Extensive knowledge of microbial metabolism has been earned through more than a century of reductionist study. There is now basic understanding of how cultivated bacteria transduce the chemical energy of a growth substrate into the work and biosynthetic processes that underlie both survival and replication. This includes an appreciation that microorganisms can metabolize many chemical substances that exist only due to the efforts of synthetic chemists (34). These direct observations of microbial metabolism, and the molecular diversity inferred from microbial genome sequencing, point to the great breadth of microbial metabolism that exists in nature.
In this age of genomics and the inherently new perceptions of metabolic networks thus engendered (101), it is worth pointing out the obvious: microbial cells are not made up of information; they are made up of atoms. The atoms in a microbial cell are determined only partly by genome-encoded transporters; they are also determined by the extracellular environment. For example, Deinococcus radiodurans accumulates more or less of the elements iron and manganese, depending on their relative concentrations in the extracellular environment. If the manganese concentration is above a threshold, D. radiodurans will oxidize its carbon source, glucose, via the glycolytic pathway (145). Below the threshold concentration, the pentose phosphate pathway is used.
Substitution of manganese for iron is also important in the bacterial pathogen Borrelia burgdorferi, the causative agent of Lyme disease. The B. burgdorferi genome project has revealed the absence of iron-containing membrane proteins and annotated genes encoding proteins such as superoxide dismutase that are predicted to contain manganese rather than iron (97). The absence of iron and iron-containing proteins is proposed to allow B. burgdorferi to survive in a host that restricts iron, an action that normally limits microbial growth and thus prevents infection. In this way, the B. burgdorferi genome may have evolved by eliminating the requirement for what is usually an essential element.
Furthermore, an element required by some bacteria may be toxic to others. For example, tungsten is a required nutrient for some hyperthermophilic archaea (64) but 300 strains of iron-oxidizing bacteria are strongly inhibited by sodium tungstate (125). So, if we wish to study how the genome directs the cell, we need to know what atoms are present in the vicinity of the cell and how the cells respond to complex mixtures of the chemical elements and compounds.
In the period from 1930 to 1970, there was a strong interest in how biological systems respond holistically to the chemical elements, largely in the context of studies on microbial, plant, and animal nutrition (106, 118, 121). This led to the identification of major elements that commonly participate in metabolic processes within diverse microbiological systems, specifically H, C, O, N, P, S, Cl, K, Na, Ca, Mg, Se, Zn, Fe, Mn, Cu, Co, Ni, and Mo. In the last 70 years, much effort was expended to establish the precise mechanisms by which these elements mediate specific biological functions. However, with this expansion of knowledge, information has become fragmented into separate domains. For example, more recent reviews have focused on such topics as the microbial disposition of (i) heavy metals (91); (ii) metalloid compounds (8, 42, 88); and (iii) specific classes of organic compounds, including organosulfur compounds (61) and nitrogen heterocyclic compounds (40).
Most recently, focus on the complete constellation of elements found in microbes has taken on a new imperative in the light of efforts in genomics-based reconstruction of the cellular machinery, microbial remediation of complex environmental mixtures, and understanding microbial individuality. Reductionism has brought us to the point that this information can be interfaced with genomics, but the effort will further require a compilation of how microbes respond to all of the chemical elements. There have been reviews that address the issue of element essentiality, transformation, and toxicity more broadly (42, 113); the present review seeks to extend those efforts. This review does not discuss in any detail the role of transition metals in microbial metabolism, a topic that has been covered in numerous books and reviews (72, 86, 137). Rather, we focus on integrating research on the most common biological elements and those that are most rare, but for which biological roles have recently been identified.
INTERPLAY AMONG ELEMENTS
A microorganism's genes persist under selective pressure to facilitate the cell's survival in a complex and often changing chemical environment. Genes relevant to the acquisition of carbon, nitrogen, sulfur, phosphorus, major cations, chloride, zinc, and transition metals function in concert; all of those needs must be met for survival of the organism. Genomics is increasingly revealing this interplay. For example, an operon in Salmonella enterica serovar Typhimurium involved in magnesium uptake is regulated by the pho system, which is involved in the cell's response to phosphate starvation (28). This seems perplexing at first consideration, but makes sense when one considers cell chemistry holistically. The many intracellular molecules containing the pyrophosphate group, such as ATP, exist mainly complexed with Mg2+. In fact, in vitro kinase assays use Mg-ATP as the phosphoryl-donating substrate (87).
Another example of how the genome and environment interact is differential metal binding to enzymes. Dai et al. (27) have shown that an isolated enzyme, the product of a single bacterial gene, has alternate enzymatic activities depending on whether the enzyme complexes Fe2+ or Co2+. Both of the reactions are physiologically relevant. Thus, one must look to what Da Silva and Williams call the metallome when considering the function of specific genes within a bacterium's genome (28). The metallome is the complete complement of the alkali elements, alkali earth elements, zinc, transition metals, and metalloid elements; collectively this consists of as many as 50 elements. The present review focuses on the metallome, excluding the biologically common transition metals as mentioned above, and what is known about how cells respond to, and metabolize, those elements and their compounds.
CATALOGING INFORMATION ON MICROBIAL INTERACTION WITH THE ELEMENTS
Increasingly, efforts are emerging to depict metabolism in the context of the full constellation of chemical elements. A recent book, The Biological Chemistry of the Elements: the Inorganic Chemistry of Life (28), discusses the properties of the elements in the context of their selection and use by biological systems, but the focus is on mammalian biochemistry. In his book Post-Genome Informatics, M. Kanehisa discusses the flow of information in biological systems, from (i) the elements, (ii) to compounds, (iii) to genomes, (iv) to enzymes, and (v) to organisms (58). A World Wide Web database developed by Kanehisa and his coworkers, KEGG (44), shows a periodic table with links from 21 chemical elements to information on biological interactions with those elements that includes enzymes, transporters, and metabolic maps. A search of the related LIGAND chemical database on 21 June 2002 provided information on 43 chemical elements.
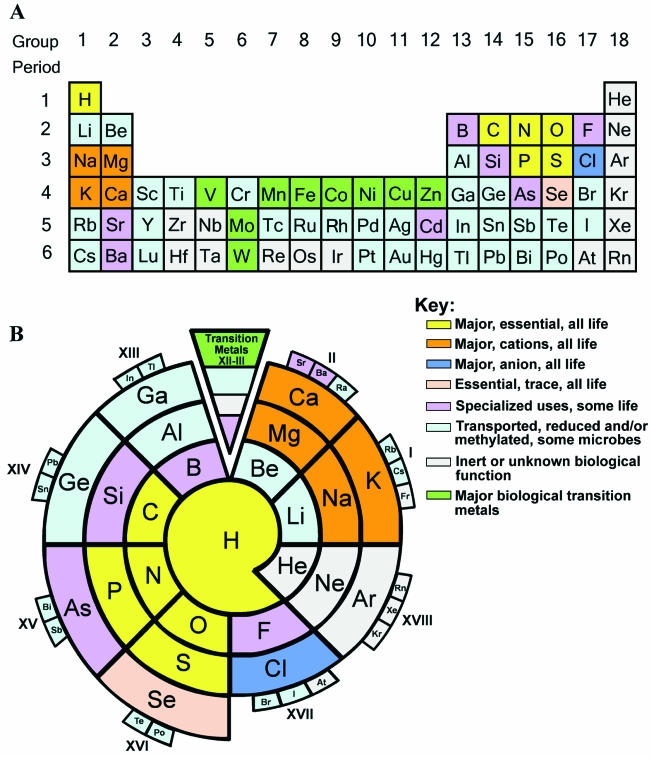
In another example, the World Wide Web-based University of Minnesota Biocatalysis/Biodegradation Database (UM-BBD) has focused on cataloging the diversity of microbial transformation reactions derived from published scientific literature (34). The UM-BBD's organizational framework is centered on depicting enzymatic transformations of distinct chemical elements and functional groups. The chemical functional groups, as defined by more than a century of research in synthetic chemistry, are collections of atoms that undergo specific chemical reactions such as reduction and/or oxidation, addition and/or elimination, or hydrolysis. Most metabolism databases deal almost exclusively with only a limited number of chemical elements, principally carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur. In contrast, the UM-BBD has long included information on the microbial metabolism of inorganic and organic substances containing mercury, arsenic, silicon, tin, lead, selenium, tellurium, fluorine, chlorine, and bromine. Most recently, the UM-BBD has been expanded to contain information on microbial interactions with 77 chemical elements. This information can be accessed holistically via a standard periodic table representation of the chemical elements (Fig. 1A). For each of the 77 chemical elements covered, a hypertext link takes the user to further information on the microbial biochemistry of that element.
