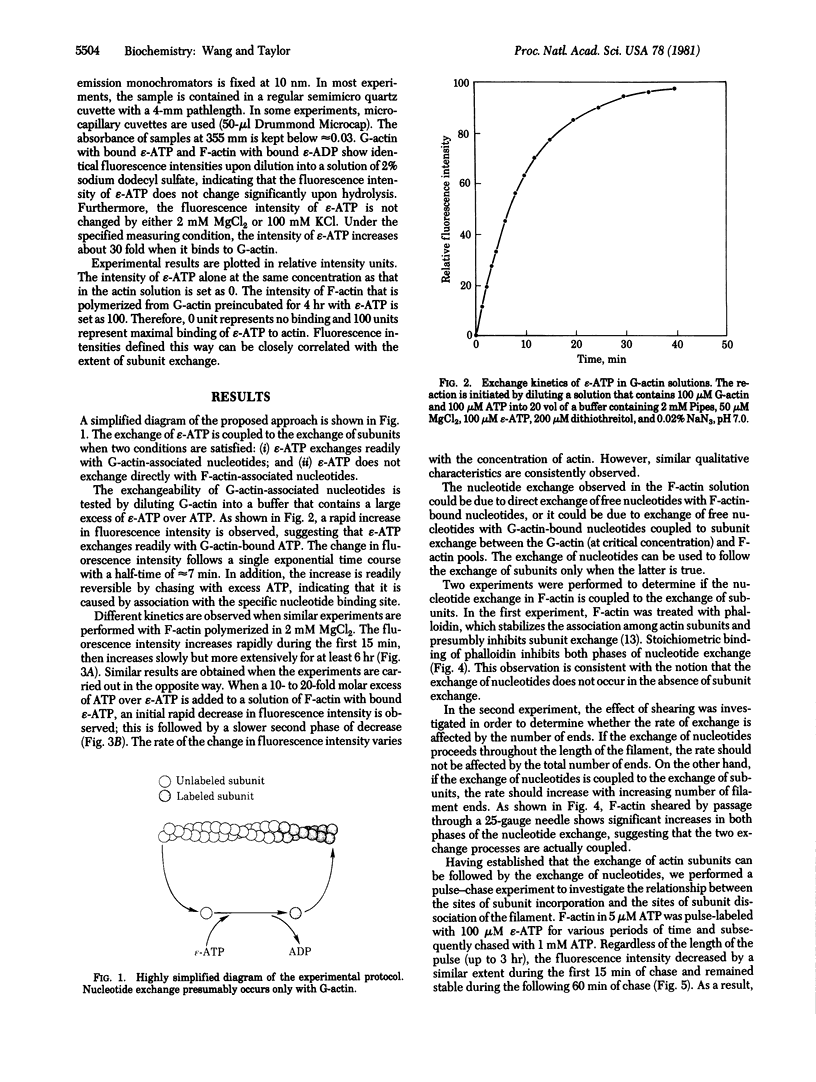
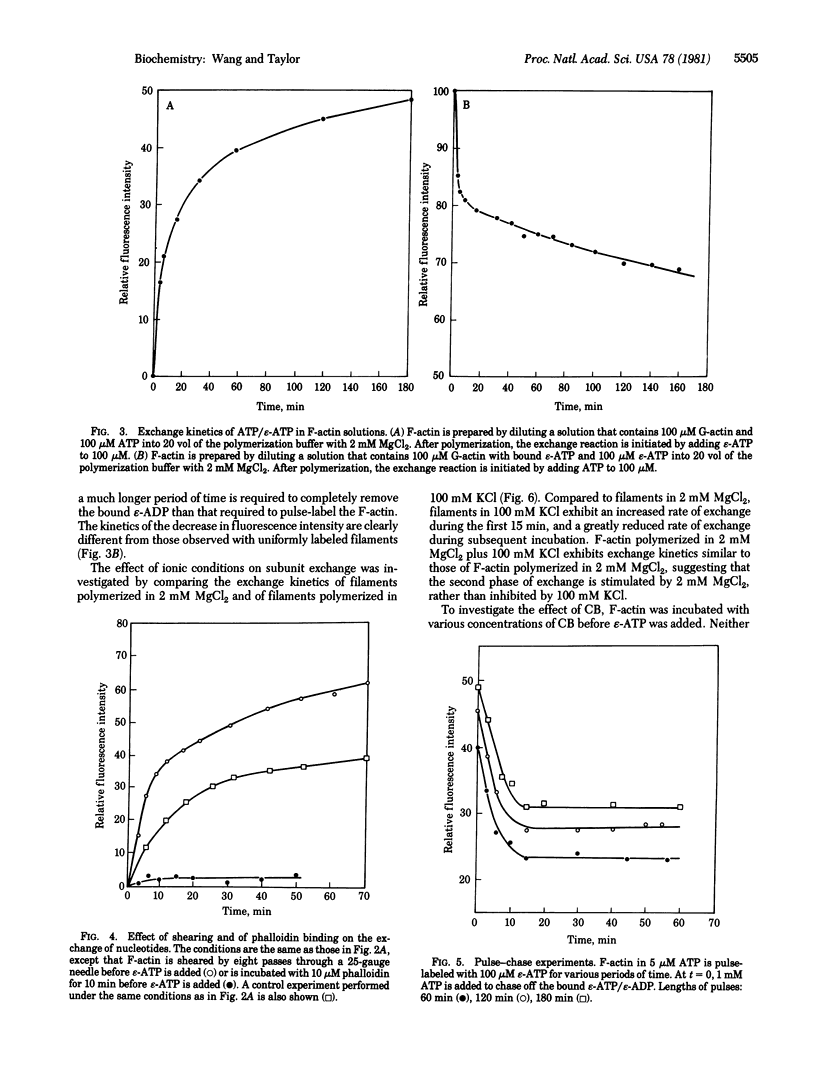
The fluorescent analog of ATP 1-N6-ethenoadenosine 5'-triphosphate (epsilon-ATP) exchanges readily with nucleotides bound to G-actin. The exchange can be observed by measuring the fluorescence intensity, which increases significantly when epsilon-ATP binds to actin. When excess epsilon-ATP is added to a solution of F-actin, a continuous increase in fluorescence intensity is observed, indicating that the nucleotides bound to F-actin are directly or indirectly exchangeable. The kinetics of exchange consist of a fast phase and a slow phase. Both phases are stimulated by shearing and are inhibited by phalloidin treatment, suggesting that the exchange of nucleotides is coupled to the exchange of subunits. Therefore, the exchange reaction can be used as a convenient, nonperturbing tool to study the exchange of free actin subunits with subunits in actin filaments. The exchange of actin subunits was characterized by a pulse-chase experiment. The results suggest that actin subunits assemble and disassemble through the same end of the filament during the fast phase of exchange but through opposite ends of the filament during the slow phase. In addition, the slow phase of exchange is inhibited in the absence of millimolar magnesium ions, but is not significantly affected by cytochalasin B at concentrations between 0.1 and 10 microM. These observations are discussed in relation to possible mechanisms of subunit exchange in steady-state F-actin solutions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner S. L., Korn E. D. Substoichiometric concentrations of cytochalasin D inhibit actin polymerization. Additional evidence for an F-actin treadmill. J Biol Chem. 1979 Oct 25;254(20):9982–9985. [PubMed] [Google Scholar]
- Brenner S. L., Korn E. D. The effects of cytochalasins on actin polymerization and actin ATPase provide insights into the mechanism of polymerization. J Biol Chem. 1980 Feb 10;255(3):841–844. [PubMed] [Google Scholar]
- Brown S. S., Spudich J. A. Mechanism of action of cytochalasin: evidence that it binds to actin filament ends. J Cell Biol. 1981 Mar;88(3):487–491. doi: 10.1083/jcb.88.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai M., Oosawa F. Behavior of divalent cations and nucleotides bound to F-actin. Biochim Biophys Acta. 1969 Feb 25;172(2):300–310. doi: 10.1016/0005-2728(69)90072-3. [DOI] [PubMed] [Google Scholar]
- Kitagawa S., Drabikowski W., Gergely J. Exchange and release of the bound nucleotide of F-actin. Arch Biochem Biophys. 1968 May;125(2):706–714. doi: 10.1016/0003-9861(68)90627-9. [DOI] [PubMed] [Google Scholar]
