Abstract
Long residence times of probiotics in the intestinal tract would prolong their potential beneficial health effects and assist colonization. This study investigated the colonization potential of Lactobacillus casei Shirota in mouse intestine by using 5 (and 6)-carboxyfluorescein diacetate, succinimidyl ester (cFDA-SE) for assessment of doubling times in different parts of the intestine. The amounts of intestinal water overlying the surfaces of the duodenum, jejunum, ileum, and colon in BALB/c mice were 34.4 ± 2.9, 58.8 ± 6.8, 21.6 ± 2.2, and 8.0 ± 1.0 mg, respectively. Based on the residual concentrations of cFDA-SE-labeled lactobacilli on intestinal mucosal surfaces, the average half times for the wash-out of lactobacilli fed were estimated at 3.98, 1.55, 1.34, and 2.48 days in the duodenum, jejunum, ileum, and colon, respectively. The average doubling times of the lactobacilli, estimated from the residual fluorescent levels of surface-adhered cells, were 4.10, 4.78, 4.56, and 5.59 days in the duodenum, jejunum, ileum, and colon, respectively. It is estimated that the lactobacilli would have to achieve an average doubling time of 1.03 to 2.04 days to colonize the various sections of the mouse intestinal tract more permanently.
Probiotic intestinal bacteria beneficially influence the health of the host by modulating the metabolic activities, immunity, and microbiota in the host's intestine (7, 12). Lactobacilli have been used as antigen and cytokine delivery vehicles for oral immunization and disease treatment (11, 16). Probiotic bacteria are selected for their beneficial health properties as well as their ability to tolerate intestinal conditions and achieve high growth rates in culture (13). However, no probiotic lactobacilli used in clinical trials and commercial production have been demonstrated to persist in fecal samples for more than a few weeks after their administration has been stopped (4, 5, 14, 15). Such an effect is termed colonization resistance. The ability of exogenously administered probiotics to adhere to the mucosal cells and multiply in the intestinal tract has been questioned (2). There are recent reports on the recovery of consumed lactobacilli from human colonic biopsies after discontinuation of probiotic administration (1, 3, 18), thus providing direct evidence that probiotic lactobacilli are able to temporary colonize colonic mucosae. Prolonged adhesion and colonization of probiotic bacteria on intestinal mucosal surfaces could favor probiotic effects. The aim of this study was to understand the growth and colonization of lactobacilli in the intestinal tract, using the mouse as the model system.
MATERIALS AND METHODS
Preparation of fluorogenic dye.
Five (and 6)-carboxyfluorescein diacetate, succinimidyl ester (cFDA-SE), is a nonfluorescent membrane-permeative ester which nonspecific prokaryotic and eukaryotic intracellular esterases convert to a fluorescent derivative that in turn is then covalently linked to intracellular proteins via the probe's succinimidyl group (19). cFDA-SE (2 mg) (Molecular Probes) was prepared according to the method of Logan and coworkers (8) with slight modifications. In brief, a 100 μM stock solution of cFDA-SE was prepared by being first dissolved in dimethyl sulfoxide (20 μl) (Merck, Darmstadt, Germany) and then further diluted in ethanol (1 ml; reagent grade). This solution was then filter sterilized (0.2-μm-pore-size Acrodisc filter; Gelman) before being aliquoted and stored at −20°C.
Culture and labeling of bacteria with fluorescent probe.
Lactobacillus casei Shirota (Yakult Singapore Pte. Ltd.) was grown overnight at 37°C in Mann Rogosa Sharpe (MRS) broth (Bio-Rad, Marnes-LaCoquette, France). The bacterial culture was centrifuged at 3,000 × g for 10 min, and the pellet was washed twice in sterile phosphate-buffered saline (PBS). The pellet was then adjusted to give a cell concentration of 1010 CFU ml−1 prior to labeling with cFDA-SE (50 μM) at 37°C for 20 min. Fluorescent labeling was terminated by pelleting the bacteria, washing twice in PBS to remove excess cFDA-SE, and resuspending the pellet in PBS. The flow cytometry profile showed that about 99% of the cells were labeled. A doubling in the cell concentration resulted in reduction of the median fluorescence intensity to half (9, 17). Thus, the generation time (td) could be determined from the fluorescence intensity of the cells as td = ln2 × (time interval)/(change in ln fluorescence intensity), where ln is the natural log value.
