Abstract
This work was aimed at producing a sourdough bread that is tolerated by celiac sprue (CS) patients. Selected sourdough lactobacilli had specialized peptidases capable of hydrolyzing Pro-rich peptides, including the 33-mer peptide, the most potent inducer of gut-derived human T-cell lines in CS patients. This epitope, the most important in CS, was hydrolyzed completely after treatment with cells and their cytoplasmic extracts (CE). A sourdough made from a mixture of wheat (30%) and nontoxic oat, millet, and buckwheat flours was started with lactobacilli. After 24 h of fermentation, wheat gliadins and low-molecular-mass, alcohol-soluble polypeptides were hydrolyzed almost totally. Proteins were extracted from sourdough and used to produce a peptic-tryptic digest for in vitro agglutination tests on K 562(S) subclone cells of human origin. The minimal agglutinating activity was ca. 250 times higher than that of doughs chemically acidified or started with baker's yeast. Two types of bread, containing ca. 2 g of gluten, were produced with baker's yeast or lactobacilli and CE and used for an in vivo double-blind acute challenge of CS patients. Thirteen of the 17 patients showed a marked alteration of intestinal permeability after ingestion of baker's yeast bread. When fed the sourdough bread, the same 13 patients had values for excreted rhamnose and lactulose that did not differ significantly from the baseline values. The other 4 of the 17 CS patients did not respond to gluten after ingesting the baker's yeast or sourdough bread. These results showed that a bread biotechnology that uses selected lactobacilli, nontoxic flours, and a long fermentation time is a novel tool for decreasing the level of gluten intolerance in humans.
Celiac sprue (CS), also known as celiac disease or gluten-sensitive enteropathy, is one of the most common food intolerances, occurring in 1 out of every 130 to 300 persons of the European (42) and U.S. (20) populations. In South America, North Africa, and Asia, CS is generally underdiagnosed (21). The epidemiological distribution of CS is conceptualized by the iceberg model introduced by Logan in 1992 (32). The prevalence of CS corresponds to the overall size of the iceberg, which is influenced by the frequency of the predisposing genotypes in the population. The “water line,” namely, the ratio of diagnosed to undiagnosed cases, depends on several factors such as awareness of CS, availability of diagnostic facilities, and variations in clinical intensity (21). The clinical manifestations of CS vary markedly with the age of the patient, the duration and extent of the disease, and the presence of extraintestinal pathology. CS is subdivided into typical (e.g., chronic diarrhea, anorexia); atypical, which is secondary to malabsorption (e.g., sideropenic anemia) or independent of malabsorption (e.g., dermatitis herpetiformis); and asymptomatic (silent) forms.
CS is an autoimmune disease of the small intestinal mucosa in genetically susceptible persons. Upon ingestion of gluten, these patients suffer from self-perpetuating mucosal inflammation characterized by progressive loss of absorptive villi and hyperplasia of the crypts. During endoluminal proteolytic digestion, prolamins of wheat (α-, β-, γ-, and ω-gliadin subgroups), rye (e.g., secalin), and barley (e.g., hordein) release a family of Pro- and Gln-rich polypeptides that are responsible for an inappropriate T-cell-mediated immune response (41). A 33-mer peptide was shown to be a potent inducer of gut-derived human T-cell lines in 14 of 14 CS patients (40). Other peptides, such as fragment 31-43 of A-gliadin, caused an inflammatory response of the small intestinal mucosa (5, 8, 36) without a T-cell-mediated response (35). The large proportion and location of proline residues in the amino acid sequences of these toxic peptides make them extremely resistant to further proteolysis (2, 11, 27). Proline is unique among the 20 amino acids because of its cyclic structure. This specific conformation imposes many restrictions on the structural aspects of peptides and proteins and confers particular biological properties. To adequately deal with such peptides, a group of specific peptidases are necessary to hydrolyze peptide bonds in which a proline residue occurs as a potential substrate. Recently, Shan et al. (40) showed that the 33-mer peptide could be hydrolyzed by exposure to a prolyl-endopeptidase of Flavobacterium meningosepticum, suggesting a strategy for an oral peptidase supplement therapy for CS patients.
Total lifelong avoidance of gluten ingestion remains the cornerstone of CS treatment. The National Food Authority has recently redefined the term “gluten free,” which now means absolutely no gluten (21). Therefore, other flours, e.g., from oats, rice, maize, millet, and buckwheat, that do not contain sequences homologous to the 33-mer gliadin or, more generally, that were proven to be nontoxic are used in various formulae as substitutes for wheat flour in the daily diet of CS patients. Since the diet of CS patients must be free of wheat products for life, efforts to reduce the human intolerance to cereals are of a great medical, nutritional, and economic interest.
Previously, we selected four sourdough lactobacilli that showed considerable hydrolysis of albumin, globulin, and gliadin fractions during wheat sourdough fermentation (18). These lactobacilli had the capacity to hydrolyze the 31-43 fragment of A-gliadin in vitro and, after hydrolysis, greatly reduced the agglutination of K 562(S) subclone cells of human myelogenous leukemia origin by a toxic peptic-tryptic (PT) digest of gliadins. On the basis of these preliminary and promising results, we further investigated a novel bread biotechnology for decreasing the level of gluten intolerance in humans.
This article describes the hydrolysis of various Pro-rich peptides, including the 33-mer peptide, by selected lactobacilli and the production of sourdoughs made from a mixture of wheat and nontoxic (26, 34) oat, buckwheat, and millet flours that were characterized by almost complete hydrolysis of the gliadin fractions. Agglutination testing of K 562(S) cells and an acute in vivo challenge showed improved tolerance of breads containing 30% wheat flour.
MATERIALS AND METHODS
Microorganisms, culture conditions, and subcellular fractionation.
Lactobacillus alimentarius 15M, L. brevis 14G, L. sanfranciscensis 7A, and L. hilgardii 51B were selected previously on the basis of their ability to hydrolyze gliadin fractions of wheat sourdoughs (18) and used in this study. The strains were routinely propagated for 24 h at 30°C (L. alimentarius 15M, L. brevis 14G, and L. sanfranciscensis 7A) or 37°C (L. hilgardii 51B) in modified MRS broth (Oxoid, Basingstoke, Hampshire, England) with the addition of fresh yeast extract (5%, vol/vol) and 28 mM maltose at a final pH of 5.6. When used for enzyme assays, sourdough fermentation, and subcellular fractionation, Lactobacillus cells were incubated until the late exponential phase of growth (optical density at 620 nm, ca. 2.5) was reached (ca. 12 h).
Twelve-hour-old cells of lactobacilli cultivated in modified MRS broth were used for subcellular fractionation by lysozyme treatment in 50 mM Tris-HCl buffer (pH 7.5) containing 24% (wt/vol) sucrose to produce the cytoplasmic extract (CE) (24). The only modification was that spheroplasts resuspended in isotonic buffer were sonicated by four cycles (10 s each) (Sony Prep model 150; Sanyo, Tokyo, Japan) to recover CE.
Enzyme assays.
All of the synthetic substrates used were from Sigma Chemical Co., St. Louis, Mo., except for fragment 62-75 (P-Q-P-Q-L-P-Y-P-Q-P-Q-S-F-P) of A-gliadin (41) and the 33-mer (L-Q-L-Q-P-F-P-Q-P-Q-L-P-Y-P-Q-P-Q-L-P-Y-P-Q-P-Q-L-P-Y-P-Q-P-Q-P-F) peptide (40), which were chemically synthesized by the Neosystem Laboratoire (Strasbourg, France). The enzyme reaction mixture contained 160 μl of 20 mM phosphate buffer (pH 7.0), 75 μl of substrate, 4 μl of NaN3 (0.05% final concentration), and 25 μl of cells (109 CFU/ml) of each Lactobacillus species. The cell aliquots were reduced to 12.5 μl when CE (12.5 μl) was added to the reaction mixture. CE preparations corresponded to those extracted from 109 cells/ml and were standardized to a protein concentration of 2.42 mg/ml. Peptidase activity on para-nitroanilide (p-NA) substrates was determined by measuring the absorbance at 410 nm (24). Peptidase activity on Z-Gly-Pro-NH-trifluoromethylcoumarin was determined by measuring the fluorescence at excitation and emission wavelengths of 400 and 505 nm, respectively. Hydrolysis of di- and tripeptides was determined by a BioChrom 30 series amino acid analyzer (BioChrom Ltd., Cambridge Science Park, England) (see below). The hydrolysis of longer peptides was analyzed by reversed-phase fast-performance liquid chromatography (RP-FPLC) with a PepRPC HR 5/5 column and FPLC equipment with a UV detector operating at 214 nm (Amersham Pharmacia Biotech, Uppsala, Sweden). Elution was at a flow rate of 0.5 ml/min with a linear gradient (0 to 100%) of acetonitrile in 0.1% trifluoroacetic acid (22).