FIG. 1.
Periodic representations of the elements. (A) Conventional table with linear columns and rows; the lanthanide and actinide elements, present in the on-line version, have been omitted for clarity of presentation. (B) Spiral representation of the elements which clusters elements that are prominent in biological systems. For on-line versions, see http://umbbd.ahc.umn.edu/periodic/.
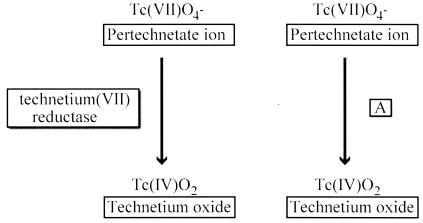
In the case of metals and metalloid elements, element pages describe how each undergoes oxidation, reduction, or perhaps coordination by specific microbes or their proteins. In the case of reactions, there are links to specific reaction pages. An example of a reaction page that is accessible from the element page for the radioactive element technetium is shown in Fig. 2. Technetium was first characterized in 1937, having been made in quantities sufficient to study via a newly built cyclotron (6). Yet, microbes are now known to change the oxidation state of the pertechnetate anion (70), showing the plasticity of biological systems to handle new chemical elements. The potential for microbes to reduce technetium to less-mobile oxidation states may have an impact on long-term environmental health, as isotopes such as technetium-99 have half-lives as long as 212,000 years.
FIG. 2.
Part of a pathway map for technetium (found at http://umbbd.ahc.umn.edu/tc/tc_map.html). Two reactions are shown. One is directly catalyzed by the enzyme technetium(VII) reductase, and the other is indirectly coupled to iron(III) reduction, and thus is labeled with an A to indicate nonspecificity.
MICROBIALLY FOCUSED DEPICTION OF THE ELEMENTS AND THEIR METABOLISM
A variety of schemes have evolved to arrange the chemical elements in a meaningful array (80), but the forerunner of the modern periodic table of the elements is generally credited to Sergei Mendeleyev (6). In 1869, Mendeleyev organized the elements into columns and rows for the purpose of teaching the properties of the elements to a chemistry class at St. Petersburg University. This fundamental organization of the chemical elements has persisted to the present as one of the most well-known and successful schemata in science. The organization imposed by Mendeleyev's periodic table facilitated the discovery of concepts related to chemical bonding, electron shells, electronic orbitals, and ultimately quantum theory.
The most widely used format for the periodic table of the elements was not developed with biology in mind, and thus different renderings may offer a better linkage between chemical properties and biological function. One such permutation presented here is a spiral representation of the chemical elements (Fig. 1B). A spiral elemental chart is not new and in fact dates back to the 1880s (80). However, it has been elaborated on here to best illustrate the biological roles and connectedness of the elements for their functions in microorganisms. For example, the periodicity of 8 focuses attention on the lighter elements, and this is further emphasized by putting biologically less relevant heavy elements in smaller boxes on the periphery. Moreover, the current spiral depiction juxtaposes hydrogen with elements to which it is commonly bonded in biological systems: carbon, nitrogen, oxygen, phosphorus, and sulfur. The lighter elements that run through the spiral from the lower left to upper right are most abundant in biology. Those from the lower right (the noble elements) to the upper left (Al, Ga, and Ge) are not abundant in biological systems. While the transition metals are not shown individually in Fig. 1B due to the emphasis of the present review, the World Wide Web version provides a link to detailed information on transition metals in prokaryotes.
A key feature of the spiral elemental diagram depicted here (Fig. 1B) is the centrality of hydrogen, in sharp contrast to the isolation of hydrogen in the upper left corner of the standard periodic table (Fig. 1A). Hydrogen is central to microbiological systems because 60% of the cell mass is H2O, most microbial enzymes effect H+ transfer, H+ gradients are widely used to generate ATP, H− is a cellular two-electron transfer currency, and H bonding is crucial for the stability of major biomacromolecules. Until recently, hydrogenases (enzymes that carry out the equilibration of H+ and H2) were thought to be relegated largely to extremophilic prokaryotes and several mesophilic bacteria. Now, with broad scale genome sequencing, many prokaryotes are shown to contain hydrogenases, although the physiological importance of these enzymes is not always known (136).
The other major elements of the cell, C, O, N, S, and P, are clustered together with hydrogen in the spiral elemental diagram. These elements comprise approximately 97% of an Escherichia coli cell and are essential elements in all prokaryotes that have been analyzed. They are often bonded together in structural (lipids), catalytic (enzymes), and metabolic (intermediates in catabolism or biosynthesis) compounds. Thus, it is logical to have H, C, O, N, S, and P clustered in any elemental diagram where the focus is their biological relevance.
The spiral elemental diagram also clusters together the major elemental cations found in a microbial cell: Na+, K+, Mg2+, and Ca2+. While the abundance of these cations varies enormously inside prokaryotic cells, they are all important to cell function. Magnesium is typically the most abundant divalent cation inside prokaryotic cells (14). In addition to its previously mentioned role of coordinating to phosphoryl oxygen atoms, magnesium serves as a cofactor for numerous enzymes, in the maintenance of pH balance, and in iron transport and metabolism (22). The major magnesium transporter in Salmonella, CorA, has gene homologs that are widespread throughout the genomes of bacteria and archaea, suggesting that magnesium ion transport is important throughout prokaryotic systems (59). Moreover, Salmonella mutants defective in Mg2+-dependent regulation and transport are hypersensitive to Fe2+-mediated oxidative killing. Ca2+ is much less abundant in bacteria than Mg2+, being present in only low-micromolar concentrations. As a result, the present understanding of calcium transport and metabolism is less developed, but genomics has added new information. Recent observations of bound Ca2+ ions in the X-ray structure of E. coli transmembrane transporter MthK initially led to the suggestion that this was a Ca2+-gated K+ channel (56), but since affinities of MthK for Ca2+ are in the millimolar range this conclusion is controversial (14). Historically, calcium has been considered a minor cation, largely involved in coordinating to some extracellular enzymes (66) and in specialized functions like sporulation (19). This perception has been changing. Analysis of sequences from a range of prokaryotes has revealed many putative EF-hand calcium-binding proteins that resemble an important class of calcium-binding proteins common in eukaryotes (83). In at least one example, a putative prokaryotic EF protein was demonstrated to coordinate calcium and have an important physiological role. Rhizobium etli produces the calcium protein calsymin. It contains six EF-hand motifs and is proposed to be important in the formation of nodules on plants.
Among monovalent cations, K+ is the most prevalent species, being present at 300 mM in E. coli (14). The structure of a bacterial potassium transporter from Streptomyces lividans was resolved to a 3.2-Å resolution (32). This will allow a more accurate determination of the physiological function of prokaryotic genes identified as encoding transmembrane cation transporters. Sodium is often excluded from prokaryotic cells. However, sodium ions that do enter can participate with Na+/H+ antiporters to prevent overalkalinization of the cytoplasm under conditions of stress (14).
While major biological elements are generally the lighter elements, the lightest alkali metal and alkali earth elements, lithium and beryllium, are not generally used biologically by bacterial cells and, in fact, show some toxicity at moderate to high concentrations. Beryllium has been implicated in enzyme inactivation and malfunction (29, 74, 85, 99). Some of the toxic effects induced by beryllium may be due to spurious binding of Be2+ to sites normally occupied by Mg2+ and Fe3+ (75, 76). Lithium toxicity varies among microorganisms, and in this context media containing lithium have been used for the selective growth of bacteria such as Bifidobacterium spp. (68). In E. coli, lithium detoxification is at least partly mediated by Li+ efflux via an Na+/H+ antiporter (51). Despite its toxicity, Li+ can substitute for Na+ in the cotransport of amino acids and some sugars in some bacteria (23, 71, 122, 129, 131, 132, 133). Li+ can also replace Na+ in driving the flagellar motor of Vibrio alginolyticus (69).