To culture the lactobacilli under anaerobic conditions, the culture flasks were incubated in an anaerobic cabinet (Concept Plus; Innovative Biotech, Cheshire, United Kingdom).
Animals.
Seven-week-old female BALB/c mice were obtained from the Laboratory Animals Centre, National University of Singapore, and maintained at the Animal Holding Unit of the Department of Microbiology, National University of Singapore. They had free access to a standard mouse diet and water. A group of 12 mice were orally dosed with approximately 109 cFDA-SE-labeled lactobacilli by orogastric intubation. Another group of 12 mice that had been orally fed with sterile PBS served as controls. The food and water intakes for the experimental and control batches of mice were measured daily. In addition, the daily production of feces was also measured and collected for mucin extraction. Groups of three mice each were sacrificed on days 1, 2, 4, and 6 after dosing by CO2 asphyxiation, and the duodenum, jejunum, ileum, and colon were extracted from each mouse. Individual sections were cut longitudinally, and any visible residual food particles or fecal material were removed. For the determination of the intestinal water volume, each intestinal part was blotted gently with preweighed filter paper (90-nm-diameter; Whatman, Kent, United Kingdom), and the increase in the weight of the filter paper was taken as the intestinal water volume of the respective intestinal portion. The various intestinal parts were then examined for the presence of adhering cFDA-SE-labeled L. casei. This was performed by adding 150 μl of PBS to every 1.0 cm of tissue and dislodging microbes from the mucosal surface of the tissues with the aid of a plunger from a syringe (1.0 ml; Terumo, Tokyo, Japan). Cell extracts were fixed with formaldehyde (0.75%, vol/vol) prior to flow cytometry analysis.
Flow cytometry analysis.
Enumeration of cFDA-SE-stained L. casei Shirota from cell extracts was conducted on an Epics Elite flow sorter (Coulter, Miami, Fla.) at a 488-nm excitation wavelength with a 15-mW argon laser with a 75-mm sort sense flow cell at 82.7 kPa of pressure. Upon excitation at 488 nm in the flow cytometer, cFDA-SE gives a maximal emission signal in the green at 518 nm. Data were recorded in the FCS2.0 file format by using Coulter Epics Elite (version 4.01) software and were then analyzed and converted into plots by using WinMDI (J. Trotter, Scripps Research Institute, La Jolla, Calif.). The sample volume was estimated from the counts of the reference glass beads included in the sample.
Extraction of mucus from fecal material.
Mucus was isolated from murine feces by extraction and dual ethanol precipitation by a modification of the method of Miller and Hoskins (10). In short, fecal samples collected daily were diluted four times in PBS containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 2 mM iodoacetamide, and 10 mM EDTA), and the mixture was slowly stirred at 4°C for 1 h. The suspension was centrifuged at 15,000 × g at 4°C for 30 min, and the supernatant was collected. Ethanol was added to the supernatant to a final concentration of 70%. This was then centrifuged at 10,000 × g at 4°C for 30 min, and the precipitate was collected. The pellet was dissolved in Milli-Q water and precipitated again with ethanol. The extracted mucus was then lyophilized and weighed to determine the amount of mucus excreted daily.
RESULTS
The amounts of intestinal water overlying the duodenum, jejunum, ileum, and colon were found to be 34.4 ± 2.9, 58.8 ± 6.8, 21.6 ± 2.2, and 8.0 ± 1.0 mg, respectively. The mice consumed 6.6 ± 0.7 g of feed and water per animal per day and excreted 1.2 ± 0.3 g of feces per animal per day. The mucus content of the feces was 0.9 ± 0.1 mg/g of feces; 1.1 ± 0.2 mg of mucus was dislodged from the intestinal surface of a mouse per day.
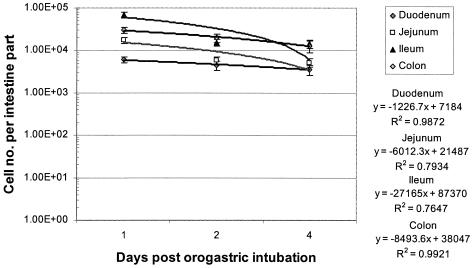
The rate of wash-out of the lactobacilli from the intestinal mucosal surface was estimated from the slope of the plots of the residual concentration of lactobacilli labeled with cFDA-SE (total fluorescent cells irrespective of the intensity) extracted from the mucosal surface versus the time after orogastric intubation (Fig. 1). The half times for the wash-out of lactobacilli were determined to be 3.98, 1.55, 1.34, and 2.48 days in the duodenum, jejunum, ileum, and colon, respectively.
