Background: The Golgi docking mechanisms for transport vesicles carrying glycosyltransferases are largely unknown.
Results: C1GalT1 utilizes GM130-GRASP65 when GRASP65 is available but GM130-Giantin without GRASP65, whereas C2GnT-M employs Giantin for Golgi targeting.
Conclusion: The Golgi-targeting mechanism is glycosyltransferase-specific.
Significance: Understanding the Golgi-targeting mechanisms of glycosyltransferases may help uncover altered glycosylation in some diseases.
Keywords: Glycobiology, Glycosylation, Glycosyltransferases, Golgi, Trafficking, ER-to-Golgi Trafficking, Golgi Docking, Golgins
Abstract
Glycosylation of secreted and membrane-bound mucins is carried out by glycosyltransferases localized to specific Golgi compartments according to the step in which each enzyme participates. However, the Golgi-targeting mechanisms of these enzymes are not clear. Herein, we investigate the Golgi-targeting mechanisms of core 1 β3 galactosyltransferase (C1GalT1) and core 2 β1,6-N-acetylglucosaminyltransferase-2 or mucus type (C2GnT-M), which participate in the early O-glycosylation steps. siRNAs, co-immunoprecipitation, and confocal fluorescence microscopy were employed to identify the golgins involved in the Golgi docking of vesicular complexes (VCs) that carry these two enzymes. We have found that these VCs use different golgins for docking: C2GnT-M-carrying VC (C2GnT-M-VC) utilizes Giantin, whereas C1GalT1-VC employs GM130-GRASP65 complex. However, in the absence of GRASP65, C1GalT1-VC utilizes GM130-Giantin complex. Also, we have found that these VCs are 1.1–1.2 μm in diameter, specific for each enzyme, and independent of coat protein complex II and I (COPII and COPI). These two fluorescently tagged enzymes exhibit different fluorescence recovery times in the Golgi after photobleaching. Thus, novel enzyme-specific Golgi-targeting mechanisms are employed by glycosyltransferases, and multiple Golgi docking strategies are utilized by C1GalT1.
Introduction
Mucin-type glycans are carbohydrates linked O-glycosidically via N-acetyl-galactosamine (GalNAc) to Ser/Thr. They are found primarily in membrane-bound and secreted mucins. Membrane-bound mucins play key roles in cell-cell interactions involved in cellular immunity and cancer metastasis. Secreted mucins serve to protect mucus-secretory epithelium by retention of water and entrapment and clearance of inhaled and ingested pathogens. The functions of mucins mainly reside in these glycans. Mucin-type glycans are synthesized in the Golgi apparatus as catalyzed in a template-independent fashion by glycosyltransferases (GTs)2 localized to the Golgi compartments according to the biosynthetic steps in which they participate. Following the formation of GalNAc-O-Ser/Thr catalyzed by peptidyl GalNAc transferases (1), core 1 (Galβ1–3GalNAc) is generated by C1GalT1 (2). Then, core 1 is converted to core 2, Galβ1–3(GlcNAcβ1–6)GalNAc, by core 2 enzymes, which include C2GnT-1, C2GnT-M, and C2GnT-3. These core structures serve as the anchors for elaboration of many biologically important carbohydrate structures. Therefore, these two enzymes control the biological functions of mucins. Loss of C1GalT1 is embryonic lethal (4), and loss of C2GnT-M leads to development of colitis and colon cancer (5, 6).
Golgi GTs are synthesized in the ER, packaged in vesicles, and then transported to and retained in the Golgi. Significant progress has been made in elucidating the molecular determinants of the GTs in their Golgi-targeting (7, 8) and recycling processes (9). Golgi matrix proteins serve as the docking sites for many Golgi-targeting vesicles and are localized to the cis- and medial-Golgi (10). For example, Giantin and GM130 have been shown to be involved in the docking of transport vesicles containing secreted proteins, such as vesicular stomatitis virus protein (11). Both golgins are effectors of Rab proteins (12, 13) and may be responsible for SNARE-dependent fusion of transport vesicles to the cis-Golgi (14). Giantin is a 400-kDa dimeric protein, disulfide-bonded in the small Golgi lumenal domain, with an extended coiled-coil structure in the cytoplasm (15). GM130 is a segmented coiled-coil dimer whose C-terminal region is tethered to the Golgi (16) through interaction with the Golgi peripheral membrane protein GRASP65 (17, 18). To date, very little is known about the contribution of the golgins to the Golgi targeting of GTs. In this study, we examine the Golgi-targeting mechanisms of core 1 and core 2 enzymes. We focus on the identification of the golgins (Giantin, GM130, GRASP65, and p115) involved in the docking of the vesicular complexes (VCs) that carry C1GalT1 and C2GnT-M, which are localized to the cis- (19) and cis-medial- (9) Golgi stacks, respectively. We have found that these GTs utilize distinct COPII- and COPI-independent VCs with a diameter of 1.1–1.2 μm, as well as distinct Golgi docking sites. Further, C1GalT1-VC uses multiple docking mechanisms.
