Background: Mature osteoclasts with a spontaneous tendency toward apoptosis resorb bone efficiently during their short lifespan.
Results: Released ATP from intracellular stores has a negative impact on the bone resorption activity of osteoclasts by altering their cytoskeletal structures.
Conclusion: ATP depletion leads to osteoclastic bone resorption.
Significance: This study provides a new direction for investigating the mechanisms involved in physiological and pathological bone resorption.
Keywords: Aging, ATP, Mitochondria, Mitochondrial DNA, Osteoclast, Tfam
Abstract
Osteoclasts, highly differentiated bone-resorbing cells of hematopoietic origin, have two conflicting tendencies: a lower capacity to survive and a higher capacity to execute energy-consuming activities such as bone resorption. Here, we report that when compared with their precursors, mature mitochondria-rich osteoclasts have lower levels of intracellular ATP, which is associated with receptor activator of nuclear factor κ-B ligand (RANKL)-induced Bcl-xL down-regulation. Severe ATP depletion, caused by disrupting mitochondrial transcription factor A (Tfam) gene, leads to increased bone-resorbing activity despite accelerated apoptosis. Although AMP-activated protein kinase (AMPK) activation by ATP depletion is not involved in the regulation of osteoclast function, the release of ATP from intracellular stores negatively regulates bone-resorbing activity through an autocrine/paracrine feedback loop by altering cytoskeletal structures. Furthermore, osteoclasts derived from aged mice exhibit reduced mitochondrial DNA (mtDNA) and intracellular ATP levels with increased bone-resorbing activity, implicating the possible involvement of age-related mitochondrial dysfunction in osteoporosis. Thus, our study provides evidence for a mechanism underlying the control of cellular functions by reciprocal changes in intracellular and extracellular ATP, which regulate the negative correlation between osteoclast survival and bone resorption.
Introduction
Osteoclasts are large, multinucleated, motile cells that are formed by fusion of hematopoietic cells of monocyte/macrophage lineage (1, 2). They are unique cells in the body that are able to degrade the extracellular bone matrix. The lifespan of mature osteoclasts is relatively short both in vitro and in vivo. Once differentiated, they rapidly die in the absence of supporting cells such as osteoblasts, bone marrow stromal cells, or growth factors such as M-CSF, RANKL,2 and IL-1 (3). Despite their short lifespan, osteoclasts execute their duties by special attachment to the bone, synthesis and secretion of hydrochloric acid and powerful proteases, internalization of extracellular bone matrix degradation products, and efficient migration along the bone surface. However, the mechanism by which apoptosis-sensitive osteoclasts complete these energy-consuming activities is poorly understood. Recently, the roles of Bcl-2 family members, including proapoptotic Bim and antiapoptotic Bcl-xL, in osteoclast apoptosis have been well studied, revealing the interesting relationship between osteoclast survival and bone resorption. Bim−/− osteoclasts showed decreased bone-resorbing activity despite prolonged survival (4), whereas Bcl-x-deficient osteoclasts exhibited increased bone resorption with accelerated apoptosis (5), implicating the existence of an as yet unidentified mechanism in the regulation of this inverse correlation.
ATP is a ubiquitous carrier of chemical energy and a building block of genetic material in all living organisms. ATP also serves as an extracellular signaling molecule involved in vascular tone, synaptic transmission, and cell death (6, 7). Cellular ATP levels have been reported to reflect cell viability, including cell survival, cell growth, and morphology (8, 9). Furthermore, it has been reported that complete ATP depletion (<5% of control) results in necrosis, and partial ATP depletion (∼10–65% of control) induces apoptosis, as evidenced by internucleosomal DNA cleavage, changes in cellular morphology, and alterations in the plasma membrane (10). Most of the ATP supply of the cell is produced in the mitochondria, and its biogenesis depends on the coordinated expression of genes in the nucleus and mitochondria.
Mitochondria have their own genomic system: namely, mitochondrial DNA (mtDNA), which is a closed-circular double-stranded DNA. mtDNA encodes 13 key subunits of the respiratory chain; therefore, mtDNA expression is critical for mitochondrial biogenesis and normal cellular function (11). mtDNA copy number is decreased not only in mtDNA mutation diseases, but also in a wide variety of acquired degenerative and ischemic diseases (12). To study the role of intracellular ATP in osteoclast function, we employed the cre-loxP recombination system to disrupt the nuclear gene for mitochondrial transcription factor A (Tfam). Tfam is necessary for mtDNA maintenance in mammals and regulates mtDNA copy number by directly binding and coating mtDNA (13, 14). In addition, Tfam is absolutely required for transcription initiation at mtDNA promoters (15). In fact, disruption of the Tfam gene causes depletion of mtDNA, loss of mitochondrial transcripts, loss of mtDNA-encoded polypeptides, severe respiratory chain deficiency, and reduced ATP production (8, 13, 16, 17).
In this study, we characterized mitochondria-rich osteoclasts as ATP-depleted cells and identified Bcl-xL as a regulator of intracellular ATP levels in mature osteoclasts. Furthermore, we demonstrated that ATP depletion following Tfam deficiency leads to increased bone-resorbing activity despite accelerated apoptosis, and the release of endogenous ATP negatively regulates osteoclast function through an autocrine/paracrine feedback loop. Osteoclasts derived from aged mice exhibited reduction of mtDNA copy number and intracellular ATP with increased bone-resorbing activity. These findings highlight a previously unknown mechanism by which intracellular ATP levels regulate the inverse correlation between osteoclast survival and bone resorption.
EXPERIMENTAL PROCEDURES
Animals
Tfamflox/flox mice of C57BL/6 background (13) were mated to cathepsin K-Cre mice, in which the Cre recombinase gene is knocked into the cathepsin K locus and is specifically expressed in osteoclasts (18). Cathepsin K-Cre+/− Tfamflox/flox (cKO) and cathepsin K-Cre−/− Tfamflox/flox (normal littermates) mice were generated by mating cathepsin K-Cre−/− Tfamflox/flox male mice with cathepsin K-Cre+/− Tfamflox/+ female mice. P2rx7−/− mice, generated as described previously (19), were obtained from The Jackson Laboratory. All animals were housed under specific pathogen-free conditions and treated with humane care under approval from the Animal Care and Use Committee of the Tokyo Metropolitan Institute of Gerontology.
Analysis of Bone Phenotype
Radiography was performed using a high-resolution soft x-ray system (Softex). Microcomputed tomography scanning was performed using a ScanXmate-A100S Scanner (Comscan Techno). Three-dimensional microstructural image data were reconstructed, and structural indices were calculated using the TRI/3D-BON software (RATOC). Bone histomorphometric analyses were performed as described (5). TRAP-positive cells were stained at pH 5.0 in the presence of l(+)-tartaric acid using naphthol AS-MX phosphate (Sigma-Aldrich) in N,N-dimethyl formamide (Wako Pure Chemical) as the substrate.
Generation of Osteoclasts and Survival/Bone Resorption Assay
Bone marrow cells were obtained from the femur and tibia of male mice, and bone marrow macrophages were cultured in α-MEM (Invitrogen) containing 10% FBS (Sigma-Aldrich) in the presence of 100 ng/ml M-CSF (R&D Systems) for 2 days. Osteoclasts were generated by stimulating bone marrow macrophages with 10 ng/ml M-CSF and 100 ng/ml RANKL (Wako Pure Chemical) for an additional 4–5 days or by the coculture system established by Takahashi et al. (20). The survival assay was performed as follows (5). After osteoclasts were generated, both RANKL and M-CSF were removed from the culture (time 0), and osteoclasts were cultured for the indicated times. The survival rate of the cells was estimated as the percentage of morphologically intact TRAP-positive multinucleated cells when compared with those at time 0. The bone resorption assay was performed as described previously (21). Briefly, osteoclasts were generated by coculturing osteoblasts and bone marrow cells on collagen gel-coated dishes in the presence of 10 nm 1α,25(OH)2vitamin D3 and 1 μm prostaglandin E2. On day 6 of culture, when osteoclasts were differentiated, the cells were dispersed by treating with 0.1% bacterial collagenase (Wako Pure Chemical) for 10 min. The cells were resuspended in α-MEM containing 10% FBS, replated on dentine slices, and cultured for 12 h. After cells were removed by treating the dentine slices with 1 m NH4OH, the resorption areas were visualized by staining with 1% toluidine blue. The resorption pit area was quantified using an image analysis system (Microanalyzer, Nihon Poladigital).
Retroviral Gene Transfer
For production of retrovirus, full-length cDNA of mouse Bcl-xL was inserted into pMx vectors. For gene silencing, an RNAi sequence was designed for the mouse Bcl-xL gene. The targeting sequence used was GCGTTCAGTGATCTAACATCC (22). The RNAi expression vector for this gene was constructed with piGENEmU6 vector (for mouse; iGENE Therapeutics). For retrovirus expressing RNAi, the U6 promotor and inserts in piGENE vectors were cloned into pMx vectors. Bone marrow-derived monocyte/macrophage precursor cells (BMMs) (5 × 106 cells) were incubated with 8 ml of retrovirus stock for 6 h in the presence of Polybrene (6 μg/ml) and recombinant mouse M-CSF (30 ng/ml). After 6 h of retrovirus infection, the medium was changed to α-MEM containing 10% FBS and M-CSF (100 ng/ml) for 48 h. After the BMMs were lifted with trypsin, the cells were selected by incubating with α-MEM, 10% FBS containing 2 mg/ml puromycin for 2 days. Puromycin-resistant cells were used for further experiments.
Adenoviral Gene Transfer
Adenoviral infection of osteoclasts was performed as reported previously (23). In short, on day 5, when osteoclasts began to appear, mouse cocultures were incubated for 1 h at 37 °C with a small amount of α-MEM containing recombinant adenoviruses at a multiplicity of infection of 100. Cells were then washed twice with PBS and further incubated with α-MEM containing 10% FBS, 10 nm 1α,25(OH)2D3, and 1 μm prostaglandin E2 at 37 °C. Experiments were performed 2–3 days after infection.
Western Blotting, Triton X-insoluble Cytoskeleton, and Immunofluorescence Staining
BMMs and osteoclasts were harvested, and cell lysates were subjected to immunoblot analysis with specific antibodies against Tfam (Aviva Systems Biology), Cre (Covance), nuclear factor of activated T cells c1 (NFATc1) (Santa Cruz Technology), v-Src (Sigma), fodrin (Millipore), proline-rich tyrosine kinase 2 (Pyk2), Bcl-xL, Bim, cleaved caspase-3, β-actin, phospho-AMPK, AMP-activated protein kinase (AMPK), and phospho-tyrosine (Cell Signaling Technology). To isolate Triton X-insoluble cytoskeletons, cells were washed once with isotonic buffer (20 mm Hepes, pH 7.5, 150 mm NaCl, 0.5 mm EDTA, 1 mm DTT, 4 mm MgCl2, 1 mm PMSF, 15 μg/ml aprotinin), treated with 0.05% Triton X-100/isotonic (+) buffer (isotonic buffer supplemented with 0.5 mm ATP, 2% BSA) for 1 min, and washed twice with isotonic (+) buffer (24, 25). For immunofluorescence staining, cells were fixed in 4% paraformaldehyde, permeabilized, and treated with the indicated specific antibodies followed by staining with Alexa Fluor 488- or 546-labeled secondary antibody (Molecular Probes). Confocal images were acquired with a Leica confocal laser-scanning unit, TCS SP5, using a 63× glycerin objective on a Leica DMI6000 microscope (Leica Microsystems).