FIG. 1.
Plots of total L. casei Shirota cell number adhered on various sections of the intestinal tract against time after orogastric intubation. Each value represents the average for three samples, and error bars indicate standard deviations.
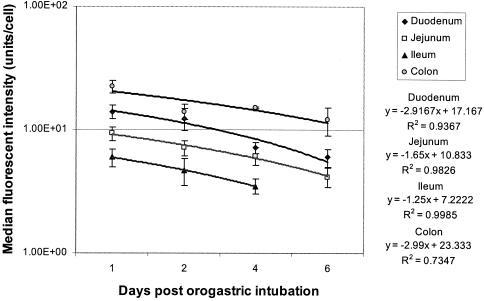
The doubling times of the lactobacilli adhered on the mucosal surfaces were estimated from the residual fluorescence intensities (median) of cells extracted at different times after orogastric intubation (Fig. 2). The growth and division of a cell would result in the dilution of the cFDA labeled protein by half. The doubling times of L. casei Shirota were 4.10, 4.78, 4.56, and 5.59 days in the duodenum, jejunum, ileum, and colon, respectively.
FIG. 2.
Plots of the residual median fluorescence intensity of L. casei Shirota adhered on various sections of the intestinal tract against time after orogastric intubation. Each value represents the average for three samples, and error bars indicate standard deviations.
The fluorescence intensity profiles of the adhered lactobacilli determined on the various days after orogastric intubation are summarized in Table 1. The fluorescence intensities are categorized into those that are higher than the median measured on day 1, those from the median intensity down to half of the median intensity measured on day 1 (expected fluorescence intensity after one cell division), those from half of the median intensity down to one-fourth of the median intensity measured on day 1 (expected fluorescence intensity after two cell divisions), and those lower than one-fourth of the median intensity measured on day 1 (expected fluorescence intensity after three cell divisions). For example, in the duodenum on day 1, 35.92% of the lactobacillus cells showed a fluorescence intensity higher than the median intensity (1.40 × 101 fluorescence units/cell), and 36.49% showed an intensity lower than 3.8 × 100 fluorescence units/cell (expected fluorescence level after three cell divisions). The bacterial cells were not uniformly labeled by cFDA-SE, probably due to the physiological status of the cells at the time of incubation with cFDA-SE. On day 6 in the duodenum, 44.4% (15.96/35.92) of the labeled lactobacilli retained the original fluorescence intensity (no cell division), whereas 13.1% of the total labeled lactobacilli had divided at least three times (49.54 − 36.49). It was calculated that on day 6 after orogastric intubation, the percentages of the lactobacilli that remained nondivided and adhered on the intestinal mucosal surfaces were 44.4, 60.5, 37.2, and 56.3% in the duodenum, jejunum, ileum, and colon, respectively. On day 6 after orogastric intubation, 13.1, 3.9, 21.6, and 21.4% of the lactobacilli fed had divided at least three times.
TABLE 1.
Fluorescence intensity profiles of lactobacilli harvested from various sections of the intestinal mucosal surface on various days after orogastric intubation of L. casei Shirota
| Section and day | % of total population with the following fluoroscence intensity (mean ± SD)a
|
|||
|---|---|---|---|---|
| <1/4 median | 1/4-1/2 median | 1/2-median | >Median | |
| Duodenum | ||||
| 1 | 36.49 ± 3.51 | 10.13 ± 1.02 | 17.46 ± 1.98 | 35.92 ± 2.47 |
| 2 | 33.04 ± 2.87 | 15.16 ± 1.48 | 19.80 ± 1.21 | 32.00 ± 2.41 |
| 4 | 37.76 ± 2.69 | 18.64 ± 1.78 | 20.02 ± 2.04 | 23.58 ± 2.84 |
| 6 | 49.54 ± 3.98 | 18.31 ± 1.20 | 16.19 ± 1.18 | 15.96 ± 1.07 |
| Jejunum | ||||
| 1 | 24.23 ± 1.79 | 12.64 ± 0.84 | 18.95 ± 1.51 | 44.18 ± 4.25 |
| 2 | 28.54 ± 2.07 | 16.53 ± 1.20 | 20.97 ± 1.99 | 33.96 ± 2.89 |
| 4 | 28.15 ± 2.10 | 18.06 ± 1.74 | 21.71 ± 2.10 | 32.08 ± 3.84 |
| 6 | 28.14 ± 3.07 | 24.51 ± 2.86 | 20.60 ± 2.51 | 26.75 ± 2.76 |
| Ileum | ||||
| 1 | 30.06 ± 2.87 | 14.73 ± 1.17 | 15.78 ± 1.72 | 39.43 ± 3.20 |
| 2 | 43.57 ± 3.51 | 24.85 ± 2.71 | 15.93 ± 1.43 | 15.65 ± 1.33 |
| 4 | 47.87 ± 3.82 | 24.18 ± 2.14 | 12.57 ± 0.94 | 15.38 ± 1.14 |
| 6 | 51.61 ± 4.01 | 22.90 ± 1.88 | 10.83 ± 0.76 | 14.66 ± 1.21 |
| Colon | ||||
