Background: Little is known about the complement evasion strategies of Enterococcus faecalis.
Results: Inactivation of tagB in E. faecalis V583 resulted in the loss of two wall teichoic acids (WTAs) associated with a strongly increased complement deposition by the lectin pathway.
Conclusion: WTA is critical for complement evasion in E. faecalis.
Significance: WTA biosynthesis may be a valuable target for novel antimicrobial agents.
Keywords: Bacterial Pathogenesis, Cell Wall, Immunology, Innate Immunity, Polysaccharide, Enterococcus faecalis, Complement System, Mannose-binding Lectin, Teichoic Acid
Abstract
The complement system is part of our first line of defense against invading pathogens. The strategies used by Enterococcus faecalis to evade recognition by human complement are incompletely understood. In this study, we identified an insertional mutant of the wall teichoic acid (WTA) synthesis gene tagB in E. faecalis V583 that exhibited an increased susceptibility to complement-mediated killing by neutrophils. Further analysis revealed that increased killing of the mutant was due to a higher rate of phagocytosis by neutrophils, which correlated with higher C3b deposition on the bacterial surface. Our studies indicated that complement activation via the lectin pathway was much stronger on the tagB mutant compared with wild type. In concordance, we found an increased binding of the key lectin pathway components mannose-binding lectin and mannose-binding lectin-associated serine protease-2 (MASP-2) on the mutant. To understand the mechanism of lectin pathway inhibition by E. faecalis, we purified and characterized cell wall carbohydrates of E. faecalis wild type and V583ΔtagB. NMR analysis revealed that the mutant strain lacked two WTAs with a repeating unit of →6)[α-l-Rhap-(1→3)]β-d-GalpNAc-(1→5)-Rbo-1-P and →6) β-d-Glcp-(1→3) [α-d-Glcp-(1→4)]-β-d-GalpNAc-(1→5)-Rbo-1-P→, respectively (Rbo, ribitol). In addition, compositional changes in the enterococcal rhamnopolysaccharide were noticed. Our study indicates that in E. faecalis, modification of peptidoglycan by secondary cell wall polymers is critical to evade recognition by the complement system.
Introduction
Enterococcus faecalis is a Gram-positive nosocomial pathogen that is a frequent cause of infection in critically ill patients (1). Underlying malignancy, neutropenia, antineoplastic chemotherapy, and immunosuppressive medication are well characterized risk factors for invasive infections with enterococci (2–4), and the clinical outcome of invasive enterococcal infections in this patient population is frequently poor (5).
Gram-positive microorganisms are typically surrounded by a thick layer of peptidoglycan. Threading through the peptidoglycan cell wall are long anionic polymers called teichoic acids. Wall teichoic acid (WTA)4 is attached by a phosphodiester bond of its disaccharide linkage unit to a MurNAc residue of peptidoglycan. The linkage unit is usually followed by a long chain of glycerol (Gro)- or ribitol (Rbo) phosphate repeats (6, 7). Lipoteichoic acid, on the other hand, is most frequently composed of a Gro-phosphate repeating unit and inserted into the cell membrane via a glycolipid anchor. In addition, other glycopolymers may be covalently attached to peptidoglycan, and together with WTA, they represent the secondary cell wall polysaccharides. E. faecalis expresses a rhamnopolysaccharide (also called enterococcal polysaccharide antigen or Epa) as a secondary cell wall polysaccharide. This complex carbohydrate is synthesized by the epa locus and bears a strong similarity to the group antigens of other streptococci. Its functions are incompletely understood, but studies have suggested a role in adherence to and invasion of host tissues (8), biofilm formation (9), resistance to phagocytosis (10), and virulence in a mouse peritonitis model (11). WTA of E. faecalis has not been studied to date, but in other Gram-positive bacteria, WTA has been shown to have roles in cell division, autolysin activity, scaffolding of surface proteins, attachment to host cells and abiotic surfaces, and cation hemostasis (12, 13). In addition, recent studies have suggested that WTA of Staphylococcus aureus is an important ligand for molecules of the complement system (14).
The complement system is an important first line of defense against invasive infections (15, 16). Its activation results in the labeling of bacteria with opsonic molecules (C3b/iC3b) that are recognized by phagocytic cells to initiate phagocytosis. There are three pathways by which complement can recognize a foreign antigen: the classical, lectin, and alternative pathway. These pathways converge in the formation of C3 convertase enzymes that cleave the main complement protein C3. The classical pathway is initiated by binding of C1q to bacterium-bound antibody (Ab). The C1q-associated protease C1s then cleaves complement proteins C4 and C2, generating the surface-bound C3 convertase (C4b2a). In the lectin pathway, recognition occurs via large multimeric lectins that bind to conserved microbial sugar patterns (17): mannose-binding lectin (MBL), M-ficolin, L-ficolin, and H-ficolin. MBL recognizes specific carbohydrates on microbial sugars, whereas ficolins bind patterns of acetyl groups (13, 18, 19). Both MBL and ficolins are complexed to MASPs. Similar to C1s, MASP-2 can cleave C4 and C2 to generate C4b2a. Cleavage of C3 by C4b2a results in covalent attachment of C3b to the bacterial surface. The alternative pathway further amplifies the labeling of bacteria with C3b molecules. Also, the alternative pathway can be activated non-specifically through “tick-over” activation of C3 in fluid phase or via properdin (20). Multiple epidemiologic studies have shown that a deficiency of MBL predisposes to severe infection and bacteremia in neonates, neutropenic patients, and patients having undergone allogeneic stem cell or solid organ transplantation (21–24). Although numerous strategies to evade the complement system have been described for Gram-positive bacteria (25), little is known about the interaction of E. faecalis with complement.
In a recent study, we constructed a library of 177 targeted insertion mutants of genes involved in putative surface or stress-response factors in E. faecalis strain V583 (26). The mutant library was screened in an opsonophagocytic killing assay, and three mutants of genes putatively involved in WTA biosynthesis were readily killed by complement and neutrophils in the absence of specific Ab (26): tagB (EF1172), tagA (EF1173), and tagO (EF2198). Previous work has also linked EF2198 to the epa locus (27). Interestingly, an insertion mutant of EF2198 in E. faecalis OG1RF still expressed the rhamnopolysaccharide (27). Here, we describe the mechanism of increased susceptibility to complement-mediated opsonophagocytosis in the E. faecalis tagB mutant and characterize structural differences of wild type and mutant secondary cell wall polymers.
EXPERIMENTAL PROCEDURES
Bacterial Strains
E. faecalis V583 is a blood culture isolate. Chromosomal insertional mutants of the genes tagA, tagB, and tagO were constructed by homologous recombination in a previous study (26).
Growth Conditions and Medium
For isolation of enterococcal cell wall polysaccharides, E. faecalis was cultivated for 18 h at 37 °C without agitation in tryptic soy broth (Carl Roth, Karlsruhe, Germany).