EXPERIMENTAL PROCEDURES
Cell Culture and Antibodies
Panc1-bC2GnT-M (c-Myc) cells were prepared and cultured as described previously (20). Antibodies were purchased from the following suppliers: c-Myc Abs (mouse monoclonal and rabbit polyclonal) and mouse monoclonal anti-GRASP65 Abs, Santa Cruz Biotechnology; rabbit polyclonal anti-Giantin and monoclonal anti-GM130 and mouse monoclonal (anti-Sar1, anti-β-COP, and anti-C1GalT1) Abs, Abcam; mouse monoclonal anti-β-actin Ab, Sigma; HRP-conjugated secondary Abs (donkey anti-rabbit and donkey anti-mouse), Jackson ImmunoResearch Laboratories.
Plasmid Construction and Transient Transfection of HEK293 Cells
The hC2GnT-M cDNA (GenBank accession number NM_004751) was cloned by PCR into the XhoI and BamHI sites of EGFP-N1 expression vector (Clontech) to generate hC2GnT-M-pEFGP-N1. The coding region of hC1GALT1 gene (GenBank accession number NM_020156) was cloned by PCR into XhoI and BamHI sites of the pDsRed-Monomer-N1 vector (Clontech) to generate hC1GalT1-pDsRed-Monomer-N1. HEK293 cells (ATCC) were transfected with Lipofectamine 2000 and analyzed after 2–3 days of culture in DMEM plus 10% FBS and antibiotics.
siRNA Transfection
siRNAs targeting GOLGB1 (Giantin), GOLGA2 (GM130), and scrambled ON-TARGETplus SMARTpool siRNA were purchased from Dharmacon. siRNAs targeting Sar1a, Sar1b, β-COP, or GRASP65 were obtained from Santa Cruz Biotechnology. Panc1-bC2GnT-M (c-Myc) cells were transfected with 100–150 nm siRNAs using Lipofectamine RNAiMAX reagent (Invitrogen). After 2–3 days of culture, cells were analyzed for specific proteins by Western blotting.
Immunofluorescence Analysis
Panc1-bC2GnT-M (c-Myc) cells grown overnight on coverslips were fixed in 4% paraformaldehyde/PBS at room temperature for 30 min. After treatment with primary Abs (1:100) at 37 °C for 1 h, the cells were stained with DyLight 488-donkey anti-mouse Ab (green) and DyLight 594-donkey anti-rabbit Ab (red) (1:200) and mounted in ProLong Gold antifade reagent with and without DAPI (Invitrogen). hC2GnT-M-pEFGP-N1- and hC1GalT1-pDsRed-Monomer-N1-transfected HEK293 cells were cultured in a thermoregulation and CO2 regulation device and imaged live by confocal fluorescence microscopy. GFP and RFP were excited at 488 and 543 nm, respectively. One image frame was collected every 10–20 s. Over a series of experiments, the scan speed and definition of pixels were varied to maintain unsaturated images to help visualize moving vesicles. For fluorescence recovery after photobleaching experiment, part of the Golgi in GFP- and RFP-expressing live HEK293 cells was bleached using 488 nm or 543 nm laser pulse, respectively. After five iterations, images were acquired every 8 s using confocal fluorescence imaging. Fluorescence values in the bleached area and an adjacent nonbleached area were measured using National Institutes of Health ImageJ. The fluorescence recovery was calculated as the ratio of bleached to adjacent areas normalized to the pre-bleach and immediate post-bleach values. Stained or live cells were viewed under a Zeiss 510 Meta confocal laser scanning microscope (63× 1.4 N/A oil for stained and 20× 0.5 N/A air objectives for live). Images were analyzed using Zeiss 510 software. For some figures, image analysis was performed using Adobe Photoshop and ImageJ. Supplemental Movies S1–S3 were processed by Windows Live Movie Maker.
Immunoprecipitation
Immunoprecipitation and identification of proteins in the pulldowns were carried out as described previously (9).
Statistical Analysis
The data are shown as average of three experiments, mean ± S.E. Significance of the difference in means was analyzed by the Student's t test.
