Abstract
An experimental protocol to validate secondary-model application to foods was suggested. Escherichia coli, Listeria monocytogenes, Bacillus cereus, Clostridium perfringens, and Salmonella were observed in various food categories, such as meat, dairy, egg, or seafood products. The secondary model validated in this study was based on the gamma concept, in which the environmental factors temperature, pH, and water activity (aw) were introduced as individual terms with microbe-dependent parameters, and the effect of foodstuffs on the growth rates of these species was described with a food- and microbe-dependent parameter. This food-oriented approach was carried out by challenge testing, generally at 15 and 10°C for L. monocytogenes, E. coli, B. cereus, and Salmonella and at 25 and 20°C for C. perfringens. About 222 kinetics in foods were generated. The results were compared to simulations generated by existing software, such as PMP. The bias factor was also calculated. The methodology to obtain a food-dependent parameter (fitting step) and therefore to compare results given by models with new independent data (validation step) is discussed in regard to its food safety application. The proposed methods were used within the French national program of predictive microbiology, Sym′Previus, to include challenge test results in the database and to obtain predictive models designed for microbial growth in food products.
Predictive microbiology has proven its value for a useful model-based description of microbial growth in foods ever since its development (18, 19). Data used in building a model are usually acquired in laboratory media. However, the predictions agree more or less successfully with observations of food products (6, 36), and validation of the model proves to be necessary in such cases. Salter et al. (31) underlined the importance of good prediction for food safety, although their model was not validated in their paper. Indeed, models should be validated for prediction in the product in question, to allow for risk assessment (26). This is all the more important when creating a software application (9, 16), such as the French national program of predictive microbiology, Sym′Previus (14). In this research program, industry, public, university, and technical center laboratories first constructed a parameter database covering 50 bacterial strains of five species grown in laboratory medium (20, 21). The pathogenic bacteria selected were Escherichia coli, Listeria monocytogenes, Bacillus cereus, Clostridium perfringens, and Salmonella. This initial work led to a model describing growth rates versus temperature, pH, and water activity.
The objective of this study was to develop a methodology to use these first results in foods. Thus, challenge tests were carried out, and then kinetics were analyzed to (i) obtain medium-dependent parameters and (ii) validate complete models. Since temperature is the major factor of interest in the food industry (18), the studies reported focused on that aspect.
MATERIALS AND METHODS
Previous results.
Strain-dependent parameters for the growth model were obtained in laboratory media (see the appendix). A cardinal-value model was used, with modules of temperature (T), pH, and water activity (aw); the parameters of this model are the cardinal values Tmin, Topt, Tmax, pHmin, pHopt, pHmax, aw(min), and aw(opt), where the subscript “min” indicates the theoretical minimal value allowing growth, the subscript “max” indicates the theoretical maximal value allowing growth, and the subscript “opt” indicates the optimal value for which growth is maximal (28). All of these parameters were therefore determined for each strain studied, resulting in a strain-dependent model for the prediction of growth in laboratory medium (20, 21).
Strains and media.
The food products and bacteria studied were selected according to food safety concerns, especially those of France (Table 1). For a given species, representative strains were chosen, whose cardinal values had been obtained in the earlier step of the Sym′Previus program (20, 21): 16 strains of L. monocytogenes from sausage (3 strains), seafood (2 strains), dairy products (6 strains), poultry (1 strain), and food plants (4 strains); 10 strains of E. coli from a meat product (1 strain), bovine feces (3 strains), dairy products (3 strains), and human isolates (3 strains); 10 strains of B. cereus from seafood (1 strain), dairy products (6 strains), egg or egg-based products (2 strains), and pasta (1 strain); 5 strains of C. perfringens from pork (1 strain), dairy products (3 strains), and poultry (1 strain); and 9 strains of Salmonella from sausage and pork meat (2 strains), dairy products (1 strain), poultry (1 strain), dairy plants (3 strains), and bakery products (2 strains). The study of the effect of temperature on growth rates demonstrated that intraspecies variability was low compared to uncertainty (21), and only one strain was retained for the validation study. In this way, a single strain was selected to perform challenge tests for each bacterial species. This selection was based principally on the strain's food origin.
TABLE 1.
