Abstract
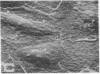
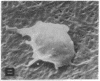
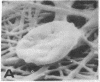
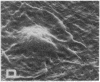
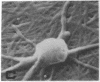
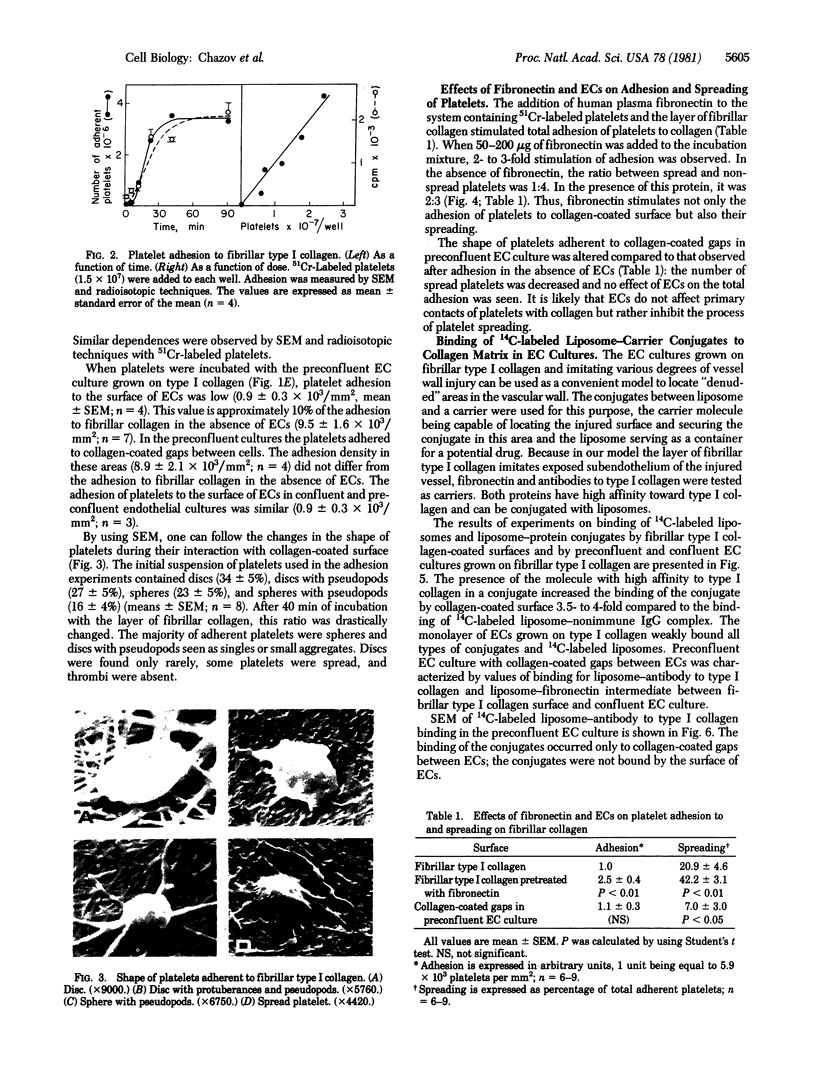
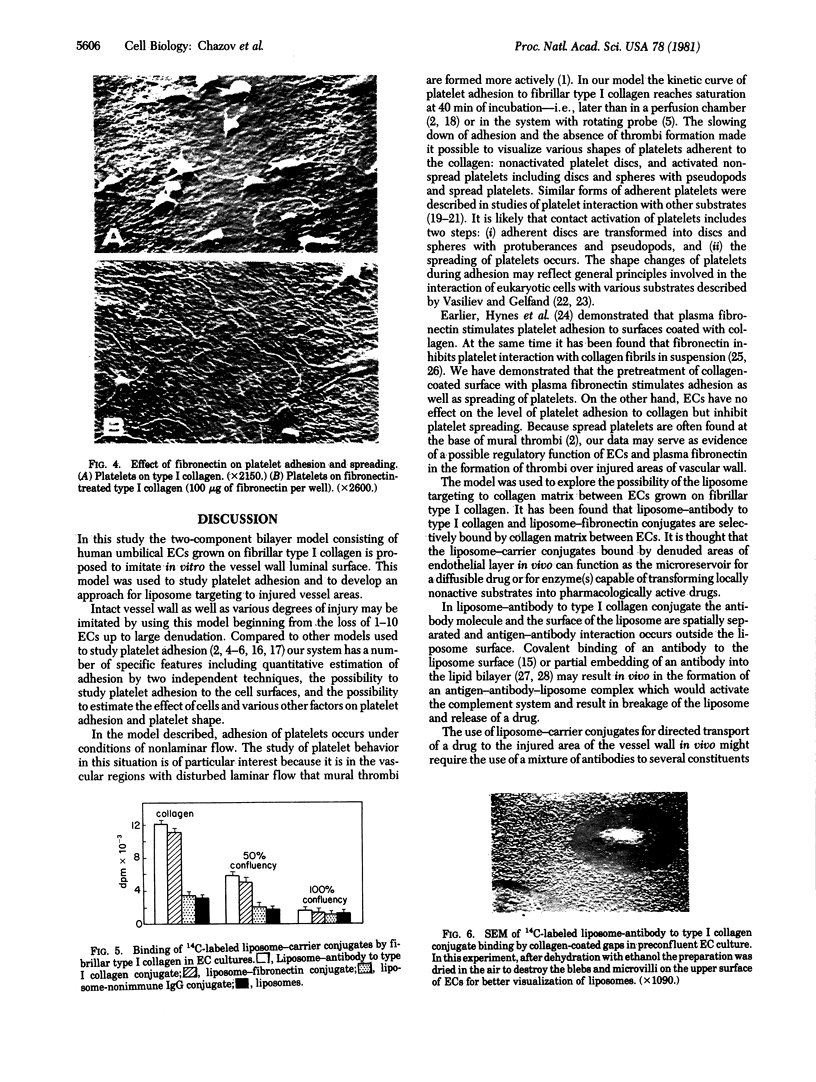
Human umbilical endothelial cells (ECs) were grown on fibrillar type I collagen in 16.4-mm multiwell tissue culture plates. Human platelets were added to the wells, and platelet adhesion to collagen was examined by scanning electron microscopy and radioisotopic technique in the absence of ECs and in preconfluent and confluent EC cultures. Single adherent platelets of different shapes as well as small aggregates were seen on collagen surface. Human plasma fibronectin added to the system stimulated platelet adhesion and their spreading on collagen. ECs had no effect on the percentage of platelets adherent to collagen-coated gaps in preconfluent culture but decreased the number of spread platelets. It is demonstrated that collagen-coated gaps can bind 14C-labeled liposome--antibody and 14-C-labeled liposome--fibronectin conjugates. ECs grown on fibrillar collagen are suggested as useful models for screening of antiplatelet drugs and for the study of drug targeting to the areas of vascular injury for prevention of thrombosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Zacharski L. R., Widirstky S. T., Rosenstein R., Zaitlin L. M., Burgess D. R. Transformation and motility of human platelets: details of the shape change and release reaction observed by optical and electron microscopy. J Cell Biol. 1979 Oct;83(1):126–142. doi: 10.1083/jcb.83.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensusan H. B., Koh T. L., Henry K. G., Murray B. A., Culp L. A. Evidence that fibronectin is the collagen receptor on platelet membranes. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5864–5868. doi: 10.1073/pnas.75.12.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave J. P., Blondowska D., Richardson M., Kinlough-Rathbone R. L., Packham M. A., Mustard J. F. Quantitative radioisotopic measurement and scanning electron microscopic study of platelet adherence to a collagen-coated surface and to subendothelium with a rotating probe device. J Lab Clin Med. 1979 Jan;93(1):60–70. [PubMed] [Google Scholar]