RESULTS AND DISCUSSION
Different Golgi Docking Mechanisms for C2GnT-M and C1GalT1
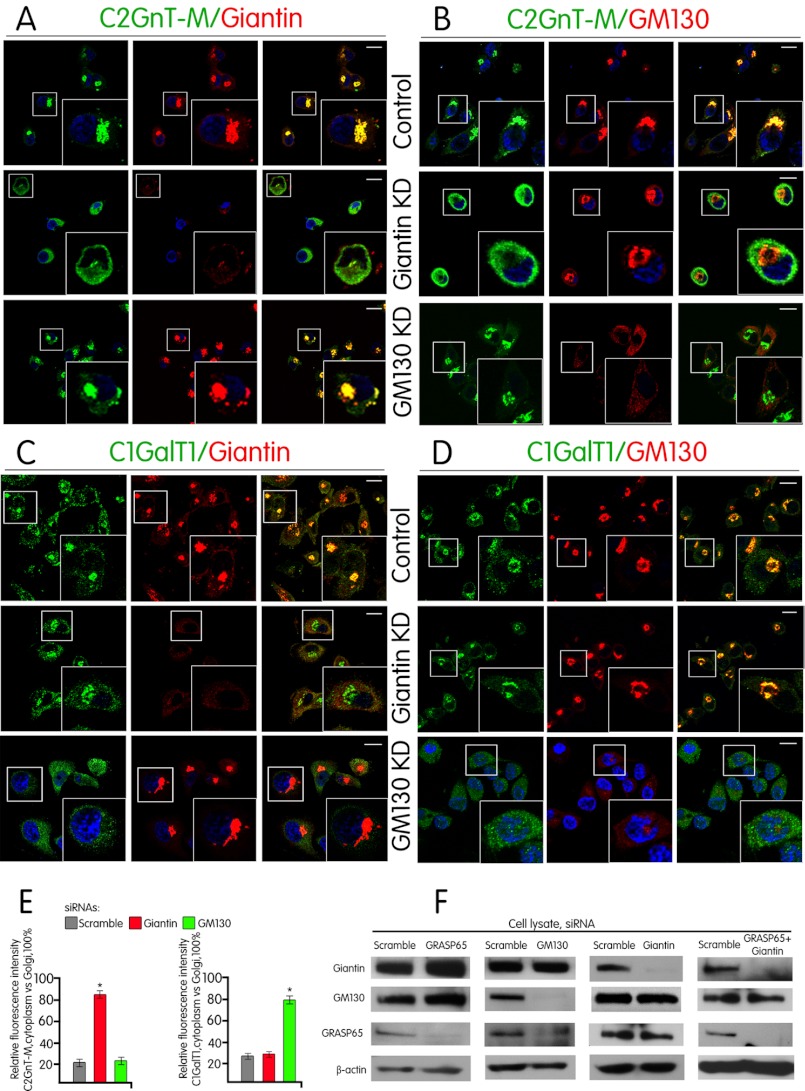
We initiated the study to examine the possible involvement of Giantin and GM130 in the Golgi targeting of C1GalT1-VC and C2GnT-M-VC. We found that knockdown (KD) of Giantin prevented Golgi localization of C2GnT-M (Fig. 1A) without affecting Golgi morphology as shown by immunofluorescence staining of GM130 (Fig. 1B). Further, Golgi morphology (21) and localization of C2GnT-M were not affected by GM130 KD (Fig. 1, A and B). Quantification of the average fluorescence of cytoplasm/Golgi ratio of C2GnT-M in these cells showed an increase from 21% in control to 84% in Giantin KD cells but no change in GM130 KD cells (Fig. 1E). These data suggest that Giantin and not GM130 is involved in the Golgi docking of C2GnT-M-VC.
FIGURE 1.
C2GnT-M-VCs utilize Giantin, whereas C1GalT1-VCs employ GM130 for Golgi targeting. A–D, confocal immunofluorescence images of Panc1-bC2GnT-M (c-Myc) cells labeled with green (anti-c-Myc Ab (A and B) anti-C1GalT1 Ab (C and D)) and red (anti-Giantin Abs (A and C) and anti-GM130 Ab (B and D)) fluorescence after treatment with scramble siRNA or siRNAs specific for Giantin or GM130. Representative cells showing KD of Giantin or GM130 are in the second or third row, respectively, of A and B or C and D. White boxes indicate areas enlarged and shown in the inset. Scale bar, 10 μm. E, quantification of C2GnT-M and C1GalT1 immunofluorescence signals of non-Golgi versus Golgi (= 100%) in cells treated with scramble siRNA or protein-specific siRNA as shown in A and B and in C and D. *, p < 0.001. F, Giantin, GM130, and GRASP65 Western blots of the lysates of Panc1-bC2GnT-M (c-Myc) cells treated with scramble siRNA or siRNA specific for Giantin, GM130, GRASP65, or GRASP65 plus Giantin.
