Background: Determinants of membrane protein dual topological orientation are not known.
Results: Dual topological conformers of lactose permease co-exist in proportion to membrane phospholipid composition. Post-assembly changes in phospholipid composition alter the proportional amounts.
Conclusion: Dual conformations of membrane proteins are determined during and post-assembly by lipid-protein interactions.
Significance: A thermodynamic model explains the existence of multiple conformers of membrane proteins in eukaryotic cells.
Keywords: Escherichia coli, Membrane Proteins, Phosphatidylethanolamine, Protein Folding, Transporters, Dual Topology, Lactose Permease, Lipid-dependent Topogenesis, membrane Protein Topology
Abstract
The mechanism by which membrane proteins exhibit structural and functional duality in the same membrane or different membranes is unknown. We posit that such duality is determined by both the protein sequence and the membrane lipid composition wherein a spatial or temporal change in the latter can result in a post-assembly change in protein structure and function. To investigate whether co-existence of multiple topological conformers is dependent on the membrane lipid composition, we determined the topological organization of lactose permease in an Escherichia coli model cell system in which phosphatidylethanolamine membrane content can be systematically varied. At intermediate levels of phosphatidylethanolamine a mixture of native and topologically mis-oriented conformers co-existed. There was no threshold level of phosphatidylethanolamine determining a sharp transition from one conformer to the other. Co-existing conformers were not in rapid equilibrium at a static lipid composition indicating that duality of topology is established during an early folding step. Depletion of intermediate levels of phosphatidylethanolamine after final protein assembly resulted in complete mis-orientation of the native conformer. Combined with previous results, such topological dynamics are reversible in both directions. We propose a thermodynamically based model for how lipid-protein interactions can result in a mixed topological organization and how changes in lipid composition can result in changes in the ratio of topologically distinct conformers of proteins. These observations demonstrate a potential lipid-dependent biological switch for generating dynamic structural and functional heterogeneity for a protein within the same membrane or between different membranes in more complex eukaryotic cells.
Introduction
The folding of membrane proteins usually results in a uniform topological orientation of transmembrane domains (TMs)3 initially orchestrated co-translationally through coordinated translocation and membrane integration events. The ribosome-translocon complex directs membrane insertion of hydrophobic TMs whose orientation is determined by topogenic signals decoded according to the positive-inside rule (1, 2). Because membrane proteins and the unique membrane lipid composition of each organism have co-evolved to guarantee proper protein topogenesis, assembly, and function, the involvement of specific lipids in topological decisions only becomes evident when membrane lipid composition is manipulated. Such genetic manipulation of Escherichia coli membrane lipid composition (3, 4) established that membrane lipid composition in combination with membrane protein sequence are co-determinants of the topological organization of several polytopic membrane proteins (5–8). The N-terminal six-TM α-helical bundle of lactose permease (LacY) (Fig. 1) and the N-terminal α-helical hairpin of phenylalanine permease and γ-aminobutyrate permease are topologically mis-oriented with loss of energy-dependent uphill transport but retention of energy independent downhill transport when assembled in E. coli membranes lacking the major phospholipid phosphatidylethanolamine (PE) (5, 7, 8).
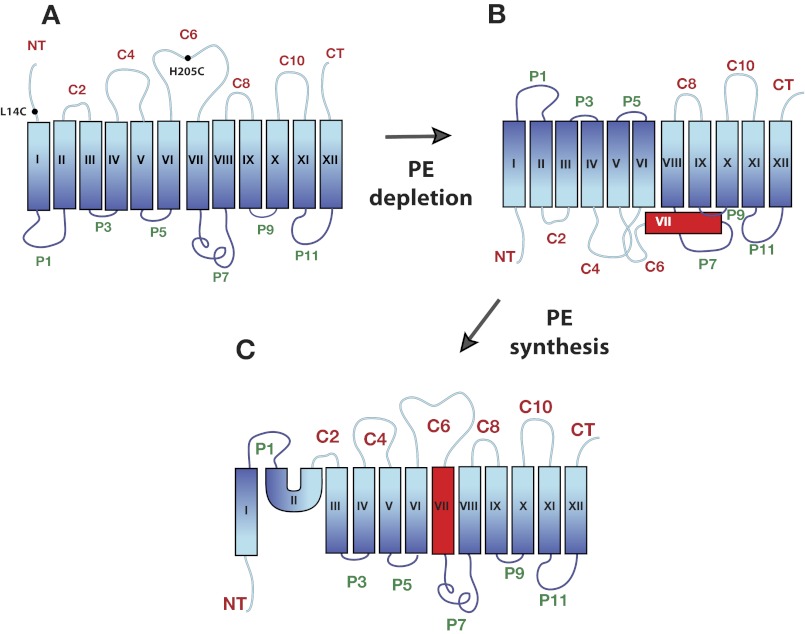
FIGURE 1.
TM orientation for LacY as a function of membrane lipid composition. The cytoplasm is at the top of each figure. TMs are labeled in Roman numerals. Extramembrane domains are N-terminal (NT), C-terminal (CT), or labeled sequentially and prefaced with their cytoplasmic (C) or periplasmic (P) location in PE-containing cells. A, topology of LacY as previously reported (5) assembled in PE-containing cells. The approximate positions are shown in NT and C6 of diagnostic single cysteine replacements (●) in a cysteine-less derivative of LacY used for topological determinations. B, topological inversion of the N-terminal six-TM helical bundle of LacY as previously determined (13) after assembly in PE-lacking cells or after depletion of PE (as demonstrated herein). The exposure of TM VII to the periplasm (not demonstrated herein) is required for inversion of topology. C, topological organization of LacY after induction of PE post-assembly of LacY in cells lacking PE (13).
Substitution of zwitterionic PE with net neutral foreign lipids (9–12), which also dilutes the high negative membrane surface charge contributed by the remaining phosphatidylglycerol and cardiolipin, prevents topological mis-orientation of LacY. The final topological organization is also dependent on the net charge of the cytoplasmic surface of the N-terminal domains in a position-independent manner (6, 13). Therefore, charge interactions between the membrane surface and normally cytoplasmic domains directly influences final topological organization (14). The influence of lipids on TM topology occurs not only co-translationally during initial membrane insertion but post-translationally either early after release from the translocon followed by initial folding steps or after complete folding into a compact structure (5, 7, 13). In the latter case mis-oriented LacY initially assembled in the absence of PE refolds with near native TM orientation (Fig. 1) and regain of uphill transport activity with induction of PE synthesis post-assembly.
If topological organization is a highly cooperative process, then a sharp transition between possible topological states and no mixture of topological orientations should be observed as a function of changing membrane lipid composition. For a less cooperative process, intermediate and mixed topological states may exist within the same membrane. In this report we show that at intermediate PE levels, LacY exists in at least two topologically distinct and stable conformations in the same cell, and further lowering of PE levels results in a post-assembly complete conversion of native LacY to its inverted conformation. Therefore, a mixture of topologically oriented forms is dependent on both protein sequence (6, 15, 16) and membrane lipid composition and topological inversions of TMs are reversible in both directions.
The existence of dual topologies for proteins within the same membrane or in different subcellular membranes raises provocative questions concerning the mechanism of membrane protein assembly (17, 18). How is the ratio of two different orientations of the same protein controlled and at what step of protein synthesis and assembly is dual topology established? At present there is no mechanistic understanding of how such duality in structure is achieved. Current hypotheses are primarily focused at the point of initial membrane insertion guided by the translocon. To our knowledge this article is the first to address these questions and demonstrate the involvement of membrane lipid composition as a determinant of dual topological organization for a membrane protein. We demonstrate that the proportion of multiple topological conformers of a membrane protein is determined by the membrane lipid composition at the time of initial protein folding and can change post-assembly in proportion to the changes in the membrane lipid composition. Examples exist in nature of proteins that exhibit topological and functional duality. Our results provide a potential molecular mechanism for generation of multiple topological arrangements for the same protein within the same membrane and open an intriguing possibility that multiple topological arrangements of membrane protein can be interchanged post-insertionally either during movement of proteins between different intracellular membranes or laterally between lipid microdomains. This novel mechanism also suggests that the shift of equilibrium between prion-like protein folding and misfolding pathways in amyloidogenesis can be simply caused by changes in local lipid environment.
