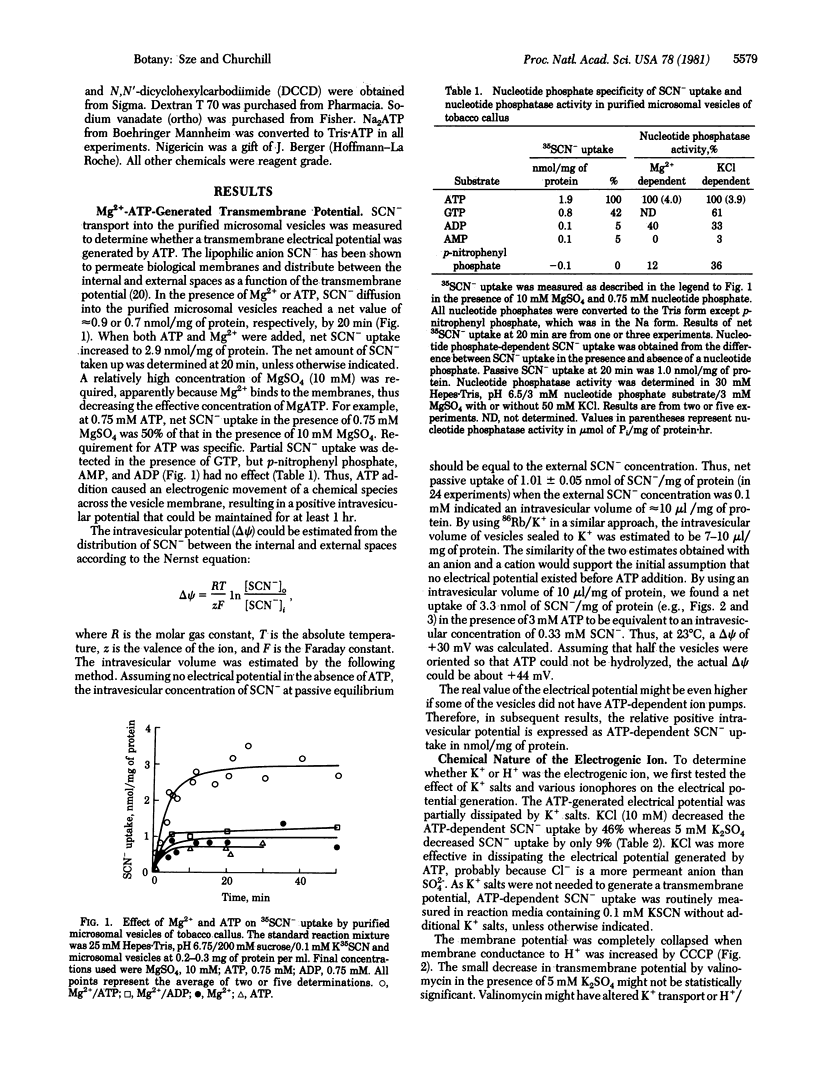
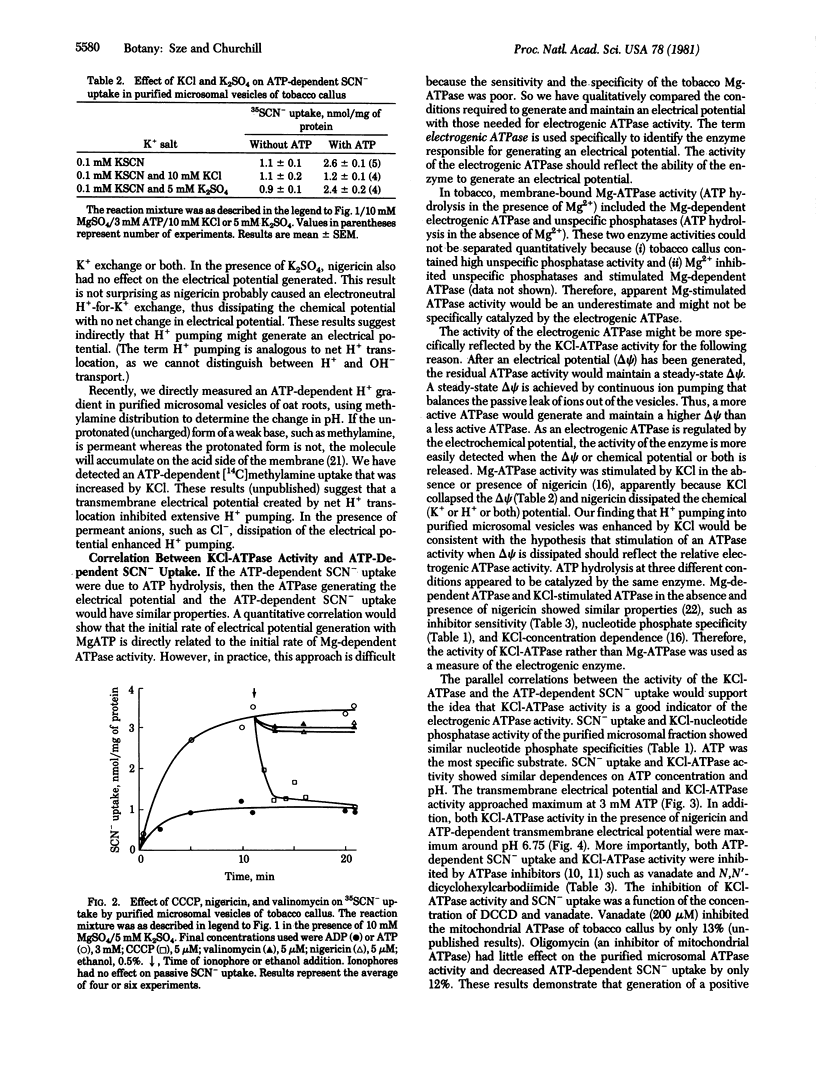
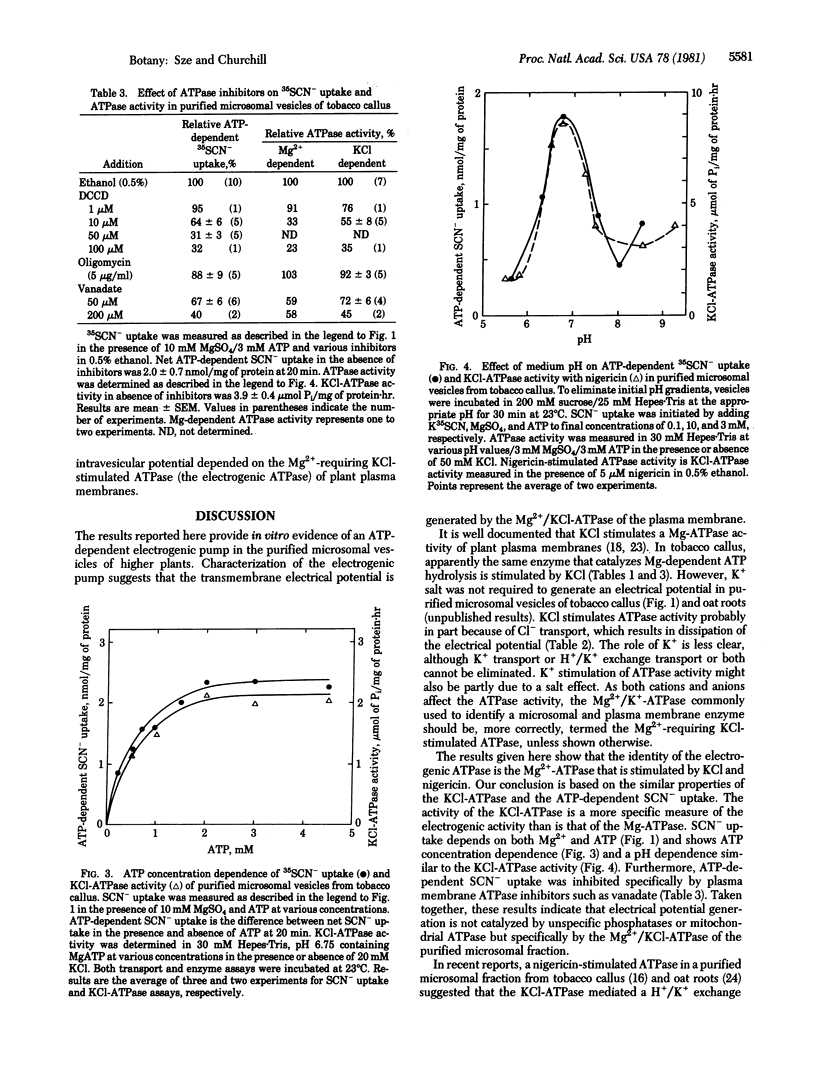
Abstract
The function of the Mg2+-requiring KCl-stimulated ATPase (ATP phosphohydrolase, EC 3.6.1.3) of higher plants in active ion transport was investigated by using a purified microsomal fraction containing sealed plasma membrane vesicles. (Sze, H. (1980) Proc. Natl. Acad. Sci. USA 77, 5904-5908). A transmembrane electrical potential (+30 to +44 mV), monitored by uptake of a permeant anion (35SCN-), was generated specifically by ATP in purified microsomal vesicles of tobacco callus. ATP-dependent 35SCN- uptake required Mg2+, was optimal at pH 6.75, and showed similar ATP concentration dependence as the Mg2+-requiring KCl-stimulated ATPase activity. Plasma membrane ATPase inhibitors (N,N′-dicyclohexylcarbodiimide and vanadate) inhibited generation of the ATP-dependent electrical potential. A proton conductor (carbonyl cyanide m-chlorophenylhydrazone), but not a K+ ionophore (valinomycin), completely collapsed the electrical potential. The results provide in vitro evidence that the Mg2+/KCl-ATPase of higher plants is an electrogenic pump. These results are consistent with the hypothesis that an electrogenic H+ pump is catalyzed by the plasma membrane ATPase of plants.
Keywords: microsomal vesicles, transmembrane electrical potential, thiocyanate uptake, pH gradient