| 1 | 40.70 ± 3.14 | 14.38 ± 1.13 | 21.05 ± 1.45 | 23.87 ± 1.71 |
| 2 | 37.74 ± 2.41 | 24.33 ± 2.03 | 25.00 ± 2.17 | 12.93 ± 0.72 |
| 4 | 65.00 ± 4.95 | 14.36 ± 1.24 | 7.12 ± 0.49 | 13.52 ± 1.00 |
| 6 | 62.14 ± 3.45 | 16.57 ± 1.52 | 7.86 ± 0.84 | 13.43 ± 1.17 |
The medians of the fluorescence intensities (n = 3) measured on day 1 were 1.40 × 101, 9.33 × 100, 1.87 × 101, and 2.23 × 101 U/cell in the duodenum, jejunum, ileum, and colon respectively. <1/4 median, lower than one fourth of the median fluorescence intensity measured on day 1; 1/4-1/2 median, one-fourth to one-half of the median intensity; 1/2-median, half of median to median intensity; >median, higher than median intensity.
In MRS culture medium incubated at 37°C, the doubling times of L. casei Shirota cultured under aerobic and anaerobic conditions were 1.7 and 1.2 h, respectively. The inclusion of 0.2% (wt/vol) bile (Sigma-Aldrich, St. Louis, Mo.) in MRS medium suppressed the growth of the lactobacilli for up to 4 h, but the cells remained viable, as resuspension of the bacterial cells in fresh culture medium gave viable counts on MRS agar comparable to the original cell concentrations (data not shown).
DISCUSSION
The layer of intestinal water in mice where intestinal bacteria were suspended amounted to 0.33 to 0.89% (wt/wt) of the daily feed and water consumption (in small intestine) and 0.67% (wt/wt) of daily fecal excretion (in colon). That is, there were more than 100 volume changes in the intestinal water content per day. One volume change in intestinal water would dilute the bacteria suspended in it by half. Hence, bacterial cells that did not adhere on mucosal surfaces would be diluted to an undetectable level soon after a single feeding. The observation that the average half time for the wash-out of lactobacilli fed ranged from 1.34 to 3.98 days in the various parts of the intestine suggests that the lactobacilli was able to compete with the indigenous bacteria for adhesion on the mucosal surface along the full stretch of the intestinal tract. In the small intestine, the affinity for adhesion of lactobacilli was in the order of duodenum > jejunum > ileum (half times for wash-out were 3.98, 1.55, and 1.34 days, respectively). A study using human fecal samples had demonstrated that the half time for the wash-out of Lactobacillus rhamnosus GG from the intestinal tract was about 1 day (4). The intestinal bacteria in a fecal sample are diluted by fecal material, and their counts could reach the limits of detection when the bacteria could still be detected on intestinal mucosal surfaces (1, 3). Moreover, the bacterial count of a fecal sample does not allow the differentiation of growth and colonization in the various sections of the intestine. The study of fecal samples has limitations in evaluating growth and colonization by probiotic bacteria.
About 1.1 mg of mucus per day was dislodged from intestinal surfaces and could be recovered in the feces of the mice. Lactobacilli adhered on mucosal surface would have dislodged together with mucus, and a fraction of the dislodged bacteria might have released and readhered onto the newly exposed mucosal surface.
The outcome of competition between two bacterial cells for adhesion on a receptor on a mucosal surface is determined by the ratio of the respective bacterial concentration around the receptor and the affinity of the respective bacterium for the receptor (6). A high bacterial cell concentration would ensure frequent encounters between the bacterial cells and the receptor and thus a higher chance for the bacterium to adhere onto the receptor site. A bacterial cell which has a high affinity for the receptor will not dissociate as readily to be replaced by another bacterial cell. Thus, one may select for a probiotic bacterium with very high affinity for the intestinal receptor to prevent it from being out-competed by other bacteria and washed out from the intestine. However, such a bacterium would not be released readily from the dislodged mucus layer and would be discharged as fecal material.
