Background: Macromolecular crowding and hydrophobic-hydrophilic interfaces promote amyloidogenesis.
Results: The outcome of macromolecular crowding on Aβ amyloidogenesis depends on the spatial heterogeneity of the system.
Conclusion: Viscosity dominates over the excluded volume effect only when the system contains a hydrophobic-hydrophilic interface.
Significance: Studying both interfacial and macromolecular crowding effects together is crucial to understand amyloid systems in a physiological context.
Keywords: Alzheimer's Disease, Amyloid, Biophysics, Macromolecular Crowding, Mathematical Modeling, Agitation, Surface Activity, Viscosity
Abstract
Amyloid formation and accumulation is a hallmark of protein misfolding diseases and is associated with diverse pathologies including type II diabetes and Alzheimer's disease (AD). In vitro, amyloidogenesis is widely studied in conditions that do not simulate the crowded and viscous in vivo environment. A high volume fraction of most biological fluids is occupied by various macromolecules, a phenomenon known as macromolecular crowding. For some amyloid systems (e.g. α-synuclein) and under shaking condition, the excluded volume effect of macromolecular crowding favors aggregation, whereas increased viscosity reduces the kinetics of these reactions. Amyloidogenesis can also be catalyzed by hydrophobic-hydrophilic interfaces, represented by the air-water interface in vitro and diverse heterogeneous interfaces in vivo (e.g. membranes). In this study, we investigated the effects of two different crowding polymers (dextran and Ficoll) and two different experimental conditions (with and without shaking) on the fibrilization of amyloid-β peptide, a major player in AD pathogenesis. Specifically, we demonstrate that, during macromolecular crowding, viscosity dominates over the excluded volume effect only when the system is spatially non homogeneous (i.e. an air-water interface is present). We also show that the surfactant activity of the crowding agents can critically influence the outcome of macromolecular crowding and that the structure of the amyloid species formed may depend on the polymer used. This suggests that, in vivo, the outcome of amyloidogenesis may be affected by both macromolecular crowding and spatial heterogeneity (e.g. membrane turn-over). More generally, our work suggests that any factors causing changes in crowding may be susceptibility factors in AD.
Introduction
The term amyloid refers to deposits of insoluble macromolecular aggregates from various proteinaceous species. These aggregates arise from a common underlying mechanism by which the conformational conversion (misfolding) of proteins or protein fragments leads to the formation of a pathological cross β-sheet fold (1). The diseases associated with amyloid formation, such as Alzheimer's disease (AD),5 are chronic and degenerative and their burden on human health is far-reaching. Consequently, there has been a great deal of research into the fundamental mechanisms of amyloid assembly. The hallmarks of AD pathology include the deposition, in the brain, of amyloid-β-peptide (Aβ) (e.g. mainly Aβ1–40 and Aβ1–42) in extracellular plaques and of hyperphosphorylated Tau in intracellular neurofibrillary tangles (2). Both misfolded Aβ and Tau are thought to adversely affect neuronal function (3).
Amyloidogenesis occurs via a nucleation-dependent polymerization process, beginning with a lag phase, during which the energetically unfavourable step of nucleus formation from monomers takes place (4). These nuclei then rapidly elongate, through monomer addition, to form assembly intermediates (e.g. oligomers and protofibrils) until the system reaches equilibrium between monomers and fibrils (4, 5). The identity of the toxic species remains controversial but an increasing body of evidence suggests that soluble oligomers cause cell dysfunction and death in a multitude of ways (1, 2). Despite originating from different proteins, amyloid-forming peptides share physico-chemical properties (e.g. amphiphilicity, ability to form extended β-strands) and a common cross β-sheet fold when assembled (6–9). Because of their amphiphilic character, these amyloid-forming peptides adsorb at an air-water interface (AWI; non-polar gas and polar aqueous solution) or more generally at hydrophobic-hydrophilic interfaces (HHI; gas-liquid, solid-liquid, or membranes) (9–14). Adsorption to HHI allows these amyloid-forming peptides to spatially concentrate, to align their side chains with polar and non-polar side-chains segregating on opposite sides of the β-strand, and ultimately to promote their assembly into amyloid species (13–18).
Most biological fluids, whether intra- or extracellular, contain a high total concentration of macromolecules (e.g. protein, nucleic acids, carbohydrates, and other biopolymers), occupying up to 40% of the total volume, which in turn non-specifically and sterically exclude additional macromolecules from this space (19, 20). Such a high total concentration of macromolecules has major kinetic and thermodynamic effects on biochemical reactions and this phenomenon has been defined as macromolecular crowding (20, 21). Although the excluded volume effect is key to crowding, specific and nonspecific interactions between solute and solvent, viscosity, solvent polarity, and water content also play a role (22). Volume exclusion may affect protein stability and conformation, protein-protein interactions and macromolecular equilibria (21, 23). Indeed, the reduction in volume available may shift the equilibrium of species and lead to the promotion of macromolecular aggregation. On the other hand, crowded conditions also reduce the kinetics (e.g. rate) of reactions such as amyloidogenesis due to an increase in viscosity. These effects are nonspecific, sensitive to small fluctuations and can apply to both amyloidogenic and non-amyloidogenic proteins (20). Despite the known crowding property of the intracellular environment, amyloid formation is widely studied in vitro in dilute conditions. In vitro, cellular crowded conditions can be mimicked by the addition of nonspecific model crowding agents, such as hydrophilic and neutral polysaccharides (e.g. dextran and Ficoll) (20, 24). Experiments involving several amyloidogenic proteins (α-synuclein, apolipoprotein C-II, hyper-phosphorylated Tau 244–441, and prion protein) studied predominantly under shaking conditions, demonstrated that crowding agents enhanced amyloidogenesis because the encounter of amyloid precursors was increased by the volume exclusion effect (22, 25–27). However, above a certain concentration of crowding agent the concomitant increase in viscosity decreased the diffusion rate of amyloid species (e.g. α-synuclein) so much that the overall rate of reaction was reduced (22).