A unit of enzyme activity on p-NA substrates was defined as the amount of enzyme that produced an increase in absorbance at 410 nm of 0.01/min. A unit of enzyme activity on Z-Gly-Pro-NH-trifluoromethylcoumarin substrate was the amount of enzyme that produced an increase in fluorescence of 0.1/min. A unit of enzyme activity on di-, tri-, and polypeptides was the amount of enzyme that liberates 1 μmol of substrate/min.
Sourdough fermentation.
The characteristics of wheat (Triticum aestivum), oat (Avena sativa), millet (Panicum miliaceum), and buckwheat (Fagopyrum esculentum) flours, respectively, were the following: moisture, 12.8, 8.0, 9.8, and 13.1%; protein (N × 5.70), 10.7, 13.0, 11.3, and 11.7% of dry matter (d.m.); fat, 1.8, 5.0, 3.8, and 2.3% of d.m.; ash, 0.6, 1.2, 1.7, and 1.5% of d.m.
Two hundred grams of the mixed wheat, oat, millet, and buckwheat flours, at a ratio of 3:1:4:2; 70 ml of tap water; and 30 ml of a cellular suspension containing 109 CFU of each lactic acid bacterial strain/ml (5 × 107 CFU/g of dough) were used to produce 300 g of dough (dough yield, 150) with a continuous high-speed mixer (60 × g; dough mixing time, 5 min). Sourdoughs were incubated at 37°C for 6, 12, and 24 h. Doughs were also started at 37°C for 12 h with selected lactic acid bacteria and their CE and at 37°C for 2 h with baker's yeast (2%, wt/wt) alone. The CE used corresponded to those extracted from a cell concentration of 109 CFU/g, which is the cell number reached at the end of sourdough fermentation (23). A dough produced with 200 g of the flour mixtures and 100 g of tap water, without a bacterial inoculum and containing chloramphenicol (1 mg/ml), was chemically acidified to pH 3.9 with a mixture of lactic and acetic acids at a molar ratio of 4:1, which corresponds to that usually found after sourdough fermentation (23), incubated for 24 h under the same conditions, and used as the control. When used, CE was added as a part of the tap water aliquot.
Protein extraction.
Protein fractions were extracted from sourdoughs and other samples by the method originally described by Osborne (39) and modified by Weiss et al. (47). Polyvinylpolypyrrolidone (1.5%, wt/vol) was used to remove tannins in the buckwheat flour (43, 46). An aliquot of each flour (7.5 g) or dough (12.75 g) was diluted with 30 ml of 50 mM Tris-HCl (pH 8.8) containing 1.5% (wt/vol) polyvinylpolypyrrolidone, held at 4°C for 1 h with vortexing at 15-min intervals, and centrifuged at 20,000 × g for 20 min. The supernatant contained albumins and globulins. In order to minimize cross contamination among albumins, globulins, and prolamins, the pellets were further extracted twice with 50 mM Tris-HCl (pH 8.8), and supernatants were discarded. After being washed with distilled water to remove buffer ions, the pellets were diluted with 30 ml of ethanol (75%, vol/vol), stirred at 25°C for 2 h, and centrifuged as described above. The supernatant contained prolamins. The extraction by ethanol was also repeated twice. Residual ethanol was eliminated by resuspending the pellets with distilled water and centrifugation. Finally, the pellets were diluted with 30 ml of sodium dodecyl sulfate (SDS)-dithiothreitol (DTT) buffer (50 mM Tris-HCl [pH 8.8], 1% SDS, 0.5% DTT), held for 2 h at room temperature with occasional vortexing, and centrifuged. The supernatant contained glutenins. All extracts were stored at −80°C until they were used. Protein concentrations of the fractions were determined by the Bradford method (16) by using bovine serum albumin as the standard.
2DE.
Two-dimensional gel electrophoresis (2DE) was performed with the immobiline-polyacrylamide system as described by Bjellqvist et al. (15). Aliquots of 30 to 50 μl (ca. 30 μg of protein) of prolamin fractions were used for the electrophoretic run. Isoelectric focusing was carried out on immobiline strips, providing a nonlinear pH gradient of 3 to 10 (IPG strips; Amersham Pharmacia Biotech) by IPG-phore, at 15°C. The voltages were the following: 0 to 300 V for 1 h, 300 to 500 V for 3 h, 500 to 2,000 V for 4 h, and a constant 8,000 V for 4 h. Following electrophoresis, IPG strips were equilibrated for 12 min against buffer A (6 M urea, 30% [vol/vol] glycerol, 2% [wt/vol] SDS, 0.05 M Tris-HCl [pH 6.8], 2% [wt/vol] DTT) and for 5 min against buffer B (6 M urea, 30% [vol/vol] glycerol, 2% [wt/vol] SDS, 0.05 M Tris-HCl [pH 6.8], 2.5% [wt/vol] iodoacetamide, 0.5% bromophenol blue). The second dimension was carried out in a Laemmli system (31) on 12% polyacrylamide gels (13 cm by 20 cm by 1.5 mm) at a constant current of 40 mA/gel and at 10°C for approximately 5 h, until the dye front reached the bottom of the gel. Gels were calibrated with two molecular mass markers: comigration of the extracts with human serum proteins for a molecular mass range of 200 to 10 kDa (18) and markers for 2DE (pI range, 7.6 to 3.8; molecular masse range, 17 to 89 kDa) from Sigma Chemical Co. The electrophoretic coordinates used for serum proteins were described by Bjellqvist et al. (15). Gels were silver stained as described by Hochstrasser et al. (28). The protein maps were scanned with an Image Scanner and analyzed with Image Master 2D v.3.01 computer software (Amersham Pharmacia Biotech). Four gels were analyzed, and spot intensities were normalized as reported by Bini et al. (14). Only statistically significant hydrolysis factors, where the P value was <0.05, are reported.
Determination of peptides and free amino acids.
The peptide profiles of the different protein fractions were analyzed by RP-FPLC with an acetonitrile gradient as described above (22). Total and individual free amino acids were analyzed in the water extracts of sourdoughs and chemically acidified and yeasted doughs by a BioChrom 30 series amino acid analyzer (BioChrom Ltd.) with an Na cation-exchange column (20 by 0.46 cm [inside diameter]). A standard amino acid mixture (Sigma Chemical Co.) made up of cysteic acid, methionine sulfoxide, methionine sulfone, tryptophan, and ornithine was used. Proteins and peptides were precipitated by addition of 5% (vol/vol) cold solid sulfosalicylic acid, holding at 4°C for 1 h, and centrifugation at 15,000 × g for 15 min. The supernatant was filtered through a 0.22-μm-pore-size filter (Millex-HA; Millipore S.A., Saint Quentin, France) and diluted (1:5) with sodium citrate loading buffer (0.2 M, pH 2.2). Amino acids were postcolumn derivatized with ninhydrin reagent and detected by absorbance at 440 (proline and hydroxyproline) or 570 (all the other amino acids) nm.
Agglutination test.
Albumins, globulins, prolamins, and glutenins were extracted from sourdoughs and yeasted or control doughs, freeze-dried, and pooled. Before freeze-drying, albumin and globulin fractions were dialyzed for 12 h at 4°C against distilled water (membrane cutoff, 1,000 Da) to remove excess carbohydrates that interfered with the agglutination test. Fifty-milligram portions of the pooled protein fractions were subjected to sequential PT digestion to produce the corresponding PT digest. Following production, the PT digest was heated at 100°C for 30 min to inactivate enzymes. This peptide preparation was used directly for the agglutination test.