- Lin D. C., Tobin K. D., Grumet M., Lin S. Cytochalasins inhibit nuclei-induced actin polymerization by blocking filament elongation. J Cell Biol. 1980 Feb;84(2):455–460. doi: 10.1083/jcb.84.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean-Fletcher S., Pollard T. D. Mechanism of action of cytochalasin B on actin. Cell. 1980 Jun;20(2):329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978 Jan;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Hartwig J. H., Stossel T. P. Cytochalasin B and the structure of actin gels. II. Further evidence for the splitting of F-actin by cytochalasin B. Biochim Biophys Acta. 1980 Dec 16;626(2):494–500. [PubMed] [Google Scholar]
- Miki M., Onuma H., Mihashi K. Interaction of actin water epsilon-ATP. FEBS Lett. 1974 Sep 15;46(1):17–19. doi: 10.1016/0014-5793(74)80324-8. [DOI] [PubMed] [Google Scholar]
- Moos C., Estes J. E., Eisenberg E. Exchange of F-actin-bound nucleotide in the presence and absence of myosin. Biochem Biophys Res Commun. 1966 May 3;23(3):347–351. doi: 10.1016/0006-291x(66)90553-5. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Mooseker M. S. Direct measurement of actin polymerization rate constants by electron microscopy of actin filaments nucleated by isolated microvillus cores. J Cell Biol. 1981 Mar;88(3):654–659. doi: 10.1083/jcb.88.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secrist J. A., 3rd, Barrio J. R., Leonard N. J., Weber G. Fluorescent modification of adenosine-containing coenzymes. Biological activities and spectroscopic properties. Biochemistry. 1972 Sep 12;11(19):3499–3506. doi: 10.1021/bi00769a001. [DOI] [PubMed] [Google Scholar]
- Simpson P. A., Spudich J. A. ATP-driven steady-state exchange of monomeric and filamentous actin from Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4610–4613. doi: 10.1073/pnas.77.8.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Taylor D. L., Reidler J., Spudich J. A., Stryer L. Detection of actin assembly by fluorescence energy transfer. J Cell Biol. 1981 May;89(2):362–367. doi: 10.1083/jcb.89.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames K. E., Cheung H. C., Harvey S. C. Binding of 1,N6-ethanoadenosine triphosphate to actin. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1252–1261. doi: 10.1016/0006-291x(74)90333-7. [DOI] [PubMed] [Google Scholar]
- Waechter F., Engel J. The kinetics of the exchange of G-actin-bound 1: N6-ethenoadenosine 5'-triphosphate with ATP as followed by fluorescence. Eur J Biochem. 1975 Sep 15;57(2):453–459. doi: 10.1111/j.1432-1033.1975.tb02320.x. [DOI] [PubMed] [Google Scholar]
- Wegner A. Head to tail polymerization of actin. J Mol Biol. 1976 Nov;108(1):139–150. doi: 10.1016/s0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]
- Wieland T. Modification of actins by phallotoxins. Naturwissenschaften. 1977 Jun;64(6):303–309. doi: 10.1007/BF00446784. [DOI] [PubMed] [Google Scholar]