EXPERIMENTAL PROCEDURES
Reagents
Anhydrotetracycline (aTc) was from Spectrum Chemical M.F.G. Corp. Site-directed polyclonal antibody (pAb) directed against the C terminus of LacY was made by ProSci Inc. Conformation-specific monoclonal antibody (mAb) 4B1 directed at the P7 domain of LacY was a generous gift of Dr. Ronald Kaback (University of California, Los Angeles). Biotinylated Ro09-0198 was a gift of Dr. Masato Umeda (Kyoto University, Japan). Avidin-horseradish peroxidase, Protein A/G-agarose resin, spin columns, and Super Signal West Pico chemiluminescent substrate were from Pierce Thermo Fisher Scientific. Thesit was from Fluka. 3-(N-Maleimidylpropionyl)biocytin (MPB) and 4-acetamido-4-maleimidylstilbene-2,2-disulfonic acid (AMS) were purchased from Invitrogen-Molecular Probes. Nitrocellulose sheets (0.2 μm) for immunoblotting were purchased from Schleicher and Schuell. [35S]Methionine/cysteine (Tran35S-labelTM) and [32P]PO4 were from MP Biomedicals.
Bacterial Strains and Plasmids
pT7-5 plasmids (AmpR) expressing LacY containing single amino acid replacements by cysteine in a derivative of LacY in which endogenous cysteines were replaced by serine were constructed by site-directed mutagenesis and expressed under OPtac regulation as previously described (5). Strain AT2033 (PLtetO-1-pssA+ pss93::kanR lacY::Tn9 recA srl::Tn10) in which the PE content of the cell can be regulated by the level of aTc in the growth medium was described previously (13).
Cell Growth and Regulated Expression of lacY and pssA
Cells of strain AT2033 containing different plasmids expressing derivatives of LacY were first grown overnight at 37 °C in Luria-Bertani (LB) medium supplemented with 100 μg/ml of ampicillin and 50 mm MgCl2 to support growth at residual levels of PE. Then cells were diluted to A600 of 0.05 into the same medium. Isopropyl β-d-thiogalactoside (IPTG, 1 mm) and aTc at the indicated concentrations were added to induce LacY expression and PE synthesis, respectively. Cells were cultured at 37 °C to an A600 of 0.5. Cells were examined by fluorescence microscopy, taken for lipid analysis, or subjected to topological analysis. To determine phospholipid composition cells were grown in the presence of [32P]PO4. Phospholipids were extracted and analyzed by silica gel thin-layer chromatography using the following systems: solvent 1, chloroform, methanol, water, 8.56 n NH4OH (60:37.5:3:1, v/v/v/v); and solvent 2, chloroform/methanol/acetic acid (65:25:8, v/v/v) (3, 19). Chromatography plates (Kiselgel 60 (0.25 mm) from Merck) were impregnated with boric acid (20) when used with solvent 1. Radiolabeled lipids were visualized and quantified using a Personal Molecular ImagerTM FX (Bio-Rad).
In PE depletion experiments identically induced aTc and IPTG cells were grown in parallel either with [32P]PO4 to monitor the phospholipid composition or without radiolabel to analyze LacY topology. Cells were collected by centrifugation (5 min, 15,000 × g) at A600 of 0.5–0.7 and the pellet derived from 100 ml of a 200-ml culture was analyzed for orientation of LacY initially expressed in the presence of PE. The pellet derived from the remaining 100 ml of culture was washed twice by centrifugation with pre-warmed LB medium supplemented with MgCl2 to remove IPTG and aTc. These cells were re-suspended in the same volume (100 ml) of pre-warmed medium supplemented with ampicillin and MgCl2 and after 3.5 h of growth in the absence of inducers, cells were harvested and assayed either for the orientation of LacY or phospholipid composition.
Fluorescence Microscopy and Image Processing
For fluorescence detection of PE in whole cells, strain AT2033 was induced by the addition of varying concentrations of aTc. Cells were harvested at A600 of 0.5–0.7 and examined for PE content by indirect fluorescence microscopy as described (21) with the following modifications. Cells were fixed for 20 min at room temperature in 4.4% (w/v) paraformaldehyde and then treated with lysozyme (0.5 mg/ml for 30 s) at room temperature as described (22). After several washing steps cells were affixed to poly-l-lysine-coated microscope slides and after drying were treated with biotinylated Ro09-0198 (21). The slides were subsequently washed with 0.05% Tween/PBS, and incubated in 0.5% (w/v) BSA/PBS containing 5 μg/ml of streptavidin conjugated with Alexa Fluor 430. Cell populations were viewed using both Nomarski differential interference contrast optics and epifluorescent illumination for detection of Alexa Fluor 430 (bright, green-fluorescent probe with excitation/emission maxima ∼434/539 nm) fluorescence. Fluorescence images were captured (FITC) using varying exposure times to assure linearity at the 300-ms exposure time finally used. Captured images were processed with SlideBook 5.0 software.
Transmembrane Protein Topology Mapping
Topological analysis is based on the controlled membrane permeability of a thiol-specific reagent (MPB, where maleimide is attached to biotin) and its reactivity with diagnostic cysteine residues in extramembrane domains of LacY as previously described (3, 23). Briefly whole cells (periplasmically exposed but cytoplasmic and TM cysteines protected) or sonicated cells (periplasmic and cytoplasmic exposed but TM cysteines protected) were treated with MPB at pH 7.5. To address mixed topologies, a whole cell aliquot was preincubated for 30 min at 25 °C with AMS at a final concentration of 5 mm to block periplasmic water-accessible cysteine residues exposed on the outer surface of the inner membrane (3, 5, 6). AMS was then removed by two cycles of centrifugation (3, 5). After membrane solubilization with SDS, LacY was incubated with pAb overnight at 4 °C and isolated using spin columns (6). The biotinylated LacY was then subjected to SDS-PAGE and Western blotting followed by detection with Avidin-HRP using a Fluor-S Max MultiImager (Bio-Rad). Images were processed and quantified as described (13). In all cases equal amounts of cells were processed from samples before and after sonication and the same amount of sample was applied to SDS gels. LacY displays as two major bands near the apparent 33-kDa region of SDS gels where no other bands occur based on controls using cells lacking LacY or expressing LacY without any cysteine residues (5). The intensity of biotinylated bands from the same gels was quantified using Quantity One software for scanning and analysis of the captured images (Bio-Rad).
Determination of Properly Oriented LacY by Antibody Precipitation
Properly oriented LacY was isolated by immunoprecipitation with conformation-specific mAb 4B1, which only recognizes properly oriented and functional LacY (11, 24) as previously described (25) with minor modifications. Total membranes prepared from whole cells by sonication (6) were solubilized in 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1.0 mm EDTA, 2% dodecyl maltoside as previously described (3) and incubated further with either mAb 4B1 or pAb (detects LacY irrespective of orientation or functionality) in the same buffer overnight at 4 °C. Supernatants obtained by ultracentrifugation at 4 °C and 38,000 rpm (65,000 × g) (TLA-55 Beckman Coulter rotor) for 10 min (11) were incubated with Protein A/G-agarose affinity resin on a rocking platform overnight at 4 °C. The affinity resin were collected by a 1-min centrifugation at 20,800 × gav and then washed four times using spin columns (6) with wash buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 0.2% dodecyl maltoside) followed by resuspension in 1% dodecyl maltoside and 1.0 mm DTT. After mixing with SDS sample buffer, the supernatants obtained from a brief centrifugation were analyzed by SDS-PAGE. LacY was visualized in gels stained with Coomassie Brilliant Blue R-250. The relative amounts of LacY in each lane were quantified using Quantity One software for scanning and analysis of the captured images (Bio-Rad).