K 562(S) subclone cells of human myelogenous leukemia origin from the European Collection of Cell Cultures (Salisbury, United Kingdom) were used (33). The cells were grown in RPMI medium (HyClone, Cramlington, United Kingdom) supplemented with 0.2 mM l-glutamine, 50 U of penicillin/ml, 50 mg of streptomycin/ml, and 10% (vol/vol) fetal calf serum (Flow Laboratories, Irvine, Scotland) at 37°C in a humidified atmosphere of 5% CO2 in air for 96 h. After cultivation, the human cells were harvested by centrifugation at 900 × g for 5 min, washed twice with 0.1 M phosphate-buffered saline solution (Ca2+ and Mg2+ free; pH 7.4), and resuspended at a concentration of 108/ml in the same buffer. Twenty-five microliters of this cell suspension was added to wells of a microtiter plate containing serial dilutions (0.013 to ca. 7.0 g/liter) of PT digest. The total volume in the well was 100 μl, and the mixture was held for 30 min at room temperature. Following incubation, a drop of the suspension was applied to a microscope slide to count clumped and single cells. Agglutination tests were carried out in triplicate, and photographs were taken with a Diaphot-TMD inverted microscope (Nikon Corp., Tokyo, Japan).
In vivo challenge.
A sourdough made from wheat flour alone (dough yield, 220) was fermented with lactobacilli and their CE for 24 h at 37°C under the conditions described above. Following fermentation, the wheat sourdough was mixed (dough yield, 150) with oat, millet, and buckwheat flours at the optimal ratio, allowed to ferment for 2 h, and baked at 220°C for 20 min. Another type of dough made from the same mixture of the four flours (dough yield, 150) was started with baker's yeast (2 h of fermentation) and baked under the same conditions. The two types of bread (ca. 80 g) contained ca. 2 g of gluten.
The in vivo acute challenge was based on intestinal permeability tests (25). Twenty volunteer CS patient were recruited after at least 2 years on a gluten-free diet. At the time of recruitment, criteria for admission were a negative test for anti-transglutaminase antibodies and exclusion of gluten from the diet for at least the previous 3 months. The protocol for recruitment of CS patients and the in vivo challenge were approved by the University of Naples Ethical Committee. After an overnight fast, CS patients voided and drank 220 ml of a carbohydrate solution that contained 1 g of rhamnose, 7.5 g of lactulose, and 40 g of sucrose. Sucrose was added to increase the osmolarity of the solution. Urine samples were collected for 5 h, and carbohydrates were determined by gas chromatographic analysis as trisilyl derivatives by the method of Dutton (19). Following the baseline permeability test, CS patients randomly ingested (double-blind trial) one of the two breads for 2 days and 6 h later drank the carbohydrate solution. Urine samples were collected and analyzed.
RESULTS
Hydrolysis of Pro-rich polypeptides.
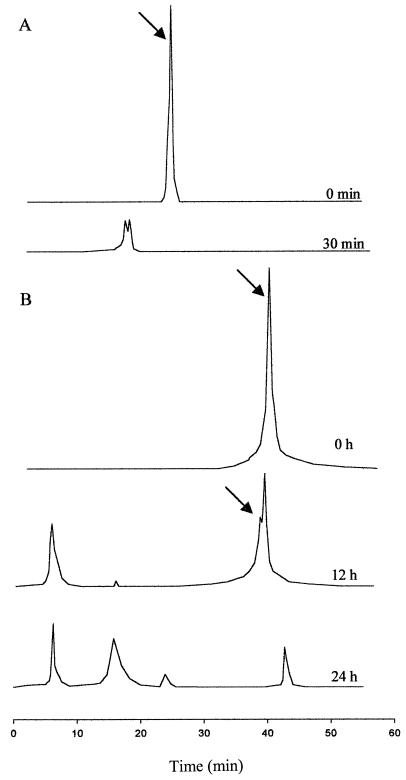
L. alimentarius 15M, L. brevis 14G, L. sanfranciscensis 7A, and L. hilgardii 51B were selected previously on the basis of their different hydrolysis profiles toward wheat gliadins (18) and used in this study. Pooled cells of the four lactobacilli (ca. 109 CFU/ml) showed the following enzyme activities: iminopeptidase on 2 mM Pro-p-NA, dipeptidyl-peptidase on 2 mM Gly-Pro-p-NA, prolyl-endopeptidase on 2 mM Z-Gly-Pro-NH-trifluoromethylcoumarin, prolidase on 2.3 mM Val-Pro, prolinase on 3 mM Pro-Gly, and aminopeptidase P on 2 mM Gly-Pro-Ala (Table 1) (17). When cells and CE were compared on these substrates, the activities did not differ significantly (data not shown). Bradykinin (P-P-G-F-S-P-F-R) and fragment 62-75 of A-gliadin (1) were hydrolyzed also. The activity on Pro-rich polypeptides was increased when cells were supplemented with the respective CE (2.42 mg of protein/ml), as a source of free peptidases. Under these conditions, the hydrolysis of peptide 62-75 of A-gliadin (450 μM) was complete in 30 min (Fig. 1A). Pooled cells and CE completely hydrolyzed the 33-mer peptide (200 μM) in 24 h (Fig. 1B). Although the hydrolysis of this longer peptide proceeded slowly compared to that of the other two oligopeptides, considerable degradation, ca. 70%, was found after 12 h. Also for this epitope, the hydrolyzing activity was ca. doubled when cells were supplemented with CE.
TABLE 1.
Enzyme activitya of selected sourdough lactobacilli on various substrates containing proline residues
| Source of enzyme activity | Substrate (concn [mM]) | Avg activity (U) ± SDa |
|---|---|---|
| Cellsb | Pro-p-NA (2) | 0.3 ± 0.01d |
| Cells | Gly-Pro-p-NA (2) | 5.2 ± 0.03 |
| Cells | Z-Gly-Pro-NH-trifluoromethyl- coumarin (2) | 12.3 ± 0.4e |
| Cells | Val-Pro (2.3) | 2.1 ± 0.03f |
| Cells | Pro-Gly (3) | 1.9 ± 0.04 |
| Cells | Gly-Pro-Ala (2) | 2.2 ± 0.02 |
| Cells | Bradykinin (0.3) | 5.5 ± 0.3 |
| Pooled cells and CEc | Bradykinin | 11.1 ± 0.3 |
| Cells | Fragment 62-75 of A-gliadin (0.45) | 9.7 ± 0.5 |
| Pooled cells and CE | Fragment 62-75 of A-gliadin | 15.0 ± 0.5 |
| Cells | 33-mer (0.200) | 0.08 ± 0.002 |
| Pooled cells and CE | 33-mer | 0.2 ± 0.01 |
Each value is the average of three enzyme assays, and standard deviations were calculated.
Aliquots (25 μl) of each cell suspension were used in the enzyme assays.
Aliquots (12.5 μl) of the pooled cells and CE of each species were used in the enzyme assays.
A unit of enzyme activity on p-NA substrates was defined as the amount of enzyme that produced an increase in absorbance at 410 nm of 0.01/min.
A unit of enzyme activity on Z-Gly-Pro-NH-trifluoromethylcoumarin was the amount of enzyme that produced an increase in fluorescence of 0.1/min.
A unit of enzyme activity on di-, tri-, and polypeptides was the amount of enzyme that liberates 1μmol of substrate/min.
FIG. 1.
Hydrolysis of peptides by pooled cells (109 CFU/ml) and CE (2.42 mg of protein/ml) of the four selected lactobacilli. (A) RP-FPLC (UV at 215 nm) trace of 450 μM fragment 62-75 of A-gliadin before and after 30 min of hydrolysis. (B) RP-FPLC (UV at 215 nm) trace of 200 μM of 33-mer peptide before and after 12 or 24 h of hydrolysis. Arrows refer to the substrate.
Proteolysis during sourdough fermentation.
