Abstract
Aquaporins are integral membrane proteins of the tonoplast and the plasma membrane that facilitate the passage of water through these membranes. Because of their potentially important role in regulating water flow in plants, studies documenting aquaporin gene expression in specialized tissues involved in water and solute transport are important. We used in situ hybridization to examine the expression pattern of the tonoplast aquaporin ZmTIP1 in different organs of maize (Zea mays L.). This tonoplast water channel is highly expressed in the root epidermis, the root endodermis, the small parenchyma cells surrounding mature xylem vessels in the root and the stem, phloem companion cells and a ring of cells around the phloem strand in the stem and the leaf sheath, and the basal endosperm transfer cells in developing kernels. We postulate that the high level of expression of ZmTIP1 in these tissues facilitates rapid flow of water through the tonoplast to permit osmotic equilibration between the cytosol and the vacuolar content, and to permit rapid transcellular water flow through living cells when required.
Long-distance transport of water and solutes occurs through xylem vessels and phloem sieve tubes that have no real membrane barriers to such transport. In contrast, water and solutes that enter these principal conduits pass through living tissues and may encounter membrane barriers when they follow the transcellular path. Cell-to-cell flow can be a major transport route for water, although the extent to which water also follows an apoplastic path is still a matter of debate and may depend on the organ or tissue, its stage of development, or its physiological state. Cell types in which transcellular flow and, therefore, transmembrane flow are limiting have been identified. For example, in roots, the Casparian strip of the endodermis is a barrier to the apoplastic route for water and ions that enter the stele (Schreiber, 1996). On the basis of results obtained from pressure-probe experiments with soybean hypocotyls, Nonami and Boyer (1993) suggested that the small xylem parenchyma cells around the vascular bundles limit the radial transport of water out of the xylem vessels. Do plants regulate the hydraulic permeability of the membranes of these cells and, if so, what mechanisms are involved?
The discovery of plant aquaporins (water-channel proteins) by Maurel et al. (1993) has given us new insights into how plants might regulate transcellular water flow and intracellular osmotic equilibration. Clearly, plants could alter both the abundance and the activity of aquaporins to modulate transmembrane water flow (for reviews, see Chrispeels and Maurel, 1994; Maurel, 1997). Aquaporins are members of a large gene family (Weig et al., 1997) and the elucidation of the physiological function(s) of the individual members will require a combination of experimental approaches, including expression studies, creation of plants in which expression is down-regulated or knocked out, and examination of water fluxes across the membranes of individual cells or vesicles derived from specific membranes.
Because of the potential role of aquaporins in regulating water flow in plants, a number of studies have focused on the sites of aquaporin gene expression. Yamamoto et al. (1991) showed that TobRB7, a putative plasma membrane aquaporin of tobacco, is highly expressed in the meristem and in the immature central cylinder of roots. We demonstrated that the Arabidopsis aquaporin γ-TIP is highly expressed in vascular bundles of roots and leaves (Ludevid et al., 1992). Yamada et al. (1995) analyzed the expression pattern of the aquaporin MIP A in roots of Mesembryanthenum crystallinum and found that this plasma membrane aquaporin is preferentially expressed in the epidermis and in the youngest portions of the xylem. Kaldenhoff et al. (1995) showed that AthH2, a plasma membrane aquaporin, is highly expressed in newly formed tissues and organs. Most recently, Sarda et al. (1997) demonstrated high expression of SunTIP7 and SunTIP20 in the guard cells of sunflower leaves. This expression pattern is in agreement with the suggestion by Maurel et al. (1997a) that TIPs play a role in osmotic equilibration of the cytoplasm.
In this paper we use in situ hybridization to examine the expression pattern of ZmTIP1, a highly expressed tonoplast aquaporin of maize, in tissues and cells involved in water and solute uptake and transport. This newly described maize tonoplast aquaporin has already been shown to be expressed in zones of cell elongation and enlargement (Chaumont et al., 1998). Here we show that this tonoplast water channel is also highly expressed in cell types that are thought to regulate water flow and/or are sites of intense solute or water transport: the root epidermis, the root endodermis, the small parenchyma cells surrounding mature xylem vessels in the root and the stem, phloem companion cells and a ring of cells around the phloem strand in the stem and the leaf sheath, the outer layer of the nucellus, and the basal endosperm transfer cells in developing kernels. To our knowledge, there is presently no evidence that plant aquaporins transport solutes. The high level of expression of ZmTIP1 in these tissues may facilitate rapid intracellular osmotic equilibration and permit rapid water flow through the vacuoles in tissues experiencing transcellular water flow. This transcellular flow may be regulated at the plasma membrane, which is less permeable to water than the tonoplast (Maurel et al., 1997b; Niemietz and Tyerman, 1997). Taken together, our results strongly suggest a role for tonoplast water channels in regulating the hydraulic permeability of the vacuolar membranes and in adjusting the water homeostasis of the protoplasm under various physiological conditions.
MATERIALS AND METHODS
Plant Material and Growth Conditions
All experiments were carried out with the inbred line of maize (Zea mays Oh43). For root studies, surface-sterilized seeds were germinated on filter paper moistened with water in the dark at 30°C for 72 h. For the analysis of other tissues, seeds were germinated and grown in a mixture of sand, peat moss, and horticultural Perlite (Aztec Perlite, Escondido, CA) containing the controlled-release fertilizer Osmocote (Scotts-Sierra, Maysville, OH). The plants were grown in a greenhouse under natural light conditions and watered daily.
Preparation of Riboprobes
The 3′-untranslated region of the ZmTIP1 cDNA (203 bp) (Chaumont et al., 1998) was subcloned in pBluescript SK to provide a template to generate sense and antisense RNA probes. The plasmid was linearized using appropriate restriction endonucleases, and digoxigenin-labeled RNA probes were prepared using a digoxigenin RNA-labeling mix (Boehringer Mannheim) and either T3 or T7 RNA polymerase (Promega). After ethanol precipitation, the probes were resuspended in 100 μL of hybridization buffer (6× SSC [1 × SSC is 150 mm NaCl, 15 mm Na3C6H5O7], 3% [w/v] SDS, 50% [v/v] formamide, and 100 μg mL−1 tRNA) and stored at −80°C before use.