Heavier metals in the alkali metal and alkali earth family are generally not prominent biologically, and their reduced importance is indicated by a smaller wedge for these elements on the spiral elemental diagram (Fig. 1B). A major impetus for the study of these elements has been the microbiological sequestration of radionuclides produced in nuclear reactors, such as cesium-137 (4, 117, 130). In general, cesium is the most toxic of the alkali metal ions to microorganisms. Cs+ influx usually occurs via monovalent cation transporters with various specificities, and the toxic effect of Cs+ may result from subsequent reduced influx or increased efflux of K+ or NH4+ (15, 57, 95, 116). Despite its toxicity, a low concentration of Cs+ stimulated growth of a bacterium in the absence of K+ (53), and Cs+ replaced K+ in the activation of some microbial enzymes (2, 50). Similarly, Sr2+ substituted for Ca2+ and Mg2+ without major cellular toxicity in processes such as spore formation (41), polysaccharide and flagellum biosynthesis (62, 104), stabilization of superficial layers (10), and enzyme activation (43, 111).
Rubidium and barium are not of concern as radioactive pollutants, but the ability of these elements to function as analogs to the lighter essential elements of their respective groups (or ions of similar valence) has been explored. No absolute requirement for rubidium in bacterial growth has been identified, but in the absence of K+, Rb+ restored normal or near-normal growth in several bacteria (17, 53, 77). Rb+ effectively substituted for K+ in the biosynthesis of a bacterial pigment (18) and for K+ or NH4+ in the activation of some bacterial and fungal enzymes (11, 135, 141). Barium is the least studied of the heavier alkali and alkali earth metals in terms of functionally replacing the lighter elements of these groups in biological systems. Compared to Sr2+, Ba2+ was a less effective substitute for Ca2+ or Mg2+ in the processes described above. However, barium sulfate does have a natural function in mechanoreceptor organelles found in members of the protozoan genus Loxodes (49), which is discussed later in this review.
Chloride, the major elemental anion in many microbes, is also the major halogen atom in biological systems. The element chlorine exists largely as chloride anion in soil and water, as well as in microbial cells. Chloride is required for the growth of at least some halophiles (105) and is thought to play an important role in osmoregulation and energy metabolism in other bacteria. As the result of human activities, chlorine oxyanions such as perchlorate, chlorate, and chlorite have increasingly appeared in the environment. Chlorine oxyanions are sometimes used as disinfectants to kill microorganisms, but some bacteria can use perchlorate as the final electron acceptor during anaerobic growth via a periplasmic perchlorate reductase and accessory enzymes (60). It has been proposed that perchlorate reductase is actually a nitrate reductase with broad specificity. However, some bacteria shown to reduce perchlorate do not use nitrate as a final electron acceptor (26).
In addition to chlorine, bacteria can incorporate other halogens (fluorine, bromine, and iodine) into metabolites, but these elements are not thought to be required for growth. These halogen atoms and chlorine are found in bactericidal halo-organic compounds and may be used by certain microorganisms in their native environments to kill competitors. For example, Hager (46) found a correlation between the antimicrobial activity of lipid extracts from 1,200 marine microorganisms and their halometabolite content. Chlorinated organics such as methyl chloride are mainly produced by soil bacteria; bromometabolites are predominantly formed by marine organisms (134). Chloro-, bromo-, and sometimes iodocompounds are formed by the action of haloperoxidases. Additionally, there are approximately one dozen fluorinated metabolites currently known to be produced by microorganisms. The mechanism by which biological fluoro-organic compounds are formed has only recently been revealed (93). The last element in the halogen series is astatine. Astatine has understandably received no biochemical attention; it is only found as a fleeting, unstable species during radioactive decay, and only 50 mg of it is estimated to exist on the Earth at any one instant (63).
The transition metals and zinc function largely as catalytic centers in enzyme catalysts. A typical bacterium will express several thousand proteins, of which approximately 30% are metalloproteins. Thus, while zinc and transition metals might comprise only 1 to 2% of the mass of a microbial cell, they are absolutely essential for cell functioning. The metallic elements are crucial catalytic centers for enzymes active in the cycling of the major elements H, O, C, N, and S (136). The major transition metals and zinc, in the general order of their prevalence in enzymes in E. coli, are Zn, Fe, Cu, Mo, Mn, Co, and Ni. The heaviest metal with well-documented functions in bacteria is tungsten (atomic number = 74; molecular weight = 183.9). Some anaerobic bacteria replace molybdenum with tungsten in some functions, probably because tungsten is much more bioavailable in certain anaerobic environments (64).
The heavy metals comprise the greatest number of elements. While they are generally not beneficial to bacterial cells, heavy metallic elements have been in the environment for billions of years, and so it is not surprising that microbes have evolved responses to their presence. A number of them are quite toxic to bacteria. Some of the most toxic elements modulate their effects by avidly binding to thiol groups inside a bacterial cell; for example, Hg2+ shows a Kd on the order of 10−52 for coordination with the thiolate anion (91). Silver, gold, and cadmium also bind avidly to thiols. Other metals show toxicity by mimicking essential elements and thus preventing normal functions mediated by those elements.
Because of this toxicity, microbes have evolved metabolic responses to the heavy, nonessential elements, but the responses differ with the element. Heavy metals often get into the cell via influx pumps for essential elements. The toxic metals then must be selectively pumped out. This mechanism comes into play with Cd, Ag, and Pb (102, 115). In some cases, heavy metals or metalloids are reduced in order to facilitate their efflux. For example, the metalloid oxyanion arsenate is reduced to arsenite to differentiate it from phosphate and allow its selective transport out of the cell (88, 114). Comparative genomic analysis has revealed the widespread occurrence of arsenate reductase in prokaryotes, suggesting that this is an ancient physiological function (88). Another widespread and relatively unique form of heavy metal detoxification is the well-studied mercuric ion operon, mer (126). This system is based on the enzymatic reduction of Hg2+ to elemental Hg. Elemental mercury has an unusually low boiling point for a metal, 357°C at atmospheric pressure, which allows it to be displaced from a microbial cell by simple volatilization.
Metal transformations are also important in microbial energy metabolism. The following metal ions or metal oxyanions are known to be or are potentially transformed when acting as a final electron acceptor during respiration by some microorganisms: Fe3+, Mn4+, vanadate, uranate, arsenate, rhodium sesquioxide, pertechnetate, chromate, molybdate, and tungstate. This has led to a conceptual appreciation that microbes “breathe” many elements and elemental oxyanions in addition to the more well-established anions such as nitrate and sulfate (72, 90). The ability to couple the concomitant oxidation and reduction of diverse organic or inorganic compounds underlies the prominent role of microbes in the geological cycles of Earth.
BIOALKYLATED ELEMENTS
Organometallic compounds are prominent intermediates in microbial metabolic pathways and include methyl-cobalamin (124) and methyl-Ni-F430 (36) for methyl group transfer and methanogenesis, respectively. Biologically common alkylated elements include nitrogen, sulfur, oxygen, and the halogens (Table 1).
TABLE 1.