Oat, millet, and buckwheat flours are considered nontoxic for CS patients (26, 34). The effects of different amounts of flours on dough leavening and texture were investigated in preliminary experiments. A mixture of wheat, oat, millet, and buckwheat flours, at a ratio 3:1:4:2, was chosen as optimal for dough production. Oat flour and especially buckwheat flour are also suitable for increasing the low concentration of methionine and lysine in wheat flour (37). Millet is suitable for improving dough texture (33). Three doughs were started with (i) lactobacilli, (ii) lactobacilli and CE, or (iii) baker's yeast. Another dough (control), without a microbial inoculum, was acidified chemically to pH 3.9. Doughs were incubated at 37°C. Sourdoughs started with lactobacilli contained ca. 109 CFU/g, presumably of each species, and had a final pH that ranged from 3.9 to 3.7, depending on the incubation time. The dough started with baker's yeast had a pH of 5.6 and contained 108 yeast cells/g and less than 106 bacteria/g (23). The total bacterial count of the control was constant at 103 CFU/g during 24 h of incubation.
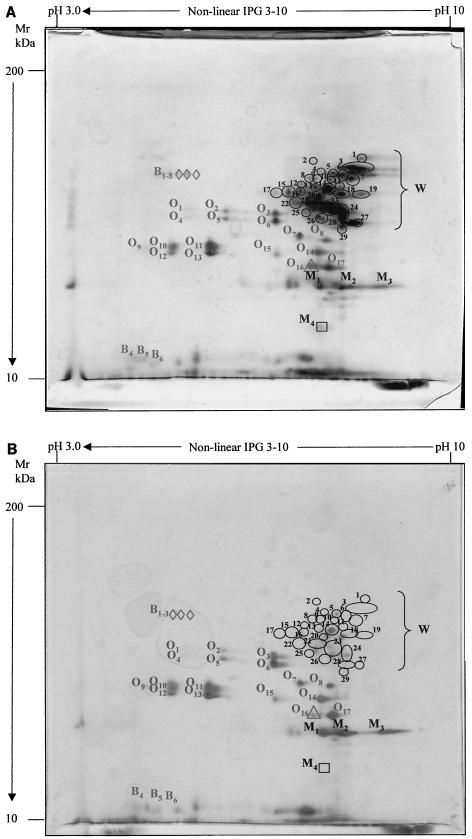
Protein fractions were selectively extracted from individual flours, sourdoughs, and yeasted and chemically acidified (control) doughs and further analyzed by 2DE. Preliminarily, 2DE gels of fermented and control doughs were compared with those of the individual flours and the map positions of the different prolamins were identified (Fig. 2A and B). As shown previously (18), the α, β, and γ fractions of wheat gliadins (28 to 50 kDa) clustered in the alkaline zone of the gel (pI 6.5 to 8.5), while the ω fractions (55 to 70 kDa) were confined to the pI 4.0 to 7.0 area (Fig. 2A). Alcohol-soluble oat avenins were located in the molecular mass range of 24.5 to 38 kDa, and this was in substantial agreement with the results of Mikola et al. (38), who found prolamins of ca. 22 to 33 kDa. As shown previously by SDS-polyacrylamide gel electrophoresis analysis (13), buckwheat prolamin fractions were spread over a wide molecular mass range (15.9 to 50 kDa) and setarins of millet were confirmed to be in the 19- to 25-kDa zone of the 2DE map (29).
FIG.2.
2DE analysis of the prolamin protein fractions of different doughs made of a mixture of wheat (30%), oat (10%), millet (40%), and buckwheat (20%) flours. Chemically acidified dough incubated for 24 h at 37°C (A) and sourdough started with selected lactic acid bacteria for 24 h (B) at 37°C were used. Prolamin polypeptides from wheat (spot W [1 to 29]), oat (spot O [1 to 17]), millet (spot M [1 to 4]), and buckwheat flours (spot B [1 to 6]) are shown. The numbered ovals, triangles, squares, and diamonds refer to hydrolyzed prolamins from wheat, oats, millet, and buckwheat, respectively. Mr, molecular mass.
After 2 h of fermentation, the dough started by baker's yeast had the same prolamin profile as the flours (data not shown). As shown previously (18), biological or chemical acidification and the related changes in redox potential caused a marked modification of the 2DE polypeptide pattern compared to the nonacidified dough; therefore, the sourdoughs fermented by lactobacilli were compared to the control to find variations due to bacterial proteolysis (Fig. 2A and B and Table 2). By this comparison, changes due to proteolysis by flour endogenous enzymes were also excluded in part (45). Prolamins from wheat flour disappeared almost completely after 24 h of proteolysis by selected lactobacilli (Fig. 2B). Twenty-one of the 29 alcohol-soluble polypeptides were characterized by hydrolysis factors of ≥90%; 4 had hydrolysis factors of 80 to 86%, and 4 were degraded by 50.0 to 71% (Table 2). Only 1 of the 17 avenin polypeptides was affected by proteolysis. One of the four alcohol-soluble polypeptides of millet flour and three of the six alcohol-soluble polypeptides of buckwheat flour were almost completely hydrolyzed, with no appreciable differences when the time of sourdough fermentation increased. On the contrary, hydrolysis of wheat prolamins increased with the duration of sourdough fermentation (Table 2). After 6 or 12 h, the hydrolysis factors of several polypeptides was still low compared to those determined after 24 h. Seventeen alcohol-soluble polypeptides showed hydrolysis factors of 0 to 20.6% only after 6 h of sourdough incubation, while at 12 h, 11 polypeptides (e.g., W12, W13, and W16) had hydrolysis factors that were still low compared to those determined after 24 h. As expected, no significant differences were found between sourdoughs started with lactobacilli alone and those started with lactobacilli and CE, probably because the hydrolysis of long-chain wheat polypeptides (molecular mass, ≥10 kDa), which are detected by 2DE, depended mainly on the activity of cell wall-associated proteinase (30). The hydrolysis of gliadins did not change when the proportions of the nontoxic flours were varied (data not shown).
TABLE 2.