Despite the extraordinary research interest that Aβ has generated over the years, because of its unequivocal role in AD, there has been no systematic investigation on the role and consequences of a crowding environment in relation to AD pathogenesis. To date, only one study investigated the effect of macromolecular crowding on Aβ, but this study focused on membrane-induced protein misfolding rather than the kinetics of amyloidogenesis in the absence of externally added catalysts (28). Here, we investigated the effect of macromolecular crowding on Aβ amyloidogenesis by using two different crowding polymers (dextran and Ficoll) and two different experimental conditions (non-shaking and shaking). We show that without shaking, viscosity impairs Aβ assembly in a threshold dependent manner, with no impairment up to 3% (w/v), but significant impairment (i.e. decreased nucleation rate and increased lag phase) at 6 and 12% polymer. Moreover, the extent of the promotion or inhibition due to macromolecular crowding was different depending on the polymer used. In contrast, with shaking, no inhibition was observed with both crowding agents accelerating Aβ nucleation. We also show that increased viscosity inhibits the fibrilization of Aβ both with and without shaking. Furthermore, polymer-induced crowding and polymer/glycerol-induced viscosity differently affect Aβ surfactant activity (the recruitment of Aβ species to the AWI) and the amount and morphology of the fibrils formed.
EXPERIMENTAL PROCEDURES
Synthetic Peptides and Reagents
Lyophilized synthetic human Aβ1–40 (EZBiolab, Carmel, IN), was purchased already purified by reverse-phase high performance liquid chromatography with a C18 column. Lyophilized and purified Aβ1–40 was resuspended in DMSO at 1.6 mm, sonicated and centrifuged for 1 h at 15,000 × g at +4 °C prior to use (to remove any pre-aggregated species). Stock solutions of amyloid peptides are widely prepared in DMSO, since DMSO maintains these peptides in a monomeric pool lacking any β-sheet secondary structures (29, 30).
Stock solutions of 300 g/liter of dextran (64 to 76 kDa) (Sigma-Aldrich, Dorset, England) and Ficoll (400 kDa) (Sigma-Aldrich), and 30% glycerol (from a 99.5% solution) (VWR, Leicestershire, England) were prepared in distilled water.
Fibrilization Experiments
30 μm Aβ1–40 (from a 1.6. mm stock in DMSO) was incubated with 125 μm ThT in PBS in a 100-μl reaction volume, in the absence or presence of various percentages (w/v) of either crowding agents (dextran or Ficoll) or glycerol. Thioflavin T (ThT) fluorescence (excitation 450 nm, emission 480 nm) was measured, in a 96-well plate (black wall, clear bottom; Greiner, Bio-One, Stonehouse, Gloucestershire, UK), at 37 °C on a BMG Polarstar plate reader (BMG Labtech, Aylesbury, Buckinghamshire, UK). Experiments were carried out with or without orbital shaking (300 s every 680 s). Control wells contained ThT and buffers (without peptide) and crowding agents or glycerol when required. The values of control wells (ThT/buffer with or without an appropriate percentage of crowding agents or glycerol) were subtracted from the values of test wells (peptide with or without crowding agents or glycerol). This ascertained that the presence of the crowding agents or glycerol did not affect ThT fluorescence. At least three independent assays were performed, with each condition assayed in duplicate. Statistical analysis was performed with the two-sample t test.
Based on the ThT data, we define empirically the elongation rate, plateau height, and lag phase of the system as follows: the elongation rate corresponds to the slope at the inflection point of the sigmoidal curve; the plateau height corresponds to an average of the highest curve values attained at the end of the experiment; the lag phase corresponds to the intercept on the time axis of the line formed tangent to the inflection point.
Surface Tension Measurement
Solutions of 12% dextran, Ficoll, or glycerol were prepared in water, and DMSO stock solutions of 1.6 mm Aβ1–40 were diluted in water to 30 μm in the absence or presence of 12% dextran, Ficoll, or glycerol. The solution was aspirated manually into a gas tight glass syringe with a 0.8 mm diameter 316 stainless steel flat-tipped needle (SGE Analytical Science, Milton Keynes, UK). The sample was delivered from the syringe by a dc motor drive to form a pendant drop of 7.5 μl. The sample and surrounding air were thermostatically controlled using a circulating water bath at 20 ± 0.1 °C. Surface tension of the 7.5 μl drop was measured by pendant drop shape analysis using a Tracker video enhanced drop tensiometer (ITConcept, Longessaigne, France) with an integrated sphere light source (tungsten bulb and diffuser) and a CCD camera attached to a telecentric lens aligned on an optical bench. A computer was used to determine the coordinates of the drop profile from the digitized 320 × 256 pixel image. The surface tension γ was determined by analyzing the profile of the drop according to the Young-Laplace equation,
and the equation describing the equilibrium between the interfacial tension and gravity for the drop.
ΔP is the pressure difference across the interface; x is the coordinate of any point on the drop; γ is the interfacial tension; R and R′ are the inside and outside radii of curvature of the interface; θ is the angle of the tangent to the drop profile; V is the volume of the fluid; ρh and ρI are the density of the air and solution (in all cases assumed to be the same as water); and g is the acceleration due to gravity. The surface tension is obtained from the best fit of the curve profile. Determination of surface tension by axisymmetric drop shape analysis is a well-established and accurate technique, enabling recording of the entire dynamic surfactant behavior without any restrictions (e.g. drop shape or size and liquid meniscus contact angle) and has been described previously (31–33). At least three independent assays were performed, with each drop done in duplicate.
Transmission Electron Microscopy
30 μm Aβ fibrilization reactions (performed as described above), without or with shaking and in the absence or presence of various percentages of crowding agents or glycerol, were harvested when the plateau phase was reached (as measured by ThT fluorescence emission). The solutions were adsorbed onto Formvar-coated 400 mesh copper grids, air dried, washed with distilled water, negatively stained with 2% aqueous uranyl acetate, and viewed with a Tecnai electron microscope (Philips).
Generation of the Theoretical Curves
The curves in Fig. 6B were generated by solving the diffusion Equation 3,
 |
with the following boundary conditions,
 |
and
The second boundary is employed to mimic the nucleation process, where a nonzero α corresponds to a nucleation process that delays the depletion of monomers at the AWI. The equation is rendered dimensionless by setting α = 1, L = 1, and
 |
The initial condition is such that m(z,t = 0) = 1. The curves in Fig. 6B depict the function shown in Equation 7.