LacY Stability and Promoter Read-through
Strain AT2033 carrying a plasmid expressing a LacY derivative with a H205C substitution under IPTG regulation was first uniformly labeled with [35S]methionine/cysteine in the presence of IPTG (LacY synthesized) and 100 ng/ml of aTc (PE synthesized to an intermediate steady-state level), and an aliquot was removed for analysis. After removal of IPTG, aTC, and radiolabel by washing cells by centrifugation, cells were resuspended in the same volume of plain LB medium containing cold methionine/cysteine (to dilute any residual radiolabel) to deplete PE in the absence of LacY induction. After 3.5 h of growth an aliquot of equal volume to the first aliquot was removed. Cells also were grown as described above except radiolabel was added only during the 3.5 h of growth without aTc and in the absence of IPTG. All aliquots used for the following analyses were of equal volumes of the cell cultures. For analysis cells were harvested by centrifugation and broken by sonication as previously described ((6)). The pellets were solubilized with 1% SDS at 37 °C (6) and subjected to overnight immunoprecipitation at 4 °C with pAb and SDS-PAGE as described above. The gels were fixed, dried, and the radiolabel incorporated into LacY was detected using Molecular Imager FX and Quantity One software (Bio-Rad) as previously described (13). All experiments described in this paper were repeated at least three times with one representative experiment shown.
RESULTS
Regulation of Cellular PE Content
The committed step to PE synthesis is catalyzed by phosphatidylserine synthase (pssA gene product) followed by phosphatidylserine decarboxylase to form PE (26). Null pssA mutants are viable but display abnormal cell division and defective secondary transporters (14). To “titrate” the effect of PE on LacY topogenesis, a plasmid copy of OPtac-lacY (i.e. IPTG controlled expression of LacY) and a chromosomal copy of PLtetO-1-pssA (i.e. aTc controlled expression of phosphatidylserine synthase) were combined in the same lacY null cell (strain AT2033 (13)). Strain AT2033 was grown in increasing amounts of aTc. As shown in Fig. 2, the PE content of the culture was regulated in a dose-dependent fashion by the level of aTc in the growth medium.
FIGURE 2.
Phospholipid composition of E. coli cells as a function of pssA gene induction. Strain AT2033 was grown to A600 of 0.5 in the presence of the indicated concentrations of aTc and [32P]PO4. Phospholipid composition using solvent 1 was quantified as described under “Experimental Procedures.” Content of PE (above each spot) is shown as mole % of major phospholipid species noted on the left. CL, cardiolipin; PG, phosphatidylglycerol.
Uniform Cell to Cell PE Content as a Function of aTc Levels
The repressor (TetR) regulating the promoter (PLtetO-1) for pssA results in a nonstochastic expression system with a dose-responsive induction of gene product in an uniform amount throughout the cells of a culture (27). However, uniform content throughout the cell culture of the enzymatic product of the resulting enzyme has not been verified. Strain AT2033 was grown in the presence of increasing amounts of aTc, harvested, fixed, and treated with biotinylated Ro09-0198, which is a cyclic peptide antibiotic highly specific for the ethanolamine phosphate head group of PE (28). Cells grown without inducer were filamentous as expected (29) and exhibited uniform slightly green fluorescence (Fig. 3A), whereas cells grown in the presence of 500 ng/ml of aTc were much shorter and exhibited uniform high green fluorescence (Fig. 3C). The cells grown with an intermediate concentration of aTc (100 ng/ml) exhibited a uniform and intermediate level of fluorescence (Fig. 3B). Thus aTc induction of the PLtetO-1-pssA gene results in an homogeneous population of E. coli cells with respect to PE content over a wide range of inducer concentration.
FIGURE 3.
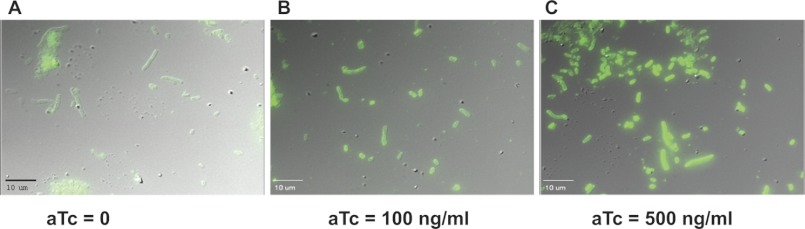
Uniform levels of PE in all cells as a function of aTc concentration. Strain AT2033 grown to A600 of 0.5 at 0 (A), 100 (B), or 500 (C) ng/ml of aTc were harvested and processed to visualize PE content by fluorescent microscopy as described under “Experimental Procedures.”
LacY Adopts Dual Topology in Cells with an Intermediate Level of PE
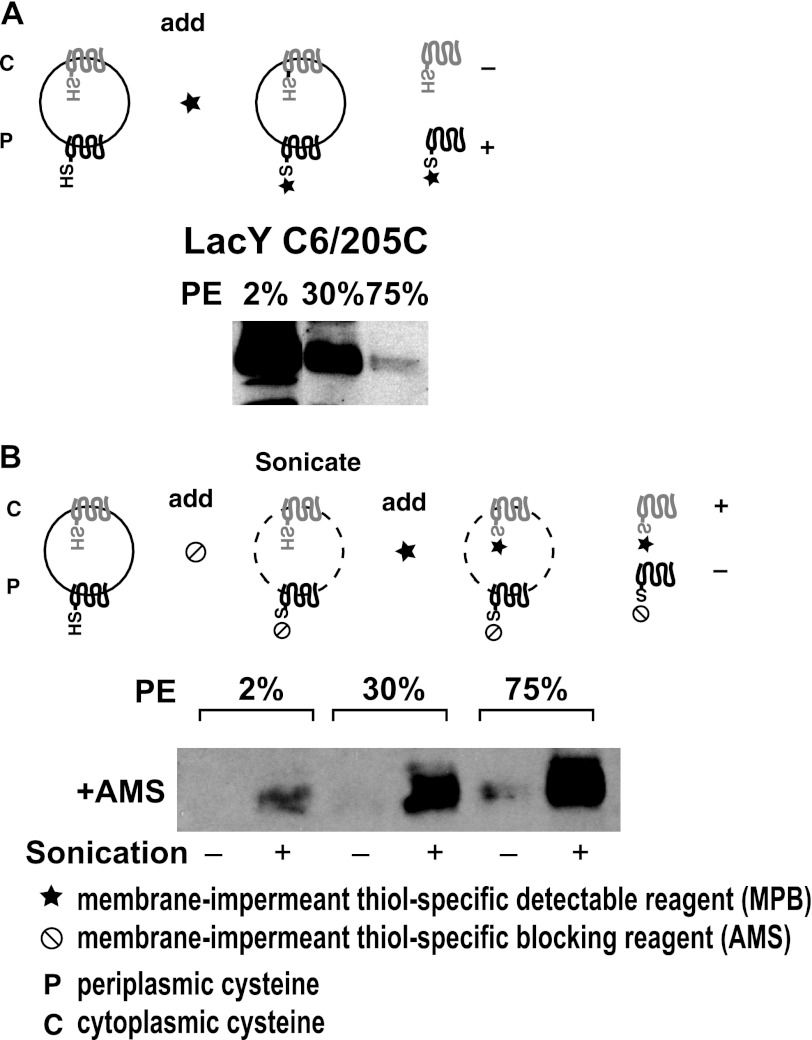
Although the topological arrangement was established for the N-terminal seven TMs of LacY (Fig. 1) after assembly in the presence and absence of wild type levels of PE (5, 13), the organization of LacY assembled at intermediate PE levels has not been investigated. Does LacY exist as a dose-dependent mixture of topologically distinct forms at intermediate PE levels or is there a defined sharp threshold level of PE marking the transition between multiple topology conformers? LacY with a single cysteine replacement at H205C (histidine 205 replaced by cysteine) in domain C6 (normally cytoplasmic, see Fig. 1) was expressed from a plasmid in strain AT2033 grown at increasing aTc concentrations. The orientation of TMs in the region of domain C6 was assessed by the single cysteine accessibility method as applied to TMs (3). Intact cells were either untreated or treated (to block all periplasmically exposed cysteines) with the membrane impermeable sulfhydryl reagent AMS. Intact untreated cells were then biotinylated by exposure to membrane impermeable sulfhydryl reagent MPB to specifically label periplasmically exposed cysteines, whereas AMS-treated cells were biotinylated before and after disruption by sonication. The protective effect of AMS for periplasmically exposed cysteines is complete and has been used to quantify the degree of mixed protein topology (6). As shown in Fig. 4A, the extent of biotinylation of H205C (i.e. periplasmically exposed) in whole cells by MPB progressively decreased with increasing PE content. Pre-treatment with AMS (Fig. 4B) almost completely blocked biotinylation in whole cells but did not block a progressive increase in biotinylation (i.e. cytoplasmically exposed) in sonicated cells with increasing levels of PE. Quantification of the biotinylation signal indicated that nearly half of LacY was labeled and nearly half was protected from labeling by MPB in cells with 30% PE, which was determined in a parallel culture containing 32PO4. Therefore, the C6 domain displays a dual orientation (both cytoplasmic and periplasmic) at intermediate PE levels and a single cytoplasmic (native) or periplasmic (inverted) orientation at high or low PE levels, respectively. Similar results were observed for a L14C (leucine 14 to cysteine) replacement in the N-terminal domain (normally cytoplasmic) of LacY (see Fig. 5 and related discussion) consistent with the N-terminal six-TM α-helical bundle of LacY adopting dual topologies in a PE dose-dependent manner. Because the varying PE content in each cell is uniform (Fig. 3), the two topological conformers of LacY co-exist in each cell membrane of the culture.