Food products and bacterial species used in the present work
| Food | Combinations studiedb
|
||||
|---|---|---|---|---|---|
| E. colia | B. cereus | Salmonella | L. monocytogenes | C. perfringens | |
| Egg cream | + | ||||
| Béchamel sauce | + | ||||
| Raw ground salmon | + | ||||
| Yogurt | + | + | + | ||
| Raw poultry meat | + | + | + | ||
| Cooked poultry meat | + | + | + | + | |
| Raw salted pork meat | + | + | + | ||
| Crab sticks | + | + | + | ||
| Chocolate cream | + | + | + | + | + |
| Sugared liquid eggs | + | + | |||
| Rice + tomato sauce | + | ||||
| Rice + milk | + | ||||
| PÂté | + | + | |||
| Raw spreadable sausage | + | + | |||
| Potted meat | + | ||||
| Pork tongue in jelly | + | ||||
| Fish dish | + | ||||
Including O157:H7 and O26.
+, studied.
For subcultures, the liquid growth medium was brain heart infusion supplemented with glucose (0.2%) and yeast extract (0.3%) and sterilized by filtration (0.2-μm pore size; Millipore).
Bacterial counts were determined by plating on selective media: Hektoen and Rambach for Salmonella; ALOA (agar Listeria [Ottaviani and Agosti]; AES Laboratoire, Bruz, France), Palcam, and Oxford for L. monocytogenes; sorbitol MacConkey agar for E. coli; Mossel for B. cereus; and tryptose-sulfite-cycloserine for Clostridium perfringens. Dilutions were made in tryptone salt broth.
Preparation of the inoculum.
Two subcultures from frozen strains were carried out successively at 37°C in brain heart infusion for 16 and 8 h, respectively. The cultures were shaken at 50 oscillations · min−1, and one final subculture was made at the product incubation temperature studied. In order to have all strains in the same physiological state, a preliminary study was performed in Bioscreen C (Labsystems, Helsinki, Finland). Turbidity was monitored over the whole growth curve of the strains at the chosen temperature. The natural logarithm of the population was calculated. On this log-transformed growth curve, t0 is the time of intersection of two straight lines, one for the exponential growth phase and one for the saturation phase [ln(N) = ln(Nmax)]. The duration of the final subculture was then chosen as t0 plus 10% in order to have cells at the end of their exponential phase.
Growth in food products (challenge tests).
Where freezing was possible, a single stock of product was used for all trials of a given experiment. Food was contaminated with an approximate inoculum level of 5 × 103 CFU/g and divided into 10-g samples. Two iterations of each experiment were performed (the second iteration was repeated twice), with at least 15 measurement points for each curve. Following the first experiment, these points were chosen at optimal time values in order to obtain an even spread of points in the growth curve for the second iteration. The two repetitions of this second experiment were conducted simultaneously. This protocol was used for all challenge test experiments throughout the study: kinetics were generally obtained at 15 and 10°C for L. monocytogenes, E. coli, B. cereus, and Salmonella and at 25 and 20°C for C. perfringens in the food products studied.
Statistical analysis.
Three different software programs were used according to what was available in each laboratory: SAS (SAS Institute Inc., Cary, N.C.), S-Plus (AT&T Bell Laboratories, Murray Hill, N.J.), and Excel (Microsoft Excel 2000). Similar results were obtained with each of these programs.
Primary and secondary models.
The primary growth model, describing the evolution of a bacterial population with time (see the appendix), was the modified logistic model proposed by Rosso (27). The population level is referred to as N.
The secondary model, describing the influences of environmental factors on the growth parameters, was based on the gamma concept (37) and was written as follows:
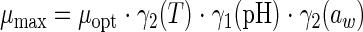 |
(1) |
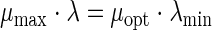 |
(2) |
where μmax and λ are the maximal specific growth rate and lag time in the specific food product at given T, pH, and aw values; μopt and λmin are values at optimal T, pH, and aw values in the specific food product. γ2(T), γ1(pH), and γ2(aw) (given in the appendix) have parameters that are considered to be independent of the growth medium (11). The matrix (food) effect is described through μopt and λmin.
RESULTS
Challenge tests.
A total of 222 kinetics of E. coli, L. monocytogenes, B. cereus, C. perfringens, and Salmonella in food products (Table 1) were generated. For example, L. monocytogenes was studied in raw ground salmon, yogurt, raw poultry meat, cooked poultry meat, raw salted pork meat, crab sticks, pÂté, raw spreadable sausage, potted meat, and pork tongue in jelly successively at 15 and 10°C. The values of pH and aw were measured for each trial. Moreover, in a few cases, additional temperature conditions were tested (for instance, 25°C for L. monocytogenes in raw ground salmon and potted meat).
