Abstract
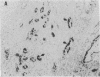
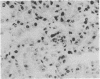
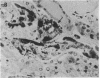
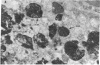
Synthetic 3-phosphocitrate, an extremely potent inhibitor of calcium phosphate crystallization as determined in a nonbiological physical-chemical assay, has many similarities to a mitochondrial factor that inhibits crystallization of nondiffracting amorphous calcium phosphate. In order to determine whether phosphocitrate can prevent uptake and crystallization of calcium phosphate in mitochondria in vivo, it was administered intraperitoneally to animals given large daily doses of calcium gluconate or parathyroid hormone, a regimen that causes massive accumulation and crystallization of calcium phosphate in the mitochondria and cytosol of renal tubule cells in vivo. Administration of phosphocitrate greatly reduced the net uptake of Ca2+ by the kidneys and prevented the appearance of apatite-like crystalline structures within the mitochondrial matrix and cytosol of renal tubule cells. Phosphocitrate, which is a poor chelator of Ca2+, did not reduce the hypercalcemia induced by either agent. These in vivo observations therefore indicate that phosphocitrate acts primarily at the cellular level to prevent the extensive accumulation of calcium phosphate in kidney cells by inhibiting the mitochondrial accumulation or crystallization of calcium phosphate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker G. L., Chen C. H., Greenawalt J. W., Lehninger A. L. Calcium phosphate granules in the hepatopancreas of the blue crab Callinectes sapidus. J Cell Biol. 1974 May;61(2):316–326. doi: 10.1083/jcb.61.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G. L., Fiskum G., Lehninger A. L. Regulation of free Ca2+ by liver mitochondria and endoplasmic reticulum. J Biol Chem. 1980 Oct 10;255(19):9009–9012. [PubMed] [Google Scholar]
- Becker G. L., Termine J. D., Eanes E. D. Comparative studies of intra- and extramitochondrial calcium phosphates from the hepatopancreas of the blue crab (Callinectes sapidus). Calcif Tissue Res. 1976 Oct 12;21(2):105–113. doi: 10.1007/BF02547386. [DOI] [PubMed] [Google Scholar]
- Duffy J. L., Suzuki Y., Churg J. Acute calcium nephropathy. Early proximal tubular changes in the rat kidney. Arch Pathol. 1971 Apr;91(4):340–350. [PubMed] [Google Scholar]
- FLEISCH H., BISAZ S. Isolation from urine of pyrophosphate, a calcification inhibitor. Am J Physiol. 1962 Oct;203:671–675. doi: 10.1152/ajplegacy.1962.203.4.671. [DOI] [PubMed] [Google Scholar]
- Fiskum G., Lehninger A. L. The mechanisms and regulation of mitochondrial Ca2+ transport. Fed Proc. 1980 May 15;39(7):2432–2436. [PubMed] [Google Scholar]
- Fleisch H. A., Russell R. G., Bisaz S., Mühlbauer R. C., Williams D. A. The inhibitory effect of phosphonates on the formation of calcium phosphate crystals in vitro and on aortic and kidney calcification in vivo. Eur J Clin Invest. 1970 Mar;1(1):12–18. doi: 10.1111/j.1365-2362.1970.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Francis M. D. The inhibition of calcium hydroxypatite crystal growth by polyphosphonates and polyphosphates. Calcif Tissue Res. 1969;3(2):151–162. doi: 10.1007/BF02058658. [DOI] [PubMed] [Google Scholar]
- GREENAWALT J. W., ROSSI C. S., LEHNINGER A. L. EFFECT OF ACTIVE ACCUMULATION OF CALCIUM AND PHOSPHATE IONS ON THE STRUCTURE OF RAT LIVER MITOCHONDRIA. J Cell Biol. 1964 Oct;23:21–38. doi: 10.1083/jcb.23.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochman N., Givelber H. Automated, simultaneous microdetermination of calcium and magnesium by atomic absorption. Clin Chem. 1970 Mar;16(3):229–234. [PubMed] [Google Scholar]
- Gron P., Hay D. I. Inhibition of calcium phosphate precipitation by human salivary secretions. Arch Oral Biol. 1976;21(3):201–205. doi: 10.1016/0003-9969(76)90130-8. [DOI] [PubMed] [Google Scholar]
- Howard J. E. Studies on urinary stone formation: a saga of clinical investigation. Johns Hopkins Med J. 1976 Dec;139(6):239–252. [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and biological mineralization processes: an exploration. Horiz Biochem Biophys. 1977;4:1–30. [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger A. L., Reynafarje B., Vercesi A., Tew W. P. Transport and accumulation of calcium in mitochondria. Ann N Y Acad Sci. 1978 Apr 28;307:160–176. doi: 10.1111/j.1749-6632.1978.tb41941.x. [DOI] [PubMed] [Google Scholar]
- Reddi A. H., Meyer J. L., Tew W. P., Howard J. E., Lehninger A. L. Influence of phosphocitrate, a potent inhibitor of hydroxyapatite crystal growth, on mineralization of cartilage and bone. Biochem Biophys Res Commun. 1980 Nov 17;97(1):154–159. doi: 10.1016/s0006-291x(80)80148-3. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER A. F., REAVEN E. P., REAVEN G. A comparison of renal calcification produced by parathyroid extract or calcium gluconate. Endocrinology. 1960 Dec;67:733–743. doi: 10.1210/endo-67-6-733. [DOI] [PubMed] [Google Scholar]
- Schenk R., Merz W. A., Mühlbauer R., Russell R. G., Fleisch H. Effect of ethane-1-hydroxy-1,1-diphosphonate (EHDP) and dichloromethylene diphosphonate (Cl 2 MDP) on the calcification and resorption of cartilage and bone in the tibial epiphysis and metaphysis of rats. Calcif Tissue Res. 1973 Mar 12;11(3):196–214. doi: 10.1007/BF02547219. [DOI] [PubMed] [Google Scholar]
- Schlesinger D. H., Hay D. I. Complete covalent structure of statherin, a tyrosine-rich acidic peptide which inhibits calcium phosphate precipitation from human parotid saliva. J Biol Chem. 1977 Mar 10;252(5):1689–1695. [PubMed] [Google Scholar]
- Sutor D. J., Percival J. M. Presence or absence of inhibitors of crystal growth in bile. 1. Effect of bile on the formation of calcium phosphate, a constituent of gallstones. Gut. 1976 Jul;17(7):506–510. doi: 10.1136/gut.17.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew W. P., Mahle C., Benavides J., Howard J. E., Lehninger A. L. Synthesis and characterization of phosphocitric acid, a potent inhibitor of hydroxylapatite crystal growth. Biochemistry. 1980 Apr 29;19(9):1983–1988. doi: 10.1021/bi00550a039. [DOI] [PubMed] [Google Scholar]
- Thomas R. S., Greenawalt J. W. Microincineration, electron microscopy, and electron diffraction of calcium phosphate-loaded mitochondria. J Cell Biol. 1968 Oct;39(1):55–76. doi: 10.1083/jcb.39.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]