GM130 is known to play an essential role in mediating fast and complete incorporation of ER-to-Golgi carriers into the Golgi stacks (22) independent of Giantin (23). We found that KD of GM130 prevented Golgi targeting of C1GalT1 in Panc1-bC2GnT-M (c-Myc) cells (Fig. 1, C and D). However, KD of Giantin did not affect Golgi localization of C1GalT1 (Fig. 1D). Quantification of the average fluorescence of the cytoplasm/Golgi ratio of C1GalT1 in these cells showed a 50% increase in GM130 KD cells over the control cells but no change in Giantin KD cells (Fig. 1E). The data indicate that GM130 and not Giantin is involved in the docking of C1GalT1-VC.
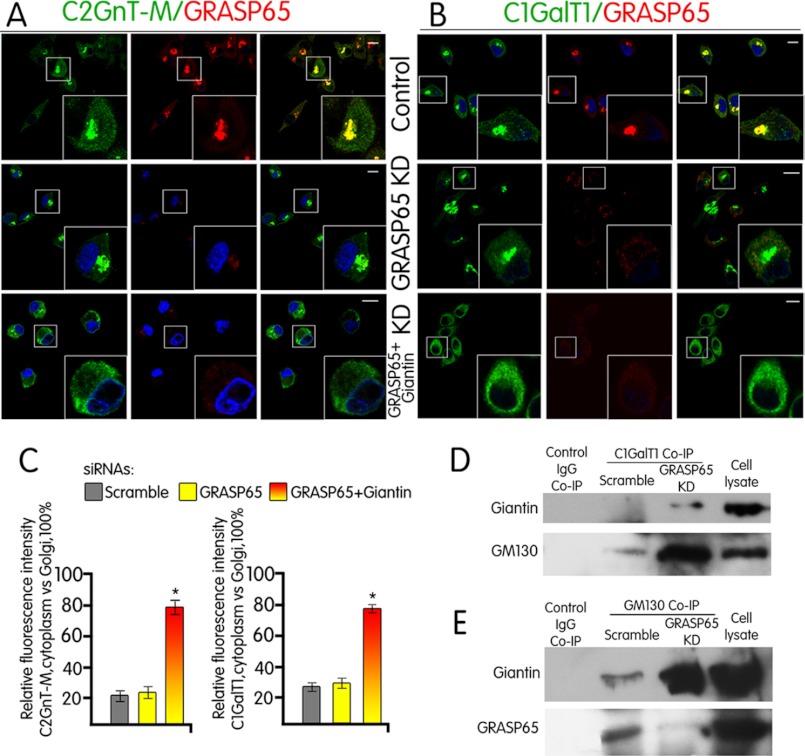
It has been well established that GM130 is localized to the Golgi by binding to a Golgi membrane-associated matrix protein GRASP65 (17, 18). To confirm that GRASP65 participates in GM130-mediated docking of the C1GalT1-VC, we examined the Golgi localization of C1GalT1 after depletion of GRASP65. To our surprise, C1GalT1 was still localized to the Golgi after KD of GRASP65 (Fig. 2B). The results suggest that C1GalT1 acquires an alternative Golgi docking mechanism in the absence of GRASP65. After a series of experiments, we found that KD of GM130 led to reduced GRASP65 (Fig. 1F), which confirmed the published result (24) that without its binding partner GM130, GRASP65 was degraded. Further, KD of GRASP65 resulted in an increase of not only Giantin and GM130 (Fig. 1F) but also complexes of both C1GalT1-GM130-Giantin and GM130-Giantin (Fig. 2, D and E). These results led us to hypothesize that these two Golgi matrix proteins were involved in the Golgi docking of C1GalT1-VC when GRASP65 was not available. The hypothesis was proven by the failure of C1GalT1 to target to the Golgi after KD of both GRASP65 and Giantin (Fig. 2, B and C). We also found that prior to GRASP65 KD, Giantin was not pulled down by anti-C1GalT1 Ab (Fig. 2D), suggesting that formation of the C1GalT1-GM130-Giantin complex occurred after KD of GRASP65. Further, in the cells treated with scramble siRNA, GRASP65 was pulled down by anti-GM130 Ab (Fig. 2E), confirming that GM130 forms a complex with GRASP65 in Panc1-bC2GnT-M (c-Myc) cells when GRASP65 was present. The Giantin-independent nature of the GM130-GRASP65 complex was confirmed by the lack of effect of Giantin KD on GRASP65 and GM130 (Fig. 1F). Also, KD of Giantin plus GRASP65 did not affect the amount of GM130 (Fig. 1F) but prevented Golgi localization of C1GalT1, confirming that GM130 alone could not serve as the Golgi docking site for C1GalT1-VC. We also noticed that the Golgi morphology was not affected under this condition (data not shown). Taken together, the results indicate that GM130-GRASP65 serves as the docking site for C1GalT1-VC when GRASP65 is available, and in the absence of GRASP65, C1GalT1-VC is targeted to the Golgi using the GM130-Giantin complex for docking.
FIGURE 2.
