Abstract
Background
Previous studies have demonstrated that claudin-6 functions as a cancer suppressor in human MCF-7 breast cancer cells. The growth inhibitory effect could be attributed to inhibition of cell proliferation and induction of apoptosis. The purpose of the current study was to examine the involvement of apoptosis signal-regulating kinase 1 (ASK1) in the anticancer effect of claudin-6.
Methods
Immunohistochemical analysis was performed to evaluate the ASK1 protein expression and the correlation between ASK1, claudin-6 and clinicopathological features in 85 samples of breast invasive ductal carcinomas (IDC). Western blotting and RT-PCR was carried out to examine the expression of ASK1 and claudin-6 in MCF-7 cell clones transfected with claudin-6.
Results
Immunohistochemical analysis showed that ASK1 expression was significantly related with that of claudin-6 in breast invasive ductal carcinomas (P < 0.05). In addition, a positive correlation between ASK1 and C-erb B 2 protein expression was identified (P < 0.05). Western blotting and RT-PCR consistently revealed that the level of ASK1 protein and mRNA was upregulated in MCF-7 cell clones transfected with claudin-6.
Conclusions
Our data suggests, for the first time, that the ASK1 signal may play a positive role in the inhibitory effect of claudin-6 in breast cancer.
Virtual Slides
The virtual slide(s) for this article can be found here: http://www.diagnosticpathology.diagnomx.eu/vs/1200314318763661
Keywords: Breast invasive ductal carcinomas, ASK1, Apoptosis, Tight junction
Background
Breast cancer is one of the most frequent and deadly cancers in women [1]. Emerging evidence points toward a pivotal role of tight junction (TJ) in mediating tumorigenic growth of breast cancer [2,3]. TJs are junctional complexes which mediate cell-to-cell adhesion in epithelial and endothelial cellular sheets [4], and which affect cell polarity and tight junction formation [5].
Claudins (CLDNs) constitute a family of integral membrane proteins and have been identified as prominent structural components of TJ strands [5,6]. The CLDNs which include 27 members at least [7], encode 20-27 kDa proteins with four transmembrane domains and two extracellular loops [8]. The expression of CLDNs is often different in various types of human tumor [9,10]. Many studies have demonstrated that claudins may participate in several signal transduction pathways [11-15]. For instance, inhibition of c-jun NH2-terinal kinase (JNK) and p38 mitogen-activated protein kinase (p38 MAPK) selectively modulates the expression of claudin-4, -8 and −9 to enhance TJ barrier function in mammary epithelial cells [16], And p38 MAPK activity is involved in the epithelial barrier dysfunction in which claudin-7 protein plays a major role [17]. It is well known that apoptosis signal-regulating kinase 1 (ASK1) phosphorylates and actives both p38 and JNK pathway [18]. ASK1 is a member of the MAPKKK family and functions as a promoting apoptosis gene in response to common pro-apoptosis stresses [19]. However, there is little knowledge about the relationship of ASK1 and claudins, especially claudin-6.
In our previous study, we found that claudin-6 is preferentially expressed in mammary epithelial cells and functions as a potential breast cancer suppressor gene [20], which is supported by the follow-up study of Osanai [21]. Recently, we have discovered that the low level expression of claudin-6 gene contributed to malignant progression of breast cancer [22]. A previous study has described that breast cancer tissues also expressed lower levels of ASK1 compared with normal mammary tissues [23]. Therefore, the purpose of the current study is to discover the relationship between ASK1 and claudin-6 in breast cancer and to explore the pathways involves the activation of ASK1.
Methods
Patients and tissue samples
The breast samples were obtained from 2006–2010 in the Jilin Oil Field General Hospital in Songyuan, Jilin province, China. A total of 85 breast invasive ductal carcinomas (IDC) aged 26 to 77 with a mean age of 51 were included in this study. The study was approved by the Ethics Committee of Jilin University. Clinicopathological features of 85 IDC samples are summarized in Table 1.
Table 1.