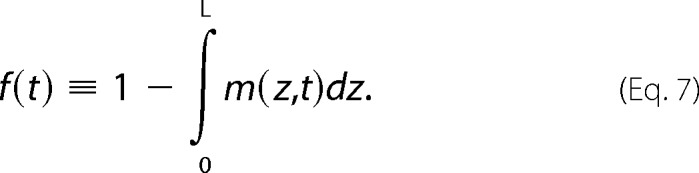 |
The three different diffusion constants are D = 3, 1, 0.5. The curves shown in Fig. 6C were generated similarly, except that the function f(t) is modified to Equation 8,
 |
with θν being 1, 0.9, or 0.8 as D goes from 3 to 0.5.
FIGURE 6.
Theoretical model linking macromolecular crowding and kinetics of amyloidogenesis. A, schematic showing the adsorbed layer at the AWI, where all the amyloid species (from monomers to fibrils) are located. The monomers in the bulk have to diffuse to the adsorbed layer in order to fibrilize. B, schematic showing the adsorbed layer of amyloid species (from monomers to fibrils) at the AWI and dextran in the bulk solution (left panel). Since dextran does not interact with the adsorbed layer, the crowding agent only affects the viscosity of the solution and as a result, the dynamics of fibrilization is slowed down (right panel). C, schematic showing the adsorbed layer of amyloid species (from monomers to fibrils) at the AWI and Ficoll at the AWI and in the bulk solution (left panel). Since Ficoll is surface active, it interacts with the adsorbed layer and as a result, decreases the kinetics of fibrilization and the amount of fibrils at the AWI (right panel). See “Experimental Procedures” on how the curves were generated.
RESULTS
To investigate the effect of macromolecular crowding on Aβ fibrilization, we compared the relative effects of two types of crowding agent, dextran, and Ficoll, on Aβ fibrilization. Dextran 70 is a polymer of d-glucopyranose (α-d-1,6-glucose-linked glucan), which behaves as a quasi random coil due to its flexibility and linearity (<5% branching) (34–37). Ficoll 400 is a highly cross-linked and extensively branched copolymer of sucrose and epichlorohydrin, which is compact and behaves as semi-rigid spheres (36). Both dextran and Ficoll are hydrophilic, neutral, inert, and non-ionized polymers. Compared with other polymers commonly used in macromolecular crowding studies (e.g. polyethylene glycol), dextran and Ficoll are more closely related to the types of macromolecules found physiologically in a cellular environment and also have been shown not to interact with proteins (24).
To follow fibril formation we used the standard amyloid dye ThT, which undergoes a spectral change in its fluorescence properties (emission from 445 to 482 nm) when intercalating into stacked β-sheet amyloid structures (38). To preclude artifacts due to ThT fluorescence being affected by the crowding agents or glycerol, the values for crowding agents/glycerol-ThT (no Aβ) were subtracted from all ThT assays (with Aβ).
Without Shaking, Dextran and Ficoll either Promote or Inhibit Aβ Fibrilization in a Concentration-dependent Manner
Shaking is widely used in amyloidogenesis assays as it drastically accelerates the otherwise very slow nucleation step, in particular for some amyloid-forming proteins or peptides such as α-synuclein and Aβ (39–42). However, outside of the hydrodynamic flows introduced by physiological fluids such as the bloodstream that may play an unrecognized role in amyloidogenesis, shaking may not represent a physiologically relevant condition. Therefore, we first tested the effect of macromolecular crowding on the fibrilization of Aβ under non-shaking conditions.
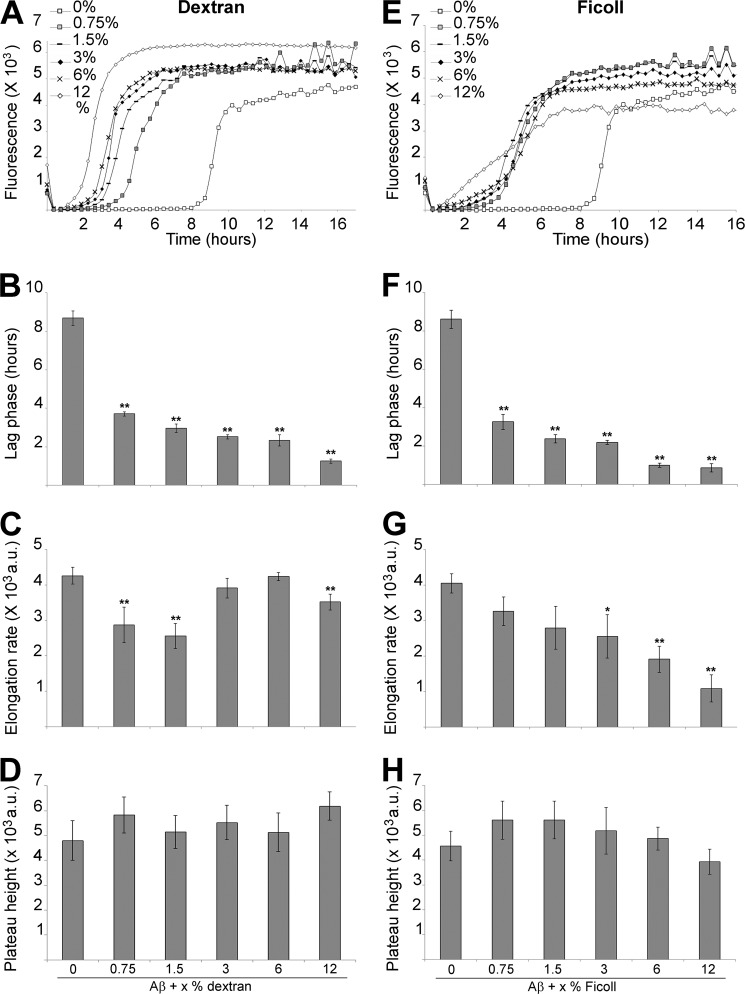
Without shaking, Aβ showed a plateau height of ∼4500 a.u. above baseline after ∼44.6 h lag phase (nucleation process), and an elongation rate of ∼140 a.u./h (Fig. 1). The addition of the highest tested percentages of both dextran and Ficoll (6 and 12%) inhibited Aβ fibril formation. The lag phase was significantly increased by the addition of dextran (∼1.2-fold for 6% and ∼1.3-fold for 12%) and Ficoll (∼1.2-fold for both %) (Fig. 1, A, B, E, and F). The elongation rate was also significantly decreased by 6 and 12% Ficoll (2.8- and 11.3-fold, respectively), while it remained unchanged with the same percentages of dextran (Fig. 1, A, C, E, and G). Aβ plateau height was unaffected by most percentages of dextran, albeit with a significant 1.2-fold increase with 0.75%, but was significantly reduced by 1.5 to 12% Ficoll in a concentration-dependent manner (1.2-, 1.7-, 2.6-, and 9.7-fold, respectively) (Fig. 1, A, D, E, and H). To ascertain that the addition of high percentages of agents did not affect the ThT fluorescence of a fibrilar sample, we added 12% glycerol, dextran, Ficoll, or 12% PBS (i.e. the volume equivalent of added agent) to a 30 μm Aβ reaction at plateau (equivalent to a reaction with 0% crowding agents at 140 h in Fig. 1A). None of the agents added affected the ThT fluorescence of such reaction (data not shown). Thus the measured kinetics, in non-shaking conditions, show that increased lag phase (both dextran and Ficoll), decreased rate (Ficoll), and decreased plateau (Ficoll) are all significant consequences of crowding agents at the highest percentages used.