Determination of μopt and λmin parameters.
The general model (equation 1) was studied more precisely with temperature, and validation was consequently performed on this factor. Growth was monitored in a food product at a fixed temperature; a preliminary study (data not presented) showed that this temperature had to be close to Topt (to obtain a correct μopt estimate), although not too high (to avoid null lag time and to prevent product modifications). The values of pH and aw were measured. When all three growth curve trials were produced, the modified logistic model was fitted to the data.
Values of pH and aw were considered to be constant for a given food product, since they were not deliberately modified. Therefore, a reduced version of the secondary model was proposed:
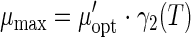 |
(3) |
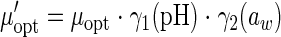 |
(4) |
Since the parameters in γ2(T) are independent of the growth medium (11), the values obtained in laboratory medium were used. Therefore, μ′opt was the parameter that needed to be adjusted to adapt the model to the product. Regression was only carried out using the temperature module, leading to an estimation of μ′opt. This new parameter represented pH and water activity effects, along with the food effect (equation 4). Using this reduced model (equation 3), simulations of growth could be produced at a given temperature in a given food product, where the μ′opt value of this product was used, assuming pH and aw values to be identical at all temperatures. Similarly, a value of λ′min was used instead of λmin.
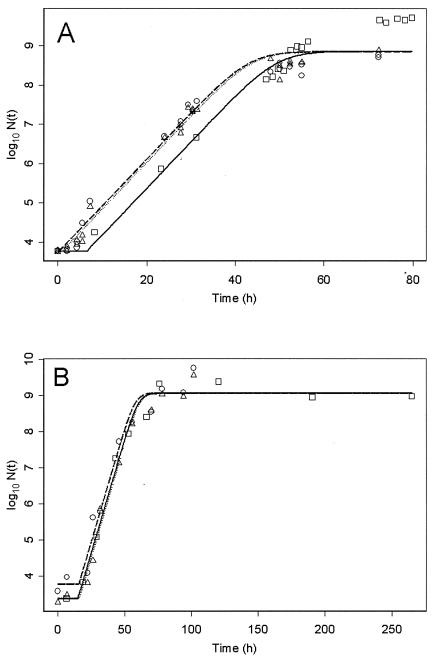
Examples of μ′opt determination with E. coli in cooked poultry meat and B. cereus in crab sticks are shown in Fig. 1. When adjusting the model, a common value of μmax over the three trials was computed (as for maximal population, Nmax), whereas there was a different lag time, λ (as for inoculum size, N0), for each trial. The final λ was chosen as the minimum of these three values, allowing λ′min calculation using the temperature module. Eventually, μ′opt and λ′min were obtained, allowing the model to be completed for the food product. As an example, parameters for an E. coli strain growing in cooked poultry meat (as shown in Fig. 1A) are given in Tables 2, 3, and 4. In Table 2, the results of the regression on the three trials are presented. Cardinal-model calculations under the experimental conditions are shown in Table 3, and the consequent parameter estimations are given in Table 4.
FIG. 1.
Growth of an E. coli O26 strain in cooked poultry meat (A) and of a B. cereus strain in crab sticks (B) at 15°C. The points represent observed data (squares, first trial; circles, second trial; triangles, third trial), and the lines represent adjusted primary model (continuous line, first trial; dashed line, second trial; dotted line, third trial). The models are adjusted with common μmax and Nmax for all trials, but with one λ and one N0 for each separate experiment.
TABLE 2.
Example of parameter estimation for an E. coli O26 strain growing in cooked poultry meata
| Parameter | Regression results |
|---|---|
| μmax | 0.273 h−1 |
| log10(N0)1 | 3.77 |
| log10(N0)2 | 3.79 |
| log10(N0)3 | 3.79 |
| λ1 | 6.48 h |
| λ2 | 0.46 h |
| λ3 | 1.13 h |
| log10(Nmax) | 8.86 |
Results of the regression presented in Fig. 1.
TABLE 3.
Example of parameter estimation for an E. coli O26 strain growing in cooked poultry meata
TABLE 4.