Properties of alcohol-soluble polypeptides hydrolyzed by selected sourdough lactic acid bacteria during fermentation of sourdoughs made from mixed floursa
| Spot desig- nationb | Estimated pI | Estimated molecular mass (kDa) | Hydrolysis factor (%)
|
||
|---|---|---|---|---|---|
| 6 h | 12 h | 24 h | |||
| W1 | 7.54 | 69.0 | 98.4 ± 2.0 | 98.0 ± 5.0 | 98.7 ± 4.3 |
| W2 | 6.75 | 68.0 | 95.0 ± 3.5 | 95.4 ± 4.8 | 95.3 ± 4.8 |
| W3 | 7.5 | 55.0 | 70.5 ± 3.0 | 80.6 ± 2.5 | 97.2 ± 5.0 |
| W4 | 6.9 | 59.0 | 10.0 ± 0.08 | 55.5 ± 2.0 | 98.7 ± 4.7 |
| W5 | 6.9 | 58.0 | 10.4 ± 1.6 | 52.0 ± 2.8 | 60.5 ± 4.0 |
| W6 | 7.4 | 56.5 | 90.7 ± 4.4 | 95.1 ± 4.3 | 98.0 ± 4.5 |
| W7 | 7.6 | 56.0 | 85.0 ± 2.8 | 90.3 ± 3.8 | 98.2 ± 4.1 |
| W8 | 6.7 | 53.0 | 18.0 ± 0.3 | 85.5 ± 4.1 | 97.5 ± 4.8 |
| W9 | 6.8 | 52.1 | 50.2 ± 2.8 | 90.4 ± 3.7 | 97.0 ± 4.0 |
| W10 | 7.0 | 49.0 | 85.5 ± 3.2 | 90.5 ± 3.8 | 95.5 ± 3.7 |
| W11 | 7.23 | 48.0 | 85.5 ± 3.7 | 91.0 ± 2.9 | 96.0 ± 2.1 |
| W12 | 6.7 | 47.0 | 0 | 52.0 ± 2.4 | 95.0 ± 3.5 |
| W13 | 6.8 | 46.9 | 0 | 45.5 ± 1.5 | 80.0 ± 2.7 |
| W14 | 7.0 | 46.5 | 0 | 50.6 ± 1.0 | 50.0 ± 2.2 |
| W15 | 6.35 | 46.0 | 15.7 ± 0.1 | 84.8 ± 3.0 | 93.2 ± 2.5 |
| W16 | 6.7 | 45.0 | 5.1 ± 0.12 | 18.5 ± 0.2 | 90.6 ± 4.1 |
| W17 | 6.0 | 44.0 | 35.0 ± 0.1 | 63.0 ± 2.4 | 95.5 ± 4.0 |
| W18 | 7.2 | 43.8 | 15.4 ± 0.08 | 47.6 ± 2.3 | 70.4 ± 3.2 |
| W19 | 7.5 | 43.0 | 80.5 ± 0.33 | 95.4 ± 4.8 | 95.0 ± 4.0 |
| W20 | 6.9 | 43.0 | 0 | 58.0 ± 2.1 | 70.8 ± 2.2 |
| W21 | 6.8 | 41.5 | 0 | 45.0 ± 2.0 | 85.9 ± 4.1 |
| W22 | 6.5 | 41.0 | 0 | 52.2 ± 1.5 | 96.0 ± 4.8 |
| W23 | 7.0 | 40.0 | 10.5 ± 0.15 | 40.5 ± 1.8 | 85.7 ± 4.0 |
| W24 | 7.4 | 38.9 | 10.0 ± 0.15 | 65.5 ± 2.4 | 80.1 ± 3.7 |
| W25 | 6.6 | 38.7 | 20.6 ± 0.2 | 62.7 ± 2.3 | 90.2 ± 4.2 |
| W26 | 6.9 | 38.0 | 10.0 ± 0.5 | 63.0 ± 2.7 | 93.8 ± 4.5 |
| W27 | 7.8 | 38.0 | 80.5 ± 4.1 | 91.6 ± 4.4 | 98.0 ± 3.2 |
| W28 | 7.4 | 38.0 | 10.7 ± 1.0 | 80.0 ± 4.6 | 97.4 ± 4.1 |
| W29 | 7.4 | 35.5 | 30.2 ± 2.0 | 83.0 ± 4.0 | 98.4 ± 3.2 |
| O1 | 5.3 | 38.5 | 0 | 0 | 0 |
| O2 | 5.5 | 38.0 | 0 | 0 | 0 |
| O3 | 6.0 | 37.5 | 0 | 0 | 0 |
| O4 | 5.3 | 37.0 | 0 | 0 | 0 |
| O5 | 5.5 | 36.3 | 0 | 0 | 0 |
| O6 | 6.0 | 36.0 | 0 | 0 | 0 |
| O7 | 6.7 | 32.8 | 0 | 0 | 0 |
| O8 | 7.0 | 32.0 | 0 | 0 | 0 |
| O9 | 5.0 | 31.0 | 0 | 0 | 0 |
| O10 | 5.1 | 31.0 | 0 | 0 | 0 |
| O11 | 5.4 | 31.0 | 0 | 0 | 0 |
| O12 | 5.1 | 29.5 | 0 | 0 | 0 |
| O13 | 5.4 | 29.5 | 0 | 0 | 0 |
| O14 | 6.0 | 29.0 | 0 | 0 | 0 |
| O15 | 6.9 | 28.0 | 0 | 0 | 0 |
| O16 | 6.8 | 24.5 | 10.1 ± 0.18 | 20.7 ± 0.3 | 35.2 ± 0.8 |
| O17 | 7.0 | 24.0 | 0 | 0 | 0 |
| M1 | 6.9 | 24.9 | 0 | 0 | 0 |
| M2 | 7.4 | 24.8 | 0 | 0 | 0 |
| M3 | 7.6 | 24.8 | 0 | 0 | 0 |
| M4 | 6.9 | 19.8 | 95.0 ± 1.8 | 95.0 ± 0.1 | 98.0 ± 1.0 |
| B1 | 5.1 | 50.0 | 95.4 ± 3.7 | 95.2 ± 4.3 | 98.0 ± 1.5 |
| B2 | 5.2 | 50.0 | 95.3 ± 4.2 | 97.8 ± 3.8 | 95.6 ± 4.6 |
| B3 | 5.3 | 50.0 | 97.8 ± 3.7 | 98.0 ± 4.5 | 98.9 ± 3.4 |
| B4 | 5.2 | 16.2 | 0 | 0 | 0 |
| B5 | 4.9 | 16.0 | 0 | 0 | 0 |
| B6 | 4.8 | 15.9 | 0 | 0 | 0 |
Analyses were performed with Image Master software (Pharmacia). Four gels of independent replicates were analyzed. For spot quantification and hydrolysis factor calculation, see Materials and Methods. All of the hydrolysis factors were calculated on the basis of the average of the spot intensities of each of four gels, and standard deviations were calculated.
Spot designations correspond to those of the gels in Fig. 2A and B. W, wheat; O, oat; M, millet; B, buck wheat.
Peptides contained in the ethanol-soluble fraction of the sourdough started with selected lactobacilli were analyzed by RP-FPLC (data not shown). This analysis also detects low-molecular-mass peptides (e.g., ≤7.5 kDa), nondetectable under our 2DE conditions, that may be hydrolysis end products of longer alcohol-soluble polypeptides, thus giving more complete information on the proteolytic activities. Compared to the RP-FPLC profile of the dough before incubation, peptides disappeared almost completely in the sourdough started for 24 h. Intermediate hydrolysis levels were found for 6 and 12 h of incubation.
Control dough showed an increase in the total free amino acid concentration with respect to the nonacidified and nonincubated dough (1,140 versus 715 mg/kg). Wheat and probably also the other flours contain endogenous proteolytic activities that are activated under acid conditions (45). The concentration of individual free amino acids in sourdoughs started with selected lactobacilli increased progressively with the incubation time. The sourdough fermented for 24 h had a concentration of total free amino acids (3,021 mg/kg) ca. three times as high as that in the control or after 6 h of incubation (1,019 mg/kg) and ca. 1.5 times as high as that after 12 h of incubation (1,849 mg/kg). Addition of the CE of sourdough lactobacilli had a significant effect on the total level of free amino acids: 2,676 versus 1,849 mg/kg in the sourdough without the addition. The major differences between the sourdough started with selected lactobacilli and chemically acidified dough incubated for 24 h involved amino acids such as Glu, Ala, Leu, Tyr, Phe, Lys, and Pro.
Agglutination test.
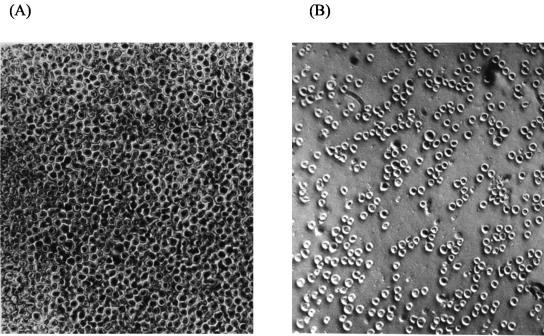
Albumins, globulins, prolamins, and glutenins were extracted from doughs, freeze-dried, and pooled. Fifty milligrams of these pooled protein preparations were subjected to PT degradation to simulate in vivo protein digestion (10). All of the protein fractions were used in this assay, which was done to include the hydrolysis products of prolamins that, after degradation, were eventually contained in the water- and SDS-DTT-soluble extracts. PT digests were used for agglutination tests with K 562(S) subclone cells of human myelogenous leukemia origin. Control dough and the dough started with baker's yeast had a minimal agglutinating activity (MAC) of 0.027 g/liter. The MAC of the PT digests from sourdoughs started with selected lactobacilli increased markedly. It was 0.875 and 7.0 g/liter for sourdoughs fermented for 12 and 24 h, respectively. The MAC of the PT digests from sourdough started with selected lactobacilli and that of the control are shown in Fig. 3. Addition of CE to the sourdough fermented for 12 h increased the MAC further (1.75 g/liter).
FIG. 3.
Agglutination test with K 562(S) cells. (A) Cells treated with the PT digest of the chemically acidified dough at a concentration of 0.027 g/liter. (B) Cells treated with the PT digest of the sourdough started with selected lactobacilli, incubated for 24 h at 37°C, at a concentration of 3.5 g/liter.