Elements known to exist in alkylated form within biological systems or produced by biological activitya
| Element | Enzyme or system | Reference |
|---|---|---|
| Specific | ||
| H | Methyl-S-coenzyme M reductase | 54 |
| C | Acetyl coenzyme A synthase | 73 |
| N | Phospholipid N-methyltransferase | 139 |
| O | S-Adenosylmethionine methyltransferase | 30 |
| F | Streptomyces sp. | 93 |
| P | Methylcobalamin | 112 |
| S | S-Adenosylmethionine methyltransferase | 5 |
| Cl | Various fungi and algae | 48 |
| Co | Methyl-B12 | 98 |
| Ni | Methyl-S-coenzyme M reductase | 36 |
| Nonspecific | ||
| Cr | Cocorrinoids (in vitro only) | 98 |
| Cu | Cocorrinoids (in vitro only) | 98 |
| Ga | Cocorrinoids (in vitro only) | 98 |
| Ge | Methylgermanium in nature; source unknown | 128 |
| As | S-Adenosylmethionine methyltransferase | 21 |
| Se | S-Adenosylmethionine methyltransferase | 100 |
| Br | Marine phytoplankton | 109 |
| Rh | Propionibacterium shermanii | 65 |
| Pd | Methylcobalamin (in vitro only) | 102 |
| Cd | Polar marine bacteria | 96 |
| In | Cocorrinoids (in vitro only) | 98 |
| Sn | Saccharomyces cerevisiae | 3 |
| Sb | Scopulariopsis brevicaulis | 55 |
| Te | Pseudomonas fluorescens K27 | 7 |
| I | S-Adenosylmethionine methyltransferase | 1 |
| Pt | Methylcobalamin (in vitro only) | 37 |
| Au | Methylcobalamin (in vitro only) | 102 |
| Hg | Desulfovibrio desulfuricans LS | 25 |
| Tl | Anaerobic bacteria from freshwater sediment | 110 |
| Pb | Bacteria from lake sediment | 108 |
| Bi | Methanobacterium formicicum | 81 |
| Po | Marine sediment | 83 |
Elements are listed in order of increasing atomic number. Specific reactions are carried out by enzymes that selectively catalyze the alkylation; others may be fortuitous.
More recently, a large number of other elements have been shown to undergo bioalkylation; most or all of these are likely the result of nonspecific biochemical transformation (Table 1). The most dramatic example of a nonspecific alkylation is the formation of methylmercury and dimethylmercury by sulfate-reducing and other bacteria in anaerobic sediments, with potentially devastating effects on human health (126). The Minamata Bay disaster in Japan was perhaps most instrumental in demonstrating the neurotoxic effects of methylmercury species (47). Although nonspecifically produced, alkylmercury species are likely not of recent origin. Some bacterial mercury resistance operons contain an organomercurial lyase gene that encodes an enzyme cleaving the carbon-mercury bond of methylmercury species to produce methane and mercury(II); the latter can be then be detoxified by mercuric reductase. Microbially generated methyl selenium and methyl tellurium species are also toxic to humans but can be microbially decomposed (42). Recent reports (8, 38, 82) have documented a large number of methylated elements, including antimony, thallium, and bismuth, emanating from anaerobic environments such as landfills (Table 1). Biomethylation of less-well-studied elements has been recently reviewed (128).
“BIOLOGICALLY INDIFFERENT” ELEMENTS FOUND TO BE BIOLOGICALLY RELEVANT
In a recent review, Beveridge et al. (9) branded some members of the periodic table as “indifferent elements,” meaning that biological systems may contain them at some low level but could very well do without them. For example, humans contain higher levels of strontium and ruthenium (two elements not known to have a physiological function) than cobalt, a known required element (35). But elements indifferent or toxic to some life forms may be critical components of others. Elements to be considered here in this context are boron, cadmium, strontium, barium, and bismuth. Molecular mechanisms by which those elements interact with biological systems are being revealed but are not broadly appreciated in the general biochemical literature.
For example, utilization of the element boron is poorly understood despite the knowledge that boron is required for proper biological function in microbes and plants. In 1934, boron was reported to be required for healthy plant growth (127). Lack of boron produces brittleness, and plants grown with excess boron have highly flexible tissues (12). These effects have only recently been explained; plant cell walls contain borate esters that cross-link pectin polysaccharides (94). With bacteria, boron salts have often been included in growth media (120). Certain Streptomyces (33) and Sorangium (52) species produce the boron-containing antibiotics boromycins and tartrolons, respectively. Cyanobacteria require boron to develop functional nitrogen-fixing heterocysts (13, 79). A compound mediating quorum sensing by bacteria has been isolated and shown to contain a furanosyl borate diester (24).
As discussed above, the heavier alkali earth metals strontium and barium are thought to be acquired by uptake systems for calcium and magnesium and to be largely indifferent in many biological systems, except for their toxicity. It is perhaps surprising that some organisms accumulate significant quantities of these elements for specific biological purposes. Protozoans of the subclass Acantharia, a sister subclass to the more well-known Radiolaria, have skeletons composed largely of strontium sulfate (103). Moreover, protozoan ciliates of the genus Loxodes have mechanoreceptor organelles composed mainly of barium sulfate (49). This suggests that these microorganisms have evolved specific mechanisms to accumulate and utilize these heavy elements.
In this context, cadmium is regarded as a toxic element, with microbial responses typically thought to be limited to detoxification via selective binding or efflux. However, it was recently discovered that growth of a marine alga is stimulated by cadmium addition to zinc-limited media (67). Under these conditions, the organism produces a cadmium-dependent carbonic anhydrase. It was proposed that the use of cadmium in this way is physiologically relevant in the native ocean environment, where competition is fierce for metals needed as enzyme cofactors (67).
Finally, bismuth is one of the heaviest naturally occurring elements and is found at a concentration of 0.1 to 0.2 mg/kg in the Earth's crust (39). While sharing chemical properties with arsenic and antimony, bismuth has more metallic character than the lighter group 15 elements (8, 39, 107). Bismuth compounds have been used medicinally for two centuries, and about half of the bismuth used commercially today is for pharmaceuticals (16). Bismuth is relatively nontoxic to humans, but it is toxic to many bacteria, including Helicobacter (Campylobacter) pylori, the causative agent of peptic ulcers and gastroduodenal infections (78, 123), and enterotoxigenic E. coli, which causes traveler's diarrhea (45, 119). Yet, little has been published on the molecular interactions of microorganisms with bismuth compared to other heavy metals such as mercury and cadmium.
Bismuth sulfite agar medium has continued to be used successfully for the selective enrichment of Salmonella species from foods (142). Woolfolk and Whiteley (140) reported the reduction of bismuth compounds to elemental bismuth with a crude extract from Micrococcus lactilyticus, but analytical data were not presented; it was stated that a “…dark brown suspended material, probably elemental bismuth, accumulated.” Moreover, at least two other groups have reported formation of “bismuth mirrors,” also suggesting microbially mediated reduction of bismuth ions to metallic bismuth (31, 89). Novick and Roth reported bismuth resistance and sensitivity conferred by penicillinase plasmids in Staphylococcus aureus and mapped the general location of putative bismuth resistance genes on the plasmid (92). More recent research has shown that bismuth ions can induce the cadmium and arsenical-antimonial resistance operons (143, 144), but no evidence has been presented that this induction translates into bismuth resistance. Most recently, the CadC repressor that controls cadmium detoxification in Staphylococcus has been shown to bind near-stoichiometric quantities of bismuth (20). The physiological significance of bismuth binding is unclear.
CONCLUSIONS AND FUTURE PROSPECTS
Prokaryotes have existed on Earth for at least 3.6 billion years and are still the most successful life form based on total biomass and metabolic flux. During that time, how many elements might microbes have “learned” to interact with? There are 92 naturally occurring elements (6); the transuranic elements are largely created via anthropogenic nuclear reaction processes, and most have fleeting lifetimes. Currently, interactions with 77 elements, of which 74 are naturally occurring, are depicted on the UM-BBD. Of those not yet depicted on the UM-BBD, some are clearly not biologically relevant (e.g., astatine, as discussed earlier). Some elements, such as the noble gases, may be excluded from inquiry by some because of their chemical inertness. However, xenon has found use in the laboratory for doping the surfaces of proteins in X-ray crystallographic analysis (138). Might nature have found some use for noble gases? Moreover, it seems likely that we will find new biological requirements for specific elements, based on the relatively recent findings described above with the elements boron, strontium, barium, cadmium, and bismuth. In the context of microbial genomics, we need to be mindful that novel genes in newly sequenced microbes may do more than encode another kinase or phosphatase, but rather may be involved in interactions with the environment that are new and wonderful to us, but long “known” to prokaryotes.
Acknowledgments
We thank Steven Toeniskoetter and Jennifer Dommer for helping to create the on-line Biochemical Periodic Table. We acknowledge Simon Silver and John Carlis for helpful discussion. The assistance of Trevor Wennblom in drawing the figures for the manuscript is appreciated.
This work was supported under grant number DE-FG02-01ER63268 from the Office of Science's Office of Biological and Environmental Research, U.S. Department of Energy.