Example of parameter estimation for an E. coli O26 strain growing in cooked poultry meata
| Parameter | Value |
|---|---|
| μ′opt = μmax/γ2(T) | 3.74 h−1 |
| λ′min = min(λ1, λ2, λ3) · γ2(T) | 0.034 h |
| μopt = μ′opt/[γ1(pH) · γ2(aw)] | 4.247 h−1 |
| λmin = λ′min · γ1(pH) · γ2(aw) | 0.030 h |
| GT′opt = ln(2)/μ′opt | 11.1 min |
| GTopt = ln(2)/μopt | 9.8 min |
Derived calculations.
As a result, a full model can be used for a given strain (where its cardinal values Tmin, Topt, and Tmax are known) growing in a given product (with known μ′opt and λ′min values) in a given environment (temperature). Values of growth rate (μmax) and lag time (λ) can be obtained for a new temperature condition with the reduced secondary model, and a predicted growth curve can subsequently be drawn using the primary model.
Validation of model.
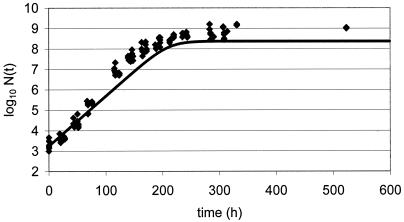
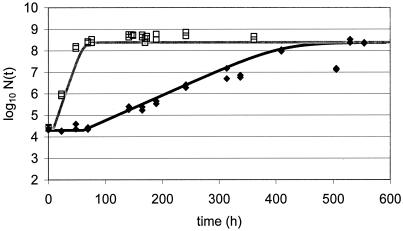
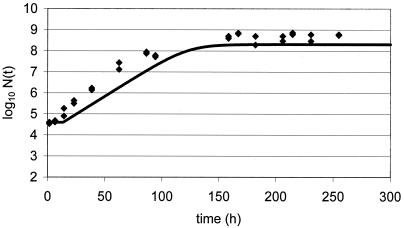
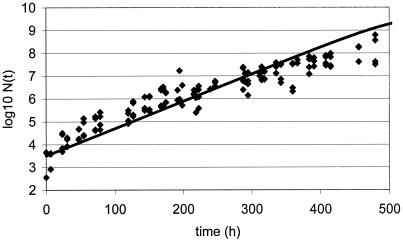
New data were acquired for the food product studied under other growth conditions chosen as being close to real storage conditions for the product yet still permitting growth. Since a full model was available and all parameters were known, a prediction of growth could be made once temperature, pH, and aw were measured or set. Challenge tests conducted with L. monocytogenes at 10°C in chocolate cream (Fig. 2), in raw poultry meat (Fig. 3), in smoked salmon (Fig. 4), in potted meat (Fig. 5), and in crab sticks (Fig. 6) are presented as illustrations. The observed kinetics were compared to simulations with the model. The results were found to be satisfactory [the discrepancy between observed and predicted log(N) was <1 log unit] in 80% of food-bacteria associations (Table 1). However, as illustrated in Fig. 5 and 6, some combinations would require further work.
FIG. 2.
Validation of L. monocytogenes model at 10°C: growth in chocolate cream (diamonds) compared to simulation of growth in chocolate cream (line).
FIG. 3.
Validation of L. monocytogenes model at 9°C: growth in raw poultry meat (diamonds) compared to simulation of growth in raw poultry meat (line).
FIG. 4.
Validation of L. monocytogenes model: growth in smoked salmon at 10 (diamonds) and at 25°C (squares) compared to simulations of growth in smoked salmon at 10 (solid line) and at 25°C (shaded line).
FIG. 5.
Validation of L. monocytogenes model at 10°C: growth in potted meat (diamonds) compared to simulation of growth in potted meat (line).
FIG. 6.
Validation of L. monocytogenes model at 10°C: growth in crab sticks (diamonds) compared to simulation of growth in crab sticks (line).
Among the environmental factors, only temperature was modified, as this was the parameter the model sought to validate. This process only validates predictions of the temperature effect: currently, there is no validation of the pH and aw modules.