Tissue Preparation
Maize tissues were fixed in 50% (v/v) ethanol with 5% (v/v) acetic acid and 3.7% (v/v) formaldehyde (Huijser et al., 1992) at room temperature for 3 to 4.5 h with occasional degassing under vacuum for 15 min. After fixation, the tissues were dehydrated through an alcohol series. Ethanol was gradually replaced by Histoclear (National Diagnostics, Manville, NJ) and Paraplast Plus (Sigma) chips were added. Tissues were incubated at 60°C for 2 h before the Histoclear/Paraplast mix was replaced by melted Paraplast. After five to six changes of Paraplast followed by a 3-h incubation at 60°C, tissues were finally embedded in Paraplast Plus blocks and stored at 4°C before sectioning.
The embedded tissues were sectioned into 8- to 10-μm-thick slices and placed on Superfrost/Plus slides (Fisher Scientific). Sections were dried and affixed to the slides by incubating the slides on a hot plate at 45°C for 18 h, dewaxed with Histoclear (National Diagnostics), and hydrated by passing through an alcohol series. The sections were then treated successively with 0.2 m HCl for 20 min and with 1 μg mL−1 proteinase K in 100 mm Tris-HCl pH 8.0, 50 mm EDTA pH 8.0 for 30 min. The proteinase K was blocked by incubating the tissues in 2 mg mL−1 Gly in PBS for 2 min. Subsequently, the sections were treated with 4% formaldehyde in PBS for 10 min, followed by two rinses of 5 min each in PBS and two rinses of 5 min each in water. Finally, the sections were dehydrated through an alcohol series to 100% ethanol and dried under vacuum.
In Situ Hybridization
The in situ hybridization protocol used was a modified procedure based on the work of Marrison and Leech (1994). The ZmTIP1 sense and antisense probes were hybridized to the tissue sections overnight at 50°C at a concentration of 200 to 400 ng mL−1 in 40 μL of hybridization buffer (6× SSC, 3% [w/v] SDS, 50% [v/v] formamide, and 100 μg mL−1 tRNA). After the hybridization, the coverslips were removed with gentle stirring in wash buffer (2× SSC, 50% [v/v] formamide) at room temperature and the sections were incubated two times for 90 min in wash buffer at 50°C. An RNase A treatment (10 μg mL−1 in 2× SSC) was performed at 37°C for 30 min and the slides were washed for another hour at 50°C in wash buffer. After a brief wash in TBS buffer (100 mm Tris-HCl, pH 7.5, and 400 mm NaCl), sections were incubated successively for 1 h in a blocking solution (Boehringer Mannheim, 0.5% in TBS) and 30 min in 1% (w/v) BSA, 0.3% (v/v) Triton X-100 in TBS. The sections were then incubated for 90 min in the same solution containing alkaline phosphatase-conjugated antibodies (Boehringer Mannheim) at a 1/1000 dilution. After three washes of 20 min each in 1% (w/v) BSA, 0.3% (v/v) Triton X-100 in TBS, the ZmTIP1 transcripts were detected by incubating the slides in color development solution (0.15 mg mL−1 nitroblue tetrazolium chloride and 0.075 mg mL−1 5-bromo-4-chloro-3-indolyl-phosphate in 100 mm Tris-HCl pH 9.5, 100 mm NaCl, and 50 mm MgCl2) for 16 to 36 h.
The color reaction was stopped by washing the slides two times for 5 min in water. Sections were finally dehydrated through an alcohol series to 100% ethanol and dried under vacuum.
Image Processing
Photographs of the sections were made under dark-field conditions using an Optiphot-2 light microscope (Nikon). The slides were digitized using a slide scanner (CoolScan, Nikon). Brightness and contrast were adjusted using Photoshop 3.0 (Adobe Systems, Mountain View, CA). Composite figures were prepared in Canvas 3.5 (Deneba Software, Miami, FL) and printed using a dye-sublimation color printer (Phaser IIsdx, Tektronix, Wilsonville, OR).
RESULTS
Expression of ZmTIP1 in Tips of Primary Maize Roots
Recent results from our laboratory (Chaumont et al., 1998) indicate that the tonoplast aquaporin ZmTIP1 is highly expressed in all plant organs and especially in meristematic and elongating cells and in vascular bundles. The expression in the xylem and the phloem vascular bundles suggests a possible involvement in long-distance water transport, and we therefore made a detailed study of ZmTIP1 expression in roots, leaves, stems, and flowers of maize, especially in relation to possible transporting tissues.
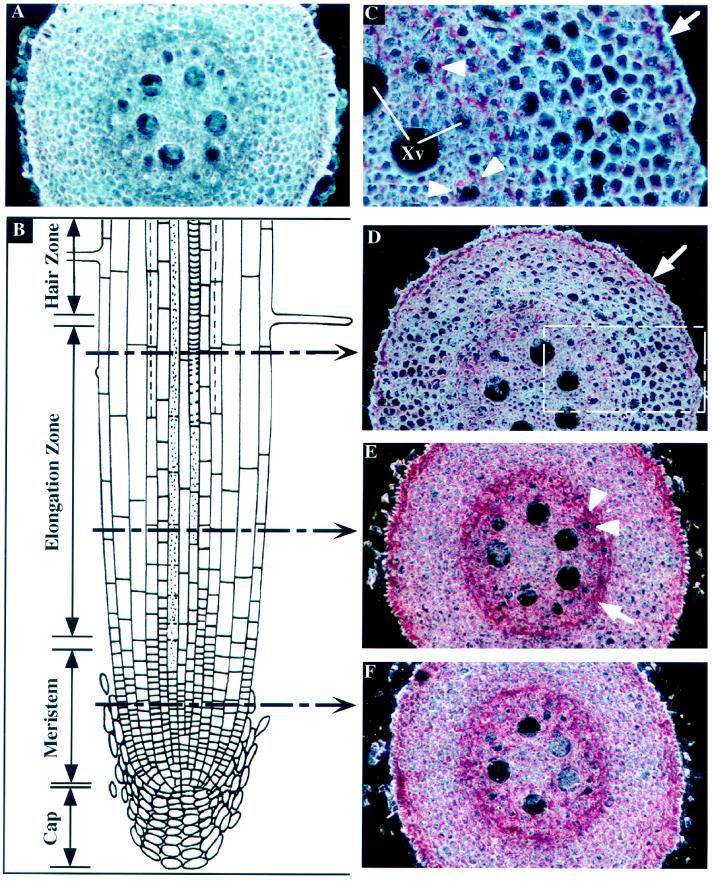
The absorption of water and solutes is one of the major functions of the root system of plants. A longitudinal section through a root tip shows successively a root cap, overlapping zones of rapid cell division and cell elongation, and a zone in which the root is covered by root hairs (Fig. 1B). To determine the level of ZmTIP1 mRNA accumulation, we probed cross-sections of roots taken at different distances from the tip with a gene-specific antisense ZmTIP1 mRNA labeled with digoxigenin. The RNA-RNA hybrids were visualized with alkaline phosphatase-conjugated antibodies to digoxigenin. The chromogen used here produced a red to purple, insoluble reaction product, and the intensity of the red color indicates the abundance of the mRNA per volume of cytoplasm. Figure 1A represents a typical result of a control hybridization with a ZmTIP1 sense probe used on a cross-section through the middle of the elongation zone. Nonspecific hybridization was very low, indicated by the faint pink color in the section shown in Figure 1A, but no specific red precipitate could be detected. A comparison of Figure 1, A and D, shows the difference between the sense probe (control) and the antisense probe.