Clinicopathological features and the expression of ASK1 in 85 IDC samples
|
Clinicopathological features |
Cases |
ASK1 expression (n) |
χ2 |
P |
|
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Age (years) |
|
|
|
0.017 |
0.896 |
| ≥45 |
58 |
18 |
40 |
||
| <45 |
27 |
8 |
19 |
||
| Histological grade |
|
|
|
0.668 |
0.414 |
| I ~ II |
21 |
8 |
13 |
||
| III |
63 |
18 |
45 |
||
| Tumor size |
|
|
|
0.21 |
0.646 |
| ≥5 cm3 |
66 |
21 |
45 |
||
| <5 cm3 |
19 |
5 |
14 |
||
| Lymph node metastasis |
|
|
|
0.527 |
0.468 |
| Positive |
41 |
11 |
30 |
||
| Negative |
44 |
15 |
29 |
||
| TNM stage |
|
|
|
0.336 |
0.562 |
| I,II |
55 |
18 |
37 |
||
| III,IV |
30 |
8 |
22 |
||
| Lesion location |
|
|
|
2.406 |
0.121 |
| Right |
45 |
10 |
35 |
||
| Left |
34 |
13 |
21 |
||
| ER |
|
|
|
0.105 |
0.746 |
| Positive |
37 |
12 |
25 |
||
| Negative |
48 |
14 |
34 |
||
| PR |
|
|
|
0.425 |
0.515 |
| Positive |
38 |
13 |
25 |
||
| Negative |
47 |
13 |
34 |
||
| C-erb B-2 |
|
|
|
5.747 |
0.017 |
| Positive |
27 |
13 |
14 |
||
| Negative | 58 | 13 | 45 | ||
Statistical analyzed by Pearson’s chi-square test.
Cell culture
Human breast cancer cell line MCF-7 cell clones expressing an vector pcDNA3.1 (+) or claudin-6 were cultured as previously described [22].
Quantitative RT-PCR
Total RNA was extracted from clone cells using TRIzol (Invitrogen, USA) following the manufacturer’s instructions. One microgram of total RNA was subjected to reverse transcription to synthesize cDNA using the M-MuLV reverse transcriptase (TaKaRa, Japan) at 42°C for 1 hour, and 0.5 ug cDNA was used for PCR. ASK1 and claudin-6 were amplified along with GAPDH as an endogenous control following the instructions of Premix LA Taq Kit (TaKaRa, Japan). The PCR reaction conditions and the primer sequences of ASK1, claudin-6 and GAPDH are shown in Table 2. After electrophoresis, the gel was captured and analyzed by the image system (Syngene, Cambridge, UK).
Table 2.
Primers used for PCR
| Gene | Sequence of primers | Size of PCR(bp) | Denaturation temperature (°C) | Cycles | |
|---|---|---|---|---|---|
| ASK1 |
sense |
5’-TTCACACAAAACGGATGTAACATT-3’ |
198 |
56 |
30 |
| antisense |
5’-CCTAAACAGTTATGGTCACATTTTGG-3’ |
||||
| Claudin-6 |
sense |
5’-TTCATCGGCAACAGCATCGT-3’ |
345 |
58 |
35 |
| antisense |
5’-GGTTATAGAAGTCCCGGATGA-3’ |
||||
| GAPDH |
sense |
5’-TGTTGCCATCAATGACCCCTT-3’ |
202 |
56 |
25 |
| antisense | 5’-CTCCACGACGTACTCAGCG-3’ | ||||
Western blotting analysis
The Western blotting analyses were performed as described previously [22]. Primary antibodies included ASK1 (rabbit polyclonal antibody, 1:1000, Bioworlde Technology), claudin-6 (rabbit polyclonal antibody, 1:1000, Bioworlde Technology) and β-actin (mouse polyclonal antibody, 1:1000, Santa Cruz). Secondary antibodies for the detection: anti-rabbit IgG (1:2000, Proteintech Group) and anti-mouse IgG (1:2000, Proteintech Group).
