Abstract
Ultrasound-activated microbubbles were used as actuators to deform microvessels for quantifying microvessel relaxation timescales at megahertz frequencies. Venules containing ultrasound contrast microbubbles were insonified by short 1 MHz ultrasound pulses. Vessel wall forced-deformations were on the same microsecond timescale as microbubble oscillations. The subsequent relaxation of the vessel was recorded by high-speed photomicrography. The tissue was modeled as a simple Voigt solid. Relaxation time constants were measured to be on the order of ∼10 μs. The correlation coefficients between the model and 38 data sets were never lower than 0.85, suggesting this model is sufficient for modeling tissue relaxation at these frequencies. The results place a bound on potential numerical values for viscosity and elasticity of venules.
Ultrasound contrast microbubbles are micron-size gas bubbles that are used in both diagnostic and therapeutic ultrasound applications.1, 2, 3 Microbubbles are unique amongst contrast agents in that they remain within the blood vessels after being injected intravenously, and they undergo complex translational and vibrational motion when subjected to an ultrasound pulse.4 In particular, nonlinear oscillations have been exploited to develop new ultrasound imaging modalities such as pulse inversion and subharmonic imaging. Microbubbles have also been investigated for drug and gene delivery through tumor microvessels or the blood brain barrier.5 Upon insonation, their interactions with microvessels can increase vascular permeability and facilitate the delivery of therapeutic materials.6
In order to exploit the physical and functional properties of microbubbles for diagnostic and therapeutic applications, it is important to understand how microbubbles respond to megahertz-ultrasound pulses in the microvasculature. Experimental evidence suggests that the bubble dynamics depends on the mechanical properties of the microvessels.7, 8, 9, 10 Modeling such behavior requires knowledge of the viscoelastic properties of the vessel connected with the surrounding tissue. Efforts to generate appropriate models are ongoing; most models assume linear elasticity only,11, 12, 13 although at least one model includes viscosity.14
Most living tissues exhibit viscoelastic properties, which are frequency dependent.15 Previous studies demonstrated that microvessels deform on the same microsecond time scale as bubble oscillation driven by megahertz-frequency ultrasound.10, 16 The objective of this paper is to constrain the possible viscoelastic values under these conditions. Although the mechanical properties of living tissues have been widely studied, only a few studies have been published at megahertz frequencies; these studies extract the parameters by measuring the complex reflection coefficient from megahertz acoustic shear waves.17, 18 In this letter, we propose a method using ultrasound-activated microbubbles as actuators to induce microvessel deformation at the megahertz frequency, and estimating the characteristic time constant of the vessel based on the relaxation of the deformed vessels. In our experiments, tissue relaxation was quantified using high-speed photomicrography.
Ex vivo rat mesentery was used as our tissue model. It provides good optical transparency for optically monitoring the bubble and vessel dynamics. The tissue sample preparation method is described elsewhere.9, 10 Briefly, the rat mesentery was surgically exposed and perfused by a Krebs-Ringer solution to flush out the blood. The solution was used to maintain the physiological condition of the tissue sample. Then the entire mesentery (with intestine) was excised. All experiments were completed within 4 h. It is important to keep the tissue fresh so that postmortem tissue degradation does not alter the mechanical properties. A selected well-vascularized segment of the mesentery was sandwiched between two plates with a window for ultrasound exposure and microscopic observation. Care was taken to mount the tissue such that it resembled its in situ dimensions. Commercially available microbubbles (Definity®, Lantheus Medical Imaging, North Billerica, MA) diluted by a Krebs-Ringer solution were injected by a syringe pump into selected mesentery segments. The microbubbles were perfluoropropane gas bubbles stabilized by lipid shells with average diameters of 1.1–3.3 μm. Venules with diameters of 10–80 μm were selected for this study, motivated by the following: Our previous experiments suggest that venules respond more than arterioles to nearby microbubble oscillations (most probably due to the relative lack of supporting structure), which allows easier quantification of vessel displacements.9, 10 Also, Kobayashi et al.19 showed that ultrasound-activated microbubble induced endothelial cell damage in rat mesenteries was dominant in venules.