Figure 1.
In situ localization of ZmTIP1 mRNA in maize root tip. Transverse sections of the root tip were hybridized with ZmTIP1 sense (A) or antisense (C–F) digoxigenin-labeled RNA probes and photographed under dark-field conditions. The transcript signal is red. A, Control transverse section in the middle of the elongation zone hybridized with a ZmTIP1 sense probe. B, Schematic representation of a longitudinal section of a root tip. Discontinuous arrows indicate the approximate sites of transverse sections presented in D, E, and F. C, High magnification of the area boxed in D. Arrow indicates the expression of ZmTIP1 in epidermal cells. Arrowheads indicate the probe accumulation in the parenchyma cells that surround the small early metaxylem vessels. Xv, Xylem vessels. D, Transverse section at the end of the elongation zone. Arrow indicates the expression of ZmTIP1 in epidermal cells. E, Transverse section in the middle of the elongation zone. Arrow indicates ZmTIP1 expression in the endodermis/pericycle. Arrowheads indicate the probe accumulation around the early metaxylem vessels. F, Transverse sections in the meristematic zone.
To analyze the changes in ZmTIP1 expression, we made transverse sections of different regions of the root, including the meristematic zone (Fig. 1F) and the beginning (Fig. 1E) and the end (Fig. 1D) of the elongation zone. In the meristem itself close to the tip (Fig. 1F), the probe was detected in all cells, but some differences in signal intensities were observed. Cells of the epidermis and a ring of cells at the interface of the cortex and the stele, which likely represent the maturing endodermis/pericycle, contained higher signal density than did cortical cells.
At the beginning of the elongation zone (Fig. 1E), the signal was still observed in elongating cortical cells and in the root epidermis, but a higher level of transcripts was observed in the endodermis/pericycle cell layers (Fig. 1E, arrow). More interestingly, the probe was also concentrated in parenchyma cells adjacent to the small, early metaxylem vessels (Fig. 1E, white arrowheads) and not next to the bigger, late metaxylem vessels.
At the end of the elongation zone (Fig. 1D), the cortical cells are elongated and the vacuole occupies most of the intracellular volume. The signal intensity was much lower in the cortex and the endodermis/pericycle but remained strong in the epidermis layer (Fig. 1D, arrow). The area that is boxed in Figure 1D is shown at higher magnification in Figure 1C. At this magnification the greater expression in the epidermis is clearly visible (arrow in Fig. 1C). The probe was also detected in the cytoplasmic part of some cortical cells. A careful examination of the probe concentration in the stele revealed some accumulation in the parenchyma cells that surround the small (and functional) early metaxylem vessels (Fig. 1C, arrowheads) but not around the large ones. At this level, only a weak accumulation of ZmTIP1 transcripts was found in the endodermal cells.
Expression of ZmTIP1 in Mature Maize Root
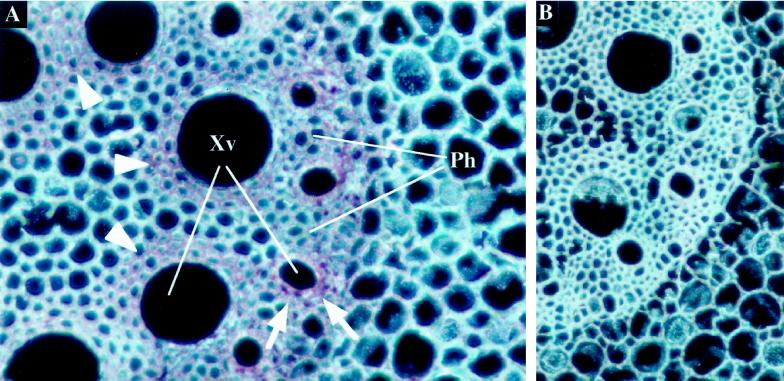
To find out if expression in mature sections of the root might be different from sections close to the tip, we probed transverse sections of mature maize roots, about 12 cm from the root tip, with a ZmTIP1 probe (Fig. 2). The most striking aspect of the distribution of ZmTIP1 aquaporin expression at this greater distance from the tip was the high level of signal in the parenchyma cells that surround the early and late metaxylem (Fig. 2A). In late metaxylem, expression of ZmTIP1 was localized in the two or three layers of cells surrounding the vessel and forming the xylem parenchyma (Fig. 2A, arrowheads). Probe accumulation was also strong in the parenchyma of the early metaxylem vessels (Fig. 2A, arrows) and was limited there to the first layer of cells. A weak signal was also detected in the endodermal cells. Figure 2B represents another control experiment carried out with a ZmTIP1 sense probe and shows that no signal was detected in these conditions. At 12 cm from the root tip there is no epidermis (the cells have died and been sloughed off) and the outermost cell layer of the root now consists of a hypodermis (Varney et al., 1993), in which we detected no accumulation of ZmTIP1 mRNA (data not shown).
Figure 2.
In situ localization of ZmTIP1 mRNA in mature maize root. Transverse sections of the root (10–12 cm from the tip) were hybridized with ZmTIP1 antisense (A) or sense (B) digoxigenin-labeled RNA probes and photographed under dark-field conditions. The transcript signal is red. A, Expression of ZmTIP1 in the parenchyma cells of early (arrows) and late (arrowheads) xylem vessels. Xv, Xylem vessels; Ph, phloem strand. B, Control section hybridized with a ZmTIP1 sense probe.