C1GalT1-VCs utilize GM130 and GRASP65 in the presence of GRASP65 but GM130 and Giantin in the absence of GRASP65 for Golgi targetting. A and B, confocal immunofluorescence images of Panc1-bC2GnT-M (c-Myc) cells labeled with green (anti-c-Myc Ab (A) anti-C1GalT1 Ab (B)) and red (anti-GRASP65 Ab) fluorescence after treatment with scramble siRNA or siRNA specific for GRASP65 or GRASP65+Giantin siRNAs are shown. Scale bar, 10 μm. C, quantification of C2GnT-M and C1GalT1 immunofluorescence signals of non-Golgi versus Golgi (= 100%) in cells treated with scramble siRNA or protein-specific siRNA as shown in A and B. *, p < 0.001. D, Giantin and GM130 Western blots of the C1GalT1 immunoprecipitates from the lysates of Panc1-bC2GnT-M (c-Myc) cells treated with scramble or GRASP65-specific siRNA. Knockdown of GRASP65 induced the formation of C1GalT1-Giantin-GM130 complex. E, Giantin and GRASP65 Western blot of the GM130 immunoprecipitate from the lysates of Panc1-bC2GnT-M (c-Myc) cells treated with scramble or GRASP65-specific siRNA. GM130 forms a complex with GRASP65, as shown in the control cells, and KD of GRASP65 increased GM130-Giantin complex. Equal amounts of proteins in the lysates were used for co-immunoprecipitation (Co-IP) as shown by the β-actin blot in Fig. 1F.
Next, we examined the dynamic intracellular transport of C2GnT-M and C1GalT1 to the Golgi using time-lapse imaging in live HEK293 cells that expressed C2GnT-M-GFP and C1GalT1-RFP. As shown in Fig. 3A and supplemental Movie S1, C2GnT-M-VCs and C1GalT1-VCs were segregated at the cell periphery and on their way to the Golgi. To quantify the kinetics of the formation and fusion of individual VCs, we analyzed 90 cells from three independent experiments and found that C2GnT-M-VCs and C1GalT1-VCs were originated in the cytosol and disappeared at the Golgi surface within 60–145 s. The shapes of these VCs ranged from round to oval with an average diameter of 1.1 ± 0.3 μm for C2GnT-M-VC and 1.2 ± 0.2 μm for C1GalT1-VC. The sizes of these VCs were quite different from the 50–70-nm sizes reported for COPI- and COPII-coated vesicles (25–27). It was noted that only 6% of GFP- and RFP-carrying vesicles were totally colocalized and 7.5% of them were partially colocalized, whereas the majority (86.5%) was segregated (Fig. 3B).
FIGURE 3.
A, direct visualization of C2GnT-M-GFP-VC and C1GalT1-RFP-VC dynamics in live HEK293 cells. Individual images from the movie of cells expressing C2GnT-M-GFP and C1GalT1-RFP over a time frame of 00.00–73.76 s are shown; the positions of anterograde ER-to-Golgi spots are indicated by arrows. Note that C2GnT-M-GFP-VCs and C1GalT1-RFP-VCs originating from opposite sides of cell periphery move toward and fuse with Golgi. The arrows at 73.76 s indicate the trajectory of Golgi-targeting C2GnT-M-GFP-VC (1) and C1GalT1-RFP-VC (2). Scale bar, 5 μm. B, quantification of colocalization of the anterograde ER-to-Golgi spots of C2GnT-M- and C1GalT1-VCs. Note that in 86.5% of the vesicles, these two VCs were not colocalized. *, p < 0.001. C–E, time-dependent recovery of green (C2GnT-M-GFP) and red (C1GalT1-RFP) fluorescence in the Golgi after photobleaching. A part of the Golgi (white box in C and highlighted in D) was bleached using the 488- and 543-nm laser pulse, respectively. The images of fluorescence signal were recorded every 8 s to determine recovery of fluorescence intensity. Restoration of C2GnT-M-GFP fluorescence was completed at 168 s, whereas recovery of C1GalT1-RFP was substantially delayed. Representative images of the indicated times are shown in D. E, quantification of C2GnT-M-GFP and C1GalT1-RFP within 216 s after photobleaching. The ratio of fluorescence (bleached/unbleached) was measured every 24 s, and values were normalized to those of the pre-bleached area. F, proposed model of the interaction of distinct VCs with cis-Golgi membrane before and after GRASP65 depletion. C2GnT-M-VCs reach the Golgi through association with Giantin, whereas C1GalT1-VCs use the GM130-GRASP65 complex. In the absence of GRASP65, C1GalT1-VCs switch to the GM130-Giantin site for Golgi docking. This new association accompanies elevated amounts of GM130 and Giantin. For simplicity, Giantin, GM130, and GRASP65 are shown as monomers. Red stars indicate complex formed between golgins and C2GnT-M-VC or C1GalT1-VC.