REFERENCES
- 1.Amachi, S., Y. Kamagata, T. Kanagawa, and Y. Muramatsu. 2001. Bacteria mediate methylation of iodine in marine and terrestrial environments. Appl. Environ. Microbiol. 67:2718-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, C. E., and S. L. Mowbray. 2002. Activation of ribokinase by monovalent cations. J. Mol. Biol. 315:409-419. [DOI] [PubMed] [Google Scholar]
- 3.Ashby, J., and P. J. Craig. 1987. Biomethylation of tin(II) complexes in the presence of pure strains of Saccharomyces cerevisiae. Appl. Organomet. Chem. 1:275-279. [Google Scholar]
- 4.Avery, S. V. 1995. Caesium accumulation by microorganisms: uptake mechanisms, cation competition, compartmentalization and toxicity. J. Ind. Microbiol. 14:76-84. [DOI] [PubMed] [Google Scholar]
- 5.Balish, E., and S. K. Shapiro. 1967. Methionine biosynthesis in Escherichia coli: induction and repression of methylmethionine (or adenosylmethionine):homocysteine methyltransferase. Arch. Biochem. Biophys. 119:62-68. [DOI] [PubMed] [Google Scholar]
- 6.Ball, P. 2002. The ingredients: a guided tour of the elements. Oxford University Press, Oxford, United Kingdom.
- 7.Basnayake, R. S. T., J. H. Bius, O. M. Akpolat, and T. G. Chasteen. 2001. Production of dimethyl telluride and elemental tellurium by bacteria amended with tellurite or tellurate. Appl. Organomet. Chem. 15:499-510. [Google Scholar]
- 8.Bentley, R., and T. G. Chasteen. 2002. Microbial methylation of metalloids: arsenic, antimony, and bismuth. Microbiol. Mol. Biol. Rev. 66:250-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beveridge, T. J., M. N. Hughes, H. Lee, K. T. Leung, R. K. Poole, I. Savvaides, S. Silver, and J. T. Trevors. 1997. Metal-microbe interactions: contemporary approaches. Adv. Microbiol. Physiol. 38:177-243. [DOI] [PubMed] [Google Scholar]
- 10.Beveridge, T. J., and R. G. E. Murray. 1976. Dependence of the superficial layers of Spirillum putridiconchylium on Ca2+ or Sr2+. Can. J. Microbiol. 22:1233-1244. [DOI] [PubMed] [Google Scholar]
- 11.Black, S. 1951. Yeast aldehyde dehydrogenase. Arch. Biochem. Biophys. 34:86-97. [DOI] [PubMed] [Google Scholar]
- 12.Blevins, D. G., and K. M. Lukaszewski. 1994. Proposed physiologic functions of boron in plants pertinent to animal and human metabolism. Environ. Health Perspect. 102:31-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonilla, I., M. Garcia-Gonzalez, and P. Mateo. 1990. Boron requirement in cyanobacteria. Plant Physiol. 94:1554-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Booth, I. R., M. D. Edwards, and S. Miller. 2003. Bacterial ion channels. Biochemistry 42:10045-10053. [DOI] [PubMed] [Google Scholar]
- 15.Bossenmeyer, D., A. Schlosser, and E. P. Bakker. 1989. Specific transport via the Escherichia coli Kup (TrkD) K+ uptake system. J. Bacteriol. 171:2219-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briand, G. G., and N. Burford. 1999. Bismuth compounds and preparations with biological or medicinal relevance. Chem. Rev. 99:2601-2658. [DOI] [PubMed] [Google Scholar]
- 17.Brown, G. R., and S. P. Cummings. 2001. Potassium uptake and retention by Oceanomonas baumannii at low water activity in the presence of phenol. FEMS Microbiol. Lett. 205:37-41. [DOI] [PubMed] [Google Scholar]
- 18.Bruce, D. L., and D. C. Duff. 1968. Requirement of potassium or rubidium for biosynthesis of pigment by Serratia marcescens. J. Bacteriol. 96:278-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke, W. F., Jr., and B. S. Slinker. 1982. Extracellular exonuclease as a stage 0 biochemical marker in Bacillus subtilis sporulation. J. Bacteriol. 149:768-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Busenlehner, L. S., J. L. Apuy, and D. P. Giedroc. 2002. Characterization of a metalloregulatory bismuth(III) site in Staphylococcus aureus pI258 CadC repressor. J. Biol. Inorg. Chem. 7:551-559. [DOI] [PubMed] [Google Scholar]
- 21.Challenger, F. 1978. Biosynthesis of organometallic and organometalloid compounds, p. 1-22. In F. E. Brinckman and J. M. Bellama (ed.), Organometals and organometalloids: occurrence and fate in the environment. American Chemical Society, Washington, D.C.
- 22.Chamnongpol, S., and E. A. Groisman. 2002. Mg2+ homeostasis and avoidance of metal toxicity. Mol. Microbiol. 44:561-571. [DOI] [PubMed] [Google Scholar]
- 23.Chen, C.-C., T. Tsuchiya, Y. Yamane, J. M. Wood, and T. H. Wilson. 1985. Na+ (Li+)-proline cotransport in Escherichia coli. J. Membr. Biol. 84:157-164. [DOI] [PubMed] [Google Scholar]
- 24.Chen, X., S. Schauder, N. Potier, A. Van Dorsselaer, I. Pelczer, B. Bassler, and F. Hughson. 2002. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415:545-549. [DOI] [PubMed] [Google Scholar]
- 25.Choi, S.-C., and R. Bartha. 1993. Cobalamin-mediated mercury methylation by Desulfovibrio desulfuricans LS. Appl. Environ. Microbiol. 59:290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coates, J. D., U. Michaelidou, R. A. Bruce, S. M. O'Connor, J. N. Crespi, and L. A. Achenbach. 1999. Ubiquity and diversity of dissimilatory (per)chlorate-reducing bacteria. Appl. Environ. Microbiol. 65:5234-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai, Y., P. C. Wensink, and R. H. Abeles. 1999. One protein, two enzymes. J. Biol. Chem. 274:1193-1195. [DOI] [PubMed] [Google Scholar]
- 28.Da Silva, J. J. R., and R. J. P. Williams. 2001. The biological chemistry of the elements: the inorganic chemistry of life, 2nd ed. Oxford University Press, Oxford, United Kingdom.
- 29.Daugherty, J. P., W. F. Kraemer, and J. G. Joshi. 1975. Purification and properties of phosphoglucomutase from Fleischmann's yeast. Eur. J. Biochem. 57:115-126. [DOI] [PubMed] [Google Scholar]
- 30.Dhar, K., and J. P. Rosazza. 2000. Purification and characterization of Streptomyces griseus catechol O-methyltransferase. Appl. Environ. Microbiol. 66:4877-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domenico, P., J. Reich, W. Madonia, and B. A. Cunha. 1996. Resistance to bismuth among Gram-negative bacteria is dependent upon iron and its uptake. J. Antimicrob. Chemother. 38:1031-1040. [DOI] [PubMed] [Google Scholar]
- 32.Doyle, D. A., J. M. Cabral, R. A. Pfuetzner, A. L. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280:69-77. [DOI] [PubMed] [Google Scholar]
- 33.Dunitz, J., D. Hawley, D. Miklos, D. White, Y. Berlin, R. Marusic, and V. Prelog. 1971. Structure of boromycin. Helv. Chim. Acta 54:1709-1713. [DOI] [PubMed] [Google Scholar]
- 34.Ellis, L. B. M., B. K. Hou, W. Kang, and L. P. Wackett. 2003. The University of Minnesota Biocatalysis/Biodegradation Database: post-genomic data mining. Nucleic Acids Res. 31:262-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emsley, J. 1989. The elements. Oxford University Press, Oxford, United Kingdom.