Expression of ZmTIP1 in Maize Stem
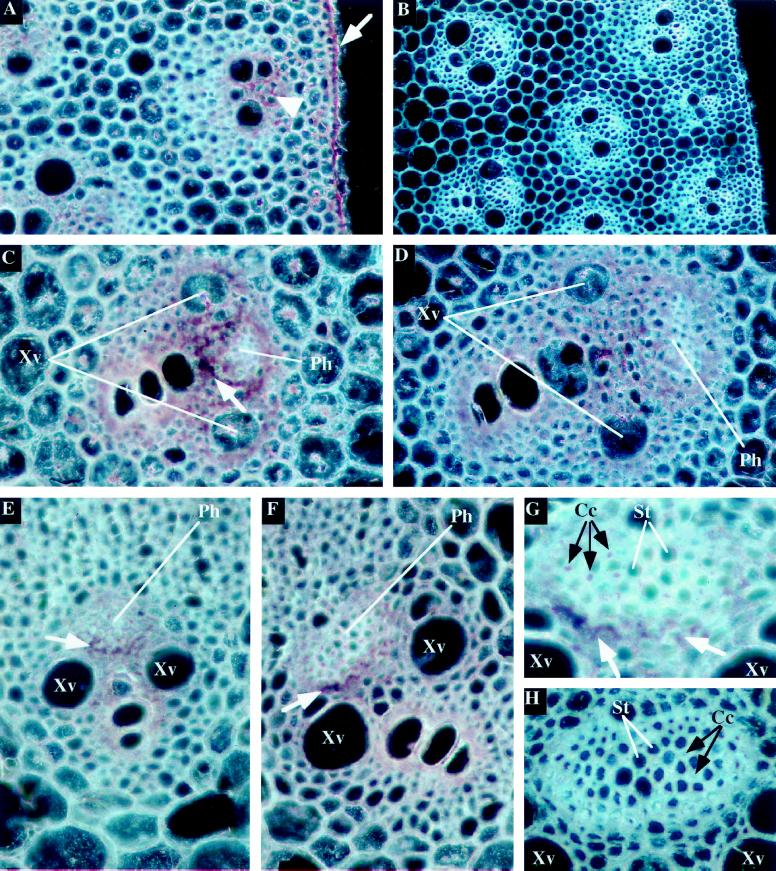
To determine whether this aquaporin is expressed in similar cell types in the stem as in the root, we analyzed the sites of expression of the ZmTIP1 in mid-mature stems about 1.5 cm in diameter. A transverse section hybridized with sense probe (control) is shown in Figure 3B. Hybridization of the ZmTIP1 antisense probe to a transverse stem section resulted in a high intensity of staining in the epidermal cells (Fig. 3A, arrow). The vascular bundles also had a high level of ZmTIP1 transcripts (Fig. 3A, arrowhead). There was some stain in the cortex cells but these cells appear less intensely stained than the vascular bundles because they are large and vacuolated. Whether the expression of ZmTIP1 (expressed per volume of cytoplasm) is actually less in the cortex than in the vascular bundles cannot be determined.
Figure 3.
In situ localization of ZmTIP1 mRNA in the vascular bundles and epidermis of stems and in the vascular bundles of leaves. Transverse sections of stems (A–D) and leaf sheaths (E–H) of 5-week-old maize plants were hybridized with ZmTIP1 antisense (A, C, D, E, F, and G) or sense (B and H) digoxigenin-labeled RNA probes and photographed under dark-field conditions. The transcript signal is red. Cc, Companion cells; Ph, phloem strand; St, sieve tubes; Xv, xylem vessels. A, Expression of ZmTIP1 in the epidermis (arrow) and in cells close to the vascular bundles (arrowhead) of maize stem. B, Control section of maize stem hybridized with a ZmTIP1 sense probe. C, Expression of ZmTIP1 in cells close to a peripheral vascular bundle of the stem. Arrow indicates the high accumulation of ZmTIP1 transcripts in parenchyma cells around the phloem bundle. D, Expression of ZmTIP1 in cells close to a central vascular bundle of the stem. E and F, Expression of ZmTIP1 in cells close to small (E) and large (F) vascular bundles of maize leaf. Arrows indicate the high concentration of the probe in parenchyma cells located between the phloem strand and the xylem vessels. G, High magnification of the phloem strand presented in F showing expression of ZmTIP1 in parenchyma cells (white arrows) and companion cells (black arrows). H, Control section of a leaf phloem strand hybridized with a ZmTIP1 sense probe.
The maize stem has a well-defined gradient of size and maturation of both the vascular bundles and the cortical cells (Fig. 3B). Near the center of the stem the cortical cells are larger than at the periphery and the vessels in the central bundles are also much larger than in the peripheral bundles (Fig. 3B). Panels C and D both show more centrally located larger vascular bundles; the bundle shown in D was closer to the center than the one shown in C. As in the roots, the parenchyma that surrounds the xylem vessels was intensely stained, whereas the region of the phloem was relatively unstained. This picture is clearly seen in both bundles. A ring of parenchyma cells around the phloem bundle is clearly stained more intensely in Fig. 3C (arrow) than in Fig. 3D.
Expression of ZmTIP1 in Maize Leaves
The expression pattern of ZmTIP1 in maize leaves resembles closely the pattern observed in the stem. The images shown here are from tissue sections close to the top of the leaf sheath. In either the small (Fig. 3E) or the larger vascular bundles (Fig. 3F), the ZmTIP1 transcripts were abundant throughout the xylem parenchyma and around the phloem strands. A ring of cells surrounding the phloem strand exhibited the highest concentration of the probe in the cells facing the xylem strand (Fig. 3, E and F, arrows). Figure 3G represents a higher magnification of the phloem strand shown in Figure 3F and confirms that the ZmTIP1 transcripts are especially abundant in the parenchyma cells between the xylem vessel and the phloem strand (Fig. 3G, arrows). At this magnification, it was possible to observe some punctate signals in the phloem strand that may represent ZmTIP1 expression in the phloem companion cells (Fig. 3G). Figure 3H represents the result of a hybridization with a ZmTIP1 sense probe on a leaf phloem bundle and shows that no specific signal was observed in these conditions.