The prepared tissue sample was placed into a tank filled with degassed Krebs-Ringer buffer at room temperature, and aligned at the con-focal plane of the microscope objective and an ultrasound transducer positioned opposite the objective. The transducer, coupled to a water cone, was driven with a one-cycle, 1 MHz sinusoidal pulse generated by an amplified function generator signal. Due to the ringing of the transducer and reflection of the pulse off the microscope objective, the total length of the ultrasound pulse (including both incident and reflected wave) is ∼8 μs.10 The peak negative pressure (PNP) of the pulse used in this study was 1.5, 2.1 or 2.8 MPa. The transducer was synchronized with an ultra-high speed camera which is capable of capturing 14 image frames with a minimum shutter speed of 5 ns. Illumination for ultra-high speed imaging was provided by a high-intensity, point-source flash lamp coupled to an optical fiber. The duration of the flash light measured to 50% of light output was 24 μs. In this study, 12 high-speed image frames were captured at each trigger event with the exposure time of each frame increased from 50 ns to the maximum of 2.5 μs to compensate for the decrease of the flash lamp light intensity over time; the increased exposure time did not lead to blurring of the images, as demonstrated later. The interframe times of the 12 frames also varied so that 4 or 5 of the 12 frames recorded the dynamic response of the microvessel before and during ultrasound excitation, and the remaining frames recorded the tissue response after ultrasound exposure. The arrival time of the ultrasound pulse at the tissue sample was set to be zero. The time point for each frame was defined as the end time point when it was captured.
Vessel wall displacements were quantified as changes to its diameter at the point that had the maximum displacement in reference to its initial diameter. To be consistent with convention, a decrease in vessel diameter was defined as negative. During bubble collapse, the vessel wall and surrounding tissue was displaced toward the lumen. The vessel relaxation process was considered to start from the time point when the recorded vessel displacement reached its most negative . Only image sequences that captured more than four frames during the relaxation process were selected for analysis.
An appropriate rheological model is needed to estimate the viscoelastic properties of the tissue. We chose the standard Voigt model, consisting of an elastic spring and a viscous dashpot in parallel. This model was selected because previous work had shown that it is appropriate for liquid-like solid, such as soft tissue, from a few hundred kilohertz20 to low-megahertz frequency range;21 further, it fits our data well, as demonstrated later. The governing equation for this model during the relaxation process is
| (1) |
where D is the vessel displacement, τ is the relaxation time constant, k is the elastic constant, and η is the viscosity coefficient. The time constant is thus the ratio of the viscous to elastic constants. It was determined by fitting the data with a single time constant exponential decay using a custom program based on the "fminsearch" function in matlab (Mathworks Inc., Sherborn, MA, USA), which performs an unconstrained nonlinear minimization of the SSR (sum of squared residuals). A two-tailed Student's t-test was used to determine whether there is a significant statistical difference (p < 0.05) in the time constant among different groups.
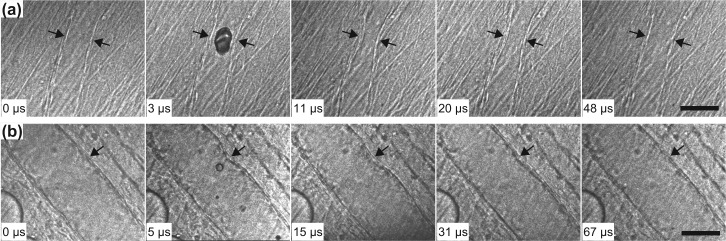
Figure 1 displays two examples of the high-speed image sequences. Figure 1a shows the dynamics of a relatively small microvessel with its initial static diameter of 30 μm. In this case, microbubble oscillation induced nearly axial-symmetrical displacement of the vessel wall. Frame 1 recorded the vessel at its initial state before the arrival of the ultrasound pulse. Frame 2 was captured during ultrasound exposure, showing a microbubble at some stage during its growth in the microvessel. In Frame 3, the vessel had reached its maximum invagination . Note that at , the bubble had already collapsed and the ultrasound pulse had ended. Consistent with our previous observations, the vessel deformed on the same microsecond timescale as bubble oscillation. Additional details of the bubble dynamics during ultrasound exposure were reported previously.9, 10 The recovery of the vessel itself was recorded here in the following frames, as representatively shown in frames captured at 11 μs, 20 μs, and 48 μs. Figure 1b shows the dynamics of a larger microvessel. Similar vessel dynamics were observed, with the difference being that vessel deformation and recovery was observed on only the side nearest to the microbubble. Note that no blurring of the images was clearly identifiable, suggesting that the exposure time selected for each frame was appropriate.