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke N. E., Hodges T. K. Comparison of Reductions in Adenosine Triphosphate Content, Plasma Membrane-associated Adenosine Triphosphatase Activity, and Potassium Absorption in Oat Roots by Diethylstilbestrol. Plant Physiol. 1979 Jan;63(1):53–56. doi: 10.1104/pp.63.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman J. M., Lafayette P. R., Gronewald J. W., Hanson J. B. Effect of ATPase inhibitors on cell potential and k influx in corn roots. Plant Physiol. 1980 Jun;65(6):1139–1145. doi: 10.1104/pp.65.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronewald J. W., Cheeseman J. M., Hanson J. B. Comparison of the responses of corn root tissue to fusicoccin and washing. Plant Physiol. 1979 Feb;63(2):255–259. doi: 10.1104/pp.63.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin W. Potassium and Phosphate Uptake in Corn Roots: Further Evidence for an Electrogenic H/K Exchanger and an OH/Pi Antiporter. Plant Physiol. 1979 May;63(5):952–955. doi: 10.1104/pp.63.5.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- Nelson N., Eytan E., Notsani B. E., Sigrist H., Sigrist-Nelson K., Gitler C. Isolation of a chloroplast N,N'-dicyclohexylcarbodiimide-binding proteolipid, active in proton translocation. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2375–2378. doi: 10.1073/pnas.74.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin D. S., Spanswick R. M. Labeling and isolation of plasma membranes from corn leaf protoplasts. Plant Physiol. 