Sera for Complement Binding Studies
Normal pooled human serum was obtained from healthy volunteers who gave informed consent. Baby rabbit serum and C1q-depleted serum were purchased from Cedarlane Laboratories and Quidel, respectively. Heat inactivation of serum was performed by incubation for 20 min at 56 °C. Serum was depleted of specific Ab to E. faecalis V583 by absorption with bacterial cells. Therefore, a 5-ml overnight culture of WT bacteria was centrifuged, and the pellet was suspended in 1 ml of normal pooled human serum in the presence of 5 mm EDTA (to prevent complement activation). Bacteria were incubated with normal pooled human serum for 60 min at 4 °C. After absorption, bacteria were pelleted by centrifugation, and the absorbed serum was passed through a 0.2-μm filter. The serum was supplemented with 10 mm MgCl2 and CaCl2 to restore sufficient concentrations of the respective cation.
Hemolytic Assays
To analyze classical pathway activation, sheep erythrocytes (Alsever) were first opsonized with anti-sheep erythrocyte IgM. Pre-opsonized erythrocytes were incubated with serum in veronal-buffered saline containing 0.5 mm CaCl2 and 0.25 mm MgCl2 for 30 min at 37 °C. For alternative pathway hemolysis, serum was incubated with rabbit erythrocytes (Alsever) in veronal-buffered saline with 5 mm MgCl2 and 10 mm EGTA for 60 min at 37 °C. Samples were centrifuged, and hemolysis was measured by monitoring the absorbance of the supernatants at an optical density of 405 nm.
Opsonophagocytic Killing Assay
Bacterial opsonophagocytosis by human neutrophils was measured as described elsewhere (28). Briefly, bacterial strains were grown to mid-logarithmic phase (A600 = 0.4) in tryptic soy broth and diluted with RPMI supplemented with 15% fetal calf serum. White blood cells (WBCs) were purified from the blood of healthy volunteers by sedimentation with heparin-dextran buffer, and the remaining erythrocytes were removed by hypotonic lysis in 1% NH4Cl solution. Baby rabbit serum (diluted 1:15) served as a complement source. To remove natural Abs against target strains, the serum was absorbed with E. faecalis V583 as described above. Rabbit serum raised against heat-killed E. faecalis V583 at a dilution of 1:2500 served as Ab source. In control tubes, Ab, complement, or WBCs were omitted from the assay. For the measurement of opsonophagocytosis, equal volumes of 2.5 × 106 WBCs, 2.5 × 106 CFUs of bacteria, complement, and heat-inactivated immune rabbit serum were combined. After 90 min of incubation, the reaction was stopped at 4 °C, and viable cells were enumerated after overnight culture on tryptic soy agar.
Phagocytosis Assay
The phagocytosis assay was performed as described with modifications (29). Serum was diluted in RPMI with 0.05% human serum albumin and incubated with 2.5 × 105 freshly isolated human neutrophils and 2.5 × 106 FITC-labeled E. faecalis for 15 min at 37 °C while shaking at 750 rpm. The reaction was stopped by adding 1% ice-cold paraformaldehyde. Phagocytosis was analyzed by flow cytometry (FACSCalibur; Becton Dickinson). Neutrophils were gated based on their forward/side scatter properties, and the mean fluorescence of 10,000 gated neutrophils was measured.
Complement Detection on E. faecalis
E. faecalis strains were grown on TSA plates overnight. Bacteria were scraped from the plate and resuspended in HEPES2+ buffer (20 mm HEPES, 140 mm NaCl, 5 mm CaCl2, 2.5 mm MgCl2) with 0.1% BSA to an A600 of 1. Washed bacteria (12.5 × 105) were incubated with serum or purified MBL (a kind gift from Professor A. Ezekowitz) for 20 min at 37 °C while shaking at 900 rpm. To specifically analyze the alternative pathway, incubations were carried out in Hepes-MgEGTA (20 mm HEPES, 5 mm MgCl2, 10 mm EGTA, pH 7.5). After the serum incubations, bacteria were washed with PBS supplemented with 0.1% BSA and incubated with the primary antibody for 30 min at 4 °C. Bacteria were washed and incubated with the FITC-labeled secondary antibody for 30 min at 4 °C. After another wash step, bacteria were suspended in PBS and analyzed by flow cytometry. Goat anti-human C3 (Protos), mouse anti-human C4/C4d (Quidel), goat anti-human MASP-2 (Santa Cruz Biotechnology), goat anti-human MBL (Santa Cruz Biotechnology), and mouse anti-L-ficolin (kindly provided by Professor P. Garred (30)) were used as primary antibodies and FITC-conjugated goat anti-mouse IgG (Dako) or FITC-conjugated rabbit anti-goat IgG (Dako) were used as secondary antibodies. Flow cytometry analysis was carried out on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). Bacteria were gated according to their forward and sideward scatter properties. The fluorescence of unlabeled bacteria was set to base line. For each sample, we analyzed 10,000 bacteria and plotted the mean fluorescence. The data were analyzed using the Cellquest Pro software (Becton Dickinson).
Isolation of Cell Wall Polysaccharide
After broth culture, bacteria were washed, resuspended in digestion buffer (PBS plus 20 mm CaCl2, 20 mm MgCl2, NaN3 0.05%), and cleaved enzymatically with mutanolysin (0.01 mg/ml) and lysozyme (0.5 mg/ml) at 37 °C for 18 h. Afterward, insoluble material was removed by centrifugation, and the supernatant was treated with nucleases (DNase I and RNase, final concentration, 0.1 mg/ml) for 18 h at 37 °C. Proteins were degraded by digestion with proteinase K (0.1 mg/ml) for 8 h at 56 °C. The supernatant was extensively dialyzed (10 kDa molecular mass cutoff) against H2O and lyophilized. For size exclusion chromatography (SEC), the sample was dissolved in 50 mm ammonium carbonate buffer (pH 8.8; NaN3 0.02%) and applied on a Sephacryl S 200 column (1.6 × 100 cm, GE Healthcare). Hexose and phosphorus content were measured as described elsewhere (31), and positive fractions eluting at a Kave of 0.29 and 0.31, respectively, were combined, dialyzed, and lyophilized. The resulting material was dissolved in 20 mm NaHCO3 (pH 8.0, NaN3 0.02%) and subjected to anion-exchange chromatography (Q Sepharose FF, GE Healthcare). To cleave phosphodiester bonds, a 10-mg sample was dissolved in 50 μl of 48% hydrofluoric acid and incubated at 4 °C for 2 days. The material was neutralized and separated by SEC on Sephadex G50 (1.6 × 100 cm column, Bio-Rad). Fractions of the lowest molecular weight were further purified by SEC on Biogel P2 (1 × 120 cm column, Bio-Rad), followed by high-performance anion-exchange chromatography (Dionex) applying a CarboPak PA 100 column (9 × 250 mm) and an ED50 electrochemical detector (Dionex). Data analysis was performed using the Chromeleon software (version 6.6).
Isolation of WTA
WTA was extracted from the cell wall as described elsewhere (32).
General and Analytical Chemical Methods
Qualitative and quantitative analyses of neutral sugars were performed by gas chromatography (GC) of the hydrolyzed and peracetylated alditol acetates as described elsewhere (28, 33). GC separations were conducted with an Agilent GC System (6890N) equipped with a poly-(5%-diphenyl-95%-dimethyl)-siloxan SPB-5-capillary column (30 m, inner diameter × 0.32 mm). Signals were detected by flame ionization and analyzed with the Agilent ChemStation software (Version B 01.01). The absolute configuration of sugars was determined by GC of peracetylated (S)-2-butylglycosides. SDS-PAGE was performed as described elsewhere (28). In brief, cell wall fragments were separated using a 10% Nupage Novex BisTris gel in SDS MES running buffer (Invitrogen), and gels were stained with periodic acid Schiff's reagent or Stains-All (Sigma Aldrich).