FIGURE 4.
Detection of dual topologies for LacY as a function of membrane PE content. Radiolabeled ([32P]PO4) and unlabeled cells expressing LacY with a H205C replacement in C6 were grown in parallel at the indicated aTc levels to result in negligible, intermediate, and fully induced PE levels as determined by analysis of the labeled culture. The nonradiolabeled culture was analyzed for LacY topology using a two-step labeling protocol. Intact cells were labeled with MPB (A) or pretreated with AMS followed by treatment of intact and sonicated cell with MPB (B) as described under “Experimental Procedures.” The diagrams indicate the resulting biotinylation (+) of LacY.
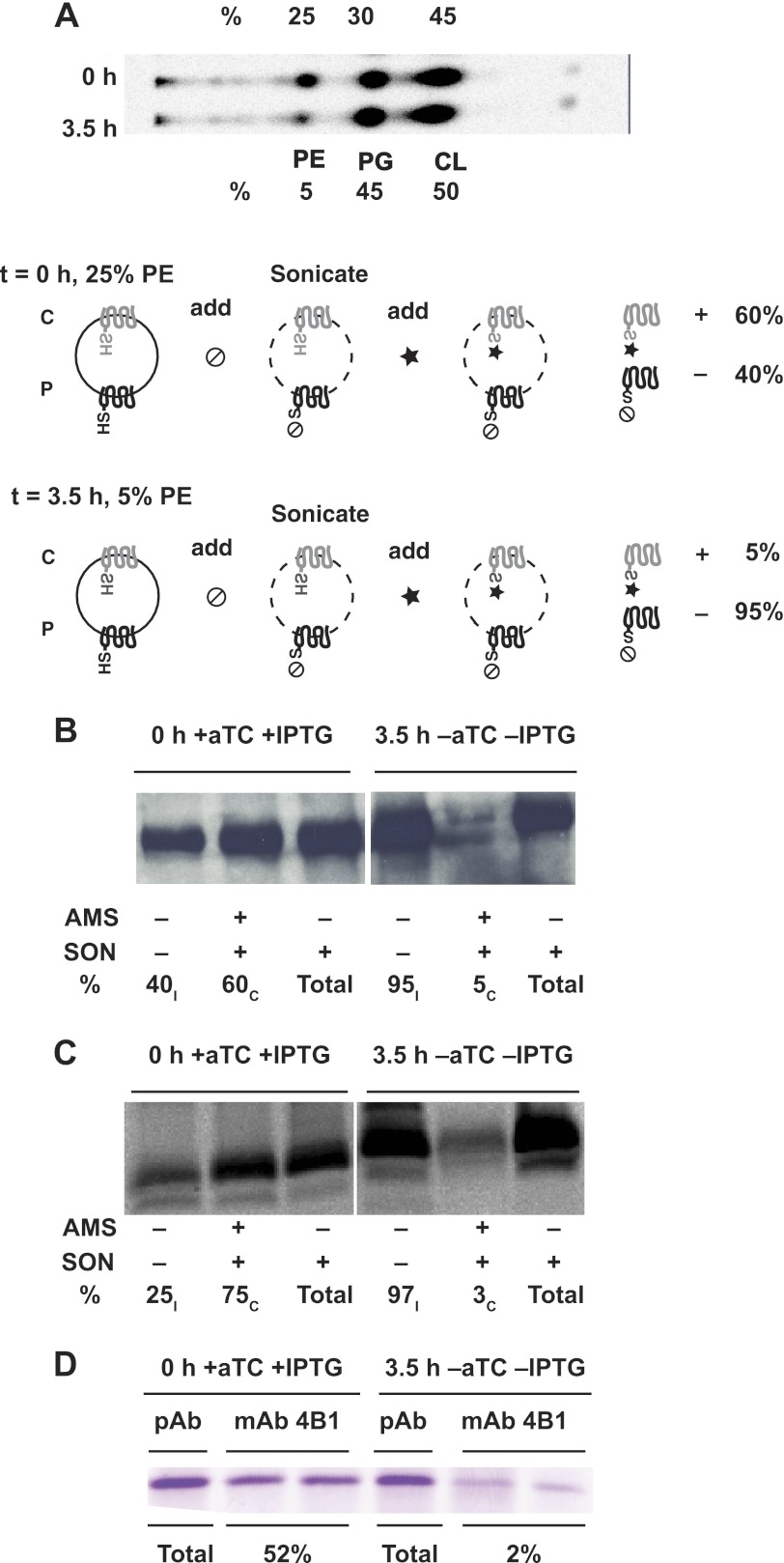
FIGURE 5.
Elimination of PE triggers post-insertional inversion of LacY. Cells expressing LacY with either a H205C replacement in C6 or a L14C replacement in N-terminal were cultivated first in the presence of 100 ng/ml of aTc to adjust PE content to an intermediate level. Cells were switched to growth for 3.5 h without IPTG and aTc to deplete PE in the absence of newly synthesized LacY. A, phospholipids were radiolabeled ([32P]PO4) during growth with IPTG and 100 ng/ml of aTc (top lane). Cells were switched to growth in the absence of IPTG and aTc but in the presence of [32P]PO4 for 3.5 h (bottom lane). Phospholipid composition expressed as mole % was determined using solvent system 2 as described under “Experimental Procedures.” B and C, intact whole cells grown in parallel without [32P]PO4 were treated with MPB as described under “Experimental Procedures” without sonication (SON, −), prelabeled (+) or not (−) with AMS, or after sonication (SON, +). Labeling was performed for H205C (C6, B) and L14C (NT, C) replacements, either after initial assembly of LacY in cells containing ∼25% PE (0 h + aTc + IPTG) or after cessation of PE and LacY synthesis for 3.5 h (3.5 h −aTc, −IPTG). The ratio of inverted (subscript I) and correct (subscript C) was calculated from the intensity of biotinylated bands by using Quantity One software for scanning and analysis of the captured images (Bio-Rad). Each lane represents aliquots of an equal volume of cell culture. D, cells were grown and harvested as indicated in B and C. Aliquots of equal volumes of cell culture were subjected to immunoprecipitation by pAb (total LacY) or mAb 4B1 (properly oriented LacY) as described under “Experimental Procedures.” Gel bands at the position of LacY after SDS-PAGE were quantified (mAb 4B1) and expressed relative to the band at the far left (pAb). The two bands under mAb4B1 represent duplicate samples.