When the medians of the fluorescence intensity profiles determined on day 1 were taken as the first generation (original population), 37.2 to 60.5% (in the various sections of the intestine) of the original population that had adhered onto the intestinal surface remained attached and undivided on day 6 after orogastric intubation (Table 1). These cells that had remained undivided on day 6 could have lost their viability after passing through the stomach and intestinal tract. Among the dividing cells, 3.9 to 21.6% of the total adhered population were found in their fourth generation (three cell division) on day 6 after orogastric intubation. This study has clearly demonstrated that a large part of the L. casei Shirota fed was able to grow and divide in the intestinal environment; however, the rate of cell division was low.
The average doubling times of the lactobacilli measured in the duodenum, jejunum, ileum, and colon were 4.10, 4.78, 4.56, and 5.59 days, respectively. These values are low compared with doubling times of the bacterium cultured in MRS medium under aerobic (1.7 h) and anaerobic (1.2 h) conditions. The nutritional composition of the feed for mice is well balanced and rich; it is the intestinal content and conditions that suppressed the growth of the lactobacilli. A bile concentration of 0.2% (wt/vol) in MRS medium was found to prevent the growth of the lactobacilli for at least 4 h, although the bacterial cells remained viable after 4 h of incubation. Bile tolerance is a selection criterion for probiotics (13); however, only those bacteria that are able to divide fast enough to maintain high local cell concentrations are able to colonize mucosal surfaces. This should be the critical criterion in the selection of probiotics.
It is interesting that the medians of the fluorescence intensities measured on day 1 in various sections of the intestine were not the same (Fig. 2). The differences in the fluorescence intensity of the original lactobacillus population were probably due to the physiological status of the cells at the time of incubation with cFDA-SE. Various quantities of cFDA-SE might have been taken up by the lactobacillus cells, converted to the fluorescent derivative, and covalently linked to intracellular proteins with different efficiencies. This observation suggests that lactobacilli in different physiological states preferentially adhere to the various sections of the intestinal tract. This may have implications for the preparation of probiotics for selective adhesion on specific sections of the intestinal tract.
The process of washing out of a bacterium from the intestinal mucosal surface can be described by the following relationship: wash-out rate = specific growth rate − dilution rate. The dilution factor involves the rate at which the intestinal content passes through and the ability of the bacterium to adhere to the mucosal surface. The effective dilution rate could be estimated from the values of the wash-out rate and the specific growth rate, which were determined experimentally. For example, in the duodenum the average wash-out rate was ln2/3.98 or 0.17 day−1, and the specific growth rate was ln2/4.10 or 0.17 day−1. Thus, the calculated effective dilution rate was 0.34 day−1. In order to persist on a mucosal surface (i.e., wash-out rate = 0), the lactobacilli would need to grow and divide at an average specific growth rate that equals the effective dilution rate (0.34 day−1). This specific growth rate represents a doubling time of 2.04 days in the environment of the duodenum. In order to permanently colonize the jejunum, ileum, and colon, the lactobacilli would need to divide at doubling times of 1.17, 1.03, and 1.72 days, respectively.
This study suggests that in addition to direct competition between intestinal bacteria for adhesion, the rate of growth and division, or generation time, determines the ability of probiotics to colonize and persist on mucosal surfaces. These are factors that will also have an impact on probiotic efficacy, and thus such factors should be included in the selection criteria for future probiotics.