Methylation analysis was carried out by analyzing the partially methylated alditol acetates of the hydrofluoric acid-treated material by GC-MS (34). Briefly, 150 μg of the hydrofluoric acid-treated lyophilized material was methylated with methyl iodide in water-free dimethyl sulfoxide (stored over molecular sieve 4 Å) with addition of powdered NaOH. The mixture was kept for 1 h at 20 °C with stirring. Then, the methylated polysaccharides were extracted three times with 2 ml of chloroform, dried, and hydrolyzed with 4 m CF3COOH for 4 h at 100 °C. Subsequently, the material was evaporated with deionized H2O to remove residual CF3COOH and reduced with sodium borodeuteride (18 h). Peracetylation was performed as described above, followed by GC-MS analysis.
Nuclear Magnetic Resonance Spectroscopy
NMR spectra were recorded on an Avance III Bruker 700 MHz Ultrashield Plus spectrometer in 99.98% 2H2O at 27 °C using internal acetone (δH, 2.225; δC, 31.45) and external aqueous 85% H3PO4 (δP 0.0) as reference. All NMR experiments (1H, 13C, and 31P, as well as COSY, TOCSY, NOESY, HMQC, and HMBC) were performed using Bruker software as described previously (28).
RESULTS
Mutation of Teichoic Acid Glycerol (Tag) Genes in E. faecalis Increases Susceptibility to Opsonophagocytosis
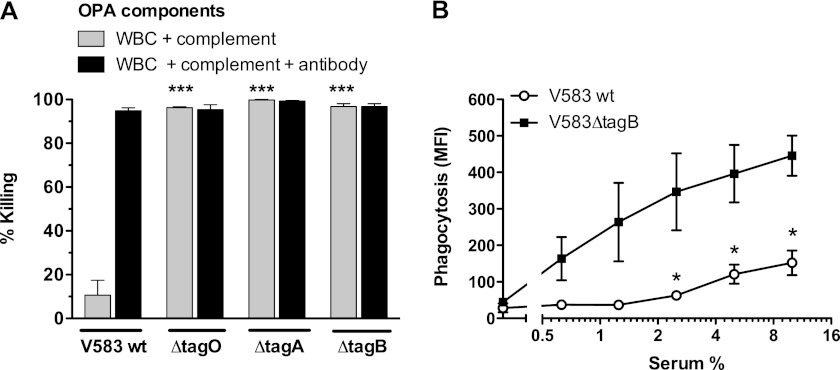
Insertional mutants of genes with high homology to tagO, tagA, and tagB were studied in a heterologous opsonophagocytic killing assay using 1.7% baby rabbit serum, depleted of specific Ab by absorption to the target strain, as complement source (Fig. 1 and Table 1). As shown previously for E. faecalis 12030 (35), E. faecalis V583 wild type was resistant to phagocytic killing by human neutrophils alone (data not shown) or in combination with complement (Fig. 1A). In the presence of specific Ab together with complement, however, bacteria were killed by phagocytes (Fig. 1A). In contrast, insertional mutants of E. faecalis in tagO, tagA, and tagB were highly susceptible to complement-mediated phagocytic killing (Fig. 1A). Because tagO has also been implicated in the biosynthesis of Epa in E. faecalis, we chose to perform further phenotypic analysis with the insertion mutant of the tagB gene that encodes for the enzyme that putatively completes the synthesis of the linkage unit of WTA.
FIGURE 1.
Opsonophagocytosis and complement deposition of E. faecalis mutants in WTA biosynthesis. A, opsonophagocytic killing of E. faecalis V583 wild type and WTA biosynthesis mutants in the presence of 1.7% absorbed baby rabbit serum in combination with human WBCs, or combined with WBC plus specific rabbit Ab. Percentage of killing was determined as a relative to colony-forming units from control tubes containing bacteria and neutrophils only. B, phagocytosis of FITC-labeled bacteria after 15 min of incubation with absorbed human serum. Error bars represent the mean ± S.E. of three separate experiments using different donors. *, p < 0.05 for mutant versus wild type (two-tailed Student's t test). ***, p < 0.001 (one-way analysis of variance with Dunnett's multiple comparison test for comparison with E. faecalis V583 wild type). MFI, mean fluorescence intensity.
TABLE 1.
Analysis of homologies of Bacillus subtilis W23 and S. aureus COL teichoic acid biosynthesis genes to E. faecalis V583 by the BLAST algorithm (Id, indentity; Si, similarity)
|
B. subtilis |
S. aureus |
Function | ||||
|---|---|---|---|---|---|---|
| % Id | % Si | % Id | % Si | |||
| EF2198 | tagO | 24 | 40 | 41 | 65 | UDP-N-acetylglucosamine:undecaprenyl-P N-acetylglucosaminyl-1-P transferase |
| EF1173 | tagA | 38 | 60 | 33 | 59 | N-Acetylglucosaminyl-diphospho-undecaprenol N-acetyl-mannosaminyl-transferase |
| EF1172 | tagB | 28 | 48 | 26 | 47 | Glycerophosphotransferase |
First, we compared wild type and tagB mutant bacteria in a phagocytosis assay using human neutrophils and pre-absorbed human serum. Previously, we confirmed by the hemolytic complement assays that the pre-absorption step did not affect complement activity of the serum (supplemental Fig. 1). FITC-labeled E. faecalis cells were incubated with pre-absorbed human serum and human neutrophils and bacterial uptake by neutrophils was analyzed by flow cytometry. At concentrations up to 10% absorbed serum, the rate of phagocytosed E. faecalis V583ΔtagB was significantly higher compared with wild type bacteria (Fig. 1B). In the presence of non-absorbed serum, on the other hand, no clear difference in phagocytosis was observed (supplemental Fig. 2).
Inactivation of tagB in E. faecalis V583 Leads to Increased C3b Deposition
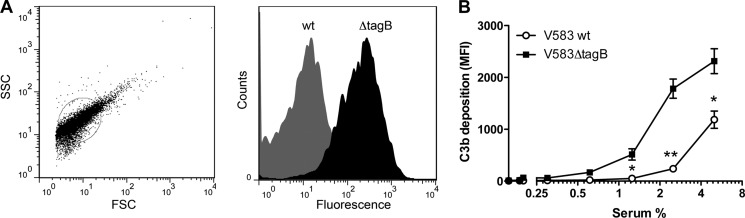
To test whether higher phagocytic uptake of E. faecalis V583ΔtagB was due to increased complement deposition, we determined the amount of C3b deposited on bacterial cells. Therefore, bacteria were incubated with pre-absorbed human serum for 20 min at 37 °C to allow complement deposition. Then, surface-bound C3b was detected by specific antibodies and flow cytometry. Bacteria were gated based on their forward/side scatter properties, and the mean fluorescence of 10,000 bacteria was analyzed (Fig. 2A). Compared with wild type bacteria, we observed much higher levels of C3b bound to E. faecalis V583ΔtagB in absorbed serum (Fig. 2, A and B). Again, no differences were observed in non-absorbed serum (supplemental Fig. 2).