- 36.Ermler U., W. Grabarse, S. Shima, M. Goubeaud, and R. K. Thauer. 1997. Crystal structure of methyl-coenzyme M reductase: the key enzyme of biological methane formation. Science 278:1457-1462. [DOI] [PubMed] [Google Scholar]
- 37.Fanchiang, Y.-T., W. P. Ridley, and J. M. Wood. 1979. Methylation of platinum complexes by methylcobalamin. J. Am. Chem. Soc. 101:1443-1447. [Google Scholar]
- 38.Feldmann, J., and A. V. Hirner. 1995. Occurrence of volatile metal and metalloid species in landfill and sewage gases. Int. J. Environ. Anal. Chem. 60:339-359. [Google Scholar]
- 39.Feldmann, J., E. M. Krupp, D. Glindemann, A. V. Hirner, and W. R. Cullen. 1999. Methylated bismuth in the environment. Appl. Organomet. Chem. 13:739-748. [Google Scholar]
- 40.Fetzner, S. 2000. Enzymes involved in the aerobic bacterial degradation of N-heteroaromatic compounds: molybdenum hydroxylases and ring-opening 2,4-dioxygenases. Naturwissenschaften 87:59-69. [DOI] [PubMed] [Google Scholar]
- 41.Foerster, H. F., and J. W. Foster. 1966. Endotrophic calcium, strontium, and barium spores of Bacillus megaterium and Bacillus cereus. J. Bacteriol. 91:1333-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gadd, G. M. 1993. Microbial formation and transformation of organometallic and organometalloid compounds. FEMS Microbiol. Rev. 11:297-316. [Google Scholar]
- 43.Goodwin, M. G., A. Avezoux, S. L. Dales, and C. Anthony. 1996. Reconstitution of the quinoprotein methanol dehydrogenase from inactive Ca2+-free enzyme with Ca2+, Sr2+, or Ba2+. Biochem. J. 319:839-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goto, S., Y. Okuno, M. Hattori, T. Nishioka, and M. Kanehisa. 2002. LIGAND: database of chemical compounds and reactions in biological pathways. Nucleic Acids Res. 30:402-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graham, D. Y., M. K. Estes, and L. O. Gentry. 1983. Double-blind comparison of bismuth subsalicylate and placebo in the prevention of enterotoxigenic Escherichia coli-induced diarrhea in volunteers. Gastroenterology 85:1017-1022. [PubMed] [Google Scholar]
- 46.Hager, L. P. 1982. Mother nature likes some halogenated compounds. Basic Life Sci. 19:415-429. [DOI] [PubMed] [Google Scholar]
- 47.Harada, M. 1995. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 25:1-24. [DOI] [PubMed] [Google Scholar]
- 48.Harper, D. B. 1993. Biogenesis and metabolic role of halomethanes in fungi and plants. Metal Ions Biol. Syst. 29:345-388.
- 49.Hubert , G., N. Reider, G. Schmitt, and W. Send. 1975. Accumulation of barium in Mueller’s bodies of the Loxodidae (Ciliata, Holotricha). Z. Naturforsch. Sect. C. Biosci. 30:422-423
- 50.Hunaiti, A. R., and P. E. Kolattukudy. 1982. Isolation and characterization of an acyl-coenzyme A carboxylase from an erythromycin-producing Streptomyces erythraeus. Arch. Biochem. Biophys. 216:362-371. [DOI] [PubMed] [Google Scholar]
- 51.Inaba, K., T. Kuroda, T. Shimamoto, T. Kayahara, M. Tsuda, and T. Tsuchiya. 1994. Lithium toxicity and Na+(Li+)/H+ antiporter in Escherichia coli. Biol. Pharm. Bull. 17:395-398. [DOI] [PubMed] [Google Scholar]
- 52.Irschik, H., D. Schummer, K. Gerth, G. Hofle, and H. Reichenbach. 1995. The tartrolons, new boron-containing antibiotics from a myxobacterium, Sorangium cellulosum. J. Antibiotics 48:26-30. [DOI] [PubMed] [Google Scholar]
- 53.Jasper, P. 1978. Potassium transport system of Rhodopseudomonas capsulata. J. Bacteriol. 133:1314-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaun, B. 1993. Methane formation by methanogenic bacteria: redox chemistry of coenzyme F430. Metal Ions Biol. Syst. 29::287-337
- 55.Jenkins, R. O., P. J. Craig, W. Goessler, D. Miller, N. Ostah, and K. J. Irgolic. 1998. Biomethylation of inorganic antimony compounds by an aerobic fungus: Scopulariopsis brevicaulis. Environ. Sci. Technol. 32:882-885. [Google Scholar]
- 56.Jiang, Y. X., A. Lee, J. Y. Chen, M. Cadene, B. T. Chait, and R. MacKinnon. 2002. Crystal structure and mechanism of a calcium-gated potassium channel. Nature 417:515-522. [DOI] [PubMed] [Google Scholar]
- 57.Jung, K., M. Krabusch, and K. Altendorf. 2001. Cs+ induces the kdp operon of Escherichia coli by lowering the intracellular K+ concentration. J. Bacteriol. 183:3800-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanehisa, M. 2001. Post-genome informatics. Oxford University Press, Oxford, United Kingdom.
- 59.Kehres, D. G., C. H. Lawyer, and M. E. Maguire. 1998. The CorA magnesium transporter gene family. Microb. Comp. Genomics 3:151-169. [DOI] [PubMed] [Google Scholar]
- 60.Kengen, S. W., G. B. Rikken, W. R. Hagen, C. G. van Ginkel, and A. J. Stams. 1999. Purification and characterization of (per)chlorate reductase from the chlorate-respiring strain GR-1. J. Bacteriol. 181:6706-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kertesz, M. A. 2000. Riding the sulfur cycle: metabolism of sulfonates and sulfate esters in gram-negative bacteria. FEMS Microbiol. Rev. 24:135-175. [DOI] [PubMed] [Google Scholar]
- 62.Kim, S. H., S. Ramaswamy, and J. Downard. 1999. Regulated exopolysaccharide production in Myxococcus xanthus. J. Bacteriol. 181:1496-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirk, K. L. 1991. Biochemistry of the elemental halogens and inorganic halides. Plenum Press, New York, N.Y.
- 64.Kletzin, A., and M. W. Adams. 1996. Tungsten in biological systems. FEMS Microbiol. Rev. 18:5-63. [DOI] [PubMed] [Google Scholar]
- 65.Koppenhagen, V. B., B. Elsenhans, and F. Wagner. 1974. Methylrhodibalamin and 5′-deoxyadenosylrhodibalamin, the rhodium analogues of methylcobalamin and cobalamin coenzyme. J. Biol. Chem. 249:6532-6540. [PubMed] [Google Scholar]
- 66.Kuhn, S., and P. Fortnagel. 1993. Molecular cloning and nucleotide sequence of the gene encoding a calcium-dependent exoproteinase from Bacillus megaterium ATCC 14581. J. Gen. Microbiol. 139:39-47. [DOI] [PubMed] [Google Scholar]
- 67.Lane, T. W., and F. M. Morel. 2000. A biological function for cadmium in marine diatoms. Proc. Natl. Acad. Sci. USA 97:4627-4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lapierre, L., P. Undeland, and L. J. Cox. 1992. Lithium chloride-sodium propionate agar for the enumeration of bifidobacteria in fermented dairy products. J. Dairy Sci. 75:1192-1196. [DOI] [PubMed] [Google Scholar]
- 69.Liu, J. Z., M. Dapice, and S. Khan. 1990. Ion selectivity of the Vibrio alginolyticus flagellar motor. J. Bacteriol. 172:5236-5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lloyd, J. R., V. A. Sole, C. V. Van Praagh, and D. R. Lovley. 2000. Direct and Fe(II)-mediated reduction of technetium by Fe(III)-reducing bacteria. Appl. Environ. Microbiol. 66:3743-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lopilato, J., T. Tsuchiya, and T. H. Wilson. 1978. Role of Na+ and Li+ in thiomethylgalactoside transport by the melibiose transport system of Escherichia coli. J. Bacteriol. 134:147-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lovely, D. R. 2000. Environmental microbe-metal interactions. ASM Press, Washington, D.C.