FIGURE 1.
The effect of high concentrations of crowding agents on Aβ fibrilization under non-agitating conditions. 30 μm Aβ, in 165 μm ThT and PBS, was incubated under non-agitating conditions in absence or presence of 0.75 to 12% dextran (A–D) or Ficoll (E–H). Changes in ThT fluorescence were monitored (A and E) with the lag phase of fibrilization (B and F), the elongation rate (C and G) and plateau height (D and H) depicted. **, p < 0.03 and *, p < 0.05 when compared with Aβ without crowding agents. a.u.: arbitrary units. The mean of at least three independent assays is shown. Error bars represent ± S.E.
At lower percentages (0.75 to 3%), the addition of both crowding agents did not cause any significant changes in Aβ lag phase (Fig. 1, A, B, E, and F). However, Aβ elongation rate was significantly increased (∼1.8-fold on average) by these percentages of dextran (Fig. 1, A, C, E, and G). Although the addition of the same concentrations of Ficoll did not show any statistical significance, Aβ elongation rate followed the same trend (increase) as that in the presence of dextran.
When even lower percentages of dextran and Ficoll (0.18 and 0.37%) were tested, Aβ nucleation was promoted with a significant decrease in lag phase (1.1-fold with 0.18% dextran, and 1.2- and 1.1-fold for 0.18 and 0.37% Ficoll, respectively) (Fig. 2, A and B). The elongation rate was also significantly increased by 0.18% Ficoll (1.3-fold) (Fig. 2, A and C). As for the higher percentages of dextran, the plateau height remained unchanged. However the plateau height was significantly decreased by 0.18 and 0.37% Ficoll (1.2- and 1.4-fold, respectively) (Fig. 2, A and D). We observed inter-batch differences in Aβ fibrilization kinetics, discernible in a comparison of Figs. 1 and 2, as has been previously described (43, 44).
FIGURE 2.
The effect of low concentrations of crowding agents on Aβ fibrilization under non-agitating conditions. 30 μm Aβ, in 165 μm ThT and PBS, was incubated under non-agitating conditions in absence or presence of 0.18 and 0.37% dextran or Ficoll. Changes in ThT fluorescence were monitored (A) with the lag phase of fibrilization (B), the elongation rate (C) and plateau height (D) depicted. **, p < 0.03 and *, p < 0.05 when compared with Aβ without crowding agents. a.u.: arbitrary units. The mean of at least three independent assays is shown. Error bars represent ± S.E.
Altogether the data from Figs. 1 and 2, under non-shaking conditions, show that the effects of dextran and Ficoll on the kinetics of Aβ fibrilization are highly dependent on the percentage of crowding agents used, with high percentages inhibiting amyloidogenesis and low percentages promoting it.
With Shaking, both Dextran and Ficoll Promote Aβ Nucleation in a Concentration-dependent Manner
Since shaking has been widely used in the study of macromolecular crowding on amyloidogenesis, we investigated the effect of dextran and Ficoll on Aβ fibrilization when the reaction was submitted to orbital shaking (Fig. 3) (39–42).
FIGURE 3.
The effect of crowding agents on Aβ fibrilization under agitating conditions. 30 μm Aβ, in 165 μm ThT and PBS, was incubated under agitating conditions in absence or presence of 0.75 to 12% dextran (A–D) or Ficoll (E–H). Changes in ThT fluorescence were monitored (A and E) with the lag phase of fibrilization (B and F), the elongation rate (C and G) and plateau height (D and H) depicted. ** p < 0.03 and * p < 0.05 when compared with Aβ without crowding agents. a.u.: arbitrary units. The mean of at least three independent assays is shown. Error bars represent ± S.E.