Predicted μmax (or equivalent generation time, GT) was compared to the observed value, which was calculated from the growth curve using the modified logistic model. The indices proposed by Ross (25) and modified by Baranyi et al. (2) were used here. The bias factor is defined as follows:
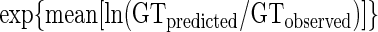 |
(5) |
The accuracy factor is defined in the following way:
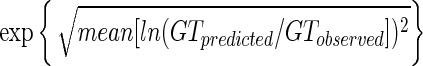 |
(6) |
The bias and accuracy factors were computed for the predictions of this model compared to the three trials of new experimental data, and predictions using the Pathogen Modeling Program (PMP) (U.S. Department of Agriculture, Wyndmoor, Pa. [http://www.arserrc.gov/mfs/pathogen.htm]) were also performed (for the PMP model, see reference 4). The results for L. monocytogenes in various products are shown in Table 5. Predictions using the present model were favorable. Some bias factors indicate slightly fail-dangerous predictions, although not too far above the acceptable level of 1.15 recommended by Ross et al. (26). For example, the highest bias factor was ∼1.3 for raw poultry meat data. As can be seen in Fig. 3, this results in a slight difference between predicted and observed growth (<1 log unit at the end of the exponential phase). Accuracy factor values are generally <1.3.
TABLE 5.
Bias and accuracy factors for generation times of L. monocytogenes in food products using the Sym′Previus model (developed in this paper) and using PMP (4)
| Food product | Bias factor
|
Accuracy factor
|
||
|---|---|---|---|---|
| Sym′Previus model | PMP | Sym′Previus model | PMP | |
| Chocolate cream | 1.073 | 0.407 | 1.084 | 2.461 |
| Raw poultry meat | 1.298 | 1.064 | 1.299 | 1.065 |
| Smoked salmon (10°C) | 0.855 | 0.299 | 1.169 | 3.349 |
| Smoked salmon (25°C) | 1.223 | 0.409 | 1.223 | 2.446 |
| Potted meat | 1.130 | 0.997 | 1.134 | 1.089 |
| Crab sticks | 0.910 | 0.184 | 1.099 | 5.436 |
| All | 1.098 | 0.540 | 1.180 | 2.452 |
DISCUSSION
Part of the purpose of the Sym′Previus project was to establish reproducible methodologies in laboratory medium, as well as in food products. Thus, many laboratories were able to obtain comparable growth data thanks to precise experimental protocols, which they were then able to analyze in a common way using analysis protocols. It is now easy to add new data to the database and to use this standardized methodology outside the program. Furthermore, an important amount of uniform results was included in the Sym′Previus database.
These results indicate that the model gives correct predictions of the effect of temperature on food products. Reliable simulations of growth can be obtained, representing useful complements to experimental assays. L. monocytogenes was chosen to illustrate the utilization of this model, but the behaviors of the other pathogens studied could also be predicted. It was therefore concluded that choosing cardinal models was interesting, and furthermore, they are easy to use and have biologically meaningful parameters. Moreover, the hypothesis of the non-food-dependent parameters Tmin, Topt, and Tmax (11) was confirmed by our results.
Temperature has been the main factor studied so far. However, the methodology could easily be adapted to study other factors more precisely. For example, if a new product formulation changed its pH, a process of μopt calculation and model validation could be conducted, along the lines of what has been done in this program. Similarly, much effort has been invested in growth rate modeling, although a simple lag time model has been assumed in this study. However, the form of the model makes it suitable for improvements without invalidating what has been done here. Hence, lag time modeling represents a future step in the program. Since the last subculture was carried out at the temperature of the challenge test, the lag time was reduced, which led to a “fail-safe” prediction. To be closer to the industrial context, other scenarios will be performed.
Validation is an essential step after modeling. The first stage of validation, when proposing a new type of model, is often internal validation (34), which means validation is performed on the same data used for building the model (23, 24). However, further external validation, using new data not used for fitting the model, would appear to be essential to confirm the robustness of the model (10).
Predictive models are often built on data obtained in laboratory medium. Extrapolation to predictions in food products is not straightforward (8, 15) because of the complexity of these media (35). Models take a limited number of factors into account compared to the numerous factors influencing growth in food products; this phenomenon has been named “completeness error” (19). Therefore, a good way of validating a model is to compare its prediction to data obtained for food products.
Food data used for validation were sometimes taken from published results (4, 12, 17). This is an easier way of validating a model than conducting new experiments on food products. However, it is often difficult to use published results for comparison with model predictions (16, 34). Conditions of growth are sometimes not precisely described, and it is necessary to make assumptions about some factors (3, 30, 32). Some models incorporate a new factor for which few data (or even no data) have been published; therefore, validations have to be made with a level 0 for such a factor (5, 33).