FIGURE 2.
Increased C3b deposition on E. faecalis V583ΔtagB. C3b deposition on enterococcal bacterial cells measured by flow cytometry after incubation with human absorbed serum. A, left: dot plot showing bacterial cells and how they were gated for fluorescence analyses. Right: representative histogram of C3b deposition analysis (2.5% serum). B, C3b deposition on E. faecalis V583 wild type and ΔtagB in absorbed serum. Error bars represent the mean ± S.E. of three separate experiments. *, p < 0.05; **, p < 0.005 for mutant versus wild type (two-tailed Student's t test). MFI, mean fluorescence intensity.
The E. faecalis tagB Mutant Activates Lectin Pathway Activation
Next we examined which complement pathway triggered increased C3b deposition on E. faecalis V583ΔtagB. To this end, we first analyzed C3b deposition via the alternative pathway using EGTA to chelate calcium ions necessary for classical and lectin pathway activation. Here we did not observe increased C3b deposition on E. faecalis V583ΔtagB (Fig. 3A). Then, we analyzed deposition of C4b on the bacterial surface (in the presence of calcium) and observed higher C4b levels on V583ΔtagB cells than on wild type bacteria (Fig. 3B). Both experiments exclude a role for the alternative pathway. When C3b deposition was analyzed in C1q-depleted human serum (abolishing classical pathway activation) (Fig. 3C), we still found an increased C3b deposition on E. faecalis V583ΔtagB, indicating that the tagB mutant activated the lectin pathway.
FIGURE 3.
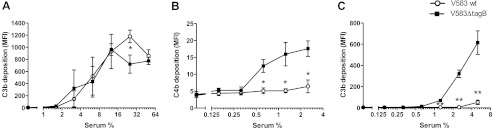
Increased C3b deposition on E. faecalis V583ΔtagB is mediated via the lectin pathway. A, C3b deposition on E. faecalis in human absorbed serum via the alternative pathway. B, C4b deposition on E. faecalis in human absorbed serum. C, C3b deposition on E. faecalis cells in C1q-depleted human serum. Error bars represent the mean ± S.E. of three separate experiments. *, p < 0.05; **, p < 0.005 for mutant versus wild type (two-tailed Student's t test). MFI, mean fluorescence intensity.
The E. faecalis tagB Mutant Strongly Binds MBL
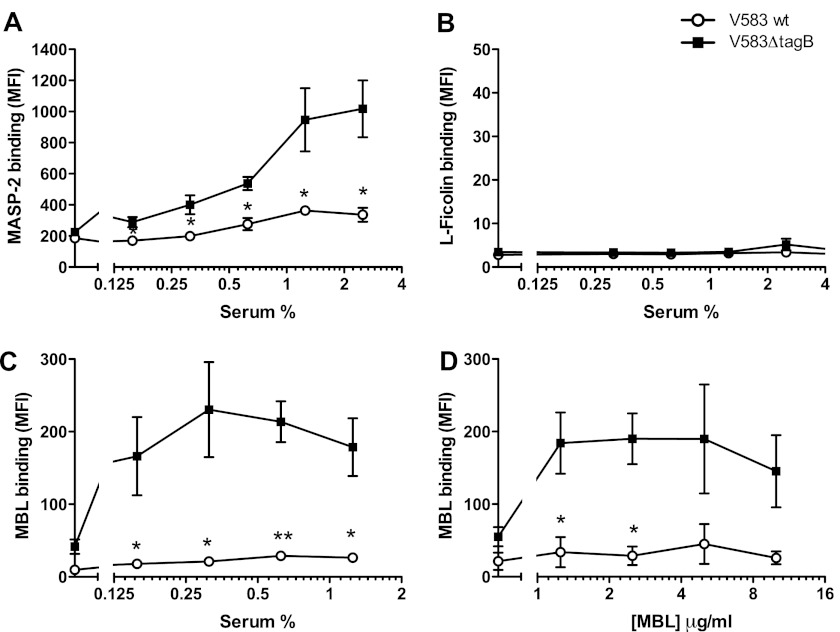
To pinpoint the differences to lectin pathway activation, we checked whether binding of the MBL- and ficolin-associated serine protease MASP-2 was altered on the mutant. Indeed, after incubating bacteria with absorbed serum, we found much more MASP-2 associated with E. faecalis V583ΔtagB compared with wild type E. faecalis (Fig. 4A). To analyze whether differential binding of the lectins may cause these differences, we studied binding of L-ficolin and MBL (Fig. 4, B and C). We could not detect L-ficolin binding to either E. faecalis wild type or the tagB mutant (Fig. 4B). However, MBL binding was strongly increased on E. faecalis V583ΔtagB compared with the wild type strain (Fig. 4C). In concordance, when bacteria were incubated with purified MBL, we observed high binding of MBL to E. faecalis V583ΔtagB, but not to the wild type strain (Fig. 4D).
FIGURE 4.
E. faecalis V583ΔtagB cells bind higher amounts of MBL. E. faecalis was incubated with human absorbed serum and binding of MASP-2 (A), L-ficolin (B), and MBL (C) was detected. D, binding of purified MBL to E. faecalis cells. Error bars represent the mean ± S.E. of three separate experiments. *, p < 0.05; **, p < 0.005 for mutant versus wild type (two-tailed Student's t test). MFI, mean fluorescence intensity.
Biochemical Analysis of Cell Wall Components
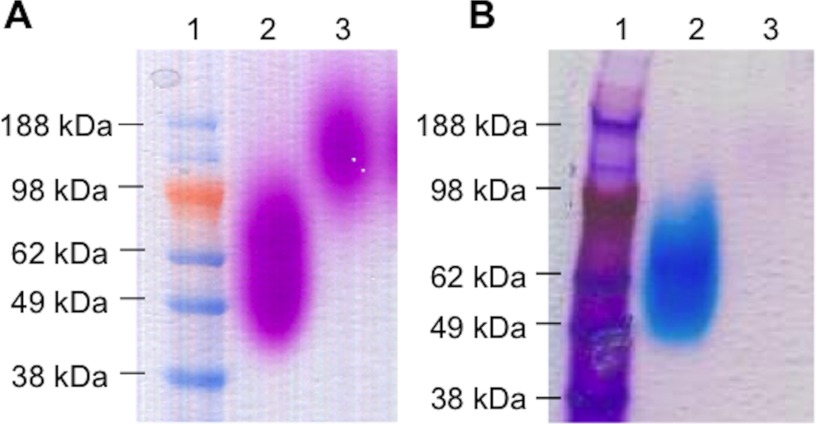
To gain insight into the mechanism of complement-resistance of E. faecalis, we characterized the structure of the cell wall-associated polysaccharides of E. faecalis V583 and the tagB mutant. Cell walls were digested by mutanolysin and lysozyme to depolymerize peptidoglycan and then separated by SDS-PAGE and stained with periodic acid Schiff's reagent and Stains All (Fig. 5). SDS-PAGE of the cell wall fragments of the wild type strain revealed a broad band at ∼60 kDa. In contrast, in E. faecalis V583ΔtagB, this band migrated distinctly more slowly and was not stained by cationic dye Stains All, suggesting a loss of negative charge motifs (Fig. 5).