Immunohistochemistry
Immunohistochemical staining was carried out as described in our previouspublications [24]. The primary antibodies against ASK1 (rabbit polyclonal antibody, 1:200, Bioworlde Technology) and claudin-6 (rabbit polyclonal antibody, 1:200, Bioworlde Technology) were used. The negative controls were handled in the same way except PBS instead of primary antibody.
Positive-staining exhibits brown staining, Claudin-6 was shown on the cell membrane and/or cytoplasm in breast cancer tissues [24], and ASK1 expressed on the breast cancer cytoplasm according to the manufacturer’s Instructions of ASK1 antibody. Immunostaining was observed under light microscopy with 400× magnification, and positive cells, negative cells and total cells of five different visual fields were numbered in each specimen. Scoring was performed as follows: negative (−), <10% positive tumor cells; positive (+), ≥10% positive tumor cells.
Statistical analyses
All computations were carried out using the software of SPSS version 19.0 for Windows (SPSS Inc, IL, USA). Chi-Square test was used to examine categorical data. Unpaired t-tests were performed to evaluated data of target mRNA and protein. The data are presented as means ± standard deviation (SD) from at least three independent experiments. P < 0.05 was considered statistically significant.
Results
Association of ASK1 expression with the clinicopathological features of breast invasive ductal carcinomas
The clinicopathological characteristics of the patients are summarized in Table 1. In order to investigate whether ASK1 protein expression was associated with clinicopathological features of patients of breast cancer, we correlated immunohistochemical ASK1 staining results with clinicopathological features. In this study, ASK1 protein was evaluated in the cytoplasm of breast cancer (Figure 1A), and the positive expression of ASK1 protein was found in 30.59% (26/85) of breast IDCs. ASK1 protein expression had no correlation with age (P = 0.896), histological grade (P = 0.414), tumor size (P = 0.646), lymph node metastasis (P = 0.468), TNM stage (P = 0.562) and lesion location (P = 0.121). But interestingly, we found that ASK1 had relationship with C-erb B 2 protein expression (P = 0.017).
Figure 1.
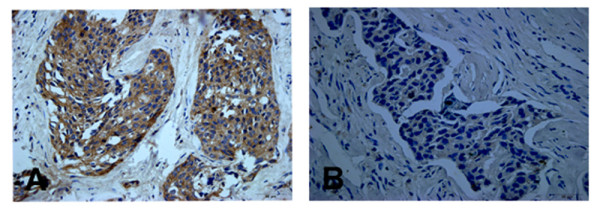
Expression and location of ASK1 in breast invasive ductal carcinoma (IDC) (Original magnification × 400). (A) ASK1 positive staining was predominant in the cytoplasm of breast carcinoma tissues. (B) ASK1 negative staining was seen in IDC tissues.
Correlation between the expression of ASK1 and claudin-6 in breast cancer tissues
We have found that the expression of claudin-6 was reduced in breast invasive ductal carcinomas [24]. The expression of claudin-6 (Figure 1A, 1B) and ASK1 (Figure 2A, 2B) was examined by immunohistochemistry, and the correlation between claudin-6 and ASK1 was analyzed by Pearson’s chi-square test. As shown in Table 3, the positive expression rate of claudin-6 was 27.09% (23/85) in IDC specimens, and cells were positive for ASK1 in 30.59% (26/85) of IDC cases. Half (13/26) of the ASK1 positive cases were positively staining for claudin-6, but only 16.95% (10/59) of ASK1 negative cases stained positively for claudin-6. Statistical analysis revealed that claudin-6 expression was positive correlated with ASK1 expression in breast invasive ductal carcinomas (P = 0.0016).
Figure 2.