Mitochondrial Fractionation
Mitochondria-enriched fractions were isolated using a mitochondria isolation kit for cultured cells (Thermo Scientific) according to the manufacturer's instructions. The pelleted mitochondria of BMMs, osteoclasts, and osteoblasts were lysed in the lysis buffer provided in the presence of protease inhibitors. The protein content of the mitochondrial fractions was expressed as the percentage of total protein of the corresponding whole-cell extract. Protein concentration was determined using the Bradford method.
ATP Assay
Prior to measurement of ATP release, culture medium was removed, cell layers were washed, and cells were incubated with serum-free α-MEM. Samples were collected after 1 h and immediately snap-frozen on dry ice for later ATP quantification (26). Intracellular ATP levels and ATP release were assayed using ATPlite (PerkinElmer Life Sciences) according to the manufacturer's instructions. Luminescence was measured using an EnVision multilabel reader (PerkinElmer Life Sciences). The standard curve for ATP was made with a series of dilutions. ATP concentrations were normalized for protein concentration determined by the Bradford method.
Electron Microscopy
Mice were transcardially perfused with 2% paraformaldehyde and 2.5% glutaraldehyde in phosphate buffer (pH 7.4). Femurs and tibiae were immersed in the same fixative for 24 h and demineralized with 10% EDTA (pH 7.4) for 1 week at 4 °C. After post-fixation with 2% OsO4 in 0.1 m phosphate buffer (pH 7.4) for 1 h at 4 °C, specimens were dehydrated in graded acetone and embedded in Epon 812 (TAAB Laboratories Equipment Ltd.). Ultrathin sections were cut using an ultramicrotome (Ultracut UCT; Leica Microsystems K.K.) and stained with uranyl acetate and lead citrate. These sections were observed under a transmission electron microscope (H-7600; Hitachi High Technologies Co.) at an accelerating voltage of 80 kV.
Real-time PCR
Total RNA was extracted with RNeasy (Qiagen), and an aliquot (1 μg) was reverse-transcribed using a QuantiTect reverse transcription kit (Qiagen) to make single-stranded cDNA. PCR was performed on a 7300 Real-Time PCR system (Applied Biosystems) using QuantiTect SYBR Green PCR master mix (Qiagen) according to the manufacturer's instructions. All reactions were run in triplicate. After data collection, the mRNA copy number of a specific gene in total RNA was calculated with a standard curve generated with serially diluted plasmids containing PCR amplicon sequences and normalized to total RNA with β-actin as an internal control. Primers for endogenous Bcl-xL were 5′-GCTGGGACACTTTTGTGGAT-3′ and 5′-TGTCTGGTCACTTCCGACTG-3′; primers for β-actin were 5′-AGATGTGGATCAGCAAGCAG-3′ and 5′-GCGCAAGTTAGGTTTTGTCA-3′; primers for Tfam were 5′-CAAAGGATGATTCGGCTCAG-3′ and 5′-AAGCTGAATATATGCCTGCTTTTC-3′; and primers for P2rx7 were 5′-CTCCAAGCTCTTCCATAAGCTC-3′ and 5′-CAGCAAGGGATCCTGGTAAA-3′.
mtDNA Quantitation
mtDNA copy number per nuclear genome in osteoclasts was quantitated as described previously (27). Total DNA purification was performed using the DNeasy kit (Qiagen). The abundance of nuclear and mitochondrial DNA was quantified by real-time PCR under standard conditions using a QuantiTect SYBR Green PCR kit (Qiagen). The primers used for mtDNA were CCTATCACCCTTGCCATCAT and GAGGCTGTTGCTTGTGTGAC. For nuclear DNA (platelet endothelial cell adhesion molecule (PECAM)), ATGGAAAGCCTGCCATCATG and TCCTTGTTGTTCAGCATCAC were used. The ratios between mtDNA and nuclear DNA concentrations were graphed.
Statistics
Statistical analyses were performed using a two-tailed unpaired Student's t test or analysis of variance analysis, and each series of experiments was repeated at least three times. Results are presented as mean ± S.D.
RESULTS
Mature Mitochondria-rich Osteoclasts Exhibit Lower Intracellular ATP Levels
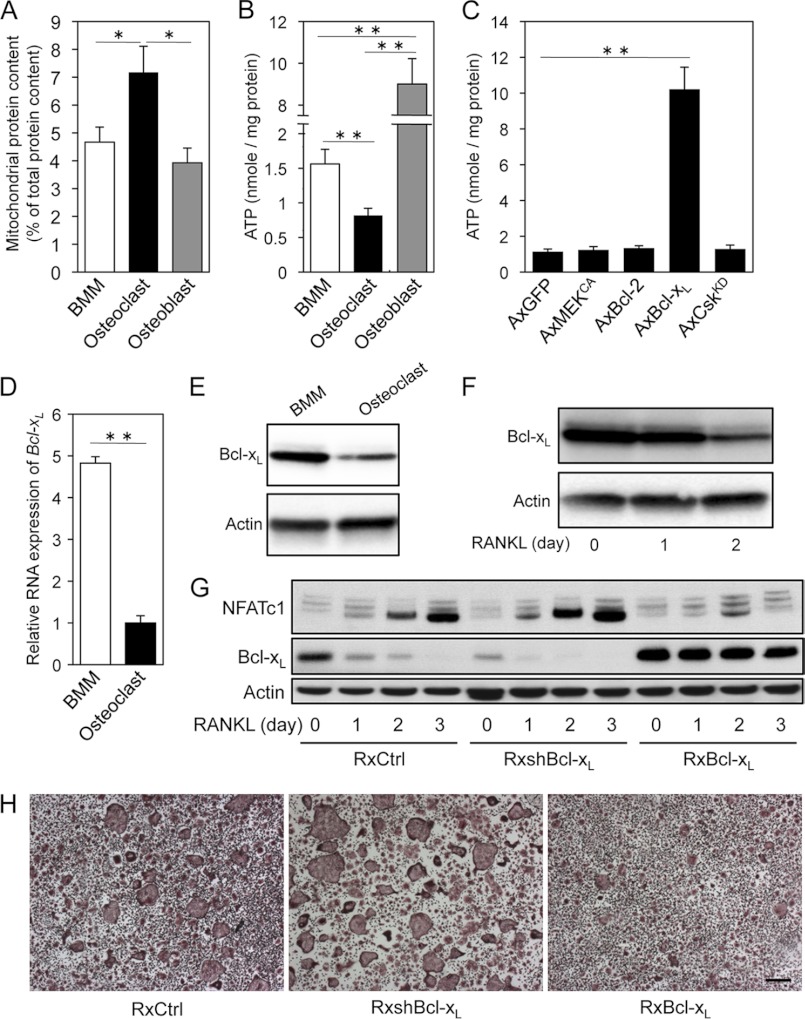
Mitochondria are responsible for metabolism or the conversion of energy and oxygen into molecules that enable cells to carry out their various functions. More mitochondria means that a cell has a higher capacity to do work. Although mature osteoclasts are characterized as mitochondria-rich cells, the precise roles of mitochondria in osteoclasts have remained largely elusive. The quantification of isolated mitochondria-enriched fractions showed that mitochondrial protein content in mature osteoclasts was higher than that in BMMs or osteoblasts (Fig. 1A). Unexpectedly, intracellular ATP levels in osteoclasts were markedly lower than those in both osteoblasts and BMMs (Fig. 1B). These findings indicate that a decline in intracellular ATP stores with an increase in mitochondrial mass occurs during osteoclast differentiation through an unknown mechanism.
FIGURE 1.
Mature osteoclasts display lower intracellular ATP levels associated with decreased expression of Bcl-xL. A, mitochondrial protein content in BMMs, osteoclasts, and osteoblasts. The protein content of the mitochondrial fractions was expressed as the percentage of total protein of the corresponding whole-cell extract. Mitochondrial protein content in mature osteoclasts was higher than that in BMMs or osteoblasts. B, intracellular ATP levels in BMMs, osteoclasts, and osteoblasts. Intracellular ATP levels in osteoclasts was markedly lower than not only osteoblasts, but also BMMs. C, effect of adenovirus-mediated expression of GFP (AxGFP; control), constitutively active MEK (AxMEKCA), Bcl-2 (AxBcl-2), Bcl-xL (AxBcl-xL), or kinase-dead Csk (AxCskKD) on intracellular ATP levels in osteoclasts. Intracellular ATP levels in osteoclasts increased dramatically upon Bcl-xL expression. D and E, real-time PCR analysis of Bcl-xL mRNA level (D) and expression of Bcl-xL protein (E) in BMMs and osteoclasts. Both mRNA and protein expression levels of Bcl-xL in mature osteoclasts were markedly lower than in BMMs. F, effect of RANKL stimulation on expression level of Bcl-xL in BMMs. Bcl-xL expression level in BMMs was gradually reduced by RANKL stimulation. G, effect of retrovirus-mediated expression of small hairpin RNA targeting Bcl-xL (RxshBcl-xL) or Bcl-xL (RxBcl-xL) on NFATc1 protein levels in BMMs stimulated with RANKL. Increase and decrease in the expression level of NFATc1 were observed in shBcl-xL and Bcl-xL transfectants, respectively. RxCtrl indicates an empty retroviral control vector. H, osteoclast differentiation from BMMs infected with RxCtrl, RxshBcl-xL, or RxBcl-xL in response to RANKL and M-CSF. Cells were stained to evaluate TRAP-positive multinucleated osteoclast formation. Scale bar: 100 μm. *, p < 0.05, **, p < 0.01.
Possible Involvement of RANKL-induced Bcl-xL Down-regulation in ATP Depletion and Osteoclast Maturation
The antiapoptotic protein Bcl-xL has been reported to regulate mitochondrial energetics through its interaction with the F1FO ATP synthase, which synthesizes ATP in the mitochondrial matrix using cytosolic ADP and phosphate as substrates (28, 29). In addition, we previously reported that overexpression of Bcl-xL, Bcl-2, and constitutively active MEK (MEKCA) strongly promotes osteoclast survival, and kinase-dead Csk (CskKD) expression increases Src kinase activity and bone-resorbing activity in osteoclasts (5, 21, 30, 31). The above mentioned data prompted us to measure total intracellular ATP levels in osteoclasts expressing these molecules. No differences in total intracellular ATP were observed among osteoclasts expressing MEKCA, Bcl-2, or CskKD. However, intracellular ATP levels in osteoclasts increased more than 9-fold upon Bcl-xL expression (Fig. 1C).
To elucidate the roles of intracellular ATP levels and Bcl-xL expression in osteoclastogenesis, we evaluated Bcl-xL expression in osteoclast lineage. Both Bcl-xL mRNA and protein expression levels in mature osteoclasts were markedly lower than BMMs (Fig. 1, D and E). In addition, their expression levels in BMMs were gradually reduced by RANKL stimulation (Fig. 1F). Knockdown of the Bcl-x gene through RNAi promoted osteoclast differentiation upon RANKL stimulation, in line with an increase in the expression level of NFATc1, the key transcription factor of osteoclastogenesis (32). On the other hand, Bcl-xL overexpression delayed the induction of NFATc1 by RANKL, which paralleled the reduced number of TRAP-positive multinucleated osteoclasts (Fig. 1, G and H). There was no significant difference in M-CSF-dependent proliferation of precursor cells (data not shown). Collectively, these data strongly indicate that lower cellular ATP levels associated with decreased expression of Bcl-xL are important for osteoclast maturation.