In vivo acute challenge.
Two types of bread (ca. 80 g) were made from the mixture of wheat, oat, millet, and buckwheat flours, started with baker's yeast or lactobacilli and CE, and used for an in vivo acute challenge. Each bread contained ca. 2 g of gluten.
The in vivo challenge was based on intestinal permeability tests (25). Twenty volunteer CS patients were recruited after at least 2 years on a gluten-free diet. Of the 20 patients, 3 were excluded from the challenge because they ingested gluten after recruitment and had baseline intestinal permeability values in the pathological range. Thirteen of the 17 CS patients showed a marked alteration of intestinal permeability after ingestion of the bread started with baker's yeast (Table 3). In these patients, absorption of rhamnose decreased more than 40%, that of lactulose in most of the cases at least doubled, and the lactulose/rhamnose ratio increased strongly. When the same 13 CS patients ingested the same dose of gluten in breads started with lactobacilli and CE, they showed rhamnose and lactulose absorption values and a lactulose/rhamnose ratio that did not differ significantly from the baseline values. After ingesting the baker's yeast bread, the other 4 of the 17 CS patients (no. 7, 9, 16, and 17; Table 3) did not apparently respond with a marked increase in intestinal permeability; they showed a decrease of ca. one-third in the amount of rhamnose absorbed, while the lactulose absorption and lactulose/rhamnose ratio did not change. The same four CS patients did not respond to gluten after ingesting the sourdough bread.
TABLE 3.
In vivo acute challenge based on intestinal permeability of CS patientsg
| Patient no. | Baselinea
|
Baker's yeastb
|
Sourdoughc
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| R | L | L/R | R | L | L/R | R | L | L/R | |
| 1 | 1.99 | 0.07 | 0.035 | 0.68 | 0.11 | 0.16 | 1.10 | 0.05 | 0.044 |
| 2 | 2.9 | 0.06 | 0.02 | 0.99 | 0.21 | 0.21 | 1.89 | 0.08 | 0.04 |
| 3 | 2.32 | 0.116 | 0.05 | 0.355 | 0.11 | 0.31 | 1.71 | 0.134 | 0.078 |
| 4 | 1.75 | 0.121 | 0.069 | 0.625 | 0.162 | 0.26 | 1.37 | 0.07 | 0.05 |
| 5 | 1.89 | 0.02 | 0.01 | 0.45 | 0.09 | 0.2 | 1.68 | 0.071 | 0.04 |
| 6 | 0.59 | 0.041 | 0.069 | 1.11 | 0.4 | 0.36 | 0.38 | 0.044 | 0.11 |
| 7 | 1.98 | 0.061 | 0.033 | 0.69 | 0.032 | 0.046 | 0.80 | 0.005 | 0.04 |
| 8 | 2.45 | 0.072 | 0.03 | 0.78 | 0.07 | 0.09 | 1.78 | 0.04 | 0.022 |
| 9 | 0.98 | 0.12 | 0.12 | 0.662 | 0.203 | 0.307 | 1.20 | 0.17 | 0.142 |
| 10 | 1.88 | 0.030 | 0.015 | 0.770 | 0.400 | 0.500 | 2.200 | 0.070 | 0.030 |
| 11 | 1.660 | 0.080 | 0.050 | 0.900 | 0.100 | 0.110 | 1.200 | 0.090 | 0.075 |
| 12 | 3.100 | 0.030 | 0.001 | 1.440 | 0.500 | 0.340 | 1.890 | 0.100 | 0.050 |
| 13 | 2.100 | 0.040 | 0.020 | 1.100 | 0.700 | 0.600 | 1.700 | 0.010 | 0.005 |
| 14 | 2.500 | 0.060 | 0.024 | 1.000 | 0.100 | 0.100 | 3.000 | 0.090 | 0.030 |
| 15 | 3.700 | 0.010 | 0.002 | 1.800 | 0.330 | 0.180 | 2.300 | 0.100 | 0.040 |
| 16 | 0.813 | 0.115 | 0.141 | 0.380 | 0.111 | 0.292 | 0.710 | 0.162 | 0.228 |
| 17 | 0.900 | 0.100 | 0.110 | 1.200 | 0.080 | 0.067 | 0.830 | 0.120 | 0.140 |
| Mean | 2.20d | 0.05d | 0.03d | 0.90d,e | 0.21d,e | 0.24d,e | 1.64e,f | 0.07f | 0.04e,f |
| SD | 0.739 | 0.032 | 0.022 | 0.382 | 0.199 | 0.160 | 0.656 | 0.032 | 0.026 |
Permeability after drinking the carbohydrate solution and before ingestion of breads (baseline values).
Permeability after ingestion of the bread started with baker's yeast and drinking the carbohydrate solution.
Permeability after ingestion of the bread started with lactobacilli and CE and drinking the carbohydrate solution.
Difference between baseline and baker's yeast, P ≤ 0.05.
Difference between baker's yeast and sourdough, P < 0.05.
Difference between baseline and sourdough, P ≥ 0.5.
R, urinary excretion of rhamnose (millimolar); L, urinary excretion of lactulose (millimolar); L/R, lactulose/rhamnose ratio. Differences between means were evaluated by the Student t test; individual variables were screened for normality of distribution (Kolmogoroff-Smirnoff, P > 0.5).
DISCUSSION
This work was aimed at showing the ability of sourdough lactobacilli to hydrolyze wheat prolamins extensively and at finding a novel protocol for manufacturing a sourdough bread that can be tolerated by CS patients.
In a previous paper (18), we showed that selected lactobacilli have the ability to hydrolyze either albumins, globulins, and gliadins during wheat sourdough fermentation or the 31-43 fragment of A-gliadin in vitro and that, after hydrolysis, they greatly reduced the agglutination of the K 562(S) subclone cells of human myelogenous leukemia origin by a toxic PT digest of gliadins. In this study, we first showed that the pool of L. alimentarius 15M, L. brevis 14G, L. sanfranciscensis 7A, and L. hilgardii 51B has a pattern of specialized peptidases capable of hydrolyzing all of the different peptide bonds that potentially include the imino acid proline. Overall, biologically active peptides contain a large proportion of Pro residues within the sequence, which makes them very resistant to hydrolysis by peptidases, which are not specific for Pro bonds (17, 30, 40). The hydrolysis by the four lactobacilli also concerned oligopeptides such as fragment 62-75 of A-gliadin and the 33-mer peptide. Peptide 62-75 is part of a longer fragment, 56-75, of A-gliadin that reacts with tissue transglutaminase and is one of the dominant epitopes responsible for the inappropriate T-cell-mediated immune response in CS patients (1). These hydrolyzing activities are not widespread in dairy lactic acid bacteria. Generally, pure prolyl-endopeptidases have very poor activity on long-chain peptides (30, 44). In our experiments, we used a pool of selected lactobacilli that was supplemented with a pool of their cytoplasmic enzymes.
Notwithstanding the heterogeneity of T-cell epitopes in gluten, a few epitopes appear to account for most of the α-gliadin recognition by CD4+ T cells from CS patients (1, 4). The most important is probably the 33-mer peptide, for the following reasons: (i) it remains intact despite prolonged exposure to gastric and pancreatic proteases, (ii) other patient-specific T-cell epitopes are present in its sequence, (iii) hydrolysis of the 33-mer peptide (100 μM) by small intestinal brush border membrane enzymes is less than 20% over 20 h of incubation (40), and (iv) it remains intact for a long time (ca. 24 h) in the small intestine and even at a low concentration is able to act as a potential antigen for T-cell proliferation and intestinal toxicity in genetically susceptible individuals (40). Although peptidases capable of hydrolyzing Pro- and Glu-rich peptides are located in the intestinal brush border (2, 3, 11, 12), these epitopes withstand enzymatic processing (27). To our knowledge, the only enzyme proposed as a detoxifying agent for the 33-mer peptide is the prolyl-endopeptidase from F. meningosepticum (40), which is not related to bread biotechnology. In this study, we first showed that sourdough lactobacilli have the ability to hydrolyze the 33-mer peptide extensively or almost totally during prolonged incubation (12 to 24 h).