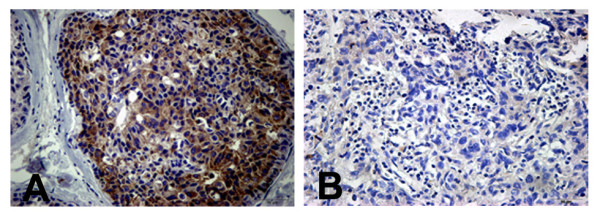
Expression and location of claudin-6 in breast invasive ductal carcinoma (IDC) (Original magnification × 400). (A) Claudin-6 was expressed in the membrane and cytoplasm of IDC tissues. (B) Claudin-6 was weakly expressed in IDC tissues.
Table 3.
The correlation between the expression of claudin-6 and ASK1 in breast invasive ductal carcinomas
|
ASK1 expression |
Cases (n,%) |
Claudin-6 expression (n,%) |
χ2 |
P |
|
|---|---|---|---|---|---|
| Positive | Negative | ||||
|
Positive |
26 (30.59%) |
13 (50%) |
13 (50%) |
9.988 |
0.0016 |
|
Negative |
59 (69.41%) |
10 (16.95%) |
49 (83.05%) |
||
| Case (n) | 85 (100%) | 23 (27.09%) | 72 (72.01%) | ||
Statistical analyzed by Pearson’s chi-square test.
Correlation between the expression of claudin-6 and ASK1 in breast cancer cells
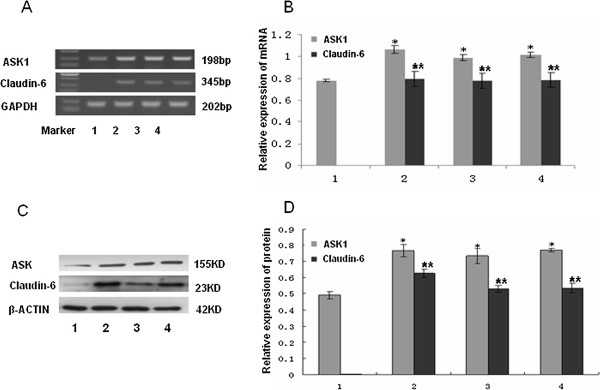
We found the correlation between claudin-6 and ASK1 expression in breast invasive ductal carcinomas tissues, but their relationship in breast cancer cell line was unknown. We used MCF-7 cells transfected with pcDNA3.1 (+) and three G418 resistant MCF-7 clones, which expressed claudin-6 stably. When claudin-6 was upregulated, ASK1 had a higher expression level than that in empty vector group (Figure 3A, 3C). Quantitative RT-PCR and western blot analysis showed that the level of claudin-6 mRNA and protein positively correlated with the level of ASK1 mRNA and protein (Figure 3B, 3D).
Figure 3.
Correlation of claudin-6 and ASK1 in breast cancer cells. Line 1 is empty vector group; lines 2, 3 and 4 are three clone groups. (A) (B) RT-PCR assay examined the mRNA levels of claudin-6 and ASK1. (C) (D) Western blot assay detected claudin-6 and ASK1 protein expression. *,**P<0.05, vs. vector group. Results are means ± standard deviation for three independent experiments.
Discussion
In the previous study, We found that the expression level of claudin-6 was lower in two human breast cancer cells (MCF-7 and BT474) and one breast cancer sample than that in normal breast tissues [20]. In addition, we also discovered the growth, migration and invasion of MCF-7 cells were inhibited by overexpression of claudin-6 [22]. One report has shown that the expression of ASK1 is lower in breast cancer tissues than that in normal tissues [23]. As we all known, ASK1 is regulated in response to various cellular stresses, including cell survival, proliferation, differentiation, and so forth. Therefore, in the current study, we attempted to elucidate the relationship between the expression of claudin-6 and ASK1 using clinicopathological features and classical prognostic factors in breast pathology, including the expression of immunohistochemical markers of prognostic significance (ER, PR, C-erb B 2) (Table 1). To the best of our knowledge, this is the first study to demonstrate an association between the protein expression of claudin-6 and ASK1 in a large series of breast invasive ductal carcinomas and the breast cancer cells.