REFERENCES
- 1.Alander, M., R. Satokari, R. Korpela, M. Saxelin, T. Vilpponen-Salmela, T. Mattila-Sandholm, and A. von Wright. 1999. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl. Environ. Microbiol. 65:351-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bezkorovainy, A. 2001. Probiotics: determinants of survival and growth in the gut. Am. J. Clin. Nutr. 73:399S-405S. [DOI] [PubMed] [Google Scholar]
- 3.Cesena, C., L. Morelli, M. Alander, T. Siljander, E. Tuomola, S. Salminen, T. Mattila-Sandholm, T. Vilpponen-Sslmela, and A. von Wright. 2001. Lactobacillus crispatus and its nonaggregating mutant in human colonisation trials. J. Dairy Sci. 84:1001-1010. [DOI] [PubMed] [Google Scholar]
- 4.Goldin, B. R., S. L. Gorbach, S. B. Saxelin, L. Gualtieri, and S. Salminen. 1992. Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig. Dis. Sci. 37:121-128. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen, C. N., V. R. Nielsen, A. E. Hayford, P. L. Moller, K. F. Michaelsen, A. Paerregaard, B. Sandstrom, M. Tvede, and M. Jakobsen. 1999. Screening of probiotic activities of 47 strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 65:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee, Y. K., C. Y. Lim, W. L. Teng, A. C. Ouwehand, E. M. Tuomola, and S. Salminen. 2000. Quantitative approach in the study of adhesion of lactic acid bacteria to intestinal cells and their competition with enterobacteria. Appl. Environ. Microbiol. 66:3692-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, Y. K., K. Nomoto, S. Salminen, and S. L. Gorbach. 1999. Handbook of probiotics, p. 211. John Wiley, New York, N.Y. 211.
- 8.Logan, R. P., A. Robins, G. A. Turner, A. Cockayne, S. P. Borriello, and C. J. Hawkey. 1998. A novel flow cytometric assay for quantitating adherence of Helicobacter pylori to gastric epithelial cells. J. Immunol. Methods 213:19-30. [DOI] [PubMed] [Google Scholar]
- 9.Lyons, A. B. 2000. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J. Immunol. Methods 243:147-154. [DOI] [PubMed] [Google Scholar]
- 10.Miller, R. S., and L. C. Hoskins. 1981. Mucin degradation in human colon ecosystems. Fecal population densities of mucin-degrading bacteria estimated by a “most probable number” method. Gastroenterology 81:759-765. [PubMed] [Google Scholar]
- 11.Pouwels, P. H., R. L. Leer, M. Shaw, M.-J. H. den Bak-Glashouwer, F. D. Tielen, E. Smit, B. Martinez, J. Jore, and P. L. Conway. 1998. Lactic acid bacteria as antigen delivery vehicles for oral immunization purposes. Int. J. Food Microbiol. 41:155-167. [DOI] [PubMed] [Google Scholar]
- 12.Salminen, S., M. C. Bouley, M. C. Boutron-Rualt, J. Cummings, A. Franck, G. Gibson, E. Isolauri, M.-C. Moreau, M. Roberfroid, and I. Rowland. 1998. Functional food science and gastrointestinal physiology and function. Br. J. Nutr. Suppl. 1:147-171. [DOI] [PubMed] [Google Scholar]
- 13.Salminen S, M. Playne, and Y. K. Lee. 2004.. Successful probiotic lactobacilli: human studies on probiotic efficacy, p. 13-32. In C. Shortt (ed.), Functional dairy products. Technomic, Lancaster, United Kingdom.
- 14.Saxelin, M. 1997. Lactobacillus GG—a human probiotic strain with thorough clinical documentation. Food Rev. Int. 13:293-313. [Google Scholar]
- 15.Spanhaak, S., R. Havenaar, and G. Schaafsma. 1998. The effect of consumption of milk fermented by Lactobacillus casei strain Shirota on the intestinal microflora and immune parameters in humans. Eur. J. Clin. Nutr. 52:899-907. [DOI] [PubMed] [Google Scholar]
- 16.Steidler, L., W. Hans, L. Schotte, S. Neirynck, F. Obermeier, W. Falk, W. Fiers, and E. Remaut. 2000. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289:1352-1355. [DOI] [PubMed] [Google Scholar]
- 17.Ueckert, J. E., G. N. von-Caron, A. P. Bos, and P. F. ter Steeg. 1997. Flow cytometric analysis of Lactobacillus plantarum to monitor lag times, cell division and injury. Lett. Appl. Microbiol. 25:295-299. [DOI] [PubMed] [Google Scholar]
- 18.von Wright, A., T. Vilpponen-Salmela, M. P. Llopis, K. Collins, B. Kiely, F. Shanahan, and C. Dunne. 2002. The survival and colonic adhesion of Bifidobacterium infantis in patients with ulcerative colitis. Int. Dairy J. 12:197-200. [Google Scholar]
- 19.Weston, S. A., and C. R. Parish. 1990. New fluorescent dyes for lymphocyte migration studies: analysis by flow cytometry and fluorescent microscopy. J. Immunol. Methods 133:87-97. [DOI] [PubMed] [Google Scholar]