To further examine whether C2GnT-M-VC or C1GalT1-VC used distinct transport mechanisms, we also monitored fluorescence recovery after photobleaching of a specific region of the Golgi in cells expressing C2GnT-M-GFP and C1GalT1-RFP (Fig. 3, C–E). The time-dependent recovery of the fluorescence in this area represented the rate of Golgi filling by C2GnT-M-GFP and C1GalT1-RFP. Fig. 3, D and E, showed distinct kinetics of fluorescence recovery; C2GnT-M-GFP fluorescence was completely restored at 168 s, whereas recovery of C1GalT1-RFP was completed at 240 s (supplemental Movies S2 and 3).
Golgi Targeting of C1GalT1 and C2GnT-M Is Independent of COPII and COPI
What remains unclear is how these Golgi-resident enzymes are transported to the Golgi. To examine whether COPII was involved in Golgi targeting of these two enzymes (28), we monitored the Golgi localization of both C1GalT1 and C2GnT-M after KD of Sar1a or Sar1b, which are GTPases regulating the assembly and disassembly of COPII coats (25). The detection of both enzymes in the Golgi (supplemental Fig. S1) suggests that Golgi targeting of both Golgi enzymes is COPII-independent. The COPII-independent ER-to-Golgi trafficking of C2GnT-M and C1GalT1 was further supported by unaltered COPII distribution after KD of Giantin or GM130 (data not shown). Our data fit well with the reported observations that non-golgin Golgi proteins are involved in the docking of COPII (29) and that 500-nm range tubular transport carriers are found shuttling between ER and Golgi (30). This transport mechanism requires intact microtubules and occurs independent of COPI or COPII coats (31, 32). However, the result is at variance with a previous study (28), which claims that COPII is involved in the Golgi targeting of GTs. In this study, the employment of a Sar1 mutant may have compromised the data interpretation because this Sar1 mutant causes alterations of the Golgi morphology.
COPI vesicles (33) and ERGIC (34) have been shown to be involved in the ER-to-Golgi transport of GTs. However, KD of either β-COP (a COPI vesicle subunit) (35) or ERGIC-53 (36) (the cargo transport receptor for glycoproteins) did not affect intra-Golgi localization of C2GnT-M (9) and C1GalT1 (supplemental Fig. S2) (data on ERGIC-53 not shown). We also performed KD of p115, a binding partner of both Giantin and GM130 (11, 16), and found that both enzymes were still in the Golgi (data not shown). Therefore, Golgi targeting of C1GalT1-VC and C2GnT-M-VC is also independent of COPI, ERGIC, and p115.
In summary, our data show that the ER-to-Golgi transportation is different for C2GnT-M and C1GalT1 by not only their VCs but also their docking sites on the Golgi (Fig. 3F). C1GalT1 is a unique GT because it is the enzyme that controls the synthesis of core 1- and core 2-associated glycans, which constitute the majority of mucin glycans. It is thus not a surprise that this enzyme has its own molecular chaperone core 1 β3-Gal-T-specific molecular chaperone (37), a unique ER-to-Golgi transport strategy, and multiple Golgi docking mechanisms. Taken together, our results have revealed novel Golgi-targeting mechanisms for different GTs. It should be mentioned that C1GalT1 lacks an N-glycosylation site, whereas bC2GnT-M is N-glycosylated (38). This information coupled with the fact that the processing of N-glycans starts at the cis-Golgi (39) provides a rationale for predicting that VCs that carry glycoproteins decorated with N-glycans use Giantin as a major docking site. On the other hand, VCs that carry glycoproteins without N-glycans or proteins without glycans may employ GM130-dependent docking site(s). The GM130-independent Golgi targeting of peptidyl GalNAc T2 (24), another Golgi GT with one N-glycosylation site (Asn-516) (European Molecular Biology Laboratory (EMBL)-Bank accession number: BC041120.1), is consistent with the hypothesis. Additional evidence that supports the hypothesis and information about the chemical nature and transport mechanisms of these VCs remain to be discovered.
Supplementary Material
This work was supported in part by the Office of Research and Development, Department of Veterans Affairs (Grant VA 1I1BX000985), the National Institutes of Health (Grants 1R21HL097238 and 2RO1HL48282) (to P. W. C.), and the State of Nebraska (Grant LB506) (to P. W. C.).

This article contains supplemental Movies S1–S3 and Figs. S1 and S2.