- 73.Lu, W. P., S. R. Harder, and S. W. Ragsdale. 1990. Controlled potential enzymology of methyl transfer reactions involved in acetyl-CoA synthesis by CO dehydrogenase and the corrinoid/iron-sulfur protein from Clostridium thermoaceticum. J. Biol. Chem. 265:3124-3133. [PubMed] [Google Scholar]
- 74.Luke, M. Z., L. Hamilton, and T. C. Hollocher. 1975. Beryllium-induced misincorporation by a DNA polymerase: a possible factor in beryllium toxicity. Biochem. Biophys. Res. Commun. 62:497-501. [DOI] [PubMed] [Google Scholar]
- 75.MacCordick, J., B. Wurz, and J. M. Hornsberger. 1975. Effect of a beryllium complex on growth of Pseudomonas fluroescens (types R and S). II. Competition with magnesium. C. R. Seances Soc. Biol. Fil. 169:421-425. [PubMed] [Google Scholar]
- 76.MacCordick, J., M. T. Youinou, and B. Wurtz. 1977. Effect of iron-beryllium antagonism on the growth of Pseudomonas fluorescens type S. Folia Microbiol. (Praha) 22:35-39. [DOI] [PubMed] [Google Scholar]
- 77.Macleod, R. A., and E. E. Snell. 1948. The effect of related ions on the potassium requirements of lactic acid bacteria. J. Biol. Chem. 176:39-52. [PubMed] [Google Scholar]
- 78.Marshall, B. J., J. A. Armstrong, G. J. Francis, N. T. Nokes, and S. H. Wee. 1987. Antibacterial action of bismuth in relation to Campylobacter pyloridis colonization and gastritis. Digestion 37(Suppl. 2):16-30. [DOI] [PubMed] [Google Scholar]
- 79.Mateo, P., I. Bonilla, E. Fernandez-Valiente, and E. Sanchez-Maeso. 1986. Essentiality of boron for dinitrogen fixation in Anabaena sp. PCC 7119. Plant Physiol. 81:430-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazurs, E. G. 1957. Types of graphic representations of the periodic system of chemical elements. LaGrange, Ill.
- 81.Michalke, K., J. Meyer, A. V. Hirner, and R. Hensel. 2002. Biomethylation of bismuth by the methanogen Methanobacterium formicicum. Appl. Organomet. Chem. 16:221-227. [Google Scholar]
- 82.Michalke, K., E. B. Wickenheiser, M. Mehring, A. V. Hirner, and R. Hensel. 2000. Production of volatile derivatives of metal(loid)s by microflora involved in anaerobic digestion of sewage sludge. Appl. Environ. Microbiol. 66:2791-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Michiels, J., C. Xi, J. Verhaert, and J. Vanderleyden. 2002. The functions of Ca2+ in bacteria: a role for EF-hand proteins? Trends Microbiol. 10:87-93. [DOI] [PubMed] [Google Scholar]
- 84.Momoshima, N., L. Song, S. Osaki, and Y. Maeda. 2001. Formation and emission of volatile polonium compound by microbial activity and polonium methylation with methylcobalamin. Environ. Sci. Technol. 35:2956-2960. [DOI] [PubMed] [Google Scholar]
- 85.Moreau, P. L., and M. F. Carlier. 1989. RecA protein-promoted cleavage of LexA repressor in the presence of ADP and structural analogues of inorganic phosphate, the fluoride complexes of aluminum and beryllium. J. Biol. Chem. 264:2302-2306. [PubMed] [Google Scholar]
- 86.Morel, F. M. M., and N. M. Price. 2003. The biogeochemical cycles of trace metals in the oceans. Science 300:944-947. [DOI] [PubMed] [Google Scholar]
- 87.Morrison, J. F., and W. W. Cleland. 1980. A kinetic method for determining dissociation constants for metal complexes of adenosine 5′-triphosphate and adenosine 5′-diphosphate. Biochemistry 19:3127-3131. [DOI] [PubMed] [Google Scholar]
- 88.Mukhopadhyay, R., B. P. Rosen, L. T. Phung, and S. Silver. 2002. Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol. Rev. 26:311-325. [DOI] [PubMed] [Google Scholar]
- 89.Nadeau, O. W., D. W. Gump, G. M. Hendricks, and D. H. Meyer. 1992. Deposition of bismuth by Yersinia enterocolitica. Med. Microbiol. Immunol. (Berlin) 181:145-152. [DOI] [PubMed] [Google Scholar]
- 90.Nealson, K. H., A. Belz, and B. McKee. 2002. Breathing metals as a way of life: geobiology in action. Antonie Leeuwenhoek 81:215-222. [DOI] [PubMed] [Google Scholar]
- 91.Nies, D. H. 1999. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 51:730-750. [DOI] [PubMed] [Google Scholar]
- 92.Novick, R. P., and C. Roth. 1968. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J. Bacteriol. 95:1335-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O'Hagan, D., C. Schaffrath, S. L. Cobb, J. T. Hamilton, and C. D. Murphy. 2002. Biochemistry: biosynthesis of an organofluorine molecule. Nature 416:279. [DOI] [PubMed] [Google Scholar]
- 94.O'Neill, M., S. Eberhard, P. Albersheim, and A. Darvill. 2001. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294:846-849. [DOI] [PubMed] [Google Scholar]
- 95.Perkins, J., and G. M. Gadd. 1995. The influence of pH and external K+ concentration on caesium toxicity and accumulation in Escherichia coli and Bacillus subtilis. J. Ind. Microbiol. 14:218-225. [DOI] [PubMed] [Google Scholar]
- 96.Pongratz, R., and K. G. Heumann. 1999. Production of methylated mercury, lead, and cadmium by marine bacteria as a significant natural source for atmospheric heavy metals in polar regions. Chemosphere 39:89-102. [Google Scholar]
- 97.Posey, J. E., and F. C. Gherardini. 2000. Lack of a role for iron in the Lyme disease pathogen. Science 288:1651-1653. [DOI] [PubMed] [Google Scholar]
- 98.Pratt, J. M. 1993. Making and breaking the co-alkyl bond in B12 derivatives. Metal Ions Biol. Syst. 29:229-286.
- 99.Price, D. J., and J. G. Joshi. 1984. Ferritin: protection of enzymatic activity against the inhibition by divalent metal ions in vitro. Toxicology 31:151-163. [DOI] [PubMed] [Google Scholar]
- 100.Ranjard, L., C. Prigent-Combaret, S. Nazaret, and B. Cournoyer. 2002. Methylation of inorganic and organic selenium by the bacterial thiopurine methyltransferase. J. Bacteriol. 184:3146-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ravasz, E., A. L. Somera, D. A. Mongru, Z. N. Oltvai, and A. L. Barabasi. 2002. Hierarchical organization of modularity in metabolic networks. Science 297:1551-1555. [DOI] [PubMed] [Google Scholar]
- 102.Ridley, W. P., L. J. Dizikes, and J. M. Wood. 1977. Biomethylation of toxic elements in the environment. Science 197:329-332. [DOI] [PubMed] [Google Scholar]
- 103.Rieder, N., H. A. Ott, P. Pfundstein, and R. Schoch. 1982. X-ray microanalysis of the mineral contents of some protozoa. J. Protozool. 29:15-18. [Google Scholar]
- 104.Robinson, J. B., O. H. Tuovinen, and W. D. Bauer. 1992. Role of divalent cations in the subunit associations of complex flagella from Rhizobium meliloti. J. Bacteriol. 174:3896-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roessler, M., and V. Muller. 2002. Chloride, a new environmental signal molecule involved in gene regulation in a moderately halophilic bacterium, Halobacillus halophilus. J. Bacteriol. 184:6207-6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rouf, M. A. 1964. Spectrochemical analysis of inorganic elements in bacteria. J. Bacteriol. 88:1545-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sadler, P. J., H. Li, and H. Sun. 1999. Coordination chemistry of metals in medicine: target sites for bismuth. Coord. Chem. Rev. 186:689-709. [Google Scholar]
- 108.Scaife, M., and J. S. Bruner. 1975. Methylation of lead in the environment. Nature 253:263-264. [DOI] [PubMed] [Google Scholar]
- 109.Scarratt, M. G., and R. M. Moore. 1998. Production of methyl bromide and methyl chloride in laboratory cultures of marine phytoplankton II. Marine Chem. 59:311-320. [Google Scholar]
- 110.Schedlbauer, O. F., and K. G. Heumann. 2000. Biomethylation of thallium by bacteria and first determination of biogenic dimethylthallium in the ocean. Appl. Organomet. Chem. 14:330-340. [Google Scholar]
- 111.Secades, P., B. Alvarez, and J. A. Guijarro. 2001. Purification and characterization of a psychrophilic, calcium-induced, growth-phase-dependent metalloprotease from the fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 67:2436-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Seto, H. 1999. Biosynthesis of the natural C-P compounds bialaphos and fosfomycin, p. 865-908. In D. Barton and K. Makanishi (ed.), Comprehensive natural products chemistry, vol. 1. Elsevier, New York, N.Y.