Osteoclast-specific Tfam Knock-out Mice Exhibit Growth Retardation with Reduced Osteoclast Number
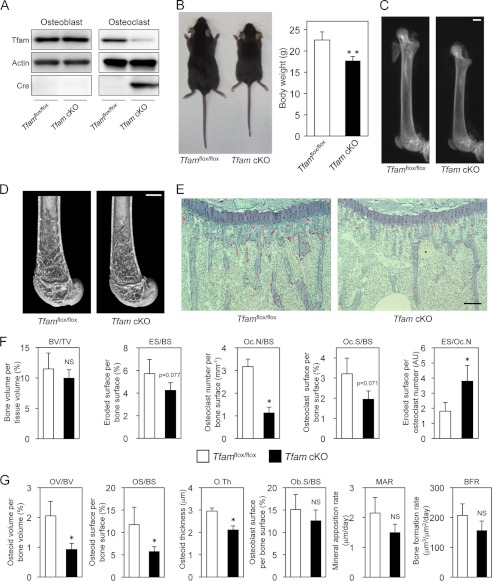
To investigate the role of intracellular ATP levels in osteoclast function in further detail, we generated osteoclast-specific Tfam conditional knock-out mice by mating Tfamflox/flox mice (13) with cathepsin K-Cre transgenic mice, in which the Cre recombinase gene was inserted into the cathepsin K locus and specifically expressed in osteoclasts (18). Tfam is ubiquitously expressed and absolutely required for mtDNA transcription. Loss of Tfam causes depletion of mtDNA, severe respiratory chain deficiency, and reduced ATP production (8, 13). The resulting cathepsin K-Cre+/−Tfamflox/flox mice (referred to herein as Tfam cKO mice) were born alive at predicted Mendelian frequencies. Tfam was markedly reduced in osteoclasts from Tfam cKO mice, whereas its expression in osteoblasts (Fig. 2A) and other tissues (data not shown) in Tfam cKO mice was comparable with that found in normal cathepsin K-Cre−/−Tfamflox/flox littermates.
FIGURE 2.
Generation and skeletal analysis of Tfam cKO mice. A, expression of Tfam, actin, and Cre proteins in osteoblasts and osteoclasts derived from Tfam cKO mice and their normal Tfamflox/flox littermates. Tfam was markedly reduced in osteoclasts derived from Tfam cKO mice. B, representative photographs and body weight of male Tfam cKO mice and their normal Tfamflox/flox littermates at 8 weeks of age. C and D, representative radiography images of the femur (C) and distal femur microcomputed tomography (D) of male Tfam cKO mice and their normal Tfamflox/flox littermates at 8 weeks of age. Scale bar: 1000 μm. E, histological analysis of proximal tibia of male Tfam cKO mice and their normal Tfamflox/flox littermates at 8 weeks of age (TRAP/hematoxylin staining). The number of TRAP-positive cells is markedly decreased in Tfam cKO mice. Scale bar: 100 μm. F, bone volume and parameters for osteoclastic bone resorption in the bone morphometric analysis of male Tfam cKO mice and their normal Tfamflox/flox littermates at 8 weeks of age (n = 5 for each genotype). Eroded surface per osteoclast (ES/Oc.N) was increased by Tfam ablation. NS, not significant; AU, arbitrary units. G, parameters for osteoblastic bone formation in the bone morphometric analysis of male Tfam cKO mice and their normal Tfamflox/flox littermates at 8 weeks of age (n = 5 for each genotype). *, p < 0.05, **, p < 0.01 versus normal Tfamflox/flox littermates.
An obvious feature of the 8-week-old male Tfam cKO mice was their reduced body size (Fig. 2B). Femoral length in male Tfam cKO mice was shorter when compared with Tfamflox/flox mice (13.29 ± 0.09 versus 15.31 ± 0.12 mm, p < 0.01; Fig. 2C). One year after birth, Tfam cKO mice were also significantly smaller than Tfamflox/flox mice (data not shown). Because Winkeler et al. (33) reported that cathepsin K is expressed in the testis, we evaluated testosterone levels in male Tfam cKO mice. We could not find any significant difference in serum testosterone levels between Tfam cKO mice and Tfamflox/flox mice (supplemental Fig. S1). Radiographic and microcomputed tomography analysis showed that there was no significant difference in bone mass between male Tfam cKO mice and Tfamflox/flox mice (Fig. 2, C and D). Interestingly, the number of TRAP-positive differentiated osteoclasts was dramatically reduced in Tfam cKO mice (Fig. 2, E and F). Despite similar expression levels of Tfam in Tfamflox/flox and Tfam cKO osteoblasts (Fig. 2A), some parameters of osteoblastic bone formation were also decreased in Tfam cKO mice (Fig. 2G). This attenuated osteoblast function may be due to the osteoclast-osteoblast coupling mechanism and contribute to the growth retardation observed in Tfam cKO mice.
ATP-depleted Tfam cKO Osteoclasts Show Increased Bone-resorbing Activity despite Accelerated Apoptosis
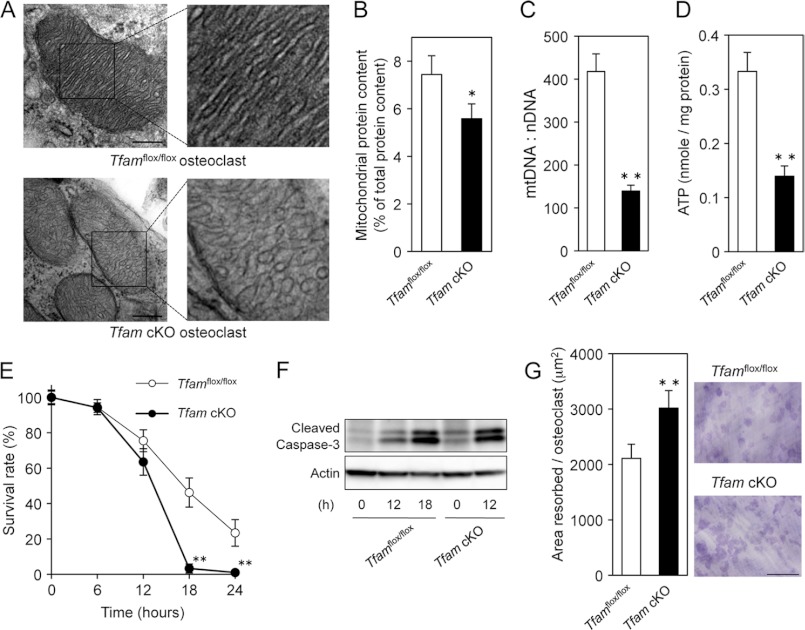
Unexpectedly, bone histomorphometric analysis showed that eroded surface per osteoclast (ES/Oc.N) was increased by Tfam ablation (Fig. 2F), indicating that Tfam cKO osteoclasts resorb bone more efficiently. To further investigate the influence of Tfam deficiency, osteoclasts were analyzed by electron microscopy using Tfamflox/flox and Tfam cKO mouse femurs. The results revealed that the normal ruffled border and sealing zone were clearly formed and faced the bone surface in Tfam cKO osteoclasts (data not shown). However, mitochondria of Tfam cKO osteoclasts appeared to exhibit disarray of mitochondrial cristae (Fig. 3A), suggestive of mitochondrial dysfunction. Given the mitochondrial morphological change in Tfam cKO mice, we analyzed the effect of Tfam deficiency on mitochondrial protein content and mtDNA levels in osteoclasts. As shown in Fig. 3B, mitochondrial protein content was slightly but significantly reduced by Tfam deficiency. Remarkably, we found that Tfam cKO osteoclasts contained only ∼100 copies of mtDNA per nuclear genome, whereas control osteoclasts contained ∼400 copies of mtDNA per nuclear genome (Fig. 3C). Consistent with these results, intracellular ATP levels in Tfam cKO osteoclasts were also significantly decreased when compared with Tfamflox/flox osteoclasts (Fig. 3D).
FIGURE 3.
Tfam cKO osteoclasts show increased bone-resorbing activity despite accelerated apoptosis. A, electron microscopy of osteoclasts derived from Tfamflox/flox and Tfam cKO mice. Mitochondria in Tfam cKO osteoclasts appeared to display abnormal internal compartmentalization. Higher magnification images of the framed areas are shown in the right panels. Swollen and disorganized cristae were observed in Tfam cKO osteoclasts. Scale bar: 100 nm. B, mitochondrial protein content in Tfamflox/flox and Tfam cKO osteoclasts. Mitochondrial protein content was slightly but significantly reduced by Tfam deficiency. C, mtDNA copy number in Tfamflox/flox and Tfam cKO osteoclasts. mtDNA copy number per nuclear genome in osteoclasts was quantitated as described under “Experimental Procedures.” Genotypes are indicated. D, intracellular ATP levels in Tfamflox/flox and Tfam cKO osteoclasts. Intracellular ATP levels in Tfam cKO osteoclasts were significantly decreased when compared with Tfamflox/flox osteoclasts. E, time course of the survival of Tfamflox/flox and Tfam cKO osteoclasts. The number of TRAP-positive viable cells remaining at the different time points is shown as a percentage of the cells at time 0. The survival rate of Tfam cKO osteoclasts was significantly lower than Tfamflox/flox osteoclasts. F, Western blotting of cleaved caspase-3 using β-actin as an internal control. Tfam cKO osteoclasts exhibited the increased amount of cleaved caspase-3. G, bone-resorbing activity of Tfamflox/flox and Tfam cKO osteoclasts. After transfer onto dentine slices, osteoclasts were further incubated for 12 h. The resorption pits were visualized by staining with 1% toluidine blue, and the resorbed area was quantified using an image analysis system. The pit-forming activity of Tfam cKO osteoclasts was significantly higher than that of Tfamflox/flox osteoclasts. Representative resorption pits, visualized by toluidine blue staining, are also shown. Scale bar: 500 μm. *, p < 0.05, **, p < 0.01 versus normal Tfamflox/flox osteoclasts.
Because massive apoptosis in Tfam knock-out embryos was reported at embryonic day 9.5 and increased apoptosis in the heart of the tissue-specific Tfam knockouts was previously reported (13, 34), we next examined the role of Tfam in osteoclast survival. Mature osteoclasts undergo spontaneous apoptosis without any cytokines or supporting cells (3). After the removal of M-CSF and RANKL, ∼80% of normal Tfamflox/flox osteoclasts died spontaneously within 24 h (Fig. 3E). ATP-depleted Tfam cKO osteoclasts disappeared more rapidly and exhibited a greater amount of cleaved caspase-3, a primary apoptosis executioner caspase, when compared with Tfamflox/flox osteoclasts, whereas Tfam deficiency increased bone-resorbing activity (Fig. 3, E–G), indicating a negative regulatory role of intracellular ATP levels in osteoclastic bone resorption. These results from our in vitro studies support our in vivo observations of decreased osteoclast number (Oc.N/BS) and increased eroded surface per osteoclast (ES/Oc.N) in Tfam cKO mice.