Expression of ZmTIP1 in Developing Maize Pistils and Kernels
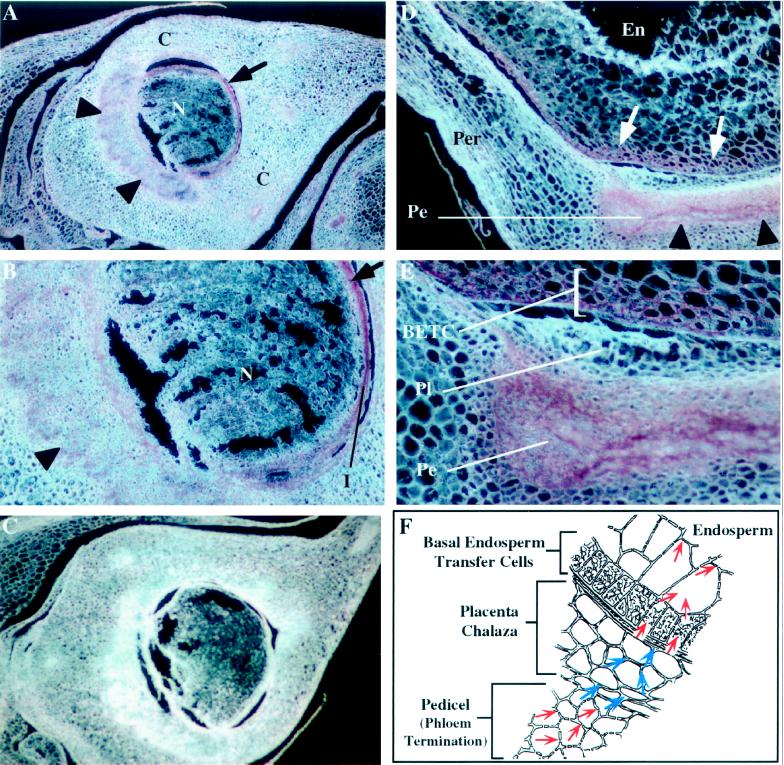
In developing maize pistils (i.e. before fertilization), a single sessile ovule consisting of a nucellus with integuments develops within the ovary made up of fused carpels. After fertilization and as the seed develops, the ovary wall will become the pericarp and the integuments will give rise to the seed coat. Each ovule develops near the end of a vascular bundle and transport of materials through this bundle will nourish the developing seed. At the stage of ovule development shown in Figure 4A, expression of ZmTIP1 can be detected in the vascular strand under the ovule (Fig. 4A, arrowheads) and in a well-defined ring of tissue at the periphery of the nucellus (Fig. 4A, arrow). Higher magnification of the developing ovule confirms the presence of the probe in the vascular tissue (Fig. 4B, arrowhead) and in the outer layer(s) of the nucellus (Fig. 4B, arrow). Again, no significant reaction product was detected in tissues hybridized with a ZmTIP1 sense probe (Fig. 4C).
Figure 4.
In situ localization of ZmTIP1 mRNA in developing pistils and caryopses of maize. Longitudinal sections of nonfertilized maize ears 7-cm long (A–C) and developing maize caryopses 14 d after pollination (D and E) were hybridized with ZmTIP1 antisense (A, B, D, and E) or sense (C) digoxigenin-labeled RNA probes and photographed under dark-field conditions. The transcript signal is red. C, Carpel wall; En, endosperm; I, integuments; N, Nucellus; Pe, Pedicel; Per, Pericarp; Pl, Placenta-chalaza. A, Section of a developing pistil showing expression of ZmTIP1 in the termination zone of the vascular bundle under the ovule (arrowheads) and around the nucellus (arrow). B, High magnification of the developing ovule presented in A showing expression of ZmTIP1 in the vascular tissue (arrowhead) and in the outer layer(s) of the nucellus (arrow). C, Control section of a developing pistil hybridized with a ZmTIP1 sense probe. D, Expression of ZmTIP1 in the basal region of the developing caryopse. The ZmTIP1 transcripts are detected in the pedicel area (arrowheads) and in a zone of the endosperm that is adjacent to the pedicel (arrows). E, High magnification of the pedicel area presented in D showing expression of ZmTIP1 in the basal endosperm transfer cells (BETC). F, Schematic representation of the three main tissues shown in E (adapted from Thorne, 1985). Red arrows within the cells indicate probable symplastic intercellular transport of assimilates and water. Blue arrows over the cell walls indicate apoplastic movement of assimilates and water.
We also determined the sites of ZmTIP1 expression in developing kernels 14 d after pollination. ZmTIP1 transcripts were detected at the base of the kernels in two distinct and well-defined tissues: the phloem termination that connects the developing kernel to the cob—this tissue is called the pedicel (Fig. 4D)—and that portion of the endosperm that is adjacent to the pedicel (Fig. 4D). This region is referred to as the basal endosperm transfer cell layer. Basal endosperm transfer cells are specialized cells thought to mediate the transfer of nutrients from the maternal tissues into the developing seed. In this tissue the highest expression is closest to the pedicel and expression diminishes in both directions as one moves away from the pedicel. No transcripts were detected in the central endosperm cells and in the pericarp (data not shown). Higher magnification (Fig. 4E) indicated that ZmTIP1 was expressed in several layers of basal endosperm transfer cells. No signal was detected in the placenta-chalaza region (Fig. 4E). Figure 4F shows a schematic representation of the three main tissues in Figure 4E: the pedicel, the placenta chalaza, and the basal endosperm transfer tissue. Red arrows within the cells indicate probable symplastic intercellular transport, whereas the blue arrows over the cell walls indicate apoplastic movement of assimilates and water (Thorne, 1985).
DISCUSSION
According to the composite transport model of Steudle (1994a, 1994b), water moves along distinct but parallel transport pathways through the apoplast and through cells (symplastic and transcellular flows) and has to overcome barriers specific for each pathway. Cellular membranes pose a major barrier to transcellular flow and Casparian strips pose a major barrier for the apoplastic flow. Since the discovery of the first tonoplast aquaporin, Arabidopsis γ-TIP, numerous other aquaporins have been found in both the plasma membrane and the vacuolar membrane of plant cells, and multiple roles for aquaporins in transmembrane water flow have been postulated (for review, see Maurel, 1997). However, because of an absence of functional studies, we do not yet understand the roles that these proteins play in the physiology of the plant. One way to approach function is to carefully study the expression patterns of the genes that encode these proteins. The expression patterns of aquaporins and putative aquaporins (major intrinsic proteins) have been studied by RNA gel-blot analysis, in situ hybridization, and expression analysis of promoter-GUS fusions in transgenic plants (see Maurel, 1997, and refs. therein). In this study we used a gene-specific probe to measure ZmTIP1 mRNA abundance and we assumed that this will translate into protein abundance, although this was not always the case.