Previously, a wheat sourdough was produced with the same Lactobacillus species; considerable, but not total, hydrolysis of gliadins was found (18). In this study, the amount of wheat flour was decreased to 30% by mixing with nontoxic (26, 34) oat, millet, and buckwheat flours (ratio, 3:1:4:2). These flours are nutritionally and technologically suitable also (33, 37). Under these conditions, we achieved almost complete hydrolysis of wheat gliadins while prolamins from oats, millet, and buckwheat were affected less or not at all. A comparison with a chemically acidified dough or with a dough started with baker's yeast alone showed that the hydrolysis was due to the proteolytic activity of sourdough lactobacilli and that prolamin fractions were not affected during dough fermentation with yeast. The great extent of hydrolysis during sourdough fermentation was confirmed on various-size, alcohol-soluble polypeptides that were analyzed by RP-FPLC and by determination of free amino acids. Addition of CE to the sourdough started with selected lactobacilli for 12 h markedly increased the concentration of free amino acids, showing a considerable activity of CE toward low- to medium-molecular-mass peptides.
Prior to initiating the in vivo acute challenge, we wanted to confirm our results based on agglutination tests. Overall, a relatively high correlation was found between the agglutination activity of cereal components against K 562(S) cells and their toxicities in clinical and in vitro trials on the basis of biopsy samples of intestinal mucosa from CS patients (6, 9, 41). The MAC of the sourdough started with selected lactobacilli was ca. 250 times higher than those of the control and of the dough started with baker's yeast. It was also confirmed that CE plays a role in the further degradation of intermediate polypeptides from gliadins, which probably still have a toxic effect.
For the in vivo challenge, a bread produced with a very long fermentation time was compared to a bread fermented for 2 h with baker's yeast. A very long fermentation time is a common feature of an ancient tradition for the production of typical wheat sourdough breads (23). After this fermentation, the structure of the dough is obviously collapsed and sourdough is traditionally reused as a starter for a new and very short (2- to 4-h) fermentation process. Under our experimental conditions, the fermented (24 h) wheat sourdough was subsequently mixed with nontoxic flours in the optimal ratio, allowed to ferment for 2 h, and baked at 220°C for 20 min. This type of bread was technologically suitable: the volume was ca. one-half of that started with baker's yeast, and the texture was comparable to that of wheat sourdough breads. To our knowledge, this is the first report of tolerance of CS patients for a bread containing 30% wheat flour on the basis of determination of intestinal permeability during an acute in vivo challenge. Thirteen of the 17 CS patients recruited showed a marked alteration of intestinal permeability after ingestion of baker's yeast bread, while when fed the sourdough bread, the same 13 patients had intestinal permeability values that did not differ significantly from the baseline values. The other four CS patients did not respond to the two types of bread.
The following multidisciplinary research efforts are currently being carried out in several directions to deal with the pathogenesis of CS: (i) engineering of gluten-free grains, (ii) search for the CS genes in humans (20), (iii) use of some protective substances (e.g., mannan and oligomers of N-acetylglucosamine) (7, 41), and (iv) use of bacterial prolyl-endopeptidase from F. meningosepticum as an oral supplementary therapy (40). None of these efforts considered strategies that are pertinent in bread biotechnology. This study shows that CS patients subjected to an acute challenge tolerated breads produced with sourdough better than those started with baker's yeast. These results showed that a bread biotechnology that uses selected lactobacilli, nontoxic flours, and a long fermentation time is a novel tool for decreasing the level of human intolerance to a certain amount of wheat flour. Work is in progress to confirm these results with a long-term in vivo challenge.
Acknowledgments
This work was supported by the Italian Ministry of University and Scientific and Technological Research (Murst), Development of Research Networks no. 488/92, cluster C06 + 07, project 6-2.2.
We thank P. F. Fox for critical revision of the paper and Giuditta Alfonsi and Valeria Ancona for technical support. M.G. thanks his father for the practical suggestions which promoted the idea of this work.
REFERENCES
- 1.Anderson, R. P., P. Degano, A. J. Godkin, D. P. Jewell, and A. V. S. Hill. 2000. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat. Med. 6:337-342. [DOI] [PubMed] [Google Scholar]
- 2.Andria, G., S. Cucchiara, B. De Vizia, G. Mazzacca, and S. Auricchio. 1980. Brush border and cytosol peptidase activities of human small intestine in normal subjects and coeliac patients. Pediatr. Res. 14:812-818. [DOI] [PubMed] [Google Scholar]
- 3.Andria, G., A. Marzi, and S. Auricchio. 1976. α-l-glutamyl-β-naphthylamide hydrolase of rabbit small intestine. Localization in the brush border and separation from other brush border peptidases. Biochim. Biophys. Acta 419:42-59. [DOI] [PubMed] [Google Scholar]
- 4.Arentz-Hansen, H., R. Korner, O. Molberg, H. Quarsten, W. Vader, Y. M. C. Kooy, K. E. A. Lundin, F. Koning, P. Roepstorff, L. M. Sollid, and S. N. McAdam. 2000. The intestinal T cell response to alpha-gliadin in adult celiac disease is focused on a single glutamine targeted by tissue transglutaminase. J. Exp. Med. 191:603-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auricchio, S., L. Maiuri, A. Picarelli, M. De Vincenzi, R. Troncone, V. Pavone, and M. Mayer. 1996. In vitro activities of A-gliadin-related synthetic peptides. Damaging effect on the atrophic celiac mucosa and activation of mucosal immune response in the treated celiac mucosa. Scand. J. Gastroenterol. 31:247-253. [DOI] [PubMed] [Google Scholar]
- 6.Auricchio, S., G. Deritis, M. De Vincenzi, V. Gentile, L. Maiuri, E. Mancini, R. Porta, and V. Raia. 1990. Amines protect in vitro the coeliac small intestine from damaging activity of gliadin peptides. Gastroenterology 99:1668-1674. [DOI] [PubMed] [Google Scholar]
- 7.Auricchio, S., G. Deritis, M. De Vincenzi, G. Magazzu, L. Maiuri, E. Mancini, M. Minetti, O. Sapora, and V. Silano. 1990. Mannan and oligomers of N-acetylglucosamine protect intestinal mucosa of coeliacs with active disease from in vitro toxicity of gliadin peptides. Gastroenterology 99:973-978. [DOI] [PubMed] [Google Scholar]
- 8.Auricchio, S., G. De Ritis, M. De Vincenzi, and V. Silano. 1985. Toxicity mechanisms of wheat and other cereals in celiac disease and related enteropathies. J. Pediatr. Gastroenterol. Nutr. 4:923-930. [DOI] [PubMed] [Google Scholar]
- 9.Auricchio, S., G. De Ritis, M. De Vincenzi, M. Minetti, O. Sapora, and V. Silano. 1984. Agglutination activity of gliadin-derived peptides from bread wheat: implications for coeliac disease pathogenesis. Biochem. Biophys. Res. Commun. 21:428-433. [DOI] [PubMed] [Google Scholar]
- 10.Auricchio, S., G. De Ritis, M. De Vincenzi, P. Occorsio, and V. Silano. 1982. Effect of gliadin peptides prepared from hexaploid and tetraploid wheat on cultures of intestine from rat fetuses and coeliac children. Pediatr. Res. 16:1004-1010. [DOI] [PubMed] [Google Scholar]