AMPK Activity Does Not Affect Osteoclast Survival and Bone Resorption
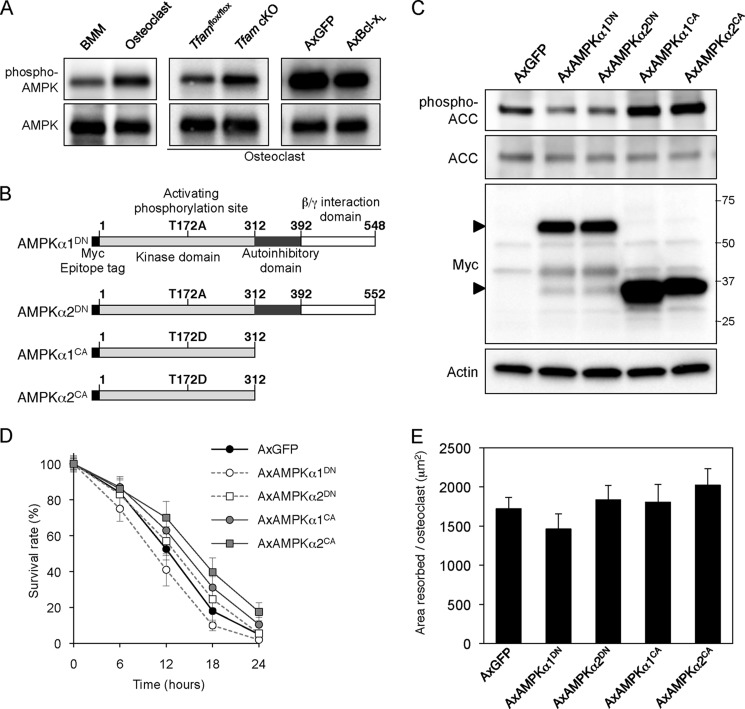
AMPK is a metabolic master switch that mediates the adaptation of the cell to variations in the nutritional environment (35). Its activity is stimulated by increases in the intracellular AMP/ATP ratio in response to stresses such as exercise, hypoxia, and glucose deprivation. Because the role of AMPK in osteoclast function has remained unexplored, we examined whether intracellular ATP levels affect AMPK activity in osteoclasts. As assessed by Western blotting, the phosphorylation level of AMPKα at Thr-172 at the activating loop was up-regulated in ATP-depleted osteoclasts after differentiation or Tfam ablation, whereas ATP-replete osteoclasts expressing Bcl-xL exhibited lower AMPK phosphorylation (Fig. 4A). To test whether modulating AMPK activity would affect osteoclast function, we used adenovirus vectors containing dominant negative (α1DN, α2DN) or constitutively active (α1CA, α2CA) forms of AMPK catalytic subunits (α1 and α2). The expression of AMPKα1DN, AMPKα2DN, AMPKα1CA, and AMPKα2CA was detected by immunoblotting using an antibody against the Myc tag. Because AMPKα1CA and AMPKα2CA are truncated mutants (Fig. 4B), they were detected as bands at a smaller molecular weight than AMPKα1DN and AMPKα2DN (Fig. 4C, third panel). As shown in Fig. 4C, top panel, the phosphorylation level of acetyl-CoA carboxylase at Ser-79, a direct target of AMPK, was modulated by adenovirus-mediated expression of the AMPK mutants. Modulation of AMPK activity did not affect osteoclast survival or bone resorption (Fig. 4, D and E), indicating that AMPK is not involved in ATP depletion-induced bone resorption.
FIGURE 4.
AMPK activity does not affect osteoclast function. A, effect of intracellular ATP level on AMPK activity. Basal activity of AMPK was up-regulated in ATP-depleted osteoclasts after differentiation (left panel) or Tfam ablation (middle panel), whereas ATP-replete osteoclasts expressing Bcl-xL exhibited lower AMPK phosphorylation (right panel). AxGFP, adenovirus-mediated expression of GFP; AxBcl-xL, adenovirus-mediated expression of Bcl-xL. B, schematic representation of AMPKα1DN, AMPKα2DN, AMPKα1CA, and AMPKα2CA. C, adenovirus-mediated expression of AMPK mutants (AxAMPK) and modulation of AMPK activity in osteoclasts. The phosphorylation level of acetyl-CoA carboxylase (ACC) at Ser-79, a direct target of AMPK, was reduced by adenovirus-mediated expression of AMPKα1DN or AMPKα2DN, whereas induction of AMPKα1CA or AMPKα2CA dramatically increased acetyl-CoA carboxylase phosphorylation. D, time course of survival of osteoclasts expressing AMPK mutants. The number of TRAP-positive viable cells remaining at the different time points is shown as a percentage of the cells at time 0. Modulation of AMPK activity did not appear to affect osteoclast survival. E, bone-resorbing activity of osteoclasts expressing AMPK mutants. After transfer onto dentine slices, osteoclasts were further incubated for 12 h. No differences in the bone-resorbing activity were observed among osteoclasts expressing AMPK mutants.
Extracellular ATP Inhibits Osteoclast Function
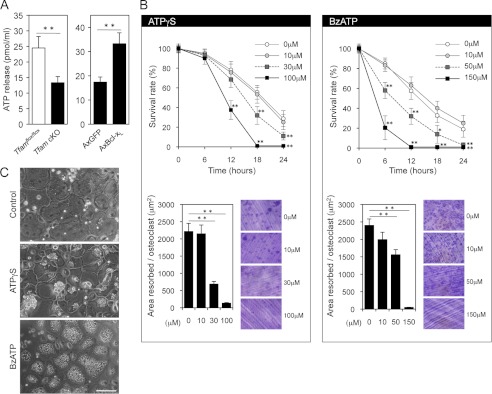
The release of ATP has been reported in a variety of cell types (6, 7, 36). To determine whether intracellular ATP levels influence constitutive ATP release from osteoclasts, extracellular ATP concentrations were measured. ATP release from ATP-depleted Tfam cKO osteoclasts was significantly reduced when compared with Tfamflox/flox osteoclasts, whereas Bcl-xL overexpression had the opposite effect (Fig. 5A). Although detailed molecular mechanisms for the release of cellular ATP have remained largely elusive, the increased ATP release appeared to occur as a result of higher intracellular ATP concentrations. We next examined the effect of extracellular ATP on osteoclast function. To avoid rapid hydrolysis of ATP by ectonucleotidases, we used the hydrolysis-resistant ATP analogue, ATPγS (37). As shown in Fig. 5B, the addition of 100 μm ATPγS dramatically reduced osteoclast survival and bone resorption. Interestingly, after stimulation with 100 μm ATPγS for 3 h, most of the osteoclasts exhibited a large vacuole formation (Fig. 5C, middle panel). Because membrane blebs or vacuoles are a typical feature of ATP-stimulated cells and are mainly caused by stimulation of the P2X7 receptor (38), we also examined the effect of BzATP, a relatively potent P2X7 agonist, on osteoclast function. Treatment with 150 μm BzATP for 30 min caused dramatic changes in osteoclast morphology with numerous large cytoplasmic vacuoles (Fig. 5C, bottom panel). Osteoclast survival and bone-resorbing activity were also strongly inhibited in the presence of 150 μm BzATP (Fig. 5B), suggesting that extracellular ATP may have inhibitory effects on morphology, survival, and the bone-resorbing activity of mature osteoclasts.
FIGURE 5.
Extracellular ATP inhibits osteoclast function. A, the effects of intracellular ATP levels on constitutive ATP release. ATP release from ATP-depleted Tfam cKO osteoclasts was reduced (left panel), whereas ATP-replete osteoclasts expressing Bcl-xL showed enhanced basal ATP release (right panel). **, p < 0.01. AxGFP, adenovirus-mediated expression of GFP; AxBcl-xL, adenovirus-mediated expression of Bcl-xL. B, the effects of ATPγS (left panel) and BzATP (right panel) on the survival and bone-resorbing activity of osteoclasts. The addition of ATPγS and BzATP dramatically reduced osteoclast survival and bone resorption in a dose-dependent manner. *, p < 0.05, **, p < 0.01 versus untreated osteoclasts. C, morphological changes in osteoclasts stimulated with 100 μm ATPγS for 3 h (middle panel) or 150 μm BzATP for 30 min (bottom panel). The treatment with these ATP analogues caused morphological changes with numerous cytoplasmic vacuoles. Scale bar: 100 μm.
The Inhibitory Effect of Extracellular ATP on Osteoclast Survival, but Not on Bone-resorbing Activity, Is Rescued by Bcl-xL-induced ATP Repletion
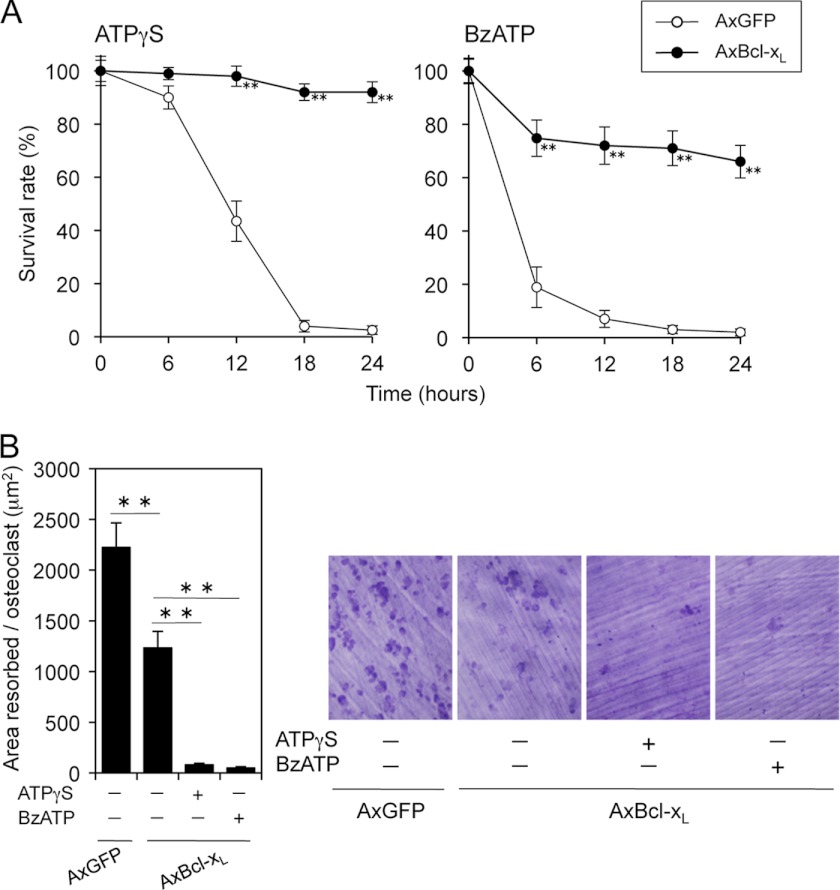
Because intracellular and extracellular ATP affect osteoclast apoptosis in opposite ways, we examined the consequences induced by intra- and extracellular ATP in the regulation of osteoclast survival. ATP repletion by Bcl-xL expression completely reversed the inhibitory effect of ATPγS on osteoclast survival (Fig. 6A, left). In addition, the overall survival curve of Bcl-xL-expressing osteoclasts showed a 30% reduction during the first 6 h after BzATP addition, after which stabilization occurred (Fig. 6A, right), suggesting that intracellular ATP may be more important than extracellular ATP in regulating osteoclast survival. Although Bcl-xL expression itself has an inhibitory effect on bone resorption (5), bone-resorbing activity of osteoclasts expressing Bcl-xL was significantly further inhibited by treatments with ATPγS or BzATP (Fig. 6B) despite the improvement of survival rate, thereby confirming that extracellular ATP has a clear inhibitory effect on osteoclastic bone resorption.