Lipid-modulated TM Molecular Switching Is Bidirectional
Inverted topology of the N-terminal α-helical bundle of LacY in PE-lacking cells can be corrected (except for the orientation of TMs I and II, see Fig. 1) by initiation of PE synthesis post-assembly of LacY (13). Due to excess levels of phosphatidylserine synthase, it can take 7 to 10 generations after cessation of pssA gene expression before the existing enzyme becomes limiting for PE synthesis (30). Over this time period, pre-existing LacY would be diluted to levels beyond detection. However, by working at a limiting level of PS synthase induction to produce an intermediate level of PE and a mixed topology for LacY, it was possible to study the orientation of extramembrane loops in response to a depletion of PE post-assembly.
LacY derivatives containing a diagnostic L14C (NT) or H205C (C6) replacement, which flank the six-TM N-terminal bundle, were subjected to topological analysis. LacY in cells with an intermediate level of PE (25%, Fig. 5A, upper lane) displayed a dual topology (Fig. 5, B (C6), and C (NT), left three lanes). 60 or 75% of cysteine at H205C (C6) or L14C (NT), respectively, was protected from biotinylation during sonication after pre-treatment of intact cells with AMS (Fig. 5, B and C, second lane versus third lane) indicating that more than half of the LacY molecules were inserted in the wild type orientation, whereas the remainder adopted the inverted topology. After continuing growth in the absence of aTc and IPTG, PE levels were reduced to 5% (Fig. 5A, lower lane). In these cells the level of LacY biotinylation was nearly the same with and without sonication and was almost completely blocked by pre-treatment of intact cells with AMS (Fig. 5, B and C, right 3 lanes). The results provide strong evidence that at an intermediate level of PE, 25 to 40% of LacY is mis-oriented and that after reduction of PE to about 5% of total phospholipid nearly all of LacY is mis-oriented. The variability in initial L14C and H205C biotinylation at intermediate PE levels is due to some variability in the intermediate PE level between experiments, which was difficult to control. The range in intermediate PE levels was between 20 and 40%, whereas the range of initially mis-oriented LacY was between 40 and 20% in several experiments. The ratios of oppositely oriented topological conformers of LacY synthesized and inserted at intermediate levels of PE were always dependent on different initial levels of PE thus further confirming that there is no sharp transition between possible topological states of the newly synthesized protein. However, after dilution of PE, the level of mis-oriented LacY was always >95% demonstrating that PE depletion always favored the inversion of LacY within the total population of LacY as is further supported by the experiment described below.
An alternative method of assessing proper topological organization and sufficiently native structure of LacY to support energy-dependent uphill transport of LacY was employed. The conformationally specific mAb 4B1 recognizes LacY via an epitope in the periplasmically exposed domain P7 (Fig. 1) (31, 32); mis-orientation of the N-terminal six-TM bundle of LacY in PE-lacking cells disrupts the native structure of P7 thus preventing recognition by mAb 4B1 (5). In addition there is a strong correlation between the proper folding of P7 and the ability of LacY to mediate uphill transport (5, 11, 24). The LacY-specific pAb directed at the C-terminal extramembrane domain recognizes total LacY irrespective of its structural organization (19, 24). The amount of LacY immunoprecipitated with mAb 4B1 changed from 52% (Fig. 5D, second and third lanes (duplicate immunoprecipitations) versus first lane), before removal of inducers, to 2% (Fig. 5D, fifth and sixth lanes versus fourth lane), after removing inducers, indicating that about half of LacY is properly folded at an intermediate level of PE and only a trace amount of LacY maintains a proper conformation in the same cells after the level of PE is reduced. As judged by immunoprecipitation with pAb (Fig. 5D, fourth lane versus first lane), the total amount of LacY after 3.5 h of growth without both inducers (i.e. after reduction in the level of PE) was not decreased significantly (<3%). Because little or no new LacY is made in the absence of IPTG (see Fig. 6 discussion below), there was no preferential degradation of either LacY conformer during this period. The changes observed after removal of inducers were a result of changes in existing native LacY organization.
FIGURE 6.
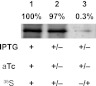
Stability of LacY and lack of promoter read-through. Cells were uniformly labeled with 35S as described under “Experimental Procedures” in the presence of aTc and IPTG (lane 1). After removing IPTG, aTc, and radiolabel, cells were grown for 3.5 h (lane 2). Cells were also grown without first in the presence of IPTG and aTc but without radiolabel and then for 3.5 h without inducers but in the presence of radiolabel (lane 3). Equal aliquots of the cell culture were subjected to immunoprecipitation with pAb and SDS-PAGE. The radiolabeled LacY bands in lanes 2 and 3 were quantified relative to lane 1 as described under “Experimental Procedures.”
LacY Stability and Lack of Promoter Read-through
Consistent with previously shown results (5, 13), LacY (based on radiolabeling) made in the presence of IPTG was stable (> 95%) after growth in the absence of IPTG (Fig. 6, lane 2 versus lane 1) and only trace amounts (<3% of total) of LacY was made during growth in the absence of aTc and IPTG (Fig. 6, lane 3). Moreover, the signal (labeled as total in Fig. 5) of biotinylated LacY in disrupted cells after growth in the absence of aTc and IPTG was nearly the same as before removing the inducers (97% in Fig. 5B and 115% in Fig. 5C). These results provide strong evidence that pre-existing LacY changes topology upon depletion of PE and coupled with the results from Fig. 5D rule out degradation of existing LacY or synthesis of new LacY during dilution of PE. Therefore, the topological orientation of the N-terminal bundle of LacY is reversible either by the addition of PE (5, 13) or depletion of PE after membrane insertion.
DISCUSSION
Herein we provide strong evidence that membrane lipid composition in concert with protein sequence can provide a mechanistic basis for the existence of multiple topologies for a single protein in the same or different membrane(s). In addition interconversion of or “flipping” of TMs within the lipid bilayer post-insertion and complete folding of a membrane protein is reversible in both directions. The novel contributions of the present report have several important implications and can be summarized as follows.
Microscopic examination of fluorescently tagged PE at different concentrations of aTc established that regulation of pssA gene expression in a dose-dependent manner results in a uniform level of PE throughout the culture rather than a population average of induced and uninduced cells as observed with promoters that exhibit stochastic induction (33, 34). Although this expression system is tightly regulated (35), to our knowledge this is the first demonstration that PLtetO-1 can be used to regulate both gene product and effective catalytic capacity in a uniform manner throughout a cell culture.
Topological heterogeneity generated co-translationally for native proteins has been observed (16, 36–41). Manipulation of protein domains (6, 15, 16) has resulted in co-existence of multiple topological arrangements for the same protein within the same membrane. As shown herein, membrane proteins can also display dual topologies dependent on membrane lipid composition thus providing molecular insight into how some proteins might exhibit multiple topological organizations within the same membrane or alternative organization in different membranes. The ratio of properly oriented and inverted LacY was dependent on the variable level of PE demonstrating that the native molar ratio of anionic to net neutral lipids is an important determinant for establishing uniform or dual protein TM topology. Membrane lipid composition has not previously been demonstrated in vivo as a possible molecular basis for dual membrane protein orientation.
Finally, the N-terminal helical bundle of LacY not only undergoes a TM reorientation to assume a near native topology associated with uphill transport when PE is re-supplied post-assembly in the absence of PE (5, 13), but also reorients to assume an inverted topology (displaying only downhill transport (13)) when PE is depleted after synthesis and insertion of LacY in its native orientation. Thus the bidirectional reversibility of the TM protein structure allows us to construct a thermodynamic model of lipid-dependent membrane protein topogenesis (see Fig. 7).
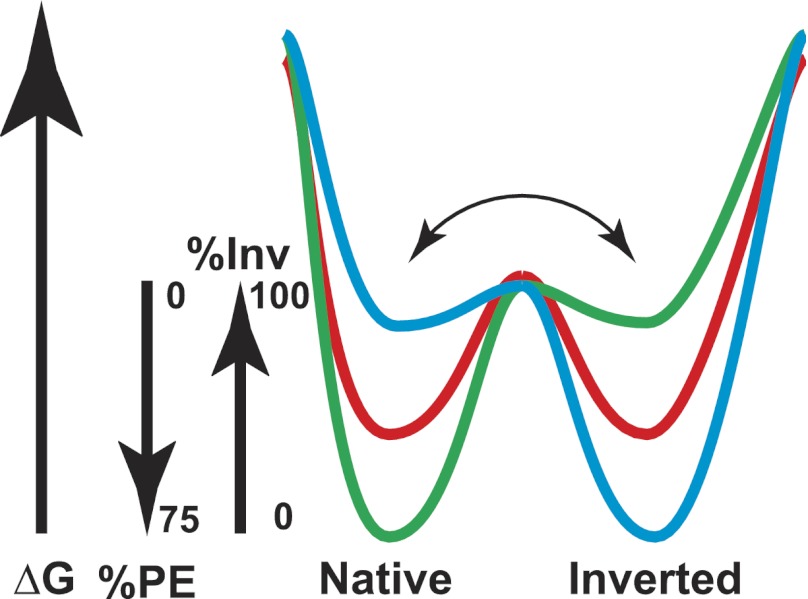
FIGURE 7.