FIGURE 5.
SDS-PAGE of enzymatically digested cell wall extracts. A, staining with periodic acid-Schiff's reagent (PAS). B, staining with Stains All. Lane 1, molecular mass standard; lane 2, cell wall extracts of E. faecalis V583 wild type; lane 3, cell wall extracts of E. faecalis V583ΔtagB.
Cell wall extracts of the E. faecalis V583 wild type and its insertional mutant V583ΔtagB were further purified by SEC and anion-exchange chromatography. In contrast to cell wall extracts from the wild type strain, extracts from the tagB mutant did not bind to Q-Sepharose, again suggesting a loss of negative charge (data not shown). Compositional analysis of the purified extracts revealed that polysaccharide of both strains contained 6-deoxy-mannose (rhamnose, Rha), glucose (Glc), 2-amino-2-deoxy-galactose (galactosamine, GalN), 2-amino-2-deoxyglucose (glucosamine, GlcN), Rbo, and phosphate. Comparison of the molar ratios of sugars, Rbo, and phosphate revealed that the V583ΔtagB polysaccharide contained ∼75% less GalN and Rbo, and ∼60% less phosphate compared with the wild type (data not shown). On 1H NMR spectroscopy, anomeric proton signals of the rhamnopolysaccharide of E. faecalis wild type and V583ΔtagB differed, but heterogeneity of the anomeric region precluded a detailed analysis without further degradation of the molecule.
Cell Wall Fragments of E. faecalis V583ΔtagB Lack WTA
To further investigate the structure of secondary cell wall polysaccharides, phosphodiester bonds were hydrolyzed with hydrofluoric acid, and the hydrolysate was fractioned by SEC. For carbohydrate material from the wild type strain, three distinct peaks were obtained (supplemental Fig. 3). For the high molecular weight and the low molecular weight material, differences between E. faecalis wild type and the tagB mutant were observed. The high molecular weight material from both wild type strain V583 and V583ΔtagB contained l-Rha, d-Glc d-GlcN, and d-GalN, but the ratios of the respective monosaccharides differed between strains (wild type 12:4:2:1; mutant 27:6:2:1) (Table 2). Methylation analysis of wild type polysaccharide revealed high amounts of 1,2-di- and 1,2,3-tri-substituted l-Rha as well as terminal d-Glc and hexosamine residues (supplemental Table 1). These findings imply a putative structure of a poly-l-Rha chain, decorated with terminal d-Glc and hexosamine residues, and suggest that the high molecular weight carbohydrate corresponds to the enterococcal rhamnopolysaccharide (27, 36–39). Cell wall extracts from V583ΔtagB completely lacked a low molecular weight material eluting near the total column volume (supplemental Fig. 3A). The corresponding material from the wild type strain was further purified by SEC, and compositional analysis confirmed the presence of d-GalN, l-Rha, d-Glc, and Rbo as typical components of a Rbo-containing teichoic acid (Table 2). Further separation by high-performance anion-exchange chromatography revealed the presence of two oligosaccharides designated OS I and OS II (supplemental Fig. 3B). NMR spectroscopy of OS I and OS II revealed a ribitol-containing trisaccharide and tetrasaccharide, respectively (supplemental data, supplemental Table 2, and supplemental Fig. 4 and Fig. 6A).
TABLE 2.
Compositional analysis of fractions obtained from SEC on Sephadex G50
In E. faecalis V583ΔtagB, the low molecular weight pool was absent. N.D., not detected. Concentrations are expressed as nmol/mg.
| Rha | Glc | Rbo | GlcN | GalN | Ala | Glu | Lys | |
|---|---|---|---|---|---|---|---|---|
| V583 WT high molecular weight | 2116 | 643 | N.D. | 280 | 154 | 112 | 28 | 28 |
| V583 WT low molecular weight | 589 | 690 | 1180 | N.D. | 1668 | 14 | 6 | 7 |
| V583ΔtagB high molecular weight | 3337 | 762 | N.D. | 268 | 123 | 129 | 30 | 28 |
FIGURE 6.
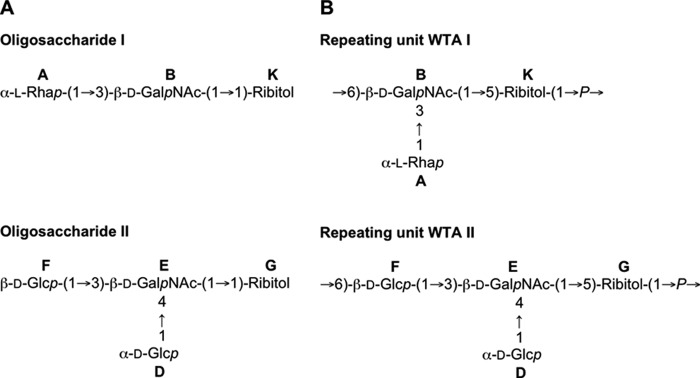
Chemical structures of teichoic acids from E. faecalis V583 wild type. A, structure of OS I and OS II. B, structure of WTA I and WTA II. A, α-l-Rha; B, β-d-GalNAc; K, Rbo; D, α-d-Glc; E, β-d-GalNAc; F, β-d-Glc; G, Rbo. For corresponding 1H and 13C NMR chemical shifts, see supplemental Tables 1 and 3. The numbering of atoms in Rbo residue is reversed in oligosaccharides compared with the polymers according to the International Union of Pure and Applied Chemistry rules.
Because the presence of ribitol was suggestive of teichoic acid, we performed additional structural investigations with WTA extracted from E. faecalis V583 wild type with 5% trichloroacetic acid. After purification by SEC, teichoic acid polymers were analyzed by NMR spectroscopy, confirming the presence of two WTAs, designated WTA I and II (supplemental Table 3). Interestingly, phosphate was attached at different locations in two oligosaccharide units: at O-6 of β-GalNAc B in the units derived from the WTA I and at O-6 of β-Glc F in the units made of WTA II. Phosphorylation caused characteristic downfield shifts of the signals of H/C-5 of the Rbo, H/C-6 of β-d-GalNAc B and β-d-Glc F. The presence of these phosphodiester bridges was confirmed by 1H-31P HMQC and HMQC-TOCSY experiments. The two WTAs were present in equal amounts (by the intensity of NMR signals). From our data, we cannot exclude the possibility that both repeating units are present in one or two WTA polymers. To date, however, WTAs with alternating repeating units have not been described (12), suggesting E. faecalis V583 expresses two WTAs in equal amounts. In summary, our structural analysis revealed the presence of two novel WTAs in E. faecalis V583 wild type with a repeating unit of →6)[α-l-Rhap-(1→3)]β-d-GalpNAc-(1→5)-Rbo-1-P→ and →6) β-d-Glcp- (1→3)[α-d-Glcp-(1→4)]-β-d-GalpNAc-(1→5)-Rbo-1-P→ (Fig. 6B).