FIGURE 6.
Adenovirus-mediated Bcl-xL (AxBcl-xL) infection reversed the inhibitory effect of extracellular ATP on osteoclast survival but not on bone-resorbing activity. A, time course of the survival of Bcl-xL-expressing osteoclasts treated with 100 μm ATPγS or 150 μm BzATP. Bcl-xL expression completely reversed the inhibitory effect of ATPγS on osteoclast survival, whereas the survival curve of Bcl-xL-expressing osteoclasts showed a 30% reduction during the first 6 h after BzATP addition, after which stabilization occurred. **, p < 0.01 versus adenovirus-mediated expression of GFP (AxGFP)-infected osteoclasts. B, bone-resorbing activity of Bcl-xL-expressing osteoclasts treated with 100 μm ATPγS or 150 μm BzATP. Bone-resorbing activity of osteoclasts expressing Bcl-xL was significantly inhibited by treatments with ATPγS or BzATP, despite the improvement of survival rate. Representative resorption pits, visualized by toluidine blue staining, are also shown. **, p < 0.01.
Extracellular ATP Alters the Pattern of Pyk2 Distribution in Osteoclasts
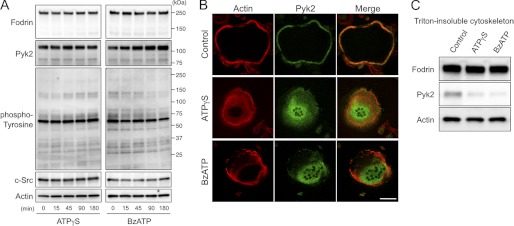
Following ATP stimulation, the 240-kDa membrane-associated cytoskeletal protein, α-fodrin, which is important for maintaining normal membrane structure and supporting cell surface protein function, was reported to be cleaved into smaller fragments, including 180, 150, and 120 kDa (39). In addition, α-fodrin degradation is proposed to contribute to cell blebbing and other morphologic changes (40). Extracellular ATP stimulation is also reported to induce c-Src activation and tyrosine phosphorylation of intracellular proteins (41, 42). However, we could not detect degradation in cytoskeletal proteins including α-fodrin, actin, c-Src, and Pyk2. Tyrosine-phosphorylated protein patterns remained unchanged upon ATPγS or BzATP stimulation (Fig. 7A). Due to dramatic morphological changes after stimulation with the ATP analogues (Fig. 5C), we further examined the effect of extracellular ATP on osteoclast actin cytoskeleton using confocal microscopy. Although the actin ring, which is a characteristic of polarized osteoclasts, was clearly formed before ATP stimulation, ATPγS- or BzATP-treated osteoclasts exhibited diffuse actin distribution (Fig. 7B). Interestingly, stimulation with ATPγS or BzATP facilitated translocation of Pyk2, a major adhesion-dependent tyrosine kinase in osteoclasts, from the periphery to the cell center (Fig. 7B). We obtained similar results using a biochemical method with Triton-insoluble cytoskeletons of osteoclasts. The amount of Pyk2 that remained associated with the cytoskeletons was reduced after treatment with ATPγS or BzATP (Fig. 7C), indicating that the inhibitory effect of extracellular ATP on bone resorption was due to altered cytoskeletal structures.
FIGURE 7.
Extracellular ATP alters Pyk2 distribution in osteoclasts. A, Western blot analysis of fodrin, Pyk2, phospho-tyrosine, c-Src, and actin levels in osteoclasts treated with ATPγS or BzATP at different time points. Degradation in cytoskeletal proteins including α-fodrin, actin, Pyk2, and c-Src was not detected, and tyrosine-phosphorylated protein patterns remained unchanged upon ATPγS (100 μm) or BzATP (150 μm) stimulation. B, double immunofluorescence staining of F-actin (red) and Pyk2 (green) in osteoclasts after stimulation of ATPγS (100 μm) or BzATP (150 μm) for 1 h. Stimulation with ATPγS or BzATP disrupted the actin ring formation of osteoclasts and facilitated translocation of Pyk2 from the periphery to the cell center. Scale bar: 50 μm. C, Western blots of Triton X-100-insoluble cytoskeletons of osteoclasts treated with ATPγS (100 μm) or BzATP (150 μm) for 1 h. Note that the amount of Pyk2 stayed associated with the cytoskeletons was reduced after ATPγS or BzATP stimulation.
Bone-resorbing Activity Is Up-regulated by Removal of Extracellular ATP or P2X7 Receptor Blockade
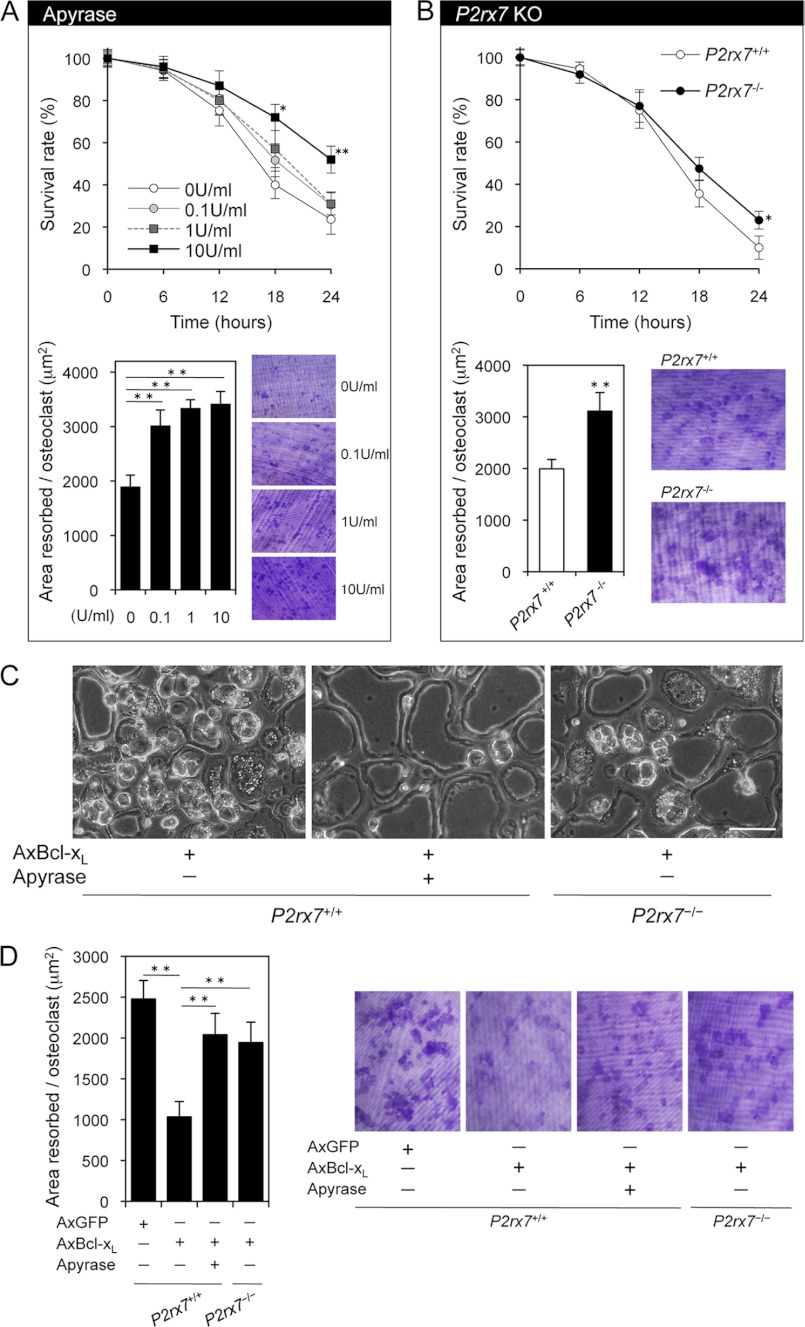
The lifetime of released ATP is short due to the presence of ecto-ATPase. To explore the physiological role of released cellular ATP, we analyzed the effect of apyrase, which hydrolyzes ATP, on osteoclast function. As shown in Fig. 8A, removal of extracellular ATP with apyrase up-regulated osteoclastic bone resorption with a slight increase in osteoclast survival. We next examined how P2X7 receptor blockade affects osteoclast function. Although Pellegatti et al. (43) reported that the P2X7 receptor drives fusion of M-CSF/RANKL-stimulated monocytes into multinucleated osteoclasts, P2rx7−/− mice exhibited a significant decrease in bone mass associated with an increased number of osteoclasts per bone surface (Oc.N/BS) (19). Because it is possible that the findings related to bone resorption in P2rx7−/− mice may be caused by alteration in other cell types that regulate osteoclast function, survival and pit formation assays were performed using osteoclasts derived from P2rx7−/− and P2rx7+/+ mice. P2rx7 deficiency increased osteoclastic bone resorption with a tendency toward increased survival (Fig. 8B). Together, these results strongly suggest that physiological concentrations of extracellular ATP can exert a negative impact on osteoclast bone-resorbing activity via purinergic receptors.
FIGURE 8.
Effects of removal of extracellular ATP or P2X7 receptor blockade on osteoclastic bone resorption. A, the effects of apyrase, which hydrolyzes extracellular ATP, on the survival and bone-resorbing activity of osteoclasts. Apyrase treatment up-regulated osteoclastic bone resorption with a slight increase in osteoclast survival. *, p < 0.05, **, p < 0.01 versus untreated osteoclasts. B, the effects of P2rx7 deficiency on the survival and bone-resorbing activity of osteoclasts. P2rx7 deficiency increased osteoclastic bone resorption with a tendency toward increased survival. *, p < 0.05, **, p < 0.01 versus normal P2rx7+/+ osteoclasts. C, morphological changes in Bcl-xL-expressing osteoclasts after a 48-h incubation. Note that Bcl-xL-induced morphological changes including large vacuoles and membrane blebs were reduced by apyrase treatment (10 units/ml) or P2rx7 deficiency. AxBcl-xL, adenovirus-mediated expression of Bcl-xL. Scale bar: 100 μm. D, bone-resorbing activity of P2rx7+/+ osteoclasts expressing Bcl-xL treated with apyrase and P2rx7−/− osteoclasts expressing Bcl-xL. The inhibitory effect of Bcl-xL expression on osteoclastic bone resorption was partially reversed by apyrase (10 units/ml) or P2rx7 deficiency. Representative resorption pits, visualized by toluidine blue staining, are also shown. AxGFP, adenovirus-mediated expression of GFP. **, p < 0.01.