Dual minima energy folding funnel for LacY as a function of membrane lipid composition. The folding of LacY to its lowest free energy state (ΔG) proceeds via a funnel-shaped energy landscape whose shape is defined by the physicochemical properties of the lipid environment (green, 75% PE; red, intermediate % PE; blue, 0% PE). The conformational space available to the population of folding proteins at a given lipid composition is defined by the funnel circumference (x axis) and the internal ΔG (y axis) of each folding intermediate. As LacY folds to lower energy conformations, it populates thermodynamic traps whose depth and shape determine the percent of the final native or inverted conformation at steady state. Membrane lipid composition affects a late folding event, which is postulated here to define a rapid equilibrium (horizontal arrow) between subsequent pathways leading to either the native or inverted conformation separated by a high thermodynamic barrier.
Current dogma assumes that the initial topology of a protein in the endoplasmic reticulum membrane accurately reflects the topology of the protein elsewhere in the cell. Counter to this dogma bidirectional post-insertional and lipid-dependent TM re-arrangement provides an unrecognized means to regulate protein function and structural organization between organelle membranes with different lipid composition during intracellular trafficking and through lateral movement of proteins in the same membrane where lipid composition is not uniform either spatially (lipid rafts) or temporally (during fusion/fission events). Therefore, TM orientation initially established in a fully mature membrane protein is dynamic rather than static and can respond to changes in membrane lipid composition in the local environment, which occurs during cell division, membrane fission and fusion, movement of proteins in and out of lipid rafts, and metabolic changes in polyphosphorylated phosphatidylinositol pools.
The existence of dual topological conformations within the same membrane raises questions concerning the mechanism of membrane protein assembly because abundant evidence indicates that final topological organization is influenced by lipid-protein and intra-protein interactions during or after exit of the polypeptide from the translocon (6, 13, 42). Final topology is dependent on the effective net positive charge of the cytoplasmically exposed protein surface and the negative charge density of the membrane bilayer surface (13, 14, 26, 43). Dual topology can result during synthesis in a native lipid composition by engineered changes in protein sequence as shown for sucrose permease of E. coli (6) and other proteins (15, 44), but the results reported here directly demonstrate that both lipid composition and protein sequence act in concert to determine the ratio of topological conformations. Although post-translational modification of proteins might result in a post-assembly change in membrane topology (45), differences in membrane lipid composition among the multiple membranes of eukaryotic cells and spatial and temporal changes in membrane lipid composition provide a means to induce multiple topological conformers.
It is possible that microdomains enriched in PE exist in the membranes that are too small to be visualized by fluorescence microscopy. However, it is unlikely that such domains account for the observed dual topology of LacY. There is no rapid equilibrium between the two topological conformations of LacY, and protein diffusion within the membrane is orders of magnitude faster than the treatment for 30 min with AMS. Therefore, dual topology is most likely established at a step or steps during initial folding where folding intermediates in multiple topological conformations are in rapid equilibrium (thus separated by a low activation energy). The lipid composition would be the determinant of the ratio of dual forms at this point of the maturation of a protein (Fig. 7). Delays in translation or insertion of TM domains may favor equilibration among different topological conformers. Stable integration of LacY into the membrane bilayer is delayed until TM VIII is synthesized (46), and the effects of PE occur at a late step of initial folding of LacY (13, 19); in both cases these events occur after the exit of the N-terminal six-TM bundle from the translocon. Shortening of the long C6 loop of LacY disrupts proper membrane insertion (47). Subsequent folding events for each intermediate conformer then would impose a high activation energy and thermodynamic block to interconversion between the two conformations resulting in stable dual conformations within the same membrane at steady state lipid composition (Fig. 7). Changing the lipid composition after final folding would reshape the energy landscape resulting in higher energy minima for the inverted conformation at high PE and the native conformation at low PE. At the same time the destabilized conformer would unfold to allow re-equilibration of conformers as determined by the new lipid composition followed by stabilization of the new ratio of conformers by subsequent folding events. Thus topological heterogeneity can arise simply through perturbations of lipid-sensitive kinetic and thermodynamic equilibriums.
The co-existence of dual topologies for membrane proteins within the same or among different subcellular membranes has received increased attention (2, 16, 18). The best documented cases for membrane proteins with dual topologies are bifunctional enzymes (see Refs. 2 and 38) and small proteins of less than 50 amino acids (36). The L envelope protein of hepatitis B virus (48) exists in two functionally distinct topological isoforms whose topological distribution varies with the endoplasmic reticulum cholesterol content (49), which reciprocally affects a translocation of positively charged domains and marginally hydrophobic segments (50). It was suggested that cholesterol can either trigger conformational changes in the translocon by affecting the fluidity of the lipid bilayer or may change the surface charge distribution of the membrane and modulate electrostatic lipid-protein interactions (50). The topological organization of a bull sperm Na+/K+-ATPase is dependent on cholesterol and sphingolipid composition after reconstitution into proteoliposomes (51) mimicking events that could be associated with protein movement in and out of lipid rafts.
To date there is no mechanistic understanding of how dual topologies are generated and maintained although most proposed mechanisms focus at the point of initial TM insertion directed by the translocon and appear to require domains possessing weak topogenic signals (16, 52). To our knowledge LacY is the first reported protein to show dual topology while possessing strong topogenic sequences. In theory all thus far described cases of topological heterogeneity of membrane proteins in prokaryotes and eukaryotes could be facilitated and controlled by the translocon and caused by delays in maturation, controlled glycosylation events, or dependent on molecular chaperones. Although the topological heterogeneity of a membrane protein could be established cotranslationally, where the nascent peptide is still engaged with translocation channel (37), the contribution of the translocon to making a topological decision is limited by time (53), the size of newly synthesized protein (54), and effective size of the translocation pore, which is still a matter of debate (55, 56). The translocon simply provides a transient folding environment that enables nascent TMs to sample alternate/possible topologies favored by the different topogenic signals for about 1 min prior to adapting an intermediate or final TM orientation (53). During this time the properties of the lipid bilayer (i.e. its hydrophobicity and surface charge nature) must influence the final decision. However, the translocon cannot be involved in post-insertional changes in protein structure and function such as movement in and out of lipid rafts or changes occurring during intracellular protein trafficking. Molecular chaperones or other cellular factors may be required in vivo to assist in post-insertional topological rearrangements, but previous in vitro results (12, 19) strongly support lipid-protein interactions as the thermodynamic driving force for such changes.
The results reported here and previously (6, 13) provide multiple plausible mechanisms for establishing and utilizing dual topologies of membrane proteins. The determinants of final and dual topology lie within both the protein sequence and the lipid environment. Therefore, dual topology can be generated or the ratio of topological conformers can be changed at any point of the vesicular intracellular protein trafficking pathway, which depends on proper orientation and exposure of protein targeting signals. It has been suggested that evolution can impart different membrane topologies on highly homologous proteins by shuffling of positively charged residues between extramembrane domains on opposite sides of the membrane (16, 57, 58). Alternatively evolutionary changes in membrane lipid composition could drive inversion of topology. Most studies on protein folding diseases have focused on alterations in protein sequences, but the involvement of a lipid component has also been reported for Alzheimer disease, diabetes, cystic fibrosis, and prion-based diseases (see Refs. 59 and 60 for list of references). We initially suggested that lipids may act as molecular chaperones in the folding of β-amyloid associated with Alzheimer disease (61). The effect of PE may be on the amyloid precursor proteases and/or the precursor itself, because PE directly affects the proteolysis of the amyloid precursor (62). PE has also been established as the sole cofactor in the generation of infectious conformation of prions (63, 64). Our results suggest possible lipid-dependent mechanisms for generating alternate non-native infectious conformers of prion-like proteins, which could overpopulate native ones during amyloidosis.