We have previously found that the expression of claudin-6 was negatively correlated with lymphatic metastasis of breast IDCs [24], but we did not found the correlation between ASK1 expression and the lymphatic metastasis (Table 1). This may be mainly due to that the ASK1 signal pathway is not the only pathway to be regulated by claudin-6. Besides that, we found the correlation of claudin-6 and ERα (p = 0.033), and also discovered that ERα regulated claudin-6 in MCF-7 cells [25]. We failed to find the correlation between ASK1 and ER. And the reason high likely is the cross-talk among different signaling pathways, as we discussed in the case of failing to discover the correlation of ASK1 and lymphatic metastasis. However, we revealed the correlation between ASK1 and C-erb B 2 (Table 1). These results indicate the role of C-erb B 2 in ASK1 signal pathway. We next analyzed the relationship of C-erb B 2 and claudin-6, but we found no relationship between them (data not shown). Therefore, these data suggest that the inhibitory effect of claudin-6 in breast cancer mainly results from the regulation of ASK1.
Besides analysis of the breast cancer tissues, we also analyzed the correlation of ASK1 and claudin-6 mRNA and protein in breast cancer cell lines. We have found claudin-6 was a anti-cancer gene in claudins family [22], and the up-regulation of claudin-6 has important clinical implication, but details of the mechanism was not clear. C-jun NH2-terinal kinase (JNK) and p38 mitogen-activated protein kinase (p38 MAPK) signal pathway played a positive role in the process of claudin-4, -8 and −9 enhancing TJ barrier function in mammary epithelial cells [16]. ASK1 actives JNK and p38 pathway and induces apoptosis in various cells through mitochondria-dependent caspase activation [18,26-28]. ASK1 activation depends on its binding proteins such as TNF receptor-associated factors2/6 [29], DAXX [30], TRADD [31], RIP1 [32], and FADD [33]. And several cellular proteins, for example, thioredoxin [26], Hsp90 [34] and 14-3-3 [35] were also reported to interact with ASK1 and inhibit ASK1 activity. Here, we demonstrated that ASK1 was upregulated when claudin-6 gene was transfected into MCF-7 cells (Figure 3). Therefore, the present study indicates that ASK1 signal participates in the pro-apoptosis function of claudin-6.
Conclusions
As a conclusion, our study suggests that the ASK1 expression is low in breast cancer, and the levels of ASK1 mRNA and protein expression are correlated with that of claudin-6. We have identified a novel mechanism responsible for the pro-apoptosis function of claudin-6, and ASK1 may become a target for breast cancer treatments. However, we still need further study to clarify the detail of this mechanism.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CQ, YG and XX carried out most of experiments, participated in the design of the study, performed the statistical analysis, and drafted the manuscript. ZL, TZ and XZ carried out part of experiments, and helped draft the manuscript. LW, MW, YL, YL and YL assisted the experiments. XX and YL helped to edit the paper. All authors have read and approved the final manuscript.
Contributor Information
Yaxiong Guo, Email: yaxiong82@126.com.
Xiaoming Xu, Email: xuxiaoming_218@yahoo.com.cn.
Zhijing Liu, Email: xiaojing_906@163.com.
Ting Zhang, Email: zhangting1987@126.com.
Xiaowei Zhang, Email: xiaowei0312@126.com.
Liping Wang, Email: jldxwlp1008@163.com.
Min Wang, Email: mindiduo@sina.com.
Yuanyuan Liu, Email: 397434658@qq.com.
Yan Lu, Email: ly19870621521@163.com.
Yunpeng Liu, Email: rhame-snow@163.com.
Chengshi Quan, Email: clondy_123@163.com.
Acknowledgements
This study was supported by National Natural Science Foundation of China (Code: 81172499) and Science and Technology Development Plan of the Office of Science and Technology Project in Jilin Province (Code: 20100731).