- GT
- glycosyltransferase
- KD
- knockdown
- VCs
- vesicular complexes
- C1GalT1
- core 1 β3 galactosyltransferase
- C2GnT-M
- core 2 β1,6-N-acetylglucosaminyltransferase-2 or mucus type
- C1GalT1-VC
- C1GalT1-carrying vesicular complex
- C2GnT-M-VC
- C2GnT-M-carrying vesicular complex
- bC2GnT-M
- bovine C2GnT-M
- hC2GnT-M
- human C2GnT-M
- ERGIC
- ER-to-Golgi intermediate compartment
- ER
- endoplasmic reticulum
- Sar1
- secretion-associated RAS-related protein 1
- COPI/II
- coat protein complex I/II
- GRASP65
- Golgi reassembly stacking protein 65
- Ab
- antibody.
REFERENCES
- 1. Hanisch F. G. (2001) O-Glycosylation of the mucin type. Biol. Chem. 382, 143–149 [DOI] [PubMed] [Google Scholar]
- 2. Ju T., Brewer K., D'Souza A., Cummings R. D., Canfield W. M. (2002) Cloning and expression of human core 1 β1,3-galactosyltransferase. J. Biol. Chem. 277, 178–186 [DOI] [PubMed] [Google Scholar]
- 3. Cheng P. W., Radhakrishnan P. (2011) in Molecular Immunology of Complex Carbohydrates-3 (Wu A. M., ed), Vol. 705 pp, 465–492, Plenum Press, New York [Google Scholar]
- 4. Xia L., Ju T., Westmuckett A., An G., Ivanciu L., McDaniel J. M., Lupu F., Cummings R. D., McEver R. P. (2004) Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. J. Cell Biol. 164, 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang M. C., Chen H. Y., Huang H. C., Huang J., Liang J. T., Shen T. L., Lin N. Y., Ho C. C., Cho I. M., Hsu S. M. (2006) C2GnT-M is down-regulated in colorectal cancer, and its re-expression causes growth inhibition of colon cancer cells. Oncogene 25, 3267–3276 [DOI] [PubMed] [Google Scholar]
- 6. Stone E. L., Ismail M. N., Lee S. H., Luu Y., Ramirez K., Haslam S. M., Ho S. B., Dell A., Fukuda M., Marth J. D. (2009) Glycosyltransferase function in core 2-type protein O-glycosylation. Mol. Cell. Biol. 29, 3770–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colley K. J. (1997) Golgi localization of glycosyltransferases: more questions than answers. Glycobiology 7, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tu L., Banfield D. K. (2010) Localization of Golgi-resident glycosyltransferases. Cell Mol. Life Sci. 67, 29–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petrosyan A., Ali M. F., Verma S. K., Cheng H., Cheng P. W. (2012) Non-muscle myosin IIA transports a Golgi glycosyltransferase to the endoplasmic reticulum by binding to its cytoplasmic tail. Int. J. Biochem. Cell Biol. 44, 1153–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Appenzeller-Herzog C., Hauri H. P. (2006) The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J. Cell Sci. 119, 2173–2183 [DOI] [PubMed] [Google Scholar]
- 11. Alvarez C., Garcia-Mata R., Hauri H. P., Sztul E. (2001) The p115-interactive proteins GM130 and giantin participate in endoplasmic reticulum-Golgi traffic. J. Biol. Chem. 276, 2693–2700 [DOI] [PubMed] [Google Scholar]
- 12. Rosing M., Ossendorf E., Rak A., Barnekow A. (2007) Giantin interacts with both the small GTPase Rab6 and Rab1. Exp. Cell Res. 313, 2318–2325 [DOI] [PubMed] [Google Scholar]
- 13. Moyer B. D., Allan B. B., Balch W. E. (2001) Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis-Golgi tethering. Traffic 2, 268–276 [DOI] [PubMed] [Google Scholar]
- 14. Shorter J., Beard M. B., Seemann J., Dirac-Svejstrup A. B., Warren G. (2002) Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J. Cell Biol. 157, 45–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Linstedt A. D., Hauri H. P. (1993) Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol. Biol. Cell 4, 679–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakamura N., Lowe M., Levine T. P., Rabouille C., Warren G. (1997) The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell 89, 445–455 [DOI] [PubMed] [Google Scholar]
- 17. Barr F. A., Puype M., Vandekerckhove J., Warren G. (1997) GRASP65, a protein involved in the stacking of Golgi cisternae. Cell 91, 253–262 [DOI] [PubMed] [Google Scholar]
- 18. Lesa G. M., Seemann J., Shorter J., Vandekerckhove J., Warren G. (2000) The amino-terminal domain of the Golgi protein Giantin interacts directly with the vesicle-tethering protein p115. J. Biol. Chem. 275, 2831–2836 [DOI] [PubMed] [Google Scholar]
- 19. Narimatsu Y., Ikehara Y., Iwasaki H., Nonomura C., Sato T., Nakanishi H., Narimatsu H. (2008) Immunocytochemical analysis for intracellular dynamics of C1GalT associated with molecular chaperone, Cosmc. Biochem. Biophys. Res. Commun. 366, 199–205 [DOI] [PubMed] [Google Scholar]