Intracellular ATP Affects Bone-resorbing Activity via Released Cellular ATP
To further confirm whether ATP release from intracellular stores affects osteoclast function, we selected Bcl-xL-overexpressing osteoclasts as a model system, which showed increased release of cellular ATP (Fig. 5A). Even after 48 h, >85% of Bcl-xL-overexpressing osteoclasts were still alive (data not shown). In addition, after a 48-h incubation, most of these cells exhibited large vacuoles and membrane blebs (Fig. 8C, left panel), possibly through continuous exposure to locally high ATP in the immediate vicinity of cell surfaces. Furthermore, these dramatic morphological changes were reduced by apyrase treatment or P2rx7 ablation (Fig. 8C, middle and right panels). Consistent with these results, the inhibitory effect of Bcl-xL expression on osteoclastic bone resorption was partially reversed by apyrase or P2rx7 deficiency (Fig. 8D). These results strongly support our hypothesis that the release of ATP from intracellular stores and autocrine/paracrine feedback through purinergic receptors control the bone-resorbing activity of mature osteoclasts.
Osteoclasts Derived from Aged Mice Display ATP Depletion with Increased Bone-resorbing Activity
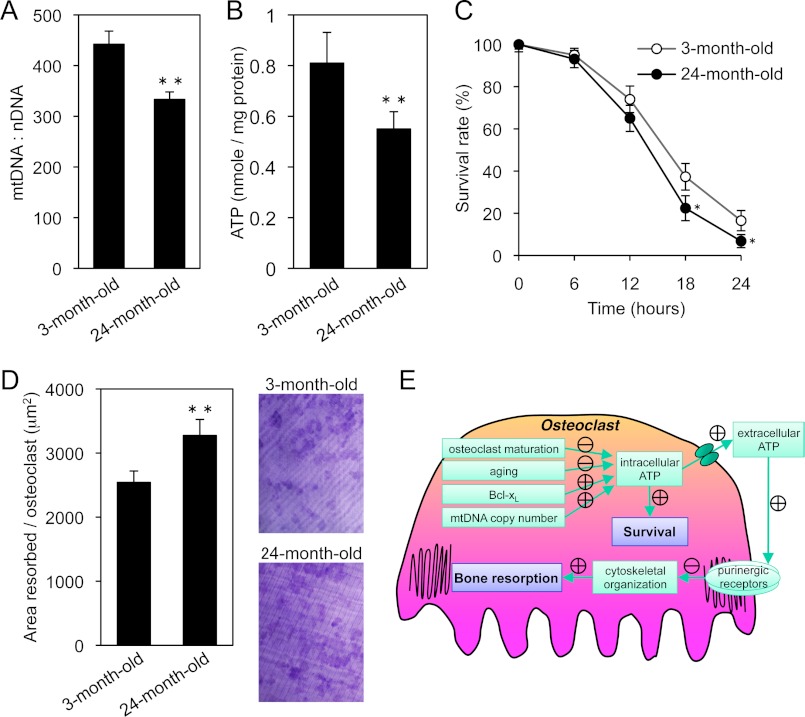
mtDNA deletions, mutations, and reduction have been reported to occur with aging in various tissues (44, 45). To determine the functional impact of these changes, we measured mtDNA copy number in osteoclasts derived from 3- and 24-month-old mice. For the old group, 24-month-old mice were used because C57BL/6 mice were already reported to lose bone continuously to 24 months of age (46). As shown in Fig. 9A, mtDNA copy number was significantly reduced without changing mitochondrial protein content in the osteoclasts of 24-month-old mice (supplemental Fig. S2A). Although the expression levels of Bcl-xL and Tfam remained unchanged with aging (supplemental Fig. S2B), intracellular ATP levels were significantly decreased in the osteoclasts of 24-month-old mice (Fig. 9B). As shown in Fig. 9, C and D, these osteoclasts exhibited increased bone resorption with a tendency toward shorter survival. Furthermore, real-time RT-PCR analysis suggested that the expression level of P2rx7 in osteoclasts was dramatically decreased when compared with BMMs (supplemental Fig. S3A). These results indicate that the reduction of P2rx7 expression during osteoclastogenesis, which led to a decreased response to external ATP, may contribute to up-regulation of bone-resorbing activity. However, we could not detect any difference in the expression level of P2rx7 in osteoclasts derived from 3- and 24-month-old mice (supplemental Fig. S3B), suggesting that age-related changes in bone-resorbing activity may be due to the decrease in mtDNA copy number and intracellular ATP, not to altered responses to extracellular ATP by aging. These results confirmed our findings that ATP depletion leads to osteoclastic bone resorption.
FIGURE 9.
Age-related changes in osteoclasts and a model for the role of intracellular ATP levels in osteoclastic bone resorption. A and B, mtDNA copy number (A) and intracellular ATP levels (B) in osteoclasts derived from 3- and 24-month-old mice. mtDNA copy number and intracellular ATP levels were significantly decreased in the osteoclasts of 24-month-old mice. C and D, time course of the survival (C) and bone-resorbing activity of osteoclasts (D) derived from 3- and 24-month-old mice. The osteoclasts of 24-month-old mice exhibited increased bone resorption with a tendency toward shorter survival. Representative resorption pits, visualized by toluidine blue staining, are also shown. E, graphic showing how osteoclasts exhibit the inverse correlation between their survival and bone-resorbing activity. In osteoclasts, intracellular ATP levels is influenced by developmental, physiological, and pathological cues. Intracellular ATP content is one of the key players in regulating osteoclast survival. On the other hand, released ATP from intracellular stores has an inhibitory effect on cytoskeletal organization and osteoclastic bone resorption via purinergic receptors. The delicate functional balance of intracellular and extracellular ATP levels regulates osteoclast function. *, p < 0.05, **, p < 0.01 versus osteoclasts derived from 3-month-old mice.
DISCUSSION
The finding that bisphosphonates directly inhibit osteoclast survival has attracted a great deal of attention to the molecular mechanism of osteoclast apoptosis, and remarkable progress has been made in the last 10 years to reveal the signaling pathways involved in the process. Recently, it was reported that nitrogen-containing bisphosphonate risedronate induces osteoclast apoptosis through the mitochondria-dependent pathway with an increased expression of Bim, a proapoptotic BH3-only Bcl-2 family member, and that the proapoptotic effect of risedronate is suppressed by Bim deletion (47). Interestingly, Bim−/− osteoclasts showed decreased bone-resorbing activity despite prolonged survival (4). In contrast, osteoclasts lacking antiapoptotic Bcl-xL exhibited the opposite pattern, namely, increased bone resorption with accelerated apoptosis (5), indicating that there may be an inverse correlation between osteoclast survival versus bone resorption. This negative relationship was also observed in Tfam cKO osteoclasts. Taken together, it is likely that osteoclasts complete their work at the sacrifice of their health.
Without ATP, life as we understand it could not exist. Even bacteria rely on an ATP molecule identical to that used in humans. One of the primary goals of the present study was to determine whether numerous high-functioning mitochondria plentifully produce ATP in osteoclasts to meet the energy demands for bone resorption, or conversely, whether osteoclasts work very hard and ATP is used up during bone resorption. We demonstrate that mature osteoclasts were depleted of ATP content when compared with BMMs, indicating that they are likely to wear out. Low intracellular ATP levels favor the release of cytochrome c, resulting in high apoptotic rates (48). It is possible that the apoptosis-sensitive phonotype of mature osteoclasts may be partly caused by ATP down-regulation. In addition, severe ATP depletion following Tfam ablation accelerated osteoclast apoptosis, whereas higher intracellular ATP concentrations by Bcl-xL expression promoted osteoclast survival and exceeded the negative effect of extracellular ATP, suggesting that intracellular ATP content is one of the key players in regulating osteoclast survival.
ATP depletion during osteoclastogenesis was found to be associated with RANKL-induced down-regulation of Bcl-xL expression. Bcl-xL overexpression failed to up-regulate osteoclastic bone resorption despite ameliorating ATP depletion. In contrast, ATP-depleted Tfam cKO osteoclasts showed increased bone resorption. This strange pattern of responses to intracellular ATP content suggests the existence of an unknown mechanism that regulates osteoclast bone-resorbing activity. One possible explanation for this strange pattern is that the metabolic stress-sensing protein kinase AMPK may play an important role in bone resorption. In fact, AMPK activity in osteoclasts was modulated in a manner opposite to intracellular ATP levels (Figs. 1, B and C, 3D, and 4A). However, changes in AMPK activity by adenovirus-mediated introduction of AMPK mutants failed to affect osteoclast survival and bone resorption. Another possible hypothesis is that released ATP from intracellular stores may have an inhibitory effect on osteoclastic bone resorption. First, we demonstrated that the steady-state level of extracellular ATP in cell cultures reflected intracellular ATP level in osteoclasts. Next, we found that high extracellular ATP concentrations strongly inhibited osteoclastic bone resorption. This inhibitory effect was caused by disruption of cytoskeletal organization including complete mislocalization of Pyk2 within cells. Pyk2 is reported to modulate integrin-dependent recruitment of cytoskeletal molecules that are important for adhesion, migration, and vesicular trafficking of mature osteoclast on bone (49), and Pyk2-null osteoclasts fail to form a podosome belt that is typically seen at the periphery of wild-type osteoclasts, resulting in a cell-autonomous defect in bone resorption (50). The above issues suggest that Pyk2 mislocalization following high extracellular ATP concentration is one of the biggest factors leading to defective bone resorption. Finally, we found that apyrase treatment or P2rx7 ablation up-regulated bone-resorbing activity. The direct functional link between intracellular ATP and osteoclastic bone resorption was clarified by our experiments with ATP-replete osteoclasts by Bcl-xL expression, in which removal of extracellular ATP or ablation of P2X7 receptor reduced deteriorative morphological changes and partially reversed the inhibitory effect on bone resorption (Fig. 8, C and D). These findings suggest that mature osteoclasts with ATP depletion resorb bone efficiently with their normal cytoskeleton, even during their short lifespan (Fig. 9E).
Cytosolic ATP concentrations are high (∼3 mm) and provide the source for extracellular ATP (51). The mechanism of nucleotide release from cells is currently not sufficiently understood. Recently, several mechanisms for ATP release from cells have been proposed, and they are activated by a wide range of stimuli (52, 53). These ATP release mechanisms include damage to the cell membrane, mechanical stress, hypoxia, inflammation, several agonists, and electrical excitation of neural tissue. In nonexcitable cells, a number of possible cellular mechanisms for ATP release have been studied, including exocytosis, gap junction hemichannels, ion channels, the cystic fibrosis transmembrane conductance regulator, and nucleotide transporters. Several properties and findings make pannexin 1 a very attractive candidate for an ATP-releasing channel. Homomeric pannexin 1 hexamers do not form gap junctions when expressed in mammalian cells and, instead, operate as plasma membrane channels (54, 55). They are activated by mechanical stress, membrane depolarization, and in a receptor-dependent manner. Despite the potential role of these channels in ATP release in numerous cell types (56), it needs to be realized that the precise mechanism of spontaneous constitutive ATP release remains unresolved and controversial.
