Abstract
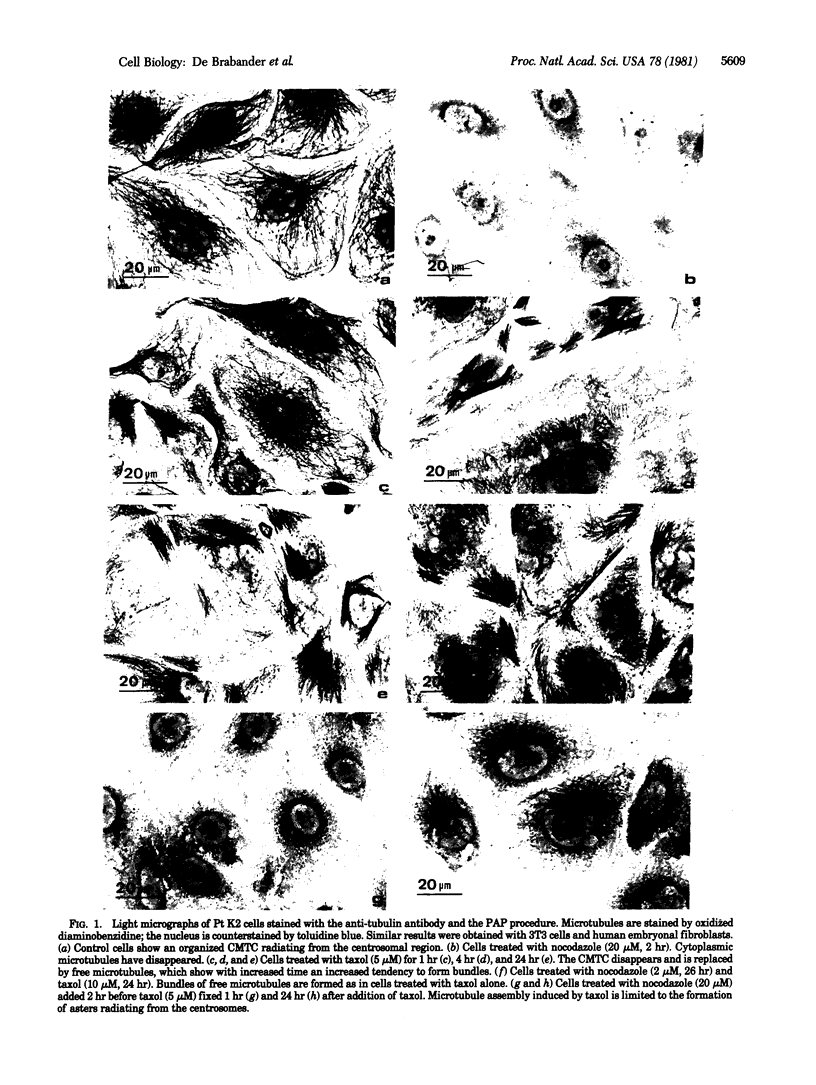
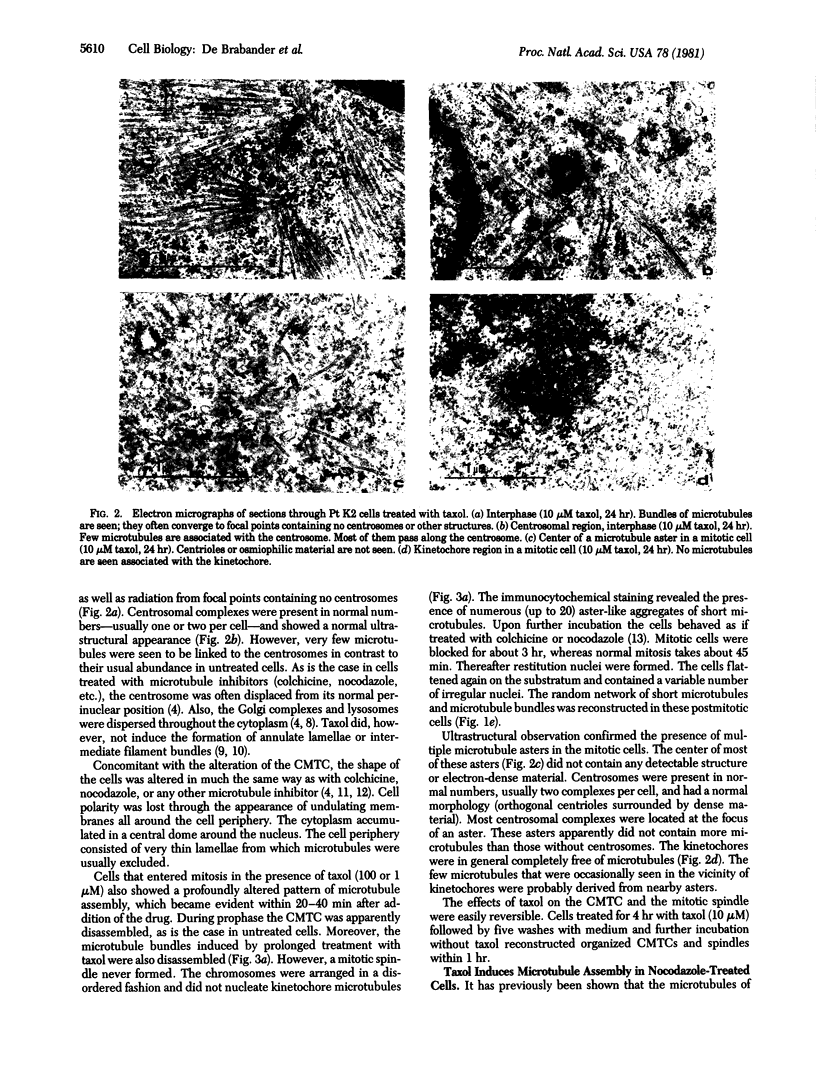
Taxol, a potent promoter of microtubule polymerization in vitro, induces massive assembly of free microtubules in cultured cells as visualized by immunocytochemistry and electron microscopy. The centrosomes and kinetochores largely lost their capacity to organize microtubule assembly, as became evident by the disappearance of the cytoplasmic microtubule complex and the mitotic spindle. The taxol-induced microtubules were partially resistant to nocodazole, an inhibitor of tubulin polymerization. Moreover, taxol induced microtubule assembly in cells pretreated with nocodazole. Increasing the ratio of nocodazole to taxol restored the ability of the centrosomes and kinetochores to specifically induce microtubule assembly in their immediate vicinity. The data suggest that taxol lowers the critical tubulin concentration in vivo as well as in vitro and that the organizing capacity of the microtubule-organizing centers depends on the cytoplasmic polymerization threshold.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergen L. G., Borisy G. G. Head-to-tail polymerization of microtubules in vitro. Electron microscope analysis of seeded assembly. J Cell Biol. 1980 Jan;84(1):141–150. doi: 10.1083/jcb.84.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brabander M. J., Van de Veire R. M., Aerts F. E., Borgers M., Janssen P. A. The effects of methyl (5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl) carbamate, (R 17934; NSC 238159), a new synthetic antitumoral drug interfering with microtubules, on mammalian cells cultured in vitro. Cancer Res. 1976 Mar;36(3):905–916. [PubMed] [Google Scholar]
- De Brabander M., Borgers M. The formation of annulated lamellae induced by the disintegration of microtubules. J Cell Sci. 1975 Nov;19(2):331–340. doi: 10.1242/jcs.19.2.331. [DOI] [PubMed] [Google Scholar]
- De Mey J., Hoebeke J., De Brabander M., Geuens G., Joniau M. Immunoperoxidase visualisation of microtubules and microtubular proteins. Nature. 1976 Nov 18;264(5583):273–275. doi: 10.1038/264273a0. [DOI] [PubMed] [Google Scholar]
