Abstract
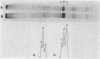
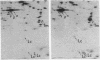
We have developed an in vitro system for the study of postnatal human muscle under standardized conditions. The technique utilizes cloning to isolate pure populations of muscle cells. By manipulating culture conditions we can maximize either proliferation or differentiation of individual clones or of clones pooled to yield mass cultures of muscle cells. The muscle phenotype is stable; cells can be stored in liquid nitrogen for long-term use without loss of proliferative or differentiative potential. We have determined proliferative capacity of muscle cells from an analysis of clonal growth kinetics; we determined differentiative capacity from morphological evidence (cell fusion, striations, contractions, and the appearance of acetylcholine receptors) and biochemical analysis of muscle protein synthesis (creatine kinase, alpha-actin, tropomyosin, and myosin light chains). Our approach eliminates the variability in cellular composition that has complicated studies of primary muscle to date. We can now study in a controlled fashion the interactions and contributions of different cell types to the development of normal and genetically dystrophic human muscle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz H., Changeux J. P. Regulation of muscle acetylcholine receptor synthesis in vitro by cyclic nucleotide derivatives. Nature. 1979 Apr 19;278(5706):749–752. doi: 10.1038/278749a0. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Enzymatic liberation of myogenic cells from adult rat muscle. Anat Rec. 1974 Dec;180(4):645–661. doi: 10.1002/ar.1091800410. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Epstein C. J. Manipulation of myogenesis in vitro: reversible inhibition by DMSO. Cell. 1979 May;17(1):95–108. doi: 10.1016/0092-8674(79)90298-8. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Kafatos F. C. Secretory kinetics in the follicular cells of silkmoths during eggshell formation. J Cell Biol. 1978 Jul;78(1):131–151. doi: 10.1083/jcb.78.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate accumulation of contractile protein mRNAs during myoblast differentiation. Dev Biol. 1979 Mar;69(1):202–216. doi: 10.1016/0012-1606(79)90286-0. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Changes in protein synthesis during myogenesis in a clonal cell line. Dev Biol. 1979 Nov;73(1):134–152. doi: 10.1016/0012-1606(79)90143-x. [DOI] [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hauschka S. D. Clonal analysis of vertebrate myogenesis. 3. Developmental changes in the muscle-colony-forming cells of the human fetal limb. Dev Biol. 1974 Apr;37(2):345–368. doi: 10.1016/0012-1606(74)90154-7. [DOI] [PubMed] [Google Scholar]
- Hauschka S. D. Clonal analysis of vertebrate myogenesis. II. Environmental influences upon human muscle differentiation. Dev Biol. 1974 Apr;37(2):329–344. doi: 10.1016/0012-1606(74)90153-5. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Jones K. W., Yaffe D. Research group on neuromuscular diseases. A report on various aspects of myogenic cell culture with particular reference to studies on the muscular dystrophies. J Neurol Sci. 1975 Sep;26(1):115–124. doi: 10.1016/0022-510x(75)90120-3. [DOI] [PubMed] [Google Scholar]
- KIELLEY W. W., HARRINGTON W. F. A model for the myosin molecule. Biochim Biophys Acta. 1960 Jul 15;41:401–421. doi: 10.1016/0006-3002(60)90037-8. [DOI] [PubMed] [Google Scholar]
- Koenig J., Vigny M. Neural induction of the 16S acetylcholinesterase in muscle cell cultures. Nature. 1978 Jan 5;271(5640):75–77. doi: 10.1038/271075a0. [DOI] [PubMed] [Google Scholar]
- Konigsberg U. R., Lipton B. H., Konigsberg I. R. The regenerative response of single mature muscle fibers isolated in vitro. Dev Biol. 1975 Aug;45(2):260–275. doi: 10.1016/0012-1606(75)90065-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Libby P., Bursztajn S., Goldberg A. L. Degradation of the acetylcholine receptor in cultured muscle cells: selective inhibitors and the fate of undegraded receptors. Cell. 1980 Feb;19(2):481–491. doi: 10.1016/0092-8674(80)90523-1. [DOI] [PubMed] [Google Scholar]
- MAURO A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961 Feb;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M. D., Brown T. H., Peacock J. H. Regulation of acetylcholine receptor levels by a cholinergic agonist in mouse muscle cell cultures. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3488–3492. doi: 10.1073/pnas.75.7.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Prives J., Silman I., Amsterdam A. Appearance and disappearance of acetycholine receptor during differentiation of chick skeletal muscle in vitro. Cell. 1976 Apr;7(4):543–550. doi: 10.1016/0092-8674(76)90204-x. [DOI] [PubMed] [Google Scholar]
- Richler C., Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970 Sep;23(1):1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- Rowland L. P. Biochemistry of muscle membranes in Duchenne muscular dystrophy. Muscle Nerve. 1980 Jan-Feb;3(1):3–20. doi: 10.1002/mus.880030103. [DOI] [PubMed] [Google Scholar]
- Rowland L. P. Pathogenesis of muscular dystrophies. Arch Neurol. 1976 May;33(5):315–321. doi: 10.1001/archneur.1976.00500050001001. [DOI] [PubMed] [Google Scholar]
- Rubenstein P. A., Spudich J. A. Actin microheterogeneity in chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Jan;74(1):120–123. doi: 10.1073/pnas.74.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Alterations of enzymatic activities during muscle differentiation in vitro. Dev Biol. 1971 May;25(1):1–29. doi: 10.1016/0012-1606(71)90017-0. [DOI] [PubMed] [Google Scholar]
- Stockdale F. E., Raman N., Baden H. Myosin light chains and the developmental origin of fast muscle. Proc Natl Acad Sci U S A. 1981 Feb;78(2):931–935. doi: 10.1073/pnas.78.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng N. N., Fiszman M. Y. Appearance of acetylcholine receptors in cultured myoblasts prior to fusion. J Supramol Struct. 1976;4(3):381–387. doi: 10.1002/jss.400040309. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1977;7(3):159–166. doi: 10.1111/j.1432-0436.1977.tb01507.x. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]