High expression of TIPs in meristematic cells and zones of cell elongation demonstrated by in situ hybridization (Yamamoto et al., 1991; Barrieu et al., 1998; Chaumont et al., 1998) is consistent with the conclusion that these proteins are needed for the biogenesis of new vacuoles and to sustain the high rate of water influx into the vacuole associated with cell enlargement. Some aquaporin genes are also highly expressed in vascular bundles and other tissues that are thought to be involved in water transport (Yamamoto et al., 1991; Ludevid et al., 1992; Kaldenhoff et al., 1995; Yamada et al., 1995; Daniels et al., 1996). These expression patterns are consistent with two postulated functions of aquaporins: a role for TIPs in intracellular osmotic equilibration, and a role for PIPs in the regulation of transcellular water transport. These two roles may intersect in cells that participate in solute transport, because solute entry into cells via channels or transporters or via a symplastic route (plasmodesmata) may necessitate osmotic equilibration between cytoplasm and vacuole and may require transcellular water flow (e.g. for phloem loading). To our knowledge there is at present no evidence that plant aquaporins participate in solute transport. Recent results (Maurel et al., 1997b; Niemietz and Tyerman, 1997) indicate that tonoplast-derived vesicles are 10 to 100 times more permeable to water than plasma membrane-derived vesicles. Given the high hydraulic conductivity of the tonoplast, the regulation of transcellular water flow is more likely to occur at the plasma membrane than at the tonoplast. The expression patterns we obtained for ZmTIP1 are discussed in this framework of postulated aquaporin functions.
The Limitations of in Situ Hybridization
In situ hybridization, which measures the abundance of mRNA, has definite advantages over promoter GUS fusions to study gene expression (Taylor, 1997). Gene-specific probes make it possible to study the expression of individual genes, and the specificity of the probe used in this work has been documented (Chaumont et al., 1998). However, it is difficult to compare different cell types, especially if they differ in cytoplasmic content or the relative volume taken up by the vacuole. Thus, the intensity of the signal reflects the abundance of cytoplasm as well as the abundance of the mRNA under study. Furthermore, abundance of mRNA does not always translate into abundance of protein, because of posttranscriptional regulation of gene expression. In addition, aquaporin activity may be regulated by posttranslational modification (Johansson et al., 1998). It is tempting to extrapolate from mRNA abundance to hydraulic conductivity of the membrane, but we must keep in mind that there are many other points of regulation.
Expression in the Epidermis
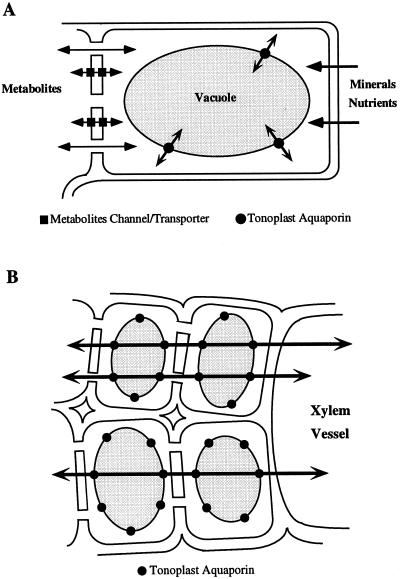
The uptake and movement of solutes and water in roots are complex processes that are still being unraveled (for review, see McCully, 1995). According to Varney and Canny (1993), water uptake is similar in the part of the root having a living epidermis (root tips and the branch roots) as in the zone of the main root where the epidermal cells have already died (Varney and Canny, 1993), suggesting that the epidermis may not be important for water uptake. The observation that ZmTIP1 is highly expressed in the epidermis of the root tip is therefore puzzling. The expression in the epidermis of the meristem and elongation zone is undoubtedly related to the need to sustain vacuolar biogenesis and the influx of water for cell elongation (see Chaumont et al., 1998). Cells that leave the meristematic zone elongate rapidly and the volume of maize root epidermal cells can increase up to 40-fold during their development (Moore and Smith, 1990). The high expression of ZmTIP1 in the epidermis could indicate a role for ZmTIP1 in osmotic equilibration of the cytoplasm. Epidermal cells are in contact with the soil solution and are involved in nutrient uptake. This uptake process and the sudden changes in water potential in the root environment may necessitate a capacity for rapid osmotic equilibration of the cytoplasm with the water from the vacuole and may be the reason for the high water permeability of the tonoplast (Maurel et al., 1997b; Niemietz and Tyerman, 1997) (see Fig. 5A). Such a role for TIPs was first suggested by Maurel et al. (1997a) for α-TIP.
Figure 5.
Schematic representation of two roles for tonoplast aquaporins. A, Tonoplast aquaporins are needed for cytoplasmic osmotic equilibration in cells that can experience rapid fluxes of metabolites or mineral nutrients. B, Tonoplast aquaporins permit rapid transcellular flow and increase the effective cross-section of the cytoplasm for symplastic flow.
Expression in the Xylem Parenchyma
One of our most striking findings was that ZmTIP1 is highly expressed in the xylem parenchyma cells of mature roots, stems, and leaves. Hydrostatic pressure drives water flow in and out of the xylem vessels and water has to go through the xylem parenchyma cells before entering the vessels. In addition, in the case of root pressure, water is thought to enter the xylem vessels because of the buildup of an osmotic gradient across the root endodermis (White et al., 1958). In stems and leaves, the small parenchyma cells surrounding the xylem vessels have an active role in establishing a water potential gradient necessary for the radial exit of water from the xylem vessels into the growing tissues (Nonami and Boyer, 1993). Moreover, the recent findings of daily embolism and repair of the water column in xylem vessels (Canny, 1997; McCully, 1997) provide an additional possible function for the xylem parenchyma cells in the control and/or the regulation of the cavitation events occurring in these vessels. Thus, the high expression of the ZmTIP1 tonoplast aquaporin in xylem parenchyma cells would facilitate a transcellular water flow and allow these cells to control water movement in and out of the xylem vessels (see Fig. 5B).
Expression in the Endodermis
The high level of expression of ZmTIP1 we observed in the endodermis/pericycle region of the root tip may be related to the function of this tissue prior to its functional differentiation. The most striking feature of the endodermis is the encrustment of the cell walls with lipid material (suberin) that forms the Casparian strip (Esau, 1977). The function of the Casparian strip in the terminal 10 cm of the root is unclear. In the root portion between 10 and 50 cm from the tip, the endodermis clearly limits radial water transport. However, closer to the tip, the endodermis appears to be quite permeable to water (Frensch et al., 1996). This high permeability may be the result of the high expression of aquaporin observed here if water transport through this cell layer is transcellular.