- Chandrakasan G., Torchia D. A., Piez K. A. Preparation of intact monomeric collagen from rat tail tendon and skin and the structure of the nonhelical ends in solution. J Biol Chem. 1976 Oct 10;251(19):6062–6067. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974 Mar;60(3):673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Ali I. U., Destree A. T., Mautner V., Perkins M. E., Senger D. R., Wagner D. D., Smith K. K. A large glycoprotein lost from the surfaces of transformed cells. Ann N Y Acad Sci. 1978 Jun 20;312:317–342. doi: 10.1111/j.1749-6632.1978.tb16811.x. [DOI] [PubMed] [Google Scholar]
- Jones P. A. Construction of an artificial blood vessel wall from cultured endothelial and smooth muscle cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1882–1886. doi: 10.1073/pnas.76.4.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legrand Y. J., Fauvel F., Kartalis G., Wautier J. L., Caen J. P. Specific and quantitative method for estimation of platelet adhesion to fibrillar collagen. J Lab Clin Med. 1979 Sep;94(3):438–446. [PubMed] [Google Scholar]
- Margolis L. B., Tikhonov A. N., Vasilieva E. Y. Platelet adhesion to fluid and solid phospholipid membranes. Cell. 1980 Jan;19(1):189–195. doi: 10.1016/0092-8674(80)90400-6. [DOI] [PubMed] [Google Scholar]
- Margolis L. B., Vasilieva E. J., Vasiliev J. M., Gelfand I. M. Upper surfaces of epithelial sheets and of fluid lipid films are nonadhesive for platelets. Proc Natl Acad Sci U S A. 1979 May;76(5):2303–2305. doi: 10.1073/pnas.76.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson M. W., Umfleet R. A. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Biol Chem. 1970 Nov 10;245(21):5728–5736. [PubMed] [Google Scholar]
- Muggli R., Baumgartner H. R., Tschopp T. B., Keller H. Automated microdensitometry and protein assays as a measure for platelet adhesion and aggregation on collagen-coated slides under controlled flow conditions. J Lab Clin Med. 1980 Feb;95(2):195–207. [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A., Kinlough-Rathbone R. L. Platelets and thrombosis in the development of atherosclerosis and its complications. Adv Exp Med Biol. 1978;102:7–30. doi: 10.1007/978-1-4757-1217-9_2. [DOI] [PubMed] [Google Scholar]