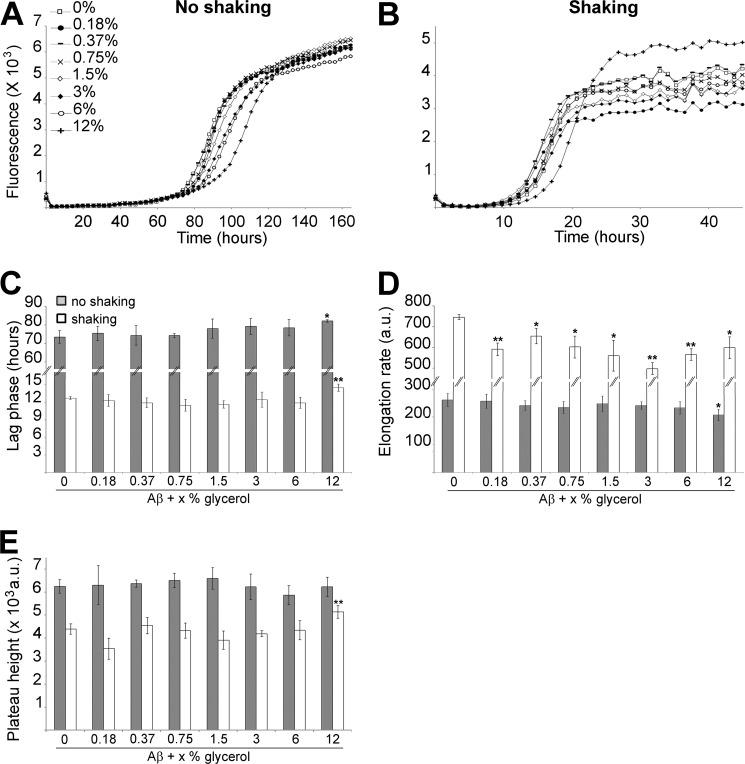
As previously observed for a variety of amyloid systems, shaking significantly accelerated Aβ fibrilization with a ∼5.1-fold decrease in lag phase and a ∼32.5-fold increase in elongation rate (compare Figs. 1 and 3) (17, 22, 25, 27, 45, 46). With shaking, both dextran and Ficoll caused a significant decrease in lag phase in a concentration-dependent manner (2.3 to 6.9 fold for dextran; 2.6 to 9.8 fold for Ficoll) (Fig. 3, A, B, E, and F). 0.75, 1.5, and 12% dextran caused a significant decrease in elongation rate (∼1.5-, 1.7- to 1.2-fold, respectively) as did 3 to 12% Ficoll (1.6- to 3.7-fold) (Fig. 3, A, C, E, and G). Both dextran and Ficoll showed non-significant effect on plateau height (Fig. 3, A, D, E, and H). Thus, both dextran and Ficoll promoted Aβ nucleation under shaking conditions.
An important observation that can be made based on the data from Figs. 1, 2, and 3 is that, although following similar trends, dextran and Ficoll affect the kinetics of Aβ fibrilization to different degrees, with Ficoll having a more pronounced effect. Under non-shaking conditions, Aβ lag phase was similarly affected by 0.75 to 12% of both crowding agents, whereas Ficoll was more efficient at decreasing the elongation rate and the plateau height than dextran (up to 11.8 and 9.6 times, respectively). In contrast, under non-shaking conditions low percentages of dextran and Ficoll were equally efficient at promoting Aβ nucleation (∼1.1–1.2-fold decrease in lag phase), but only Ficoll was able to increase the elongation rate. Under shaking conditions, Ficoll was up to 1.4 times more efficient at decreasing the lag phase than dextran.
Effects of Viscosity on Aβ Fibrilization
Fig. 4 illustrates the effects of viscosity on Aβ fibrilization. Glycerol was used as it contributes to viscosity but, being a polyol osmolyte, has a negligible crowding effect due to its small size. In both non-shaking and shaking conditions, the lag phase was significantly increased by 12% glycerol (∼1.1-fold on average) (Fig. 4, A–C). In non-shaking conditions, only 12% glycerol significantly reduced the elongation rate by 1.3-fold (Fig. 4, A and D). In contrast, every glycerol concentration tested was able to significantly reduce the elongation rate under shaking conditions (1.3-fold on average) (Fig. 4, B and D). The plateau height remained unchanged under non-shaking conditions and was only significantly increased by 12% glycerol under shaking conditions (1.2-fold) (Fig. 4, A, B, and E).
FIGURE 4.
The effect of glycerol on Aβ fibrilization. 30 μm Aβ, in 165 μm ThT and PBS, was incubated in absence or presence of 0.18 to 12% glycerol under non-agitating (A) or agitating conditions (B). Changes in ThT fluorescence were monitored (A and B) with the lag phase of fibrilization (C), the elongation rate (D) and plateau height (E) depicted. **, p < 0.03 and *, p < 0.05 when compared with Aβ without crowding agents. a.u.: arbitrary units. The mean of at least three independent assays is shown. Error bars represent ± S.E.
Ficoll Is More Surface Active than Dextran or Glycerol
We determined the ability of 12% glycerol, dextran, or Ficoll to lower the surface tension of water (Fig. 5, A and B). The surface tension of water for all solutions was immediately lowered but remained stable over the time course. The surface tension of water decreased to 67.3 ± 0.1 mN/m with glycerol, to 67.5 ± 0.1 mN/m with dextran, and to 63.0 ± 0.1 mN/m with Ficoll. The surface tension of both glycerol and dextran was statistically higher than that of Ficoll, which demonstrates that Ficoll is more surface active than glycerol or dextran.
FIGURE 5.
Glycerol, dextran, and Ficoll have different surface activities and affect differently the recruitment of Aβ to the AWI. Dynamic measurement of the surface tension for solutions of 12% glycerol, dextran, or Ficoll (A), with the surface tension at 350 s depicted (B). The surface tension for a solution of 30 μm Aβ, in absence or presence of 12% glycerol or crowding agents was also monitored over time (C), with the surface tension at the start of the reaction and at 350 s (D), the rate of decrease in surface tension (E) and the time required for this decrease (F, delay) depicted. The inset in E shows, highlighted by a gray box, the area on the curves used to calculate the rate of decrease. The graphs in A and C show 4 point moving averages of the raw data. a.u.: arbitrary units. The mean of at least three independent assays is shown. Error bars represent ± S.E. ** indicates p < 0.006 when compared with Ficoll in B and when compared with Aβ alone in D to F.