- 20. Choi K. H., Basma H., Singh J., Cheng P. W. (2005) Activation of CMV promoter-controlled glycosyltransferase and β-galactosidase glycogenes by butyrate, tricostatin A, and 5-aza-2′-deoxycytidine. Glycoconj. J. 22, 63–69 [DOI] [PubMed] [Google Scholar]
- 21. Kodani A., Sütterlin C. (2008) The Golgi protein GM130 regulates centrosome morphology and function. Mol. Biol. Cell 19, 745–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marra P., Maffucci T., Daniele T., Tullio G. D., Ikehara Y., Chan E. K., Luini A., Beznoussenko G., Mironov A., De Matteis M. A. (2001) The GM130 and GRASP65 Golgi proteins cycle through and define a subdomain of the intermediate compartment. Nat. Cell Biol. 3, 1101–1113 [DOI] [PubMed] [Google Scholar]
- 23. Weide T., Bayer M., Köster M., Siebrasse J. P., Peters R., Barnekow A. (2001) The Golgi matrix protein GM130: a specific interacting partner of the small GTPase rab1b. EMBO Rep. 2, 336–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Puthenveedu M. A., Bachert C., Puri S., Lanni F., Linstedt A. D. (2006) GM130- and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat. Cell Biol. 8, 238–248 [DOI] [PubMed] [Google Scholar]
- 25. Fromme J. C., Schekman R. (2005) COPII-coated vesicles: flexible enough for large cargo? Curr. Opin. Cell Biol. 17, 345–352 [DOI] [PubMed] [Google Scholar]
- 26. Nickel W., Brügger B., Wieland F. T. (2002) Vesicular transport: the core machinery of COPI recruitment and budding. J. Cell Sci. 115, 3235–3240 [DOI] [PubMed] [Google Scholar]
- 27. Stephens D. J., Pepperkok R. (2001) Illuminating the secretory pathway: when do we need vesicles? J. Cell Sci. 114, 1053–1059 [DOI] [PubMed] [Google Scholar]
- 28. Quintero C. A., Giraudo C. G., Villarreal M., Montich G., Maccioni H. J. (2010) Identification of a site in Sar1 involved in the interaction with the cytoplasmic tail of glycolipid glycosyltransferases. J. Biol. Chem. 285, 30340–30346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cao X., Ballew N., Barlowe C. (1998) Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 17, 2156–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fan J. Y., Roth J., Zuber C. (2003) Ultrastructural analysis of transitional endoplasmic reticulum and pre-Golgi intermediates: a highway for cars and trucks. Histochem. Cell Biol. 120, 455–463 [DOI] [PubMed] [Google Scholar]
- 31. Martínez-Alonso E., Egea G., Ballesta J., Martínez-Menárguez J. A. (2005) Structure and dynamics of the Golgi complex at 15 °C: low temperature induces the formation of Golgi-derived tubules. Traffic 6, 32–44 [DOI] [PubMed] [Google Scholar]
- 32. Simpson J. C., Nilsson T., Pepperkok R. (2006) Biogenesis of tubular ER-to-Golgi transport intermediates. Mol. Biol. Cell 17, 723–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scales S. J., Pepperkok R., Kreis T. E. (1997) Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell 90, 1137–1148 [DOI] [PubMed] [Google Scholar]
- 34. Marra P., Salvatore L., Mironov A., Jr., Di Campli A., Di Tullio G., Trucco A., Beznoussenko G., Mironov A., De Matteis M. A. (2007) The biogenesis of the Golgi ribbon: the roles of membrane input from the ER and of GM130. Mol. Biol. Cell 18, 1595–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waters M. G., Serafini T., Rothman J. E. (1991) ’Coatomer’: a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature 349, 248–251 [DOI] [PubMed] [Google Scholar]
- 36. Hauri H. P., Kappeler F., Andersson H., Appenzeller C. (2000) ERGIC-53 and traffic in the secretory pathway. J. Cell Sci. 113, 587–596 [DOI] [PubMed] [Google Scholar]
- 37. Ju T., Cummings R. D. (2002) A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β3-galactosyltransferase. Proc. Natl. Acad. Sci. U.S.A. 99, 16613–16618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singh J., Khan G. A., Kinarsky L., Cheng H., Wilken J., Choi K. H., Bedows E., Sherman S., Cheng P. W. (2004) Identification of disulfide bonds among the nine core 2 N-acetylglucosaminyltransferase-M cysteines conserved in the mucin β6-N-acetylglucosaminyltransferase family. J. Biol. Chem. 279, 38969–38977 [DOI] [PubMed] [Google Scholar]
- 39. Stanley P., Schachter H., Taniguchi N. (2009) in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E.) 2nd Ed., pp. 101–114, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.