- Porzio M. A., Pearson A. M. Improved resolution of myofibrillar proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1977 Jan 25;490(1):27–34. doi: 10.1016/0005-2795(77)90102-7. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha D., Karush F. Attachment to membranes of exogenous immunoglobulin conjugated to a hydrophobic anchor. Biochem Biophys Res Commun. 1979 Sep 27;90(2):554–560. doi: 10.1016/0006-291x(79)91271-3. [DOI] [PubMed] [Google Scholar]
- Sochynsky R. A., Boughton B. J., Burns J., Sykes B. C., McGee J. O. The effect of human fibronectin on platelet-collagen adhesion. Thromb Res. 1980 May 1;18(3-4):521–533. doi: 10.1016/0049-3848(80)90348-5. [DOI] [PubMed] [Google Scholar]
- Tangen O., Berman H. J., Marfey P. Gel filtration. A new technique for separation of blood platelets from plasma. Thromb Diath Haemorrh. 1971 Jun 30;25(2):268–278. [PubMed] [Google Scholar]
- Torchilin V. P., Khaw B. A., Smirnov V. N., Haber E. Preservation of antimyosin antibody activity after covalent coupling to liposomes. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1114–1119. doi: 10.1016/0006-291x(79)92123-5. [DOI] [PubMed] [Google Scholar]
- Torchilin V. P., Omel'yanenko V. G., Klibanov A. L., Mikhailov A. I., Gol'danskii V. I., Smirnov V. N. Incorporation of hydrophilic protein modified with hydrophobic agent into liposome membrane. Biochim Biophys Acta. 1980 Nov 18;602(3):511–521. doi: 10.1016/0005-2736(80)90330-2. [DOI] [PubMed] [Google Scholar]
- Vasiliev J. M., Gelfand I. M. Mechanisms of morphogenesis in cell cultures. Int Rev Cytol. 1977;50:159–274. doi: 10.1016/s0074-7696(08)60099-6. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Turitto V. T., Baumgartner H. R. Effect of shear rate on platelet interaction with subendothelium in citrated and native blood. I. Shear rate--dependent decrease of adhesion in von Willebrand's disease and the Bernard-Soulier syndrome. J Lab Clin Med. 1978 Nov;92(5):750–764. [PubMed] [Google Scholar]