Linking Macromolecular Crowding with the Kinetics of Amyloidogenesis (Theory)
We examined theoretically the effect of macromolecular crowding in the absence of shaking. Since amyloid-forming monomers are surface active, they have to diffuse to the surface layer (AWI) to be fibrilized (Fig. 6A), as was previously demonstrated experimentally (13–18). Let m(z,t) denotes the mass density of the monomers in solution at position z, the equation that describes the time evolution of this mass density under diffusion is in Equation 9,
 |
where D is the diffusion coefficient, which is inversely proportional to the viscosity of the solution. For simplicity, we assume that the boundary conditions are such that at m(z = L) = 0 and ∂zm(z = 0) = 0. These boundary conditions amount to the assumptions that fibrilization is fast at the AWI (at z = L), and that there are no other sources of depletion for the monomers in the system. The solution to the above Equation 9 is shown in Equation 10,
 |
where the expansion coefficients, an, are determined by the boundary conditions. Specifically,
 |
and the constant a is fixed by the initial total monomer mass. The above equation indicates that the n-th term in the expansion decays as exp(−Dn2π2t/4L2). Therefore, in the long time limit, the kinetic of fibrilization is dictated by the first term, i.e. the n = 1 term. Focusing on this limit, the evolution of the mass of fibrils, f(t), grows like in Equation 12,
 |
where fs corresponds to the total mass of fibrils allowed at the adsorbed layer at the AWI.
We then considered the effects of adding crowding agents to our system, denoting the mass of crowding agents in the system by ν. If the crowding agent is not surface active (e.g. dextran), then the AWI adsorbed layer is unlikely to be affected by the presence of the crowding agent (Fig. 6B). In other words, we can assume that fs does not depend on the mass ν. For simplicity, we assume that the viscosity is approximately proportional to ν, i.e. D = β/ν for some proportionality constant β. Then the evolution of the fibril amount is shown in Equation 13.
 |
These analytical results indicate that an increase in the amount of crowding agents, ν, leads to a quicker increase in f(t), although the total amount at long time remains the same. Since dextran shows little surface activity (Fig. 5), we expect that under the non-shaking condition, its presence would only shift the kinetics of fibrilization (slower nucleation due to the viscosity reducing monomer diffusion to the AWI), but would not affect the total amount of fibrils (plateau height unchanged) (Fig. 6B, right panel). This explains the experimental results in Fig. 1, A–D, in which the presence of high percentages of dextran significantly increased Aβ lag phase but did not affect the plateau height.
For crowding agents that are surface active, we expect that due to competitive adsorption between proteins and crowding agents, the maximum amount of adsorbed fibrils, fs, will be diminished (Fig. 6C). Denoting again the mass of crowding agent by ν, we expect that fs decreases with increasing ν. For simplicity, we assume that,
where θ is a constant that quantify how the amount of adsorbed fibrils diminishes with increasing amount of crowding agent. Assuming further that as before, D = β/ν, the evolution of the fibril amount becomes Equation 15.
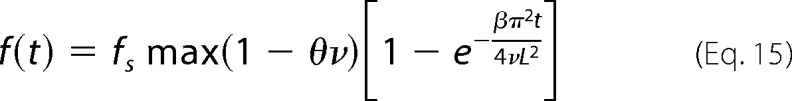 |
In this scenario, an increase in crowding agent leads to slower kinetics of fibrilization (both due to viscosity slowing diffusion to the AWI and by reducing monomer adsorption at the AWI), but also to a lower plateau height due to competition between amyloid species and crowding agents at the AWI. By comparison to dextran, Ficoll has a higher surface activity (Fig. 5). As a result, the presence of Ficoll would be expected to slow the kinetics and also decrease the final amount of fibrils (Fig. 6C, right panel). This model fits with our experimental observations under non-shaking conditions, in which high percentages of Ficoll slowed the kinetics of Aβ fibrilization (both lag phase and elongation rate) and also decreased the plateau height (Fig. 1, E–H).
Crowding-induced Viscosity Affects the Recruitment of Aβ to the AWI
Since our theoretical modeling predicted that, without shaking, viscosity should impair amyloid fibrilization due to a reduction in monomer diffusion to the AWI and that a surface active crowding agent (e.g. Ficoll) should compete with the monomers for adsorption at the AWI, we then assessed whether 12% of the crowding agents or glycerol were interfering with Aβ surface activity (Fig. 5, C and D). By itself, Aβ lowered the surface tension of water from 69.9 ± 1.7 (at 0 s) to 41.8 ± 1.2 mN/m (at 350 s), with no delay observed for the progressive decrease in surface tension. Both crowding agents and glycerol significantly affected the starting surface activity of Aβ, but only dextran significantly affected it at 350 s with the surface tension of water being increased from 41.8 (Aβ alone) to 47.0 ± 2.4 mN/m (Aβ with dextran) (Fig. 5D).
In addition to affecting the final surface activity of Aβ, the rate at which Aβ was decreasing the surface tension of water was also affected the most by 12% dextran with a ∼7.4-fold decrease (Fig. 5E). In presence of 12% glycerol, Aβ was ∼5.5-fold slower at decreasing the surface tension of water, whereas with 12% Ficoll it was only ∼1.5-fold slower.
12% dextran also significantly increased the time taken (12.64 ± 1.11 s) for the surface tension of water to be lowered by Aβ (Fig. 5F). A similar, but shorter, time delay (10.13 ± 1.09 s) was also observed in presence of 12% glycerol. In contrast, 12% Ficoll did not significantly affect the time required for Aβ to lower the surface tension (0.60 ± 0.92 s).