1980 Jun;65(6):1053–1057. doi: 10.1104/pp.65.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi-Caldogno F., de Michelis M. I., Pugliarello M. C. Evidence for an electrogenic ATPase in microsomal vesicles from pea internodes. Biochim Biophys Acta. 1981 Mar 20;642(1):37–45. doi: 10.1016/0005-2736(81)90135-8. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T., Avron M. Determination of pH in chloroplasts. I. Distribution of ( 14 C) methylamine. Eur J Biochem. 1972 Jan 31;25(1):54–63. doi: 10.1111/j.1432-1033.1972.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Scarborough G. A. Proton translocation catalyzed by the electrogenic ATPase in the plasma membrane of Neurospora. Biochemistry. 1980 Jun 24;19(13):2925–2931. doi: 10.1021/bi00554a017. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Padan E., Rottenberg H., Gromet-Elhanan Z., Avron M. Delta pH and membrane potential in bacterial chromatophores. FEBS Lett. 1974 Dec 15;49(2):174–177. doi: 10.1016/0014-5793(74)80505-3. [DOI] [PubMed] [Google Scholar]
- Sze H., Hodges T. K. Characterization of passive ion transport in plasma membrane vesicles of oat roots. Plant Physiol. 1976 Sep;58(3):304–308. doi: 10.1104/pp.58.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H. Nigericin-stimulated ATPase activity in microsomal vesicles of tobacco callus. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5904–5908. doi: 10.1073/pnas.77.10.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]