Hazama et al. (57) reported that the treatment of 0.5 mm ATP or 300 μm BzATP increased bone resorption. In their experimental system, they used human osteoclasts, which are differentiated from CD14-positive monocyte on dentin slices under osteoclast-inducing condition for 3 weeks. In addition, their bone resorption assay is performed by counting the number of pits, not by measuring the pit area. In contrast to their results, we found that the pit area was dramatically decreased after treatment of 100 μm ATPγS or 150 μm BzATP on the bone-resorbing activity by measuring the pit area using mouse osteoclasts (Fig. 5B). In addition, removal of extracellular ATP with apyrase up-regulated osteoclastic bone resorption (Fig. 8A). It should also be noted that P2rx7−/− mice exhibited a significant decrease in bone mass associated with increased number of osteoclast per bone surface (19). This is consistent with our findings that extracellular ATP has a deteriorative effect on osteoclast function. We cannot fully explain the reason for the discrepancy between our results and those of the previous study, but it is possible that human osteoclasts have a different profile of functional P2X receptors and that counting the number of pits using human osteoclasts, which require 3 weeks of culture on dentin slices, may be inappropriate to investigate the bone-resorbing activity of mature osteoclasts in vitro.
Our in vitro study showed that Tfam-deficient osteoclasts exhibited increased bone-resorbing activity and decreased survival. Due to this contrary relationship, it is possible that Tfam cKO mice develop two different phenotypes; osteoporosis is one predicted consequence of increased bone-resorbing activity, and osteopetrosis is another consequence of a reduced number of osteoclasts. According to the bone histomorphometric analysis, Oc.N/BS was dramatically reduced, whereas ES/BS and Oc.S/BS (where Oc.S. indicates osteoclast surface) were also reduced with marginal statistical difference (p < 0.1) (Fig. 2F), indicating that overall bone resorption in Tfam cKO mice was slightly reduced. Because some parameters of osteoblastic bone formation decreased and several coupling factors were reported to be involved in osteoclast-mediated promotion of osteoblast recruitment and maturation (58), we investigated the expression levels of candidate coupling factors, such as Wnt10b, BMP6, and sphingosine kinase 1 (SPHK1) in Tfam cKO osteoclasts. However, we could not find any difference in these factors (data not shown). One possible explanation for the decrease in osteoblast parameters is that dramatically reduced number of osteoclasts may lead to a decrease in the concentration of osteoclast-derived coupling factors in the bone marrow microenvironment. On the other hand, ATP released from bone cells through constitutive and inducible mechanisms is reported to promote osteogenesis in vitro (59–61). Moreover, osteogenesis and anabolic responses to mechanical loading are impaired in P2rx7−/− mice (19, 62), indicating that release of ATP into the bone extracellular fluid occurs in vivo. It is also possible that the observed reduction in osteoblast parameters and body size in Tfam cKO mice may be caused by reduced release of ATP from Tfam-deficient osteoclasts. Together, the reduced number of osteoclasts and decreased amount of osteoclast-derived coupling factors following Tfam deficiency achieve a balance between bone resorption and formation, resulting in similar bone mass.
Osteoporosis, a classical age-related disease, is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility. Most studies evaluating age-related changes in bone resorption markers observed an increase in serum and urinary indices (63–66). mtDNA deletions, mutations, and reductions have been reported to occur with aging in various tissues (44, 45). The above issues suggest that mtDNA copy number may decrease with age in osteoclasts, which might in turn contribute to the age-related increase in bone resorption. Experiments using osteoclasts derived from 3- and 24-month-old mice showed that mtDNA copy number and intracellular ATP level decreased with age. In addition, we found a positive correlation between age and bone-resorbing activity of osteoclasts. These findings from aged osteoclasts confirm our findings that lower intracellular ATP levels up-regulate osteoclastic bone resorption. Age-related mitochondrial dysfunction, such as reduced mtDNA copy number, is likely to be involved in the development of osteoporosis.
In conclusion, the results of the present study demonstrate that intracellular ATP levels play a pivotal role, not only in osteoclast apoptosis, but also in the bone-resorbing function of the cells. Further investigation of mitochondrial functions regulating the inverse correlation between osteoclast survival and bone resorption will give new insights into the molecular mechanisms regulating bone homeostasis.
Supplementary Material
Acknowledgments
Tfamflox/flox mice were kindly provided by Dr. Nils-Göran Larsson (Karolinska Institutet). Cathepsin K-Cre mice were kindly provided by Dr. Shigeaki Kato (University of Tokyo). We thank Yasuhiro Sawada (National University of Singapore), Shigeru Yamada, Sachiho Kubo, Daichi Fukunaga, Ryo Nakayama, and Naoya Murase (Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology) for helpful discussion.
This work was in part supported by grants-in-aid for scientific research from the Japan Society for the Promotion of Science.

This article contains supplemental Figs. S1–S3.
- RANKL
- receptor activator of nuclear factor κ-B ligand
- AMPK
- AMP-activated protein kinase
- cKO
- conditional knockout
- Pyk2
- proline-rich tyrosine kinase
- Tfam
- mitochondrial transcription factor A
- α-MEM
- α-minimum Eagle's medium
- ATPγS
- adenosine 5′-O-(3-thiotriphosphate)
- TRAP
- tartrate-resistant acid phosphatase
- BMM
- bone marrow-derived monocyte/macrophage precursor cell
- NFATc1
- nuclear factor of activated T cells c1
- Csk
- C-Src tyrosine kinase
- BzATP
- 2′,3′-O-(4-benzoylbenzoyl)-ATP
- Oc.N.
- osteoclast number
- ES
- eroded surface
- BS
- bone surface
- DN
- dominant negative
- CA
- constitutively active.
REFERENCES
- 1. Boyle W. J., Simonet W. S., Lacey D. L. (2003) Osteoclast differentiation and activation. Nature 423, 337–342 [DOI] [PubMed] [Google Scholar]
- 2. Negishi-Koga T., Takayanagi H. (2009) Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol. Rev. 231, 241–256 [DOI] [PubMed] [Google Scholar]
- 3. Tanaka S., Miyazaki T., Fukuda A., Akiyama T., Kadono Y., Wakeyama H., Kono S., Hoshikawa S., Nakamura M., Ohshima Y., Hikita A., Nakamura I., Nakamura K. (2006) Molecular mechanism of the life and death of the osteoclast. Ann. N.Y. Acad. Sci. 1068, 180–186 [DOI] [PubMed] [Google Scholar]
- 4. Akiyama T., Bouillet P., Miyazaki T., Kadono Y., Chikuda H., Chung U. I., Fukuda A., Hikita A., Seto H., Okada T., Inaba T., Sanjay A., Baron R., Kawaguchi H., Oda H., Nakamura K., Strasser A., Tanaka S. (2003) Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. EMBO J. 22, 6653–6664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iwasawa M., Miyazaki T., Nagase Y., Akiyama T., Kadono Y., Nakamura M., Oshima Y., Yasui T., Matsumoto T., Nakamura T., Kato S., Hennighausen L., Nakamura K., Tanaka S. (2009) The antiapoptotic protein Bcl-xL negatively regulates the bone-resorbing activity of osteoclasts in mice. J. Clin. Invest. 119, 3149–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bodin P., Burnstock G. (2001) Purinergic signaling: ATP release. Neurochem. Res. 26, 959–969 [DOI] [PubMed] [Google Scholar]
- 7. Burnstock G. (2002) Purinergic signaling and vascular cell proliferation and death. Arterioscler. Thromb. Vasc. Biol. 22, 364–373 [DOI] [PubMed] [Google Scholar]
- 8. Jeng J. Y., Yeh T. S., Lee J. W., Lin S. H., Fong T. H., Hsieh R. H. (2008) Maintenance of mitochondrial DNA copy number and expression are essential for preservation of mitochondrial function and cell growth. J. Cell. Biochem. 103, 347–357 [DOI] [PubMed] [Google Scholar]
- 9. Yi C. H., Pan H., Seebacher J., Jang I. H., Hyberts S. G., Heffron G. J., Vander Heiden M. G., Yang R., Li F., Locasale J. W., Sharfi H., Zhai B., Rodriguez-Mias R., Luithardt H., Cantley L. C., Daley G. Q., Asara J. M., Gygi S. P., Wagner G., Liu C. F., Yuan J. (2011) Metabolic regulation of protein N-α-acetylation by Bcl-xL promotes cell survival. Cell 146, 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feldenberg L. R., Thevananther S., del Rio M., de Leon M., Devarajan P. (1999) Partial ATP depletion induces Fas- and caspase-mediated apoptosis in MDCK cells. Am. J. Physiol. 276, F837–846 [DOI] [PubMed] [Google Scholar]
- 11. Gaspari M., Larsson N. G., Gustafsson C. M. (2004) The transcription machinery in mammalian mitochondria. Biochim. Biophys. Acta 1659, 148–152 [DOI] [PubMed] [Google Scholar]
- 12. Corral-Debrinski M., Shoffner J. M., Lott M. T., Wallace D. C. (1992) Association of mitochondrial DNA damage with aging and coronary atherosclerotic heart disease. Mutat. Res. 275, 169–180 [DOI] [PubMed] [Google Scholar]
- 13. Larsson N. G., Wang J., Wilhelmsson H., Oldfors A., Rustin P., Lewandoski M., Barsh G. S., Clayton D. A. (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 18, 231–236 [DOI] [PubMed] [Google Scholar]
- 14. Ekstrand M. I., Falkenberg M., Rantanen A., Park C. B., Gaspari M., Hultenby K., Rustin P., Gustafsson C. M., Larsson N. G. (2004) Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet 13, 935–944 [DOI] [PubMed] [Google Scholar]
- 15. Falkenberg M., Gaspari M., Rantanen A., Trifunovic A., Larsson N. G., Gustafsson C. M. (2002) Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nat. Genet. 31, 289–294 [DOI] [PubMed] [Google Scholar]
- 16. Wang J., Wilhelmsson H., Graff C., Li H., Oldfors A., Rustin P., Brüning J. C., Kahn C. R., Clayton D. A., Barsh G. S., Thorén P., Larsson N. G. (1999) Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat. Genet. 21, 133–137 [DOI] [PubMed] [Google Scholar]
- 17. Li H., Wang J., Wilhelmsson H., Hansson A., Thoren P., Duffy J., Rustin P., Larsson N. G. (2000) Genetic modification of survival in tissue-specific knockout mice with mitochondrial cardiomyopathy. Proc. Natl. Acad. Sci. U.S.A. 97, 3467–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakamura T., Imai Y., Matsumoto T., Sato S., Takeuchi K., Igarashi K., Harada Y., Azuma Y., Krust A., Yamamoto Y., Nishina H., Takeda S., Takayanagi H., Metzger D., Kanno J., Takaoka K., Martin T. J., Chambon P., Kato S. (2007) Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130, 811–823 [DOI] [PubMed] [Google Scholar]
- 19. Ke H. Z., Qi H., Weidema A. F., Zhang Q., Panupinthu N., Crawford D. T., Grasser W. A., Paralkar V. M., Li M., Audoly L. P., Gabel C. A., Jee W. S., Dixon S. J., Sims S. M., Thompson D. D. (2003) Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol. Endocrinol. 17, 1356–1367 [DOI] [PubMed] [Google Scholar]