Currently it is technically difficult to conduct similar experiments in eukaryotic cells because extensive genetic manipulation of membrane lipid composition is usually lethal. However, proteins that exhibit dual topology or function can be isolated and analyzed in reconstituted proteoliposome with controlled but variable lipid composition as with the above ATPase (51). We have demonstrated that the topological conformers of LacY observed in vivo form in exactly the same manner solely as a function of liposome lipid composition (12) and not as a function of the lipid composition of the cell source. Topological decisions are driven solely by the thermodynamics of lipid-protein interactions thus validating the use of purified proteins in proteoliposomes of native lipid composition to study duality of eukaryotic membrane protein structure. Therefore, this report should stimulate the further investigation of the role membrane lipid composition plays in establishing multiple topological conformations of proteins and post-assembly interconversion of different structural forms in more complex organisms.
Acknowledgments
We acknowledge Dr. Masato Umeda and Dr. Ron Kaback for the generous gift of biotinylated Ro09-0198 and mAb 4B1, respectively. We thank Zalman Vaksman for assistance with fluorescence microscopy.
This work was supported, in whole or in part, by National Institutes of Health Grant GM R37 20478 and a grant from the John Dunn Research Foundation (to W. D.).
We dedicate this work in memory of Eugene P. Kennedy (1919–2011) who first demonstrated the presence of the M protein in membranes of E. coli as the product of the lacY gene.
- TM
- transmembrane domain
- AMS
- 4-acetamido-4-maleimidylstilbene-2,2-disulfonic acid
- aTc
- anhydrotetracycline
- IPTG
- isopropyl β-d-thiogalactoside
- MPB
- 3-(N-maleimidylpropionyl)biocytin
- pAb
- polyclonal antibody
- mAb
- monoclonal antibody
- PE
- phosphatidylethanolamine.
REFERENCES
- 1. Luirink J., Yu Z., Wagner S., de Gier J. W. (2012) Biogenesis of inner membrane proteins in Escherichia coli. Biochim. Biophys. Acta 1817, 965–976 [DOI] [PubMed] [Google Scholar]
- 2. von Heijne G. (2006) Membrane-protein topology. Nat. Rev. Mol. Cell. Biol. 7, 909–918 [DOI] [PubMed] [Google Scholar]
- 3. Bogdanov M., Heacock P. N., Dowhan W. (2010) Study of polytopic membrane protein topological organization as a function of membrane lipid composition. Methods Mol. Biol. 619, 79–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dowhan W., Bogdanov M. (2011) Lipid-protein interactions as determinants of membrane protein structure and function. Biochem. Soc. Trans. 39, 767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bogdanov M., Heacock P. N., Dowhan W. (2002) A polytopic membrane protein displays a reversible topology dependent on membrane lipid composition. EMBO J. 21, 2107–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vitrac H., Bogdanov M., Heacock P., Dowhan W. (2011) Lipids and topological rules of membrane protein assembly. Balance between long- and short-range lipid-protein interactions. J. Biol. Chem. 286, 15182–15194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang W., Bogdanov M., Pi J., Pittard A. J., Dowhan W. (2003) Reversible topological organization within a polytopic membrane protein is governed by a change in membrane phospholipid composition. J. Biol. Chem. 278, 50128–50135 [DOI] [PubMed] [Google Scholar]
- 8. Zhang W., Campbell H. A., King S. C., Dowhan W. (2005) Phospholipids as determinants of membrane protein topology. Phosphatidylethanolamine is required for the proper topological organization of the γ-aminobutyric acid permease (GabP) of Escherichia coli. J. Biol. Chem. 280, 26032–26038 [DOI] [PubMed] [Google Scholar]
- 9. Wikström M., Kelly A. A., Georgiev A., Eriksson H. M., Klement M. R., Bogdanov M., Dowhan W., Wieslander A. (2009) Lipid-engineered Escherichia coli membranes reveal critical lipid headgroup size for protein function. J. Biol. Chem. 284, 954–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xie J., Bogdanov M., Heacock P., Dowhan W. (2006) Phosphatidylethanolamine and monoglucosyldiacylglycerol are interchangeable in supporting topogenesis and function of the polytopic membrane protein lactose permease. J. Biol. Chem. 281, 19172–19178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bogdanov M., Heacock P., Guan Z., Dowhan W. (2010) Plasticity of lipid-protein interactions in the function and topogenesis of the membrane protein lactose permease from Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 107, 15057–15062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang X., Bogdanov M., Dowhan W. (2002) Topology of polytopic membrane protein subdomains is dictated by membrane phospholipid composition. EMBO J. 21, 5673–5681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bogdanov M., Xie J., Heacock P., Dowhan W. (2008) To flip or not to flip. Lipid-protein charge interactions are a determinant of final membrane protein topology. J. Cell Biol. 182, 925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dowhan W., Bogdanov M. (2009) Lipid-dependent membrane protein topogenesis. Annu. Rev. Biochem. 78, 515–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nilsson I., von Heijne G. (1990) Fine-tuning the topology of a polytopic membrane protein. Role of positively and negatively charged amino acids. Cell 62, 1135–1141 [DOI] [PubMed] [Google Scholar]
- 16. Rapp M., Granseth E., Seppala S., von Heijne G. (2006) Identification and evolution of dual-topology membrane proteins. Nat. Struct. Mol. Biol. 13, 112–116 [DOI] [PubMed] [Google Scholar]
- 17. Schuldiner S. (2007) Controversy over EmrE structure. Science 317, 748–751 [DOI] [PubMed] [Google Scholar]
- 18. Schuldiner S. (2012) Undecided membrane proteins insert in random topologies. Up, down and sideways. It does not really matter. Trends Biochem. Sci. 37, 215–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bogdanov M., Dowhan W. (1998) Phospholipid-assisted protein folding. Phosphatidylethanolamine is required at a late step of the conformational maturation of the polytopic membrane protein lactose permease. EMBO J. 17, 5255–5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fine J. B., Sprecher H. (1982) Unidimensional thin-layer chromatography of phospholipids on boric acid-impregnated plates. J. Lipid Res. 23, 660–663 [PubMed] [Google Scholar]
- 21. Nishibori A., Kusaka J., Hara H., Umeda M., Matsumoto K. (2005) Phosphatidylethanolamine domains and localization of phospholipid synthases in Bacillus subtilis membranes. J. Bacteriol. 187, 2163–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pedersen L. B., Angert E. R., Setlow P. (1999) Septal localization of penicillin-binding protein 1 in Bacillus subtilis. J. Bacteriol. 181, 3201–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bogdanov M., Zhang W., Xie J., Dowhan W. (2005) Transmembrane protein topology mapping by the substituted cysteine accessibility method (SCAMTM). Application to lipid-specific membrane protein topogenesis. Methods 36, 148–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bogdanov M., Sun J., Kaback H. R., Dowhan W. (1996) A phospholipid acts as a chaperone in assembly of a membrane transport protein. J. Biol. Chem. 271, 11615–11618 [DOI] [PubMed] [Google Scholar]
- 25. Nagamori S., Smirnova I. N., Kaback H. R. (2004) Role of YidC in folding of polytopic membrane proteins. J. Cell Biol. 165, 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dowhan W. (2009) Molecular genetic approaches to defining lipid function. J. Lipid Res. 50, S305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lutz R., Bujard H. (1997) Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, TetR/O, and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 25, 1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Emoto K., Kobayashi T., Yamaji A., Aizawa H., Yahara I., Inoue K., Umeda M. (1996) Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc. Natl. Acad. Sci. U.S.A. 93, 12867–12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mileykovskaya E., Sun Q., Margolin W., Dowhan W. (1998) Localization and function of early cell division proteins in filamentous Escherichia coli cells lacking phosphatidylethanolamine. J. Bacteriol. 180, 4252–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DeChavigny A., Heacock P. N., Dowhan W. (1991) Phosphatidylethanolamine may not be essential for the viability of Escherichia coli. J. Biol. Chem. 266, 5323–5332 [PubMed] [Google Scholar]