References
- Pagani O, Senkus E, Wood W, Colleoni M, Cufer T, Kyriakides S, Costa A, Winer EP, Cardoso F. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102:456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough CD, Sherman-Baust CA, Pizer ES, Montz FJ, Im DD, Rosenshein NB, Cho KR, Riggins GJ, Morin PJ. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000;60:6281–6287. [PubMed] [Google Scholar]
- Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22:2021–2033. doi: 10.1038/sj.onc.1206199. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Matter K, Balda MS. Signaling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/S0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K, Tsukita S. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585:606–612. doi: 10.1016/j.febslet.2011.01.028. [DOI] [PubMed] [Google Scholar]
- Turksen K, Troy TC. Barriers built on claudins. J Cell Sci. 2004;117:2435–2447. doi: 10.1242/jcs.01235. [DOI] [PubMed] [Google Scholar]
- Resnick MB, Konkin T, Routhier J, Sabo E, Pricolo VE. Claudin-1 is a strong prognostic indicator in stage II colonic cancer: a tissue microarray study. Mod Pathol. 2004;18:511–518. doi: 10.1038/modpathol.3800301. [DOI] [PubMed] [Google Scholar]
- Tzelepi VN, Tsamandas AC, Vlotinou HD, Vagianos CE, Scopa CD. Tight junctions in thyroid carcinogenesis: diverse expression of claudin-1, claudin-4, claudin-7 and occludin in thyroid neoplasms. Mod Pathol. 2007;21:22–30. doi: 10.1038/modpathol.3800959. [DOI] [PubMed] [Google Scholar]
- Ikari A, Sato T, Watanabe R, Yamazaki Y, Sugatani J. Increase in claudin-2 expression by an EGFR/MEK/ERK/c-Fos pathway in lung adenocarcinoma A549 cells. Biochim Biophys Acta. 2012;1823:1110–1118. doi: 10.1016/j.bbamcr.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J. Claudin-14 regulates renal Ca(+)(+) transport in response to CaSR signaling via a novel microRNA pathway. EMBO J. 2012;31:1999–2012. doi: 10.1038/emboj.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba K, Kondo Y, Yamaguchi K, Miyake H, Fujisawa M. Inhibition of Claudin-11 and Occludin Expression in Rat Sertoli Cells by Mono-(2-Ethylhexyl) Phthalate Through p44/42 Mitogen-Activated Protein Kinase Pathway. J Androl. 2012;33:368–374. doi: 10.2164/jandrol.111.013664. [DOI] [PubMed] [Google Scholar]
- Lu Z, Ding L, Hong H, Hoggard J, Lu Q, Chen YH. Claudin-7 inhibits human lung cancer cell migration and invasion through ERK/MAPK signaling pathway. Exp Cell Res. 2011;317:1935–1946. doi: 10.1016/j.yexcr.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter Y, Goodenough D. Claudins. Current biology: CB. 2004;14:R293–R294. doi: 10.1016/j.cub.2004.03.045. [DOI] [PubMed] [Google Scholar]
- Carrozzino F, Pugnale P, Féraille E, Montesano R. Inhibition of basal p38 or JNK activity enhances epithelial barrier function through differential modulation of claudin expression. Am J Physiol Cell Physiol. 2009;297:C775–C787. doi: 10.1152/ajpcell.00084.2009. [DOI] [PubMed] [Google Scholar]
- Oshima T, Miwa H, Joh T. Aspirin induces gastric epithelial barrier dysfunction by activating p38 MAPK via claudin-7. Am J Physiol Cell Physiol. 2008;295:C800–C806. doi: 10.1152/ajpcell.00157.2008. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Choi HK, Chung KC. Dyrk1A Positively Stimulates ASK1-JNK Signaling Pathway during Apoptotic Cell Death. Exp neurobiol. 2011;20:35–44. doi: 10.5607/en.2011.20.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan C, Lu SJ. Identification of genes preferentially expressed in mammary epithelial cells of Copenhagen rat using subtractive hybridization and microarrays. Carcinogenesis. 2003;24:1593–1599. doi: 10.1093/carcin/bgg129. [DOI] [PubMed] [Google Scholar]