The methodology presented in this paper makes it necessary to conduct experiments on food products, since some parameters (μopt and λmin) are specific to a bacterial-species-growth medium combination. Data are acquired on the studied product for model building, and then new data are obtained on the product for model validation. This method is an intermediate between (i) “all laboratory media” methods (1, 7), which require further validation to be considered safe, and (ii) “all food” methods (13, 22), which are more expensive. Even so, the validation process described here currently considers a single strain per food-species combination. However, it is possible at this stage to give a rough classification of foods according to the suitability of the model for predictions in these products. Furthermore, using the standard methodology reported in this paper, new challenge tests could be performed to further validate the model. It should be noted that a complementary study of the variability of model predictions has been conducted (21). A comparison between our experimental results obtained from foods and PMP simulations (Table 5) indicated that the PMP software gave too conservative GT predictions. This result is not surprising, since PMP is not a food-oriented program. However, the comparison was made because, at the moment, this software is one of the references in predictive microbiology modeling and is freely available on the Internet.
The Sym′Previus program is still running. Further studies are planned to include new microorganisms (Staphylococcus aureus) and new factors (organic acids) or to improve models (lag time modeling, growth limits, and interactions). The existence of a standardized methodology would be extremely helpful in conducting these projects jointly in several laboratories.
Acknowledgments
This project was part of the French national predictive microbiology program, Sym′Previus. It was supported by the French Departments of Research and Agriculture.
APPENDIX
The primary model of growth used here is that of Rosso (27):
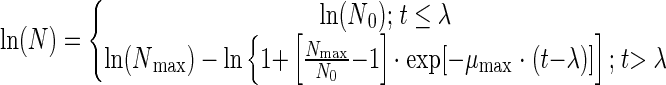 |
where t is time, N is the population level, N0 is the initial population level, Nmax is the maximal population level, λ is the lag time, and μmax is the growth rate.
The secondary model (28, 29), based on the gamma concept (37), uses individual modules for the environmental factors:
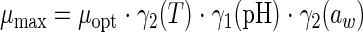 |
These modules are defined as
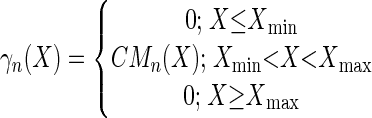 |
with
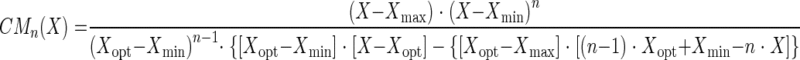 |
X corresponds to T, pH, or aw factors, with n values of 2, 1, and 2, respectively. The estimated parameters are μopt, Tmin, Topt, Tmax, pHmin, pHopt, aw(min), and aw(opt).
For the pH equation, the symmetry hypothesis (20) was assumed:
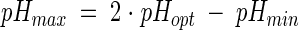 |
For the water activity, aw(max) was fixed at 1.
REFERENCES
- 1.Baranyi, J., T. P. Robinson, A. Kaloti, and B. M. Mackey. 1994. Predicting growth of Brochothrix thermosphacta at changing temperature. Int. J. Food Microbiol. 27:61-75. [DOI] [PubMed] [Google Scholar]
- 2.Baranyi, J., C. Pin, and T. Ross. 1999. Validating and comparing predictive models. Int. J. Food Microbiol. 48:159-166. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn, C. W., L. M. Curtis, L. Humpheson, C. Billon, and P. J. McClure. 1997. Development of thermal inactivation models for Salmonella enteritidis and Escherichia coli O157:H7 with temperature, pH and NaCl as controlling factors. Int. J. Food Microbiol. 38:31-44. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan, R. L., and J. G. Phillips. 1990. Response surface model for predicting the effects of temperature, pH, sodium chloride content, sodium nitrite concentration and atmosphere on the growth of Listeria monocytogenes. J. Food Prot. 53:370-376. [DOI] [PubMed] [Google Scholar]
- 5.Buchanan, R. L., and L. K. Bagi. 1994. Expansion of response surface models for the growth of Escherichia coli O157:H7 to include sodium nitrite as a variable. Int. J. Food Microbiol. 23:317-332. [DOI] [PubMed] [Google Scholar]