Figure 1.
Ultra-high speed image sequences that show microvessel deformation and recovery. (a) Vessel diameter = 30 μm; PNP = 1.5 MPa. (b) Vessel diameter = 76 μm; PNP = 2.8 MPa. The arrows point to the deformed vessel wall. The scale bar represents 50 μm. For both image sequences, frame 1 shows the initial state of the vessel; frame 2 was captured during ultrasound exposure; frame 3 presents vessel at its recorded maximum invagination, which was recorded after the ultrasound pulse ended; frames 4 and 5 show representative images from the recovery process.
Figure 2 gives the displacements of the microvessels recorded in Fig. 1. The time , when the displacements reached their maximum (negative) , is marked by the dashed line. For the small microvessel case (Fig. 1a), = 11 μs and = −14 μm; for the larger microvessel case (Fig. 1b), = 15 μs and = −9 μm. Lower bound estimates of the average speed of vessel deformation (using ) gives 1.3 m/s and 0.6 m/s for the two cases, respectively. Again, the relaxation processes were fitted to the Voigt model. For both small and larger vessel cases, the time constant was τ = 15 μs. This small time constant indicates a higher rate of change in displacement, and smaller viscous force relative to the elastic restoring force. Figure 2 also suggests that the Voigt model accurately describes the relaxation of the vessel. Although a more complicated mechanical model might result in a somewhat more accurate match, the correlation coefficients, r, between experimental data and the Voigt model were 0.96 and 0.97, respectively, for the two cases shown in Fig. 2; i.e., the fit was very good.
Figure 2.
Vessel wall displacements as a function of time for the two sequences shown in Fig. 1, respectively. The error bars are standard deviations of three repeated measurements. The solid curve is the best fit to the data using the Voigt model.
A summary of the estimated τ from 38 high-speed image sequences is shown in Fig. 3. The estimated time constants ranged from 3 to 24 μs. All of the correlation coefficients of the Voigt model to the experimental data were larger than 0.85. Figure 3 shows the time constants as a function of initial vessel size. There are three types of venules: postcapillary venules, collecting venules, and muscular venules, with their diameters 8–30 μm, 30–50 μm, and 50–250 μm, respectively.22, 23 According to this classification of venules, the data were divided into three groups based on these initial vessel diameters: 10–29, 30–49, and 50–80 μm. The mean τ for each group is shown with the error bar corresponding to the standard deviation of τ within each group. For the three groups, the means of τ were 7, 12, and 8 μs, respectively. Within the accuracy of our measurements, the time constants of the three groups were not significantly different, suggesting that the mechanical properties of these microvessels are similar (at megahertz frequencies). The scatter of τ might be associated with variation in vessel and surrounding tissue structures within each group, and also the limited number of images captured during vessel relaxation. The absolute values of for these data ranged from 3–25 μm, and we found no pronounced influence of on τ, indicating that is only a scaling factor in the model describing the displacement of the vessels. Rephrased, the extent of vessel displacement did not lead to changes of vessel mechanical properties.
Figure 3.
Relaxation time constants estimated for microvessels with different diameters. The three groups were divided based on the classification of venules. The error bars are standard deviations of the measurements within each group.
The microbubbles were injected into the microvessels under the control of a syringe pump. To estimate the contribution of different syringe pump speeds on the relaxation time constants, two different pump speeds were tested. All the data presented, thus far, were acquired with the syringe pump speed set to 50 ml/h. Additional experiments were carried out with the pump speed set to 10 ml/h. A summary of the measured relaxation time constants corresponding to the two pump speeds for vessels ranging from 20–40 μm is presented in Fig. 4. Apparently, there is a reduction in the time constant when the pump speed increases. The statistically significant means of τ at the low and high pump speeds were 26 μs and 10 μs, respectively. Previous studies on the mechanical properties of arterioles and arteries found that the static Young's modulus increased as the static transmural pressure increased.24, 25 Accordingly, changes in the measured τ at different pump speeds may be explained by the variation in the elastic modulus with changes in initial strain. Still, the time scales for τ remained on the order of 10 μs. It needs to be stressed that as the estimation of τ depends on experimental techniques and theoretical modeling, but with those caveats in mind, the results obtained here appear to be accurate to a first approximation.