Alternatively, the high expression of tonoplast aquaporin may again be related to the need for osmotic equilibration of the cytosol with vacuolar water. Cells may have to cope with rapid changes in osmotic pressure (caused by influx of ions or metabolites) in the terminal 10 cm of the root. Nutrient uptake is high in root tips, and root tips are also prime sites for phloem unloading (Oparka et al., 1994). Rapid changes in nutrient uptake as the root grows and changes in phloem unloading may result in osmotic imbalances that have to be accommodated in the cytoplasm by the rapid influx or outflux of water to and from the vacuole.
Expression in the Phloem Bundles
The phloem is the major pathway for long-distance transport of assimilates. In leaves, the entry of solutes in the sieve tubes of phloem bundles, controlled by the companion cells (for review, see Sauer, 1997), creates an osmotic pressure difference that results in rapid water entry into the sieve tubes. Assimilates and water entering the phloem strands are then transported to different parts of the plant, where they are unloaded. Köckenberger et al. (1997) recently demonstrated that water is internally recirculated between the phloem and the xylem. Phloem ends are therefore sites of rapid metabolite and water transport, although such transport may also occur all along the phloem strand. The high expression of ZmTIP1 in the cells between the phloem and the xylem strands (Fig. 3) are in agreement with the findings of Köckenberger et al. (1997) mentioned above. In developing pistils and caryopses, assimilates are unloaded from the phloem terminals located either underneath the ovule or in the pedicel. The high expression of tonoplast aquaporins in the companion cells, the cells surrounding the phloem strands and the phloem terminals, suggests an important role for tonoplast water channels in cells involved in solute transport. The changes in solute concentration that probably occur at these sites as a result of solute transport and the recirculation of water probably necessitate an increased capacity for intracellular osmotic equilibration between cytosol and vacuole. In addition, high levels of tonoplast aquaporins may facilitate transcellular water movement.
The high expression of ZmTIP1 in certain tissues of the nucellus and the developing kernels lead to a similar conclusion. The pedicel, where assimilates transported to the grain are unloaded from the phloem, is part of the maternal tissue that surrounds the developing embryo and the large, starchy endosperm. The veins reticulate and terminate within the parenchyma of the pedicel. Numerous plasmodesmata connect the cytoplasm of these parenchyma cells, providing a symplastic route for assimilates when they exit the sieve tubes and intermediary cells (Felker and Shannon, 1980; Thorne, 1985). Assimilates then enter the apoplast of the placenta-chalaza, which is also a maternal tissue, and diffuse apoplastically. Uptake of assimilates by the endosperm is finally facilitated by the conversion of the outer layer of the endosperm (aleurone layer) to transfer cells (Felker and Shannon, 1980). At this point, assimilates re-enter the symplast and are translocated throughout the endosperm. We observed high expression of ZmTIP1 in the two tissues where transport of assimilates is symplastic, and no expression in the placenta-chalaza region, where transport is apoplastic. Using promoter-GUS fusions, Ludevid et al. (1992) also observed high expression of the aquaporin γ-TIP in the pedicel of Arabidopsis. These observations confirm the idea that a high permeability of the tonoplast to water is necessary in cells that can be exposed to rapid changes in cytosolic metabolite concentration.
In conclusion, although aquaporins probably do not transport solutes, the results presented here show that a tonoplast aquaporin is abundantly expressed in those cell types where rapid transport of inorganic solutes and metabolites occurs. We postulate that such cellular activities necessitate a capacity for rapid adjustment of the water potential of the cytoplasm and that this is facilitated by the high water permeability of the tonoplast. Until we know whether transcellular water flow is regulated at the plasma membrane or at the tonoplast, we have to assume that the high level of expression of tonoplast aquaporins may also permit the rapid transcellular flow of water in these tissues.
ACKNOWLEDGMENTS
The authors are grateful to Gary Ditta, Cristina Ferrandiz, and Martin Yanofsky (University of California, San Diego) for their advice and assistance with the in situ hybridization experiments. We thank Margaret McCully (Carleton University, Ottawa) for her helpful comments about ZmTIP1 expression in roots. We are also grateful to Christophe Maurel (Centre National de la Recherche Scientifique, Gif-sur-Yvette, France) for his critical comments on the manuscript.
Abbreviation:
- MIP
major intrinsic protein
- PIP
plasma membrane intrinsic protein
- TIP
tonoplast intrinsic protein
Footnotes
This work was supported by a grant from the National Science Foundation (Cell Biology). F.C. was supported by a European Molecular Biology Organization fellowship.