- 11.Auricchio, S., L. Greco, B. De Vizia, and V. Buonocore. 1978. Dipeptidylaminopeptidase and carboxypeptidases activities of the brush border of rabbit small intestine. Gastroenterology 75:1073-1079. [PubMed] [Google Scholar]
- 12.Auricchio, S., M. Pierro, G. Andria, and G. De Ritis. 1972. Enzymatic activities of the brush border membrane of rat intestine hydrolyzing β-naphthylamides of amino acids, leucilamides and dipeptides. Biochim. Biophys. Acta 274:420-425. [DOI] [PubMed] [Google Scholar]
- 13.Bejosano, F. P., and H. Corke. 1999. Properties of protein concentrates and hydrolysates from Amaranthus and buckwheat. Ind. Crop Prod. 10:175-183. [Google Scholar]
- 14.Bini, L., B. Magi, B. Marzocchi, F. Arcuri, S. Tripodi, M. Cintorino, J. C. Sanchez, S. Frutiger, and D. Hochstrasser. 1997. Protein expression profiles in human breast ductal carcinoma and histologically normal tissue. Electrophoresis 18:2832-2841. [DOI] [PubMed] [Google Scholar]
- 15.Bjellqvist, B., G. J. Hughes, C. Pasquali, N. Paquet, F. Ravier, J. C. Sanchez, S. Frutiger, G. Hughes, V. Pallini, D. F. Hochstrasser, and P. Tosi. 1993. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 14:1023-1031. [DOI] [PubMed] [Google Scholar]
- 16.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham, D. F., and B. O'Connor. 1997. Proline specific peptidases. Biochim. Biophys. Acta 1343:160-186. [DOI] [PubMed] [Google Scholar]
- 18.Di Cagno, R., M. De Angelis, P. Lavermicocca, M. De Vincenzi, C. Giovannini, M. Faccia, and M. Gobbetti. 2002. Proteolysis by sourdough lactic acid bacteria: effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Appl. Environ. Microbiol. 68:623-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutton, G. G. S. 1973. Application of gas-liquid chromatography to carbohydrates: part I. Adv. Carbohydr. Chem. Biochem. 28:11-160. [Google Scholar]
- 20.Fasano, A., I. Berti, T. Gerarduzzi, T. Not, R. B. Colletti, S. Drago, Y. Elitsur, P. H. Green, S. Guandalini, I. D. hill, M. Pietzak, A. Ventura, M. Thorpe, D. Kryszak, F. Fornaroli, S. S. Wasserman, J. A. Murray, and K. Horvath. 2003. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch. Intern. Med. 163:286-292. [DOI] [PubMed] [Google Scholar]
- 21.Fasano, A., and C. Catassi. 2001. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology 120:636-651. [DOI] [PubMed] [Google Scholar]
- 22.Gobbetti, M., P. Ferranti, E. Smacchi, F. Goffredi, and F. Addeo. 2002. Production of angiotensin-I-converting-enzyme-inhibitory peptides in fermented milks started by Lactobacillus delbrueckii subsp. bulgaricus SS1 and Lactococcus lactis subsp. cremoris FT4. Appl. Environ. Microbiol. 66:3898-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gobbetti, M. 1998. The sourdough microflora: interactions between lactic acid bacteria and yeast. Trends Food Sci. Technol. 9:267-274. [Google Scholar]
- 24.Gobbetti, M., E. Smacchi, and A. Corsetti. 1996. The proteolytic system of Lactobacillus sanfrancisco CB1: purification and characterization of a proteinase, a dipeptidase, and an aminopeptidase. Appl. Environ. Microbiol. 62:3220-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greco, L., G. Dadamo, A. Truscelli, G. Parrilli, M. Mayer, and G. Budillon. 1991. Intestinal permeability after single dose gluten challenge in coeliac disease. Arch. Dis. Child. 66:870-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardman, C. M., and L. Fry. 1997. Absence of toxicity of oats in patients with dermatitis herpetiformis. N. Engl. J. Med. 337:1884-1887. [DOI] [PubMed] [Google Scholar]
- 27.Hausch, F., L. Shan, N. A. Santiago, G. M. Gray, and C. Khosla. 2003. Intestinal digestive resistance of immunodominant gliadin peptides. Am. J. Physiol. 283:996-1003. [DOI] [PubMed] [Google Scholar]
- 28.Hochstrasser, D. F., M. G. Harrington, A. C. Hochstrasser, M. J. Miller, and C. R. Merril. 1988. Methods for increasing the resolution of two dimensional protein electrophoresis. Anal. Biochem. 173:424-435. [DOI] [PubMed] [Google Scholar]
- 29.Kumar, K. K., and K. P. Parameswaran. 1988. Characterisation of storage protein from selected varieties of foxtail millet (Setaria italica L). J. Sci. Food Agric. 77:535-542. [Google Scholar]
- 30.Kunji, E. R. S., I. Mierau, A. Hagting, B. Poolman, and W. N. Konings. 1996. The proteolytic systems of lactic acid bacteria. Antonie Van Leeuwenhoek 70:187-221. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 32.Logan, R. F. A. 1992. Problems and pitfalls in epidemiological studies of coeliac disease. Dyn. Nutr. Res. 2:14-24. [Google Scholar]
- 33.Lorenz, K., and W. Dilsaver. 1980. Rheological properties and food applications of proso millet flours. Cereal Chem. 57:21-24. [Google Scholar]
- 34.Madara, J. L., and J. S. Trier. 1980. Structural abnormalities of jejunal epithelial cell membranes in celiac sprue. Lab. Investig. 43:254-261. [PubMed] [Google Scholar]
- 35.Maiuri, L., A. Picarelli, M. Boirivant, S. Coletta, M. C. Mazzoli, M. De Vincenzi, M. Londei, and S. Auricchio. 1996. Definition of the initial immunological modifications, upon in vitro gliadin challenge, in the small intestine of celiacs. Gastroenterology 110:1368-1378. [DOI] [PubMed] [Google Scholar]
- 36.Marsh, M. N., S. Morgan, A. Ensari, T. Wardle, R. Lobley, C. Mills, and S. Auricchio. 1995. In vivo activity of peptide 31-43, 44-55, 56-68 of alfa-gliadin in gluten sensitive enteropathy (GSE). Gastroenterology 108:A871. [Google Scholar]
- 37.Matuz, J., T. Bartók, K. Mórocz-Salamon, and L. Bóna. 2000. Structure and potential allergenic character of cereal proteins. I. Protein content and amino acid composition. Cereal Res. Commun. 28:263-270. [Google Scholar]
- 38.Mikola, M., O. Brinck, and B. L. Jones. 2001. Characterization of oat endoproteinases that hydrolyze oat avenins. Cereal Chem. 78:55-58. [Google Scholar]
- 39.Osborne, T. B. 1907. The proteins of the wheat kernel. Carnegie Institute of Washington publication 84. Judd and Detweiler, Washington, D.C.
- 40.Shan, L., O. Molberg, I. Parrot, F. Hausch, F. Filiz, G. M. Gray, L. M. Sollid, and C. Khosla. 2002. Structural basis for gluten intolerance in celiac sprue. Science 297:2275-2279. [DOI] [PubMed] [Google Scholar]
- 41.Silano, M., and M. De Vincenzi. 1999. Bioactive antinutritional peptides derived from cereal prolamins: a review. Nahrung 43:175-184. [DOI] [PubMed] [Google Scholar]
- 42.Sollid, L. 2002. Coeliac disease: dissecting a complex inflammatory disorder. Nat. Rev. Immunol. 2:647-655. [DOI] [PubMed] [Google Scholar]
- 43.Steadman, K. J., M. S. Burgoon, B. A. Lewis, S. E. Edwardson, and R. L. Obendorf. 2001. Minerals, phytic acid, tannin and rutin in buckwheat seed milling fractions. J. Sci. Food Agric. 81:1094-1100. [DOI] [PubMed] [Google Scholar]
- 44.Stepaniak, L. 1999. Purification and characterization of proline-specific peptidases from Lactococcus and Lactobacillus. Electronic Polish Agric. Univ. 2. [Online.] http://www.ejpau.media.pl/series/volume2/issue2/food/art-05.html.
- 45.Thiele, C., M. G. Ganzle, and R. F. Vogel. 2002. Contribution of sourdough lactobacilli, yeast, and cereal enzymes to the generation of amino acids in dough relevant for bread flavor. Cereal Chem. 79:45-51. [Google Scholar]
- 46.Toth, G. B., and H. Pavia. 2001. Removal of dissolved brown algal phlorotannins using insoluble polyvinylpolypyrrolidone (PVPP). J. Chem. Ecol. 27:1899-1910. [DOI] [PubMed] [Google Scholar]
- 47.Weiss, W., C. Vogelmeier, and A. Gorg. 1993. Electrophoretic characterization of wheat grain allergens from different cultivars involved in bakers' asthma. Electrophoresis 14:805-816. [DOI] [PubMed] [Google Scholar]