DISCUSSION
The complement system is a critical component of innate immunity involved in the recognition, opsonization, and killing of bacteria. In the present study, we investigated how enterococcal secondary cell wall polymers interact with the complement system. To this end, we inactivated tagB, a glycerophosphate transferase that is essential for WTA biosynthesis in E. faecalis V583, and analyzed its phenotype with regard to opsonization by the complement system and expression of secondary cell wall polymers. Disruption of tagB resulted in the loss of two teichoic acids from the cell wall as well as compositional changes in the rhamnopolysaccharide of E. faecalis. These changes in secondary cell wall polymers resulted in a net loss of negative charge, as indicated by an altered migration pattern on SDS-PAGE and binding to Q-Sepharose. Inactivation of tagB rendered mutant bacteria highly susceptible to opsonophagocytosis in the presence of serum due to elevated deposition of C3b on the bacterial surface, which was mediated by increased binding of MBL and consecutive activation of the lectin pathway. Our findings suggest that WTA and/or the enterococcal rhamnopolysaccharide mask the binding sites of MBL within the cell envelope of E. faecalis.
To aid our understanding of the structure-function relationship between enterococcal glycopolymers and complement resistance, we conducted a detailed structural analysis of the carbohydrate antigens covalently attached to peptidoglycan of the E. faecalis V583 wild type strain and its isogenic tagB mutant. Our study revealed the presence of three secondary cell wall polymers in wild type E. faecalis: the previously described rhamnopolysaccharide and two novel ribitol teichoic acids. Compared with most other Gram-positive bacteria that express substituted poly(1,5-Rbo-P) or poly(1,2-Gro-P) WTA, the repeating unit of the E. faecalis WTAs described here is substantially more complex (12). Also, the expression of two WTAs has not been documented previously in E. faecalis and is only known for Bacillus subtilis (12).
Studies in C3-depleted mice have underscored the importance of the complement system in the clearance of systemic enterococcal infections (40). The strategies of enterococci to circumvent recognition by the complement system, however, are still incompletely understood. Recently, a novel role for the extracellular serine protease GelE of E. faecalis as degrading enzyme for soluble C3 and surface-bound iC3b, resulting in a substantial reduction of phagocytosis and killing by neutrophils has been described (41). Other studies have addressed the role of enterococcal glycopolymers in immune evasion from the complement system. Arduino and co-workers (42) suggested that a carbohydrate structure is responsible for resistance to opsonization by human pooled serum in some Enterococcus faecium strains, but no further information on the nature of the carbohydrate was obtained. The role of the rhamnopolysaccharide of E. faecalis in resistance to complement-mediated opsonophagocytosis was investigated in two disruption mutants of the epa locus (10). Inactivation of epaB and epaE in E. faecalis OG1RF resulted in an altered biosynthesis of the rhamnopolysaccharide, but only the epaB mutant displayed reduced resistance to antibody-independent opsonophagocytic killing in the presence of 5% serum (10, 27). Compositional analysis of the rhamnopolysaccharide from the epaB mutant revealed that rhamnose was replaced by mannose in this strain (27). Although the investigators did not elucidate the mechanism for the increased complement susceptibility of the epaB mutant, it is tempting to speculate that mannose residues of the mutant polysaccharide served as neoepitopes for MBL. Capsule expression is another strategy of E. faecalis to evade the opsonization by the complement system (43). Although the enterococcal capsule itself does not interfere with the amount of C3b bound to the bacterial cell wall, experimental evidence suggests that C3b on encapsulated strains is not surface exposed, resulting in lower opsonophagocytosis of bacteria. Compared with the impact of inactivation of tagB in our study, however, the effect of capsule expression on complement-mediated phagocytosis was less pronounced.
Due to the pleiotropic effects of the inactivation of tagB, we were unable to pinpoint the molecular mechanism of increased MBL deposition on the surface of the mutant strain. Typically, MBL recognizes terminal monosaccharides having equatorial 3- and 4-hydroxyl groups, such as Man, Glc, ManNAc, and GlcNAc. In vitro studies have identified lipomannan, lipoteichoic acid, and peptidoglycan as bacterial glycopolymers recognized by MBL (44, 45). A recent study in S. aureus, however, raised doubts about peptidoglycan as a ligand and demonstrated that MBL primarily binds to wall teichoic acid (14).
According to our analysis, the most obvious alteration of the cell envelope of the tagB mutant was the lack of MurNAc substitution by WTA, rendering this peptidoglycan residue available for a possible interaction with MBL. As described above, in vitro binding and inhibition studies have demonstrated binding of MBL with peptidoglycan, but these studies have been hampered by impurities of peptidoglycan preparations with other components of the cell wall, including WTA (14, 45, 46). Nevertheless, further evidence for the role of acetyl moieties of peptidoglycan as a target for MBL comes from a Streptococcus suis mutant defective in N-deacetylation of peptidoglycan. This mutant bound higher amounts of C3b, and bacteria opsonized with serum were more readily killed by bone marrow-derived dendritic cells (47). The phenotype of the E. faecalis tagB mutant conflicts, however, with the observation by Park and co-workers (14), who found decreased MBL deposition in a S. aureus tagO mutant, which also does not express WTA. These differences should be interpreted with caution because the expression and structure of WTA and other secondary cell wall polysaccharides as well as the substitution pattern of peptidoglycan differs substantially between both species (12, 13, 48). For example, our structural analysis of the two WTA of E. faecalis V583 revealed a lower density of binding ligands for MBL compared with WTA from S. aureus.
Of note, we observed differences in complement binding binding between the E. faecalis wild type strain and the tagB mutant only in the absence of specific antibodies. A possible explanation for this phenomenon could be that complement deposition by the classical pathway quantitatively overrides the lectin pathway activation. An alternative hypothesis was given by Park et al. (14), who proposed that specific antibodies compete with MBL for binding sites within the cell envelope.
Teichoic acids represent as much as 60% of the molar weight of the Gram-positive cell wall and are major determinants of the net negative charge of the bacterial cell envelope (6). The absence of WTA conceivably disturbs the surface topography of the Gram-positive cell envelope and may affect the three-dimensional orientation of other enterococcal glycopolymers such as the rhamnopolysaccharide, lipoteichoic acid, or capsular polysaccharide. Binding of the polyvalent oligomer MBL, however, is highly sensitive to the scaling of its binding domains. Rearrangement of its ligands even in the nanometer range strongly affects its interaction (49). Hence, WTA may mediate resistance to MBL indirectly by acting as a scaffold for other glycopolymers.
In summary, inactivation of tagB in E. faecalis V583 results in loss of two ribitol-containing WTA from the cell envelope and secondary changes in the rhamnopolysaccharide, leading to increased susceptibility to complement deposition by the lectin pathway and increased opsonophagocytic killing of mutant bacteria. Our results suggest a critical role for secondary cell wall polysaccharides in complement evasion by E. faecalis.
Supplementary Material
Acknowledgments
We thank Professors Peter Garred and Alan Ezekowitz for providing anti-ficolin antibodies and purified MBL, respectively. We thank Heiko Käβner (NMR), Hermann Moll (GC-MS), Regina Engel (high-performance anion-exchange chromatography), Katharina Jakob (GC), and Volker Grote (HPLC) for excellent technical assistance. Furthermore, the authors thank Dr. Katarzyna Duda for valuable discussions.