Effects of Macromolecular Crowding on Aβ Fibril Morphology
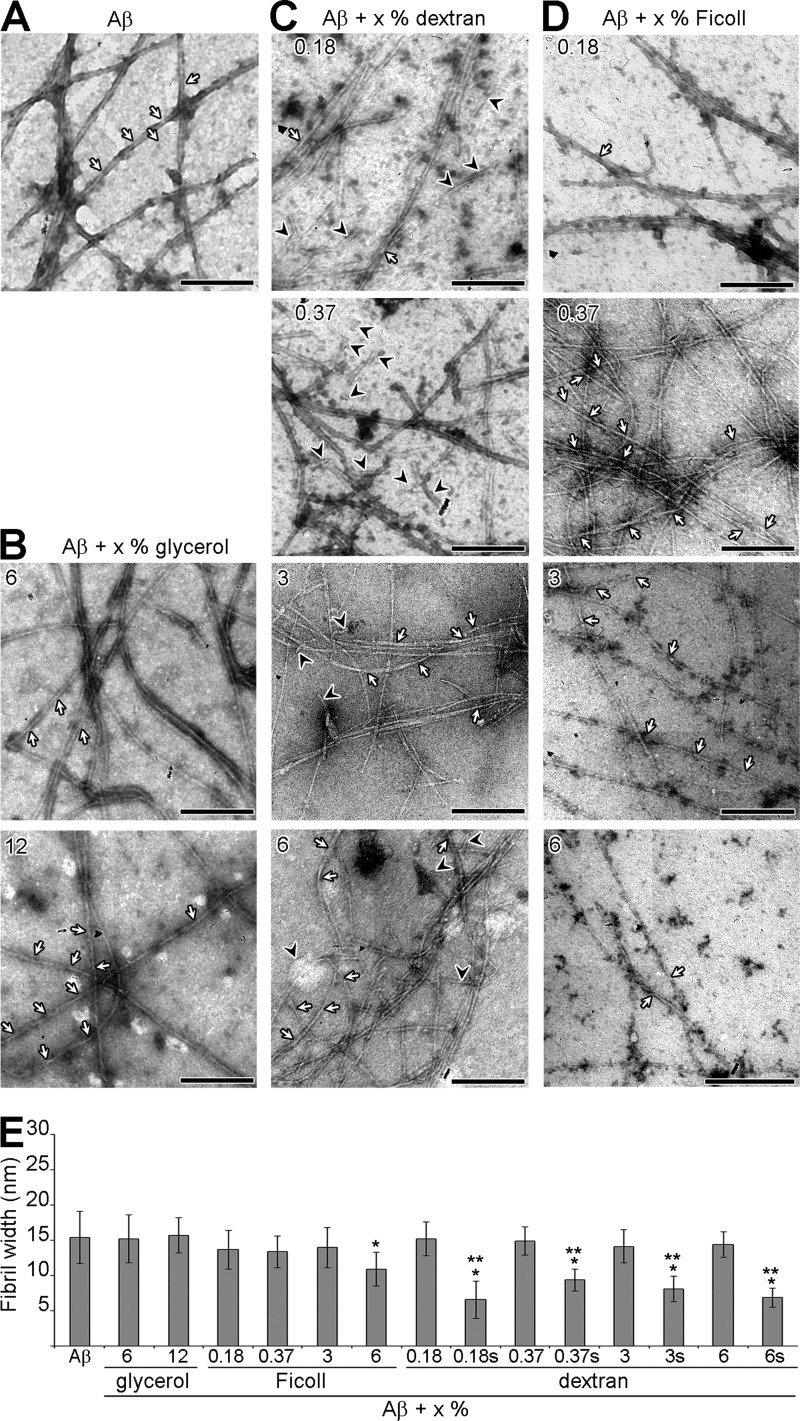
We used transmission electron microscopy to study the morphology of the fibrils formed by human Aβ incubated under different conditions. The reactions were monitored by measuring ThT fluorescence emission to ensure that the plateau was reached. The results presented in Figs. 7 and 8 indicate that neither the crowding agents nor glycerol promoted the formation of amorphous Aβ aggregates or of Aβ-crowding agents/glycerol heterocomplexes.
FIGURE 7.
The effect of crowding agents on the morphology of Aβ fibrils under non-agitating conditions. A–D, electron micrographs of negatively stained Aβ fibrilization reactions. Aβ fibrilization was performed under non-agitating conditions in absence (A) or presence of different percentages of glycerol (B), dextran (C), or Ficoll (D) until the reaction reached plateau. The white arrows indicate helical twists in the fibrils, and the black arrowheads indicate short fibrilar species. The scale bar represents 200 nm. E, the width of Aβ fibrils (n = 12), in absence or presence of glycerol, dextran, or Ficoll, was measured from electron micrographs of negatively stained reactions. s indicates short fibrilar species (as defined in the text). * indicates p < 0.004 when compared with Aβ, ** indicates p < 6 × 10−7 when compared with Aβ + x% dextran.
FIGURE 8.
The effect of crowding agents on the morphology of Aβ fibrils under agitating conditions. Electron micrographs of negatively stained Aβ fibrilization reactions. Aβ fibrilization was performed under agitating conditions in absence or presence of 12% glycerol, dextran, or Ficoll until the reaction reached plateau. The white arrows indicate helical twists in the fibrils, the black arrowheads indicate short fibrilar species, and the white arrowheads indicate short fibrils. The scale bar represents 200 nm. The width of Aβ fibrils (n = 12), in absence or presence of glycerol, dextran, or Ficoll, was measured from electron micrographs of negatively stained reactions (right panel). short indicates short fibrilar species (as defined in the text). ** indicates p < 0.02.
In the absence of shaking, Aβ formed ∼15.4 nm wide long fibrils that tended to associate together in bundles and possessed a typical helical twist (Fig. 7A, arrows; Fig. 7E). In presence of glycerol, Aβ fibrils were of normal appearance and width but did not associate in bundles (Fig. 7, B and E). Macromolecular crowding with dextran caused Αβ to form long fibrils with a normal width, albeit not associated in bundles, but also some shorter fibrilar species (Fig. 7C, arrowheads; Fig. 7E). The size of these shorter species varied between ∼40 and 150 nm in length, and their width was significantly reduced (∼1.6–2.4-fold). In contrast to dextran, the addition of 0.18% Ficoll had no significant effect on the morphology of Aβ fibrils, with long bundles of fibrils with helical twist being observed (Fig. 7, D and E). However, increasing concentrations of Ficoll led first to a decrease in fibril bundles (0.37% Ficoll) and then to an overall decrease in the number of fibrils (3 and 6% Ficoll). With 6% Ficoll, the width of the fibrils was also significantly decreased, ∼10.9 nm (Fig. 7E).
Under shaking conditions, Aβ formed ∼14.4 nm wide long fibrils that did not associate in bundles anymore but still possessed the typical amyloid helical twist (Fig. 8). We investigated the morphology of Aβ fibrils only in the presence of the highest percentage of crowding agent used in this study (12%) as these gave, for both dextran and Ficoll, the highest level of fibrilization inhibition (Fig. 3). In presence of dextran, the fibril width was significantly decreased (∼11.1 nm), and a number of short fibrilar species (∼55 to 130 nm in length, black arrowheads) with even more reduced width (∼7.6 nm) were observed. With Ficoll, the fibrils were not associating laterally with each other, as seen with Aβ alone, their width was significantly smaller (∼8.8 nm), and some short fibrilar species (∼300 to 400 nm, white arrowheads) were observed.