Figure 4.
Box plot of relaxation time constants estimated for vessels with initial diameters within the range of 20–40 μm with perfusion pump speeds of 10 or 50 ml/h. The numbers of high-speed image sequences included in these two groups were 16 and 17, respectively. A significant difference was found between these two groups (two tailed t-test, p = 0.0002).
Ultrasound based techniques have been used before for estimating tissue viscoelastic properties at high frequencies, and the scales of τ estimated in this study fits well with previous observations. Several techniques are based on the acoustic radiation force, which is a steady, time-averaged force that acts upon an object in an acoustic field.26, 27 The frequencies of radiation force based techniques are on the scale of ∼100 Hz and the associated tissue relaxation time constant is on the order of 10 ms.26, 27 To measure tissue viscoelastic properties at megahertz frequency, a reflection technique has been used by some researchers, in which the elasticity and viscosity were deduced from the measured complex reflection coefficient of shear waves at the interface between the tissue and a flat fused silica surface. Using this technique, the relaxation time constants of several soft tissues (such as liver, kidney and muscle) at frequencies ranging from 2.2 to 20 MHz were estimated to be within the range of 4 ns to 5.5 μs. Generally, most soft tissues are shear thinning materials, so that the dynamic viscosity decreases as frequency increases, while, in contrast, the elasticity was found to increase with frequency.28 As a result, the relaxation time should decrease as frequency increases. We estimated that at 1 MHz the vessel relaxation time constant was on the order of 10 μs, which is shorter than that measured by radiation force techniques (at lower frequencies), but closer to the lower bound of that estimated using shear wave reflection technique (at higher frequencies).
Direct knowledge of viscosity and elasticity would be ideal, but current knowledge of microvessel parameters is so poor that τ itself can help constrain possible values, which can give useful reference to the modeling of microbubble dynamics in microvessels. Moreover, knowledge of microvessel characteristic time scales help us better understand the physics of microbubble dynamics in microvessels observed in experimental studies. The very short time scales suggest that the energy stored in the tissue during the expansion phase of the microbubbles' motion can be 'injected' back into the liquid during microbubble collapse, contributing to the observed vessel invagination (see Fig. 1 and references 9, 10).
In summary, we presented a technique for estimating the time-dependent mechanical properties of microvessels in (optically transparent) rat mesenteries. Microbubble oscillation caused vessel deformation at the megahertz frequency, the recovery of which was observed by high-speed photomicrography. Based on the relaxation process, microvessel characteristic time scales were estimated to be on the order of tens of microseconds, which is consistent with that reported for other soft tissues at similar frequency. These results help constrain possible values of viscosity and elasticity used in the modeling of microbubble dynamics in microvessels for understanding the physics of microbubble/microvessel dynamical interactions. Future work will examine the feasibility of using targeted microbubbles and acoustic imaging to measure relaxation timescales in vivo.
Acknowledgments
The authors acknowledge Dr. Michael R. Bailey, Dr. Wayne Kreider, and Dr. Charles C. Church for many helpful discussions. They also thank Frank Starr and Brian MacConaghy for assistance with the experiments. This work was supported in part by NIH Grants EB000350 (NIBIB), AR053652 (NIAMS), and P01DK043881 (NIDDKD).