LITERATURE CITED
- Barrieu F, Thomas D, Marty-Mazars D, Charbonnier M, Marty F. Tonoplast intrinsic proteins from cauliflower (Brassica oleracea L. var. botrytis): immunological analysis, cDNA cloning and evidence for expression in meristematic tissues. Planta. 1998;204:335–344. doi: 10.1007/s004250050264. [DOI] [PubMed] [Google Scholar]
- Canny MJ. Vessel contents during transpiration: embolisms and refilling. Am J Bot. 1997;84:1223–1230. [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Herman EM, Chrispeels MJ. Characterization of a maize tonoplast aquaporin expressed in zones of cell division and elongation. Plant Physiol. 1998;117:1143–1152. doi: 10.1104/pp.117.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels MJ, Maurel C. Aquaporins: the molecular basis of facilitated water movement through living plant cells. Plant Physiol. 1994;105:9–13. doi: 10.1104/pp.105.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MJ, Chaumont F, Mirkov TE, Chrispeels MJ. Characterization of a new vacuolar membrane aquaporin sensitive to mercury at a unique site. Plant Cell. 1996;8:587–599. doi: 10.1105/tpc.8.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K (1977) Anatomy of Seed Plants. John Wiley, New York, pp 221–223
- Felker FC, Shannon JC. Movement of 14C-labeled assimilates into kernels of Zea mays L. III. An anatomical examination and microautoradiographic study of assimilate transfer. Plant Physiol. 1980;65:864–870. doi: 10.1104/pp.65.5.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frensch J, Hsiao TC, Steudle E. Water and solute transport along developing maize roots. Planta. 1996;198:348–355. [Google Scholar]
- Huijser P, Klein J, Lonnig WE, Meijer H, Saedler H, Sommer H. Bracteomania, an inflorescence anomaly, is caused by the loss of function of the MADS-box gene squamosa in Antirrhinum majus. EMBO J. 1992;11:1239–1249. doi: 10.1002/j.1460-2075.1992.tb05168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Karlsson M, Shukla VK, Chrispeels MJ, Larsson C, Kjellbom P. Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell. 1998;10:451–459. doi: 10.1105/tpc.10.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R, Kölling A, Meyers J, Karmann U, Ruppel G, Richter G. The blue light-responsive AthH2 gene of Arabidopsis thaliana is primarily expressed in expanding as well as in differentiating cells and encodes a putative channel protein of the plasmalemma. Plant J. 1995;7:97–95. doi: 10.1046/j.1365-313x.1995.07010087.x. [DOI] [PubMed] [Google Scholar]
- Köckenberger W, Pope JM, Xia Y, Jeffrey KR, Komor E, Callaghan PT. A non-invasive measurement of phloem and xylem water flow in castor bean seedlings by nuclear magnetic resonance microimaging. Planta. 1997;201:53–63. [Google Scholar]
- Ludevid D, Höfte H, Himelblau E, Chrispeels MJ. The expression pattern of the tonoplast intrinsic protein γ-TIP in Arabidopsis thaliana is correlated with cell enlargement. Plant Physiol. 1992;100:1633–1639. doi: 10.1104/pp.100.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrison JL, Leech RM. The subcellular and intra-organelle recognition of nuclear and chloroplast transcripts in developing leaf cells. Plant J. 1994;6:605–614. [Google Scholar]
- Maurel C. Aquaporins and water permeability of plant membranes. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:399–429. doi: 10.1146/annurev.arplant.48.1.399. [DOI] [PubMed] [Google Scholar]
- Maurel C, Chrispeels MJ, Lurin M, Tacnet F, Geelen D, Ripoche P, Guern J. Function and regulation of plant seed aquaporins. J Exp Bot. 1997a;48:421–430. doi: 10.1093/jxb/48.Special_Issue.421. [DOI] [PubMed] [Google Scholar]
- Maurel C, Reizer J, Schroeder JI, Chrispeels MJ. The vacuolar membrane protein γ-TIP creates water specific channels in Xenopus oocytes. EMBO J. 1993;12:2241–2247. doi: 10.1002/j.1460-2075.1993.tb05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Tacnet F, Guclu J, Guern J, Ripoche P. Purified vesicles of tobacco cell vacuolar and plasma membranes exhibit dramatically different water permeability and water channel activity. Proc Natl Acad Sci USA. 1997b;94:7103–7108. doi: 10.1073/pnas.94.13.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully M. How do real roots work? Plant Physiol. 1995;109:1–6. doi: 10.1104/pp.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully M. A microscopist's view of root water relations (abstract no. 20001) Plant Physiol. 1997;114:S-4. [Google Scholar]
- Moore R, Smith H. Morphometric analysis of epidermal differentiation in primary roots of Zea mays. Am J Bot. 1990;77:727–735. [PubMed] [Google Scholar]
- Niemietz CM, Tyerman ST. Characterization of water channels in wheat root membrane vesicles. Plant Physiol. 1997;115:561–567. doi: 10.1104/pp.115.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H, Boyer JS. Direct demonstration of a growth-induced water potential gradient. Plant Physiol. 1993;102:13–19. doi: 10.1104/pp.102.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparka KJ, Duckett CM, Prior DAM, Fisher DB. Real-time imaging of phloem unloading in the root tip of Arabidopsis. Plant J. 1994;6:759–766. [Google Scholar]
- Sarda X, Tousch D, Ferrare K, Legrand E, Dupuis JM, Casse-Delbart F, Lamaze T. Two TIP-like genes encoding aquaporins are expressed in sunflower guard cells. Plant J. 1997;12:1103–1111. doi: 10.1046/j.1365-313x.1997.12051103.x. [DOI] [PubMed] [Google Scholar]
- Sauer N. Sieve elements and companion cells: extreme division of labour. Trends Plant Sci. 1997;2:285–286. [Google Scholar]
- Schreiber L. Chemical composition of Casparian strips isolated from Clivia miniata Reg. roots: evidence for lignin. Planta. 1996;199:596–601. [Google Scholar]
- Steudle E. The regulation of plant water at the cell, tissue, and organ level: role of active processes and of compartmentation. In: Schulze E-D, editor. Flux Control in Biological Systems: From the Enzyme to the Population and Ecosystem Level. San Diego, CA: Academic Press; 1994a. pp. 237–299. [Google Scholar]
- Steudle E. Water transport across roots. Plant Soil. 1994b;167:79–90. [Google Scholar]
- Taylor CB. Promoter fusion analysis: an insufficient measure of gene expression. Plant Cell. 1997;9:273–275. [Google Scholar]
- Thorne JH. Phloem unloading of C and N assimilates in developing seeds. Annu Rev Plant Physiol Plant Mol Biol. 1985;36:317–343. [Google Scholar]
- Varney GT, Canny MJ. Rates of water uptake into the mature root system of maize plants. New Phytol. 1993;123:775–786. [Google Scholar]
- Varney GT, McCully ME, Canny MJ. Sites of entry of water into the symplast of maize roots. New Phytol. 1993;125:733–741. doi: 10.1111/j.1469-8137.1993.tb03922.x. [DOI] [PubMed] [Google Scholar]
- Weig A, Deswarte C., Chrispeels MJ (1997) The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiol 114: 1347–1357 [DOI] [PMC free article] [PubMed]
- White PR, Schuker E, Kern JR, Fuller FH. Root-pressure in gymnosperms. Science. 1958;128:308–309. doi: 10.1126/science.128.3319.308. [DOI] [PubMed] [Google Scholar]
- Yamada S, Katsuhara M, Kelly W, Michalowski C, Bohnert H. A family of transcripts encoding the water channel proteins: tissue-specific expression in the common ice plant. Plant Cell. 1995;7:1129–1142. doi: 10.1105/tpc.7.8.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto YT, Taylor CG, Acedo GN, Cheng CL, Conkling MA. Characterization of cis-acting sequences regulating root-specific gene expression in tobacco. Plant Cell. 1991;3:371–382. doi: 10.1105/tpc.3.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]