- DeBrabander M., Aerts F., Van de Veire R., Borgers M. Evidence against interconversion of microtubules and filaments. Nature. 1975 Jan 10;253(5487):119–120. doi: 10.1038/253119a0. [DOI] [PubMed] [Google Scholar]
- Goldman R. D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971 Dec;51(3):752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R. R., Borisy G. G. Quantitative initiation of microtubule assembly by chromosomes from Chinese hamster ovary cells. Exp Cell Res. 1978 May;113(2):369–374. doi: 10.1016/0014-4827(78)90377-4. [DOI] [PubMed] [Google Scholar]
- Gould R. R., Borisy G. G. The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J Cell Biol. 1977 Jun;73(3):601–615. doi: 10.1083/jcb.73.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M. W. Implications of treadmilling for the stability and polarity of actin and tubulin polymers in vivo. J Cell Biol. 1980 Jul;86(1):330–334. doi: 10.1083/jcb.86.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalewski S., Thyberg J., Lohmander S., Friberg U. Influence of colchicine and vinblastine on the golgi complex and matrix deposition in chondrocyte aggregates. An ultrastructural study. Exp Cell Res. 1975 Oct 15;95(2):440–454. doi: 10.1016/0014-4827(75)90569-8. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Cytoplasmic microtubules in tissue culture cells appear to grow from an organizing structure towards the plasma membrane. Proc Natl Acad Sci U S A. 1976 Mar;73(3):867–871. doi: 10.1073/pnas.73.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Horwitz S. B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani M. C., Taylor H. L., Wall M. E., Coggon P., McPhail A. T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971 May 5;93(9):2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Dedman J. R., Brinkley B. R., Means A. R. Tubulin and calmodulin. Effects of microtubule and microfilament inhibitors on localization in the mitotic apparatus. J Cell Biol. 1979 Jun;81(3):624–634. doi: 10.1083/jcb.81.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]