- 20. Takahashi N., Akatsu T., Udagawa N., Sasaki T., Yamaguchi A., Moseley J. M., Martin T. J., Suda T. (1988) Osteoblastic cells are involved in osteoclast formation. Endocrinology 123, 2600–2602 [DOI] [PubMed] [Google Scholar]
- 21. Miyazaki T., Katagiri H., Kanegae Y., Takayanagi H., Sawada Y., Yamamoto A., Pando M. P., Asano T., Verma I. M., Oda H., Nakamura K., Tanaka S. (2000) Reciprocal role of ERK and NF-κB pathways in survival and activation of osteoclasts. J. Cell Biol. 148, 333–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oshima Y., Akiyama T., Hikita A., Iwasawa M., Nagase Y., Nakamura M., Wakeyama H., Kawamura N., Ikeda T., Chung U. I., Hennighausen L., Kawaguchi H., Nakamura K., Tanaka S. (2008) Pivotal role of Bcl-2 family proteins in the regulation of chondrocyte apoptosis. J. Biol. Chem. 283, 26499–26508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akiyama T., Miyazaki T., Bouillet P., Nakamura K., Strasser A., Tanaka S. (2005) In vitro and in vivo assays for osteoclast apoptosis. Biol. Proced. Online 7, 48–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sawada Y., Sheetz M. P. (2002) Force transduction by Triton cytoskeletons. J. Cell Biol. 156, 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sawada Y., Tamada M., Dubin-Thaler B. J., Cherniavskaya O., Sakai R., Tanaka S., Sheetz M. P. (2006) Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Orriss I. R., Knight G. E., Utting J. C., Taylor S. E., Burnstock G., Arnett T. R. (2009) Hypoxia stimulates vesicular ATP release from rat osteoblasts. J. Cell Physiol. 220, 155–162 [DOI] [PubMed] [Google Scholar]
- 27. Chen H., Vermulst M., Wang Y. E., Chomyn A., Prolla T. A., McCaffery J. M., Chan D. C. (2010) Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141, 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alavian K. N., Li H., Collis L., Bonanni L., Zeng L., Sacchetti S., Lazrove E., Nabili P., Flaherty B., Graham M., Chen Y., Messerli S. M., Mariggio M. A., Rahner C., McNay E., Shore G. C., Smith P. J., Hardwick J. M., Jonas E. A. (2011) Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat. Cell Biol. 13, 1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Y. B., Aon M. A., Hsu Y. T., Soane L., Teng X., McCaffery J. M., Cheng W. C., Qi B., Li H., Alavian K. N., Dayhoff-Brannigan M., Zou S., Pineda F. J., O'Rourke B., Ko Y. H., Pedersen P. L., Kaczmarek L. K., Jonas E. A., Hardwick J. M. (2011) Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J. Cell Biol. 195, 263–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyazaki T., Neff L., Tanaka S., Horne W. C., Baron R. (2003) Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J. Cell Biol. 160, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nagase Y., Iwasawa M., Akiyama T., Kadono Y., Nakamura M., Oshima Y., Yasui T., Matsumoto T., Hirose J., Nakamura H., Miyamoto T., Bouillet P., Nakamura K., Tanaka S. (2009) Antiapoptotic molecule Bcl-2 regulates the differentiation, activation, and survival of both osteoblasts and osteoclasts. J. Biol. Chem. 284, 36659–36669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takayanagi H., Kim S., Koga T., Nishina H., Isshiki M., Yoshida H., Saiura A., Isobe M., Yokochi T., Inoue J., Wagner E. F., Mak T. W., Kodama T., Taniguchi T. (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 3, 889–901 [DOI] [PubMed] [Google Scholar]
- 33. Winkeler C. L., Kladney R. D., Maggi L. B., Jr., Weber J. D. (2012) Cathepsin K-Cre causes unexpected germline deletion of genes in mice. PLoS One 7, e42005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang J., Silva J. P., Gustafsson C. M., Rustin P., Larsson N. G. (2001) Increased in vivo apoptosis in cells lacking mitochondrial DNA gene expression. Proc. Natl. Acad. Sci. U.S.A. 98, 4038–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hardie D. G. (2003) Minireview: The AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 144, 5179–5183 [DOI] [PubMed] [Google Scholar]
- 36. Schwiebert E. M., Zsembery A. (2003) Extracellular ATP as a signaling molecule for epithelial cells. Biochim. Biophys. Acta 1615, 7–32 [DOI] [PubMed] [Google Scholar]
- 37. Chen Y., Corriden R., Inoue Y., Yip L., Hashiguchi N., Zinkernagel A., Nizet V., Insel P. A., Junger W. G. (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314, 1792–1795 [DOI] [PubMed] [Google Scholar]
- 38. Morelli A., Chiozzi P., Chiesa A., Ferrari D., Sanz J. M., Falzoni S., Pinton P., Rizzuto R., Olson M. F., Di Virgilio F. (2003) Extracellular ATP causes ROCK I-dependent bleb formation in P2X7-transfected HEK293 cells. Mol. Biol. Cell 14, 2655–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hwang S. M., Li J., Koo N. Y., Choi S. Y., Lee S. J., Oh S. B., Castro R., Kim J. S., Park K. (2009) Role of purinergic receptor in α-fodrin degradation in Par C5 cells. J. Dent. Res. 88, 927–932 [DOI] [PubMed] [Google Scholar]
- 40. Martin S. J., O'Brien G. A., Nishioka W. K., McGahon A. J., Mahboubi A., Saido T. C., Green D. R. (1995) Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J. Biol. Chem. 270, 6425–6428 [DOI] [PubMed] [Google Scholar]
- 41. Auger R., Motta I., Benihoud K., Ojcius D. M., Kanellopoulos J. M. (2005) A role for mitogen-activated protein kinase(Erk1/2) activation and non-selective pore formation in P2X7 receptor-mediated thymocyte death. J. Biol. Chem. 280, 28142–28151 [DOI] [PubMed] [Google Scholar]
- 42. Iglesias R., Locovei S., Roque A., Alberto A. P., Dahl G., Spray D. C., Scemes E. (2008) P2X7 receptor-Pannexin 1 complex: pharmacology and signaling. Am. J. Physiol. Cell Physiol. 295, C752–C760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pellegatti P., Falzoni S., Donvito G., Lemaire I., Di Virgilio F. (2011) P2X7 receptor drives osteoclast fusion by increasing the extracellular adenosine concentration. FASEB J. 25, 1264–1274 [DOI] [PubMed] [Google Scholar]
- 44. Wallace D. C. (1992) Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science 256, 628–632 [DOI] [PubMed] [Google Scholar]
- 45. Barazzoni R., Short K. R., Nair K. S. (2000) Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J. Biol. Chem. 275, 3343–3347 [DOI] [PubMed] [Google Scholar]
- 46. Halloran B. P., Ferguson V. L., Simske S. J., Burghardt A., Venton L. L., Majumdar S. (2002) Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J. Bone Miner Res. 17, 1044–1050 [DOI] [PubMed] [Google Scholar]
- 47. Matsumoto T., Nagase Y., Iwasawa M., Yasui T., Masuda H., Kadono Y., Nakamura K., Tanaka S. (2011) Distinguishing the proapoptotic and antiresorptive functions of risedronate in murine osteoclasts: role of the Akt pathway and the ERK/Bim axis. Arthritis Rheum. 63, 3908–3917 [DOI] [PubMed] [Google Scholar]
- 48. Izyumov D. S., Avetisyan A. V., Pletjushkina O. Y., Sakharov D. V., Wirtz K. W., Chernyak B. V., Skulachev V. P. (2004) “Wages of fear”: transient threefold decrease in intracellular ATP level imposes apoptosis. Biochim. Biophys. Acta 1658, 141–147 [DOI] [PubMed] [Google Scholar]
- 49. Duong L. T., Lakkakorpi P., Nakamura I., Rodan G. A. (2000) Integrins and signaling in osteoclast function. Matrix Biol. 19, 97–105 [DOI] [PubMed] [Google Scholar]
- 50. Gil-Henn H., Destaing O., Sims N. A., Aoki K., Alles N., Neff L., Sanjay A., Bruzzaniti A., De Camilli P., Baron R., Schlessinger J. (2007) Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2−/− mice. J. Cell Biol. 178, 1053–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miller D. S., Horowitz S. B. (1986) Intracellular compartmentalization of adenosine triphosphate. J. Biol. Chem. 261, 13911–13915 [PubMed] [Google Scholar]
- 52. Fields R. D. (2011) Nonsynaptic and nonvesicular ATP release from neurons and relevance to neuron-glia signaling. Semin. Cell Dev. Biol. 22, 214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Praetorius H. A., Leipziger J. (2009) ATP release from non-excitable cells. Purinergic Signal. 5, 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang Y., Grinspan J. B., Abrams C. K., Scherer S. S. (2007) Pannexin 1 is expressed by neurons and glia but does not form functional gap junctions. Glia 55, 46–56 [DOI] [PubMed] [Google Scholar]
- 55. Boassa D., Ambrosi C., Qiu F., Dahl G., Gaietta G., Sosinsky G. (2007) Pannexin 1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J. Biol. Chem. 282, 31733–31743 [DOI] [PubMed] [Google Scholar]
- 56. MacVicar B. A., Thompson R. J. (2010) Non-junction functions of pannexin 1 channels. Trends Neurosci. 33, 93–102 [DOI] [PubMed] [Google Scholar]
- 57. Hazama R., Qu X., Yokoyama K., Tanaka C., Kinoshita E., He J., Takahashi S., Tohyama K., Yamamura H., Tohyama Y. (2009) ATP-induced osteoclast function: the formation of sealing-zone like structure and the secretion of lytic granules via microtubule-deacetylation under the control of Syk. Genes Cells 14, 871–884 [DOI] [PubMed] [Google Scholar]
- 58. Pederson L., Ruan M., Westendorf J. J., Khosla S., Oursler M. J. (2008) Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc. Natl. Acad. Sci. U.S.A. 105, 20764–20769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Panupinthu N., Rogers J. T., Zhao L., Solano-Flores L. P., Possmayer F., Sims S. M., Dixon S. J. (2008) P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. J. Cell Biol. 181, 859–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Genetos D. C., Geist D. J., Liu D., Donahue H. J., Duncan R. L. (2005) Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J. Bone Miner. Res. 20, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Katz S., Ayala V., Santillán G., Boland R. (2011) Activation of the PI3K/Akt signaling pathway through P2Y2 receptors by extracellular ATP is involved in osteoblastic cell proliferation. Arch. Biochem. Biophys. 513, 144–152 [DOI] [PubMed] [Google Scholar]
- 62. Li J., Liu D., Ke H. Z., Duncan R. L., Turner C. H. (2005) The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J. Biol. Chem. 280, 42952–42959 [DOI] [PubMed] [Google Scholar]
- 63. Wishart J. M., Need A. G., Horowitz M., Morris H. A., Nordin B. E. (1995) Effect of age on bone density and bone turnover in men. Clin. Endocrinol. (Oxf.) 42, 141–146 [DOI] [PubMed] [Google Scholar]
- 64. Fatayerji D., Eastell R. (1999) Age-related changes in bone turnover in men. J. Bone Miner. Res. 14, 1203–1210 [DOI] [PubMed] [Google Scholar]
- 65. Gallagher J. C., Kinyamu H. K., Fowler S. E., Dawson-Hughes B., Dalsky G. P., Sherman S. S. (1998) Calciotropic hormones and bone markers in the elderly. J. Bone Miner. Res. 13, 475–482 [DOI] [PubMed] [Google Scholar]
- 66. Clarke B. L., Ebeling P. R., Jones J. D., Wahner H. W., O'Fallon W. M., Riggs B. L., Fitzpatrick L. A. (2002) Predictors of bone mineral density in aging healthy men varies by skeletal site. Calcif. Tissue Int. 70, 137–145 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.