This work was supported by the German Federal Ministry of Education and Research (BMBF 01 EO 0803). This work was also supported by the German grant (0313933) from the 1st call of the ERA-Net PathoGenoMics, a European program for cooperation and coordination of genome sequencing and functional genomics of human-pathogenic microorganisms.

This article contains supplemental data, Tables 1–3, and Figs. 1–4.
- WTA
- wall teichoic acid
- Ab
- antibody
- GC-MS
- gas chromatography-mass spectrometry
- MASP
- mannose-binding lectin serin protease
- MBL
- mannose-binding lectin
- SEC
- size exclusion chromatography
- TCA
- trichloroacetic acid
- WBC
- white blood cells
- Epa
- enterococcal polysaccharide antigen
- Rbo
- ribitol
- Rha
- rhamnose
- GalN
- galactosamine
- TOCSY
- Total Correlation Spectroscopy
- HMBC
- Heteronuclear Multiple Bond Correlation
- HQMC
- Heteronuclear Multiple Quantum Correlation
- NOESY
- Nuclear Overhauser effect spectroscopy.
REFERENCES
- 1. Vincent J. L., Rello J., Marshall J., Silva E., Anzueto A., Martin C. D., Moreno R., Lipman J., Gomersall C., Sakr Y., Reinhart K. (2009) International study of the prevalence and outcomes of infection in intensive care units. JAMA 302, 2323–2329 [DOI] [PubMed] [Google Scholar]
- 2. Ghanem G., Hachem R., Jiang Y., Chemaly R. F., Raad I. (2007) Outcomes for and risk factors associated with vancomycin-resistant Enterococcus faecalis and vancomycin-resistant Enterococcus faecium bacteremia in cancer patients. Infect Control Hosp. Epidemiol. 28, 1054–1059 [DOI] [PubMed] [Google Scholar]
- 3. DiazGranados C. A., Jernigan J. A. (2005) Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection. J. Infect. Dis. 191, 588–595 [DOI] [PubMed] [Google Scholar]
- 4. Peel T., Cheng A. C., Spelman T., Huysmans M., Spelman D. (2012) Differing risk factors for vancomycin-resistant and vancomycin-sensitive enterococcal bacteraemia. Clin. Microbiol. Infect 18, 388–394 [DOI] [PubMed] [Google Scholar]
- 5. Theilacker C., Jonas D., Huebner J., Bertz H., Kern W. V. (2009) Outcomes of invasive infection due to vancomycin-resistant Enterococcus faecium during a recent outbreak. Infection 37, 540–543 [DOI] [PubMed] [Google Scholar]
- 6. Silhavy T. J., Kahne D., Walker S. (2010) The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2, a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swoboda J. G., Campbell J., Meredith T. C., Walker S. (2010) Wall teichoic acid function, biosynthesis, and inhibition. Chembiochem. 11, 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeng J., Teng F., Weinstock G. M., Murray B. E. (2004) Translocation of Enterococcus faecalis strains across a monolayer of polarized human enterocyte-like T84 cells. J. Clin. Microbiol. 42, 1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohamed J. A., Huang W., Nallapareddy S. R., Teng F., Murray B. E. (2004) Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect Immun. 72, 3658–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teng F., Jacques-Palaz K. D., Weinstock G. M., Murray B. E. (2002) Evidence that the enterococcal polysaccharide antigen gene (epa) cluster is widespread in Enterococcus faecalis and influences resistance to phagocytic killing of E. faecalis. Infect Immun. 70, 2010–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu Y., Singh K. V., Qin X., Murray B. E., Weinstock G. M. (2000) Analysis of a gene cluster of Enterococcus faecalis involved in polysaccharide biosynthesis. Infect Immun. 68, 815–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neuhaus F. C., Baddiley J. (2003) A continuum of anionic charge: Structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67, 686–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weidenmaier C., Peschel A. (2008) Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 6, 276–287 [DOI] [PubMed] [Google Scholar]
- 14. Park K. H., Kurokawa K., Zheng L., Jung D. J., Tateishi K., Jin J. O., Ha N. C., Kang H. J., Matsushita M., Kwak J. Y., Takahashi K., Lee B. L. (2010) Human serum mannose-binding lectin senses wall teichoic acid Glycopolymer of Staphylococcus aureus, which is restricted in infancy. J. Biol. Chem. 285, 27167–27175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gros P., Milder F. J., Janssen B. J. (2008) Complement driven by conformational changes. Nat. Rev. Immunol. 8, 48–58 [DOI] [PubMed] [Google Scholar]
- 16. Ricklin D., Hajishengallis G., Yang K., Lambris J. D. (2010) Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garred P., Honoré C., Ma Y. J., Rørvig S., Cowland J., Borregaard N., Hummelshøj T. (2010) The genetics of ficolins. J. Innate Immun. 2, 3–16 [DOI] [PubMed] [Google Scholar]
- 18. Ip W. K., Takahashi K., Ezekowitz R. A., Stuart L. M. (2009) Mannose-binding lectin and innate immunity. Immunol. Rev. 230, 9–21 [DOI] [PubMed] [Google Scholar]
- 19. Krarup A., Thiel S., Hansen A., Fujita T., Jensenius J. C. (2004) L-ficolin is a pattern recognition molecule specific for acetyl groups. J. Biol. Chem. 279, 47513–47519 [DOI] [PubMed] [Google Scholar]
- 20. Kemper C., Atkinson J. P., Hourcade D. E. (2010) Properdin: Emerging roles of a pattern-recognition molecule. Annu. Rev. Immunol. 28, 131–155 [DOI] [PubMed] [Google Scholar]
- 21. Vekemans M., Robinson J., Georgala A., Heymans C., Muanza F., Paesmans M., Klastersky J., Barette M., Meuleman N., Huet F., Calandra T., Costantini S., Ferrant A., Mathissen F., Axelsen M., Marchetti O., Aoun M. (2007) Low mannose-binding lectin concentration is associated with severe infection in patients with hematological cancer who are undergoing chemotherapy. Clin. Infect. Dis. 44, 1593–1601 [DOI] [PubMed] [Google Scholar]
- 22. Schlapbach L. J., Mattmann M., Thiel S., Boillat C., Otth M., Nelle M., Wagner B., Jensenius J. C., Aebi C. (2010) Differential role of the lectin pathway of complement activation in susceptibility to neonatal sepsis. Clin. Infect Dis. 51, 153–162 [DOI] [PubMed] [Google Scholar]
- 23. Worthley D. L., Johnson D. F., Eisen D. P., Dean M. M., Heatley S. L., Tung J. P., Scott J., Padbury R. T., Harley H. A., Bardy P. G., Angus P. W., Mullighan C. G. (2009) Donor mannose-binding lectin deficiency increases the likelihood of clinically significant infection after liver transplantation. Clin. Infect Dis. 48, 410–417 [DOI] [PubMed] [Google Scholar]
- 24. Mullighan C. G., Heatley S. L., Danner S., Dean M. M., Doherty K., Hahn U., Bradstock K. F., Minchinton R., Schwarer A. P., Szer J., Bardy P. G. (2008) Mannose-binding lectin status is associated with risk of major infection following myeloablative sibling allogeneic hematopoietic stem cell transplantation. Blood 112, 2120–2128 [DOI] [PubMed] [Google Scholar]