- 6.Castillejo-Rodriguez, A. M., R. M. G. Gimeno, G. Z. Cosano, E. B. Alcala, and M. R. R. Perez. 2002. Assessment of mathematical models for predicting Staphylococcus aureus growth in cooked meat products. J. Food Prot. 65:659-665. [DOI] [PubMed] [Google Scholar]
- 7.Chhabra, A. T., W. H. Carter, R. H. Linton, and M. A. Cousin. 1999. A predictive model to determine the effects of pH, milkfat, and temperature on thermal inactivation of Listeria monocytogenes. J. Food Prot. 62:1143-1149. [DOI] [PubMed] [Google Scholar]
- 8.Dalgaard, P., and L. V. Jorgensen. 1998. Predicted and observed growth of Listeria monocytogenes in seafood challenge tests and in naturally contaminated cold-smoked salmon. Int. J. Food Microbiol. 40:105-115. [DOI] [PubMed] [Google Scholar]
- 9.Dalgaard, P., P. Buch, and S. Silberg. 2002. Seafood Spoilage Predictor—development and distribution of a product specific application software. Int. J. Food Microbiol. 73:343-349. [DOI] [PubMed] [Google Scholar]
- 10.Delignette-Muller, M. L., L. Rosso, and J. P. Flandrois. 1994. Accuracy of microbial growth predictions with square root and polynomial models. Int. J. Food Microbiol. 27:139-146. [DOI] [PubMed] [Google Scholar]
- 11.Delignette-Muller, M. L., and L. Rosso. 2000. Biological variability and exposure assessment. Int. J. Food Microbiol. 58:203-212. [DOI] [PubMed] [Google Scholar]
- 12.George, S. M., L. C. C. Richardson, and M. W. Peck. 1996. Predictive models of the effect of temperature, pH and acetic and lactic acids on the growth of Listeria monocytogenes. Int. J. Food Microbiol. 32:73-90. [DOI] [PubMed] [Google Scholar]
- 13.Koutsoumanis, K. P., P. S. Taoukis, E. H. Drosinos, and G.-J. E. Nychas. 2000. Applicability of an Arrhenius model for the combined effect of temperature and CO2 packaging on the spoilage microflora of fish. Appl. Environ. Microbiol. 66:3528-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leporq, B., J.-M. Membré, M. H. Zwietering, C. Dervin, P. Buche, and J. P. Guyonnet. 2003. The “Sym′Previus” software, a tool to support decisions to the foodstuff safety, p. 55-57. In J. F. Van Impe, A. H. Geeraerd, I. Leguerinel, and P. Mafart (ed.), Predictive modelling in foods—conference proceedings. Katholieke Universiteit Leuven/BioTeC, Quimper, France.
- 15.McClure, P. J., J. Baranyi, E. Boogard, T. M. Kelly, and T. A. Roberts. 1993. A predictive model for the combined effect of pH, sodium chloride and storage temperature on the growth of Brochothrix thermosphacta. Int. J. Food Microbiol. 19:161-178. [DOI] [PubMed] [Google Scholar]
- 16.McClure, P. J., C. W. Blackburn, M. B. Cole, P. S. Curtis, J. E. Jones, J. D. Legan, I. D. Ogden, M. W. Peck, T. A. Roberts, J. P. Sutherland, and S. J. Walker. 1994. Modelling the growth, survival and death of microorganisms in foods: the UK Food Micromodel approach. Int. J. Food Microbiol. 23:265-275. [DOI] [PubMed] [Google Scholar]
- 17.McClure, P. J., A. L. Beaumont, J. P. Sutherland, and T. A. Roberts. 1997. Predictive modelling of growth of Listeria monocytogenes. The effects on growth of NaCl, pH, storage temperature and NaNO2. Int. J. Food Microbiol. 34:221-232. [DOI] [PubMed] [Google Scholar]
- 18.McDonald, K., and D. W. Sun. 1999. Predictive food microbiology for the meat industry: a review. Int. J. Food Microbiol. 52:1-27. [DOI] [PubMed] [Google Scholar]
- 19.McMeekin, T. A., and T. Ross. 2002. Predictive microbiology: providing a knowledge-based framework for change management. Int. J. Food Microbiol. 78:133-153. [DOI] [PubMed] [Google Scholar]
- 20.Membré, J.-M., B. Leporq, M. Vialette, E. Mettler, L. Perrier, and M. H. Zwietering. 2002. Experimental protocols and strain variability of cardinal values (pH and aw) of bacteria using Bioscreen C: microbial and statistical aspects, p. 143-146. In L. Alexon, E. S. Tronrud, and K. J. Merok (ed.), Microbial adaptation to changing environments. Matforsk Norwegian Food Research Institute, Lillehammer, Norway.