- 31. Frillingos S., Wu J., Venkatesan P., Kaback H. R. (1997) Binding of ligand or monoclonal antibody 4B1 induces discrete structural changes in the lactose permease of Escherichia coli. Biochemistry 36, 6408–6414 [DOI] [PubMed] [Google Scholar]
- 32. Sun J., Wu J., Carrasco N., Kaback H. R. (1996) Identification of the epitope for monoclonal antibody 4B1 which uncouples lactose and proton translocation in the lactose permease of Escherichia coli. Biochemistry 35, 990–998 [DOI] [PubMed] [Google Scholar]
- 33. Choi P. J., Cai L., Frieda K., Xie X. S. (2008) A stochastic single-molecule event triggers phenotype switching of a bacterial cell. Science 322, 442–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siegele D. A., Hu J. C. (1997) Gene expression from plasmids containing the araBAD promoter at subsaturating inducer concentrations represents mixed populations. Proc. Natl. Acad. Sci. U.S.A. 94, 8168–8172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bertram R., Hillen W. (2008) The application of Tet repressor in prokaryotic gene regulation and expression. Microb. Biotechnol. 1, 2–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fontaine F., Fuchs R. T., Storz G. (2011) Membrane localization of small proteins in Escherichia coli. J. Biol. Chem. 286, 32464–32474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim S. J., Hegde R. S. (2002) Cotranslational partitioning of nascent prion protein into multiple populations at the translocation channel. Mol. Biol. Cell 13, 3775–3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levy D. (1996) Membrane proteins which exhibit multiple topological orientations. Essays Biochem. 31, 49–60 [PubMed] [Google Scholar]
- 39. McGinnes L. W., Reitter J. N., Gravel K., Morrison T. G. (2003) Evidence for mixed membrane topology of the Newcastle disease virus fusion protein. J. Virol. 77, 1951–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moss K., Helm A., Lu Y., Bragin A., Skach W. R. (1998) Coupled translocation events generate topological heterogeneity at the endoplasmic reticulum membrane. Mol. Biol. Cell 9, 2681–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhu Q., von Dippe P., Xing W., Levy D. (1999) Membrane topology and cell surface targeting of microsomal epoxide hydrolase. Evidence for multiple topological orientations. J. Biol. Chem. 274, 27898–27904 [DOI] [PubMed] [Google Scholar]
- 42. Seppälä S., Slusky J. S., Lloris-Garcerá P., Rapp M., von Heijne G. (2010) Control of membrane protein topology by a single C-terminal residue. Science 328, 1698–1700 [DOI] [PubMed] [Google Scholar]
- 43. Bogdanov M., Xie J., Dowhan W. (2009) Lipid-protein interactions drive membrane protein topogenesis in accordance with the positive inside rule. J. Biol. Chem. 284, 9637–9641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beltzer J. P., Fiedler K., Fuhrer C., Geffen I., Handschin C., Wessels H. P., Spiess M. (1991) Charged residues are major determinants of the transmembrane orientation of a signal-anchor sequence. J. Biol. Chem. 266, 973–978 [PubMed] [Google Scholar]
- 45. Wurie H. R., Buckett L., Zammit V. A. (2011) Evidence that diacylglycerol acyltransferase 1 (DGAT1) has dual membrane topology in the endoplasmic reticulum of HepG2 cells. J. Biol. Chem. 286, 36238–36247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nagamori S., Vázquez-Ibar J. L., Weinglass A. B., Kaback H. R. (2003) In vitro synthesis of lactose permease to probe the mechanism of membrane insertion and folding. J. Biol. Chem. 278, 14820–14826 [DOI] [PubMed] [Google Scholar]
- 47. Weinglass A. B., Kaback H. R. (2000) The central cytoplasmic loop of the major facilitator superfamily of transport proteins governs efficient membrane insertion. Proc. Natl. Acad. Sci. U.S.A. 97, 8938–8943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lambert C., Mann S., Prange R. (2004) Assessment of determinants affecting the dual topology of hepadnaviral large envelope proteins. J. Gen. Virol. 85, 1221–1225 [DOI] [PubMed] [Google Scholar]
- 49. Dorobantu C., Macovei A., Lazar C., Dwek R. A., Zitzmann N., Branza-Nichita N. (2011) Cholesterol depletion of hepatoma cells impairs hepatitis B virus envelopment by altering the topology of the large envelope protein. J. Virol. 85, 13373–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamamoto H., Fujita H., Kida Y., Sakaguchi M. (2012) Pleiotropic effects of membrane cholesterol upon translocation of protein across the endoplasmic reticulum membrane. Biochemistry 51, 3596–3605 [DOI] [PubMed] [Google Scholar]
- 51. Hickey K. D., Buhr M. M. (2011) Lipid bilayer composition affects transmembrane protein orientation and function. J. Lipids 2011, 208457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bowie J. U. (2006) Flip-flopping membrane proteins. Nat. Struct. Mol. Biol. 13, 94–96 [DOI] [PubMed] [Google Scholar]
- 53. Goder V., Junne T., Spiess M. (2004) Sec61p contributes to signal sequence orientation according to the positive-inside rule. Mol. Biol. Cell 15, 1470–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kida Y., Morimoto F., Sakaguchi M. (2007) Two translocating hydrophilic segments of a nascent chain span the ER membrane during multispanning protein topogenesis. J. Cell Biol. 179, 1441–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Skach W. R. (2009) Cellular mechanisms of membrane protein folding. Nat. Struct. Mol. Biol. 16, 606–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van den Berg B., Clemons W. M., Jr., Collinson I., Modis Y., Hartmann E., Harrison S. C., Rapoport T. A. (2004) X-ray structure of a protein-conducting channel. Nature 427, 36–44 [DOI] [PubMed] [Google Scholar]
- 57. Sääf A., Johansson M., Wallin E., von Heijne G. (1999) Divergent evolution of membrane protein topology. The Escherichia coli RnfA and RnfE homologues. Proc. Natl. Acad. Sci. U.S.A. 96, 8540–8544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rapp M., Seppälä S., Granseth E., von Heijne G. (2007) Emulating membrane protein evolution by rational design. Science 315, 1282–1284 [DOI] [PubMed] [Google Scholar]
- 59. Bogdanov M., Mileykovskaya E., Dowhan W. (2008) Lipids in the assembly of membrane proteins and organization of protein supercomplexes. Implications for lipid-linked disorders. Subcell. Biochem. 49, 197–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Diaz-Espinoza R., Soto C. (2010) Generation of prions in vitro and the protein-only hypothesis. Prion 4, 53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bogdanov M., Dowhan W. (1999) Lipid-assisted protein folding. J. Biol. Chem. 274, 36827–36830 [DOI] [PubMed] [Google Scholar]
- 62. Nesic I., Guix F. X., Vennekens K., Michaki V., Van Veldhoven P. P., Feiguin F., De Strooper B., Dotti C. G., Wahle T. (2012) Alterations in phosphatidylethanolamine levels affect the generation of Aβ. Aging Cell 11, 63–72 [DOI] [PubMed] [Google Scholar]
- 63. Deleault N. R., Piro J. R., Walsh D. J., Wang F., Ma J., Geoghegan J. C., Supattapone S. (2012) Isolation of phosphatidylethanolamine as a solitary cofactor for prion formation in the absence of nucleic acids. Proc. Natl. Acad. Sci. U.S.A. 109, 8546–8551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Deleault N. R., Walsh D. J., Piro J. R., Wang F., Wang X., Ma J., Rees J. R., Supattapone S. (2012) Cofactor molecules maintain infectious conformation and restrict strain properties in purified prions. Proc. Natl. Acad. Sci. U.S.A. 109, E1938–E1946 [DOI] [PMC free article] [PubMed] [Google Scholar]