References
- Stride E. and Saffari N., Proc. Inst. Mech. Eng. Part H: J. Eng. Med. 217(6), 429–447 (2003). 10.1243/09544110360729072 [DOI] [PubMed] [Google Scholar]
- Cosgrove D., Eur. J. Radiol. 60 (3), 324–330 (2006). 10.1016/j.ejrad.2006.06.022 [DOI] [PubMed] [Google Scholar]
- Harvey C. J., Blomley M. J. K., Eckersley R. J., and Cosgrove D. O., Eur. Radiol. 11(4), 675–689 (2001). 10.1007/s003300000624 [DOI] [PubMed] [Google Scholar]
- Qin S. P., Caskey C. F., and Ferrara K. W., Phys. Med. Biol. 54(6), R27–R57 (2009). 10.1088/0031-9155/54/6/R01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitragotri S., Nat. Rev. Drug Discovery 4(3), 255–260 (2005). 10.1038/nrd1662 [DOI] [PubMed] [Google Scholar]
- Hernot S. and Klibanov A. L., Adv. Drug Delivery Rev. 60(10), 1153–1166 (2008). 10.1016/j.addr.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey C. F., Kruse D. E., Dayton P. A., Kitano T. K., and Ferrara K. W., Appl. Phys. Lett. 88(3), 033902 (2006). 10.1063/1.2164392 [DOI] [Google Scholar]
- Garbin V., Cojoc D., Ferrari E., Di Fabrizio E., Overvelde M. L. J., van der Meer S. M., de Jong N., Lohse D., and Versluis M., Appl. Phys. Lett. 90(11), 114103 (2007). 10.1063/1.2713164 [DOI] [Google Scholar]
- Chen H., Brayman A. A., Kreider W., Bailey M. R., and Matula T. J., Ultrasound Med. Biol. 37(12), 2139–2148 (2011). 10.1016/j.ultrasmedbio.2011.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Kreider W., Brayman A. A., Bailey M. R., and Matula T. J., Phys. Rev. Lett. 106(3), 034301 (2011). 10.1103/PhysRevLett.106.034301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H., Gracewski S. M., and Dalecki D., J. Acoust. Soc. Am. 124(4), 2374–2384 (2008). 10.1121/1.2967488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S. P. and Ferrara K. W., Phys. Med. Biol. 51(20), 5065–5088 (2006). 10.1088/0031-9155/51/20/001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T. and Bull J. L., J. Biomech. Eng. 128(4), 554–563 (2006). 10.1115/1.2206200 [DOI] [PubMed] [Google Scholar]
- Freund J. B., J. Acoust. Soc. Am. 123(5), 2867–2874 (2008). 10.1121/1.2902171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. C., Biomechanics: Mechanical Properties of Living Tissues (Springer, New York, 1993), pp. 330–336. [Google Scholar]
- Caskey C. F., Stieger S. M., Qin S., Dayton P. A., and Ferrara K. W., J. Acoust. Soc. Am. 122, 1191–1200 (2007). 10.1121/1.2747204 [DOI] [PubMed] [Google Scholar]
- Frizzell L. A. and Carstensen E. L., J. Acoust. Soc. Am. 60(6), 1409–1411 (1976). 10.1121/1.381236 [DOI] [PubMed] [Google Scholar]
- Madsen E. L., Sathoff H. J., and Zagzebski J. A., J. Acoust. Soc. Am. 74(5), 1346–1355 (1983). 10.1121/1.390158 [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Yasu T., Yamada S., Kudo N., Kuroki M., Kawakami M., Miyatake K., and Saito M., Ultrasound Med. Biol. 28(7), 949–956 (2002). 10.1016/S0301-5629(02)00532-X [DOI] [PubMed] [Google Scholar]
- Catheline S., Gennisson J. L., Delon G., Fink M., Sinkus R., Abouelkaram S., and Culioli J., J. Acoust. Soc. Am. 116(6), 3734–3741 (2004). 10.1121/1.1815075 [DOI] [PubMed] [Google Scholar]
- Yang X. and Church C. C., IEEE Trans. Ultrason. Ferroelectr. Freq. Control 53(8), 1404–1411 (2006). 10.1109/TUFFC.2006.1665097 [DOI] [PubMed] [Google Scholar]
- Zhang S.-X., An Atlas of Histology (Springer, New York, 1999), pp. 126–128. [Google Scholar]
- Lee J. S., Ann. Biomed. Eng. 28(1), 1–13 (2000). 10.1114/1.249 [DOI] [PubMed] [Google Scholar]
- Bergel D. H., J. Physiol. 156(3), 445–457 (1961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayne G. T. G., Smaje L. H., and Bergel D. H., Int. J. Microcirc. 8(1), 25–42 (1989). [PubMed] [Google Scholar]
- Palmeri M. L. and Nightingale K. R., Interface Focus 1(4), 553–564 (2011). 10.1098/rsfs.2011.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erpelding T. N., Hollman K. W., and O'Donnell M., IEEE Trans. Ultrason. Ferroelectr. Freq. Control 52(6), 971–979 (2005). 10.1109/TUFFC.2005.1504019 [DOI] [PubMed] [Google Scholar]
- Nasseri S., Bilston L. E., and Phan-Thien N., Rheol. Acta 41(1–2), 180–192 (2002). 10.1007/s003970200017 [DOI] [Google Scholar]