- 25. Serruto D., Rappuoli R., Scarselli M., Gros P., van Strijp J. A. (2010) Molecular mechanisms of complement evasion: Learning from staphylococci and meningococci. Nat. Rev. Microbiol. 8, 393–399 [DOI] [PubMed] [Google Scholar]
- 26. Rigottier-Gois L., Alberti A., Houel A., Taly J. F., Palcy P., Manson J., Pinto D., Matos R. C., Carrilero L., Montero N., Tariq M., Karsens H., Repp C., Kropec A., Budin-Verneuil A., Benachour A., Sauvageot N., Bizzini A., Gilmore M. S., Bessières P., Kok J., Huebner J., Lopes F., Gonzalez-Zorn B., Hartke A., Serror P. (2011) Large-scale screening of a targeted Enterococcus faecalis mutant library identifies envelope fitness factors. PLoS One 6, e29023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teng F., Singh K. V., Bourgogne A., Zeng J., Murray B. E. (2009) Further characterization of the epa gene cluster and Epa polysaccharides of Enterococcus faecalis. Infect Immun 77, 3759–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Theilacker C., Kaczyński Z., Kropec A., Sava I., Ye L., Bychowska A., Holst O., Huebner J. (2011) Serodiversity of opsonic antibodies against Enterococcus faecalis–glycans of the cell wall revisited. PLoS One 6, e17839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rooijakkers S. H., Ruyken M., Roos A., Daha M. R., Presanis J. S., Sim R. B., van Wamel W. J., van Kessel K. P., van Strijp J. A. (2005) Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat. Immunol. 6, 920–927 [DOI] [PubMed] [Google Scholar]
- 30. Hummelshøj T., Ma Y. J., Munthe-Fog L., Bjarnsholt T., Moser C., Skjoedt M. O., Romani L., Fujita T., Endo Y., Garred P. (2012) The interaction pattern of murine serum ficolin-A with microorganisms. PLoS One 7, e38196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Theilacker C., Kaczynski Z., Kropec A., Fabretti F., Sange T., Holst O., Huebner J. (2006) Opsonic antibodies to Enterococcus faecalis strain 12030 are directed against lipoteichoic acid. Infect Immun. 74, 5703–5712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bychowska A., Theilacker C., Czerwicka M., Marszewska K., Huebner J., Holst O., Stepnowski P., Kaczyński Z. (2011) Chemical structure of wall teichoic acid isolated from Enterococcus faecium strain U0317. Carbohydr Res. 346, 2816–2819 [DOI] [PubMed] [Google Scholar]
- 33. Haseley S. R., Holst O., Brade H. (1997) Structural and serological characterisation of the O-antigenic polysaccharide of the lipopolysaccharide from Acinetobacter haemolyticus strain ATCC 17906. Eur. J. Biochem. 244, 761–766 [DOI] [PubMed] [Google Scholar]
- 34. Ciucanu I., Kerek F. (1984) A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 131, 209–217 [Google Scholar]
- 35. Theilacker C., Kropec A., Hammer F., Sava I., Wobser D., Sakinc T., Codée J. D., Hogendorf W. F., van der Marel G. A., Huebner J. (2012) Protection against Staphylococcus aureus by antibody to the polyglycerolphosphate backbone of heterologous lipoteichoic acid. J. Infect Dis. 205, 1076–1085 [DOI] [PubMed] [Google Scholar]
- 36. Bleiweis A. S., Young F. E., Krause R. M. (1967) Cell walls of group D streptococci. II. Chemical studies on the type 1 antigen purified from the autolytic digest of cell walls. J. Bacteriol. 94, 1381–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hancock L. E., Gilmore M. S. (2002) The capsular polysaccharide of Enterococcus faecalis and its relationship to other polysaccharides in the cell wall. Proc. Natl. Acad. Sci. U.S.A. 99, 1574–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elliott S. D. (1959) Group and type-specific polysaccharides of group D Streptococci. Nature 184, 1342. [DOI] [PubMed] [Google Scholar]
- 39. Elliott S. D. (1960) Type and group polysaccharides of group D streptococci. J. Exp. Med. 111, 621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leendertse M., Willems R. J., Flierman R., de Vos A. F., Bonten M. J., van der Poll T. (2010) The complement system facilitates clearance of Enterococcus faecium during murine peritonitis. J. Infect Dis. 201, 544–552 [DOI] [PubMed] [Google Scholar]
- 41. Park S. Y., Shin Y. P., Kim C. H., Park H. J., Seong Y. S., Kim B. S., Seo S. J., Lee I. H. (2008) Immune evasion of Enterococcus faecalis by an extracellular gelatinase that cleaves C3 and iC3b. J. Immunol. 181, 6328–6336 [DOI] [PubMed] [Google Scholar]
- 42. Arduino R. C., Jacques-Palaz K., Murray B. E., Rakita R. M. (1994) Resistance of Enterococcus faecium to neutrophil-mediated phagocytosis. Infect Immun. 62, 5587–5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thurlow L. R., Thomas V. C., Fleming S. D., Hancock L. E. (2009) Enterococcus faecalis capsular polysaccharide serotypes C and D and their contributions to host innate immune evasion. Infect Immun. 77, 5551–5557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Polotsky V. Y., Fischer W., Ezekowitz R. A., Joiner K. A. (1996) Interactions of human mannose-binding protein with lipoteichoic acids. Infect Immun. 64, 380–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nadesalingam J., Dodds A. W., Reid K. B., Palaniyar N. (2005) Mannose-binding lectin recognizes peptidoglycan via the N-acetyl glucosamine moiety, and inhibits ligand-induced proinflammatory effect and promotes chemokine production by macrophages. J. Immunol. 175, 1785–1794 [DOI] [PubMed] [Google Scholar]
- 46. Ma Y. G., Cho M. Y., Zhao M., Park J. W., Matsushita M., Fujita T., Lee B. L. (2004) Human mannose-binding lectin and L-ficolin function as specific pattern recognition proteins in the lectin activation pathway of complement. J. Biol. Chem. 279, 25307–25312 [DOI] [PubMed] [Google Scholar]
- 47. Lecours M. P., Gottschalk M., Houde M., Lemire P., Fittipaldi N., Segura M. (2011) Critical role for Streptococcus suis cell wall modifications and suilysin in resistance to complement-dependent killing by dendritic cells. J. Infect Dis. 204, 919–929 [DOI] [PubMed] [Google Scholar]
- 48. Vollmer W. (2008) Structural variation in the glycan strands of bacterial peptidoglycan. FEMS Microbiol. Rev. 32, 287–306 [DOI] [PubMed] [Google Scholar]
- 49. Gjelstrup L. C., Kaspersen J. D., Behrens M. A., Pedersen J. S., Thiel S., Kingshott P., Oliveira C. L., Thielens N. M., Vorup-Jensen T. (2012) The role of nanometer-scaled ligand patterns in polyvalent binding by large mannan-binding lectin oligomers. J. Immunol. 188, 1292–1306 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.