- 21.Membré, J.-M., B. Leporq, M. Vialette, E. Mettler, L. Perrier, D. Thuault, and M. H. Zwietering. 2003. Temperature effect on bacterial growth rate: quantitative microbiology approach, including cardinal values and variability estimates, to perform growth simulations on food, p. 123-125. In J. F. Van Impe, A. H. Geeraerd, I. Leguerinel, and P. Mafart (ed.), Predictive modelling in foods—conference proceedings. Katholieke Universiteit Leuven/BioTeC, Quimper, France.
- 22.Pond, T. J., D. S. Wood, I. M. Mumin, S. Barbut, and M. W. Griffiths. 2001. Modeling the survival of Escherichia coli O157:H7 in uncooked, semidry, fermented sausage. J. Food Prot. 64:759-766. [DOI] [PubMed] [Google Scholar]
- 23.Presser, K., T. Ross, and D. Ratkowsky. 1998. Modelling the growth limits (growth/no growth interface) of Escherichia coli as a function of temperature, pH, lactic acid concentration, and water activity. Appl. Environ. Microbiol. 64:1773-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratkowsky, D., and T. Ross. 1995. Modelling the bacterial growth/no growth interface. Lett. Appl. Microbiol. 20:29-33. [Google Scholar]
- 25.Ross, T. 1996. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol. 81:501-508. [DOI] [PubMed] [Google Scholar]
- 26.Ross, T., P. Dalgaard, and S. Tienungoon. 2000. Predictive modelling of the growth and survival of Listeria in fishery products. Int. J. Food Microbiol. 62:231-245. [DOI] [PubMed] [Google Scholar]
- 27.Rosso, L. 1995. Ph. D. thesis. Université Claude Bernard, Lyon, France.
- 28.Rosso, L., J. R. Lobry, S. Bajard, and J. P. Flandrois. 1995. Convenient model to describe the combined effects of temperature and pH on microbial growth. Appl. Environ. Microbiol. 61:610-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosso, L., and T. P. Robinson. 2001. A cardinal model to describe the effect of water activity on the growth of moulds. Int. J. Food Microbiol. 63:265-273. [DOI] [PubMed] [Google Scholar]
- 30.Salter, M. A., T. Ross, and T. A. McMeekin. 1998. Applicability of a model for non-pathogenic Escherichia coli for predicting the growth of pathogenic Escherichia coli. J. Appl. Microbiol. 85:357-364. [DOI] [PubMed] [Google Scholar]
- 31.Salter, M. A., D. Ratkowsky, T. Ross, and T. A. McMeekin. 2000. Modelling the combined temperature and salt (NaCl) limits for growth of a pathogenic Escherichia coli strain using nonlinear logistic regression. Int. J. Food Microbiol. 61:159-167. [DOI] [PubMed] [Google Scholar]
- 32.Sutherland, J. P., A. J. Bayliss, and D. S. Braxton. 1995. Predictive modelling of growth of Escherichia coli O157:H7: the effects of temperature, pH and sodium chloride. Int. J. Food Microbiol. 25:29-49. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland, J. P., A. J. Bayliss, D. S. Braxton, and A. L. Beaumont. 1997. Predictive modelling of Escherichia coli O157:H7: inclusion of carbon dioxide as a fourth factor in a pre-existing model. Int. J. Food Microbiol. 37:113-120. [DOI] [PubMed] [Google Scholar]
- 34.te Giffel, M. C., and M. H. Zwietering. 1999. Validation of predictive models describing the growth of Listeria monocytogenes. Int. J. Food Microbiol. 46:135-149. [DOI] [PubMed] [Google Scholar]
- 35.Tsujihata, S., E. Entani, M. Asai, Y. Tsukamoto, and M. Ohta. 1998. Mathematical modeling to predict the bactericidal effect of processed vinegar on Escherichia coli O157:H7. Int. J. Food Microbiol. 43:135-138. [DOI] [PubMed] [Google Scholar]
- 36.Walls, I., and V. N. Scott. 1996. Validation of predictive mathematical models describing the growth of Escherichia coli O157:H7 in raw ground beef. J. Food Prot. 59:1331-1335. [DOI] [PubMed] [Google Scholar]
- 37.Wijtzes, T., F. M. Rombouts, M. L. T. Kant-Muermans, K. Van't Riet, and M. H. Zwietering. 2001. Development and validation of a combined temperature, water activity, pH model for bacterial growth rate of Lactobacillus curvatus. Int. J. Food Microbiol. 63:57-64. [DOI] [PubMed] [Google Scholar]