DISCUSSION
The majority of in vitro investigations on amyloidogenesis have been performed in simple buffer systems, which do not reflect the crowded and viscous in vivo environment. However, macromolecular crowding can affect amyloid systems in at least two different ways, with the excluded volume effect favoring aggregation and increased viscosity reducing the kinetics of amyloid fibrilization. In this study, we investigated the potential effects of two crowding agents and two experimental conditions on Aβ fibrilization, a major key player in AD pathogenesis. We clearly demonstrate that the crowding concentration threshold below which the excluded volume effect dominates over viscosity is highly dependent on the amyloid system studied and the spatial heterogeneity of the system. We also show that the outcome of macromolecular crowding (fibrilization kinetics, recruitment to the AWI and morphology of the species formed) can be critically influenced by the nature and properties of the crowding agents and the variations in experimental conditions.
Without shaking, Aβ fibrilization was either promoted or inhibited by the crowding agents. Precisely, Aβ nucleation was promoted with 0.18% dextran or 0.18–0.37% Ficoll, unchanged with 0.75 and 3%, and finally inhibited with 6 and 12% crowding agents. The elongation rate went from an increase to a decrease with Ficoll (0.18% versus 6–12%) or from an increase to unchanged with dextran (0.75–3% versus 6–12%). These results suggest differential effects of the crowding agents on nucleation relative to fibril growth. It is highly unlikely that Aβ monomers interact with the crowding agent in a way that inhibits amyloid formation. Indeed, dextran and Ficoll are both hydrophilic and neutral polysaccharide polymers, which do not contain ionized groups and are surrounded by a shell of a water. The effect of electrostatic interactions between Aβ and the crowding agents can also be eliminated since Aβ is overall acidic with a pI of 5.22 and a net charge of −2.722 at pH 7. Moreover, dextran was previously shown not to affect the ionic strength or pH of a solution, and more importantly both dextran and Ficoll were shown not to interact with amyloid proteins (26, 47).
In a crowded environment, many factors can influence protein aggregation: associated states will be favored over individual molecules, decreased protein solubility will favor self-association, and viscosity will disfavor diffusion-controlled reactions (20, 21). The two formers are dependent on the excluded volume effect, which increases the effective protein concentration. With increasing concentrations of crowding agents, the promotion of fibrilization by the excluded volume approaches a limit before viscosity inhibits it. Our results, without shaking, are consistent with the excluded volume accelerating Aβ nucleation and elongation. At higher concentrations of crowding agents, viscosity dominated, first leading to a leveling off in lag phase and then to an inhibition of the reaction.
We verified the effect of viscosity by introducing glycerol to the assays. Without shaking, only 12% glycerol increased Aβ lag phase and decreased the elongation rate. With shaking, only 12% glycerol increased Aβ lag phase, whereas all the concentrations tested decreased the elongation rate. Similarly, glycerol was shown to either slow or accelerate α-synuclein fibrilization in a concentration-dependent manner (22). Increasing the glycerol concentration will proportionally increase the viscosity of the solution, which will affect the rate of diffusion of Aβ molecules and their adsorption at the AWI. In turn, this will slow down Aβ nucleation and elongation. Our data suggest that 6% glycerol represents the threshold above which viscosity will start affecting Aβ nucleation. With shaking, 12% glycerol also increased Aβ plateau height. A recent study on a 16 residue aggregate-forming model peptide showed that, in a ThT assay with shaking, 7.03% glycerol did not affect the lag phase or elongation rate but increased the plateau height, whereas 17.90% glycerol increased both the lag phase and plateau height, in a similar way to our results (48). It was suggested that glycerol affects individual peptide states differentially, with nuclei destabilization in favor of monomers and stabilization of the fibrils once formed. The increase in Aβ plateau height may be due to a glycerol-induced reduction in fibril breakage.
Our theoretical modeling predicted that the Aβ adsorbed layer at the AWI would not be affected by a non-surface active crowding agent but, that without shaking, Aβ nucleation would be slowed by high percentages of such agent, with the total number of fibrils not being affected. The effect on nucleation would be due to viscosity reducing monomer diffusion to the AWI. Experimentally we found that 12% dextran exhibited little surface activity, significantly increased Aβ final surface activity, was the most effective at delaying the lowering of surface tension by Aβ and at decreasing the rate at which it was lowered. 12% glycerol behaved identically to 12% dextran. Dextran is also more viscous than glycerol and Ficoll (22). The viscosity of 12% glycerol is ∼1.39 cp (49), 12% Ficoll 400 is ∼6.95 cp (GE Healthcare Life Sciences) and 12% dextran is ∼12.8 cp (GE Healthcare Life Sciences).
The delay and the rate decrease for lowering the surface tension induced by dextran and glycerol are both consistent with these reducing Aβ monomer diffusion to the AWI. Therefore our results, with high percentages of dextran or glycerol significantly increasing Aβ lag phase but not affecting the plateau height, can be explained by viscosity initially impeding the recruitment of Aβ monomers to the AWI but not perturbing the assembly once adsorption has taken place.
Our theoretical modeling also predicted that, without shaking, high percentages of a surface active crowding agent would slow fibrilization and decrease the final amount of fibrils. These effects would be due to competitive adsorption at the AWI between Aβ and the crowding agent reducing adsorption of every amyloid species (from monomers to fibrils). Experimentally, we found that 12% Ficoll was surface active, did not significantly affect the time required for lowering the surface tension and that it only marginally affected the rate at which the surface tension was decreased. Since both Aβ and Ficoll are surface active, we would not expect the time required and the rate for lowering the surface tension to be affected due to both molecules adsorbing at the AWI. Thus our experimental results, with high percentages of Ficoll slowing the kinetics of Aβ fibrilization and also decreasing the plateau height, can be explained by competition for the AWI between Aβ species and Ficoll.
Another factor that may contribute to the differences observed between the two