- 113.Silver, S. 1998. Genes for all metals: a bacterial view of the periodic table. J. Ind. Microbiol. Biotechnol. 20:1-12. [DOI] [PubMed] [Google Scholar]
- 114.Silver, S., and D. Keach. 1982. Energy-dependent arsenate efflux: the mechanism of plasmid-mediated resistance. Proc. Natl. Acad. Sci. USA 79:6114-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Silver, S., and M. Walderhaug. 1992. Gene regulation of plasmid- and chromosome-determined inorganic ion transport in bacteria. Microbiol. Rev. 56:195-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singh, S., S. Negri, N. Bharati, and H. N. Singh. 1994. Common nitrogen control of caesium uptake, caesium toxicity and ammonium (methylammonium) uptake in the cyanobacterium Nostoc muscorum. FEMS Microbiol. Lett. 117:243-248. [DOI] [PubMed] [Google Scholar]
- 117.Smith, M. L., H. W. Taylor, and H. D. Sharma. 1993. Comparison of the post-Chernobyl 137Cs contamination of mushrooms from eastern Europe, Sweden, and North America. Appl. Environ. Microbiol. 59:134-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sokoloff, V. P. 1954. Diagrams relating the periodic table to geochemistry. J. Chem. Educ. 31:15-17. [Google Scholar]
- 119.Sox, T. E., and C. A. Olson. 1989. Binding and killing of bacteria by bismuth subsalicylate. Antimicrob. Agents Chemother. 33:2075-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stanier, R. Y., N. J. Palleroni, and M. Duodoroff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43:159-271. [DOI] [PubMed] [Google Scholar]
- 121.Steinberg, R. A. 1938. Correlations between biological essentiality and atomic structure of the chemical elements. J. Agric. Res. 57:851-858. [Google Scholar]
- 122.Stock, J., and S. Roseman. 1971. A sodium-dependent sugar co-transport system in bacteria. Biochem. Biophys. Res. Commun. 44:133-138. [DOI] [PubMed] [Google Scholar]
- 123.Stoltenberg, M., M. Martiny, K. Sorensen, J. Rungby, and K. A. Krogfelt. 2001. Histochemical tracing of bismuth in Helicobacter pylori after in vitro exposure to bismuth citrate. Scand. J. Gastroenterol. 36:144-148. [PubMed] [Google Scholar]
- 124.Stryer, L. 1988. Biochemistry, 3rd ed. W. H. Freeman and Company, New York, N.Y.
- 125.Sugio, T., H. Kuwano, A. Negishi, T. Maeda, F. Takeuchi, and K. Kamimura. 2001. Mechanism of growth inhibition by tungsten in Acidithiobacillus ferrooxidans. Biosci. Biotechnol. Biochem. 65:555-562. [DOI] [PubMed] [Google Scholar]
- 126.Summers, A. O. 1988. Biotransformations of mercury compounds. Basic Life Sci. 45:105-109. [DOI] [PubMed] [Google Scholar]
- 127.Thatcher, R. 1934. A proposed classification of the chemical elements with respect to their function in plant nutrition. Science 79:463-466. [DOI] [PubMed] [Google Scholar]
- 128.Thayer, J. S. 2002. Biological methylation of less-studied elements. Appl. Organomet. Chem. 16:677-691. [Google Scholar]
- 129.Tokuda, H., and H. R. Kaback. 1977. Sodium-dependent methyl 1-thio-β-D-galactopyranoside transport in membrane vesicles isolated from Salmonella typhimurium. Biochemistry 16:2130-2136. [DOI] [PubMed] [Google Scholar]
- 130.Tomioka, N., H. Uchiyama, and O. Yagi. 1992. Isolation and characterization of cesium-accumulating bacteria. Appl. Environ. Microbiol. 58:1019-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tsuchiya, T., M. Oho, and S. Shiota-Nijya. 1983. Lithium ion-sugar cotransport via the melibiose transport system in Escherichia coli. J. Biol. Chem. 258:12765-12767. [PubMed] [Google Scholar]
- 132.Tsuchiya, T., Y. Yamane, S. Shiota, and T. Kawasaki. 1984. Cotransport of proline and Li+ in Escherichia coli. FEBS Lett. 168:327-330. [DOI] [PubMed] [Google Scholar]
- 133.Uratani, Y., T. Tsuchiya, Y. Akamatsu, and T. Hoshino. 1989. Na+ (Li+)/branched-chain amino acid cotransport in Pseudomonas aeruginosa. J. Membr. Biol. 107:57-62. [DOI] [PubMed] [Google Scholar]
- 134.van Pee, K. H. 2001. Microbial biosynthesis of halometabolites. Arch. Microbiol. 175:250-258. [DOI] [PubMed] [Google Scholar]
- 135.Veres, Z., I. Y. Kim, T. D. Scholz, and T. C. Stadtman. 1994. Selenophosphate synthetase. Enzyme properties and catalytic reaction. J. Biol. Chem. 269:10597-10603. [PubMed] [Google Scholar]
- 136.Vignais, P. M., B. Billoud, and J. Meyer. 2001. Classification and phylogeny of hydrogenases. FEMS Microbiol. Rev. 25:455-501. [DOI] [PubMed] [Google Scholar]
- 137.Wackett, L. P., W. H. Orme-Johnson, and C. T. Walsh. 1989. Transition metal enzymes in bacterial metabolism, p. 165-206. In T. Beveridge and R. J. Doyle (ed.), Metal ions and bacteria. Wiley-Interscience, New York, N.Y.
- 138.Whittington, D. A., A. C. Rosenzweig, C. A. Frederick, and S. J. Lippard. 2001. Xenon and halogenated alkanes track putative substrate binding cavities in the soluble methane monooxygenase hydroxylase. Biochemistry 40:3476-3482. [DOI] [PubMed] [Google Scholar]
- 139.Wilderman, P. J., A. I. Vasil, W. E. Martin, R. C. Murphy, and M. L. Vasil. 2002. Pseudomonas aeruginosa synthesizes phosphatidyl choline by use of the phosphatidylcholine synthase pathway. J. Bacteriol. 184:4792-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Woolfolk, C. A., and H. R. Whiteley. 1962. Reduction of inorganic compounds with molecular hydrogen by Micrococcus lactilyticus. J. Bacteriol. 84:647-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wootton, J. C. 1983. Re-assessment of ammonium-ion affinities of NADP-specific glutamate dehydrogenases. Activation of the Neurospora crassa enzyme by ammonium and rubidium ions. Biochem. J. 209:527-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Worcman-Barnink D., M. T. Destro, S. A. Fernandes, and M. Landgraf. 2001. Evaluation of motility enrichment on modified semi-solid Rappaport-Vassiladis medium (MSRV) for the detection of Salmonella in foods. Int. J. Food Microbiol. 64:387-393. [DOI] [PubMed] [Google Scholar]
- 143.Wu, J., and R. P. Rosen. 1993. Metalloregulated expression of the ars operon. J. Biol. Chem. 268:52-58. [PubMed] [Google Scholar]
- 144.Yoon, K. P., T. K. Misra, and S. Silver. 1991. Regulation of the cadA cadmium resistance determinant of Staphylococcus aureus plasmid pI258. J. Bacteriol. 173:7643-7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang, Y. M., T. Y. Wong, L. Y. Chen, C. S. Lin, and J. K. Liu. 2000. Induction of a futile Embden-Meyerhof-Parnas pathway in Deinococcus radiodurans by Mn: possible role of the pentose phosphate pathway in cell survival. Appl. Environ. Microbiol. 66:105-112. [DOI] [PMC free article] [PubMed] [Google Scholar]