- Osanai M, Murata M, Chiba H, Kojima T, Sawada N. Epigenetic silencing of claudin‐6 promotes anchorage‐independent growth of breast carcinoma cells. Cancer Sci. 2007;98:1557–1562. doi: 10.1111/j.1349-7006.2007.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Liu Y, Ren Y, Xu X, Yu L, Li Y, Quan C. Tight junction protein, claudin-6, down regulates the malignant phenotype of breast carcinoma. Eur J Cancer Prev. 2010;19:186–194. doi: 10.1097/CEJ.0b013e328337210e. [DOI] [PubMed] [Google Scholar]
- Won M, Park KA, Byun HS, Sohn KC, Kim YR, Jeon J, Hong JH, Park J, Seok JH, Kim JM. et al. Novel anti-apoptotic mechanism of A20 through targeting ASK1 to suppress TNF-induced JNK activation. Cell Death Differ. 2010;17:1830–1841. doi: 10.1038/cdd.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Jin H, Liu Y, Liu L, Wu Q, Guo Y, Yu L, Liu Z, Zhang T, Zhang X. et al. The expression patterns and correlations of claudin-6, methy-CpG binding protein 2, DNA methyltransferase 1, histone deacetylase 1, acetyl-histone H3 and acetyl-histone H4 and their clinicopathological significance in breast invasive ductal carcinomas. Diagn Pathol. 2012;7:33. doi: 10.1186/1746-1596-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yafang L, Qiong W, Yue R, Xiaoming X, Lina Y, Mingzi Z, Ting Z, Yulin L, Chengshi Q. Role of Estrogen Receptor-alpha in the Regulation of Claudin-6 Expression in Breast Cancer Cells. J breast cancer. 2011;14:20–27. doi: 10.4048/jbc.2011.14.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatai T, Matsuzawa A, Inoshita S, Mochida Y, Kuroda T, Sakamaki K, Kuida K, Yonehara S, Ichijo H, Takeda K. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J Biol Chem. 2000;275:26576–26581. doi: 10.1074/jbc.M003412200. [DOI] [PubMed] [Google Scholar]
- Takeda K, Matsuzawa A, Nishitoh H, Tobiume K, Kishida S, Ninomiya-Tsuji J, Matsumoto K, Ichijo H. Involvement of ASK1 in Ca2+-induced p38 MAP kinase activation. EMBO Rep. 2004;5:161–166. doi: 10.1038/sj.embor.7400072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Takeda K, Matsuzawa A, Saegusa K, Nakano H, Gohda J, Inoue J, Ichijo H. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J Biol Chem. 2005;280:37033. doi: 10.1074/jbc.M506771200. [DOI] [PubMed] [Google Scholar]
- Chang HY, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281:1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- Morgan M, Thorburn J, Pandolfi PP, Thorburn A. Nuclear and cytoplasmic shuttling of TRADD induces apoptosis via different mechanisms. J Cell Biol. 2002;157:975–984. doi: 10.1083/jcb.200204039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang H, Lin Y, Li J, Pober JS, Min W. RIP1-mediated AIP1 phosphorylation at a 14-3-3-binding site is critical for tumor necrosis factor-induced ASK1-JNK/p38 activation. J Biol Chem. 2007;282:14788–14796. doi: 10.1074/jbc.M701148200. [DOI] [PubMed] [Google Scholar]
- Juric V, Chen CC, Lau LF. TNFalpha-induced apoptosis enabled by CCN1/CYR61: pathways of reactive oxygen species generation and cytochrome c release. PLoS One. 2012;7:e31303. doi: 10.1371/journal.pone.0031303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Luo D, Miao R, Bai L, Ge Q, Sessa WC, Min W. Hsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene. 2005;24:3954–3963. doi: 10.1038/sj.onc.1208548. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen J, Fu H. Suppression of apoptosis signal-regulating kinase 1-induced cell death by 14-3-3 proteins. Proc Natl Acad Sci U S A. 1999;96:8511–8515. doi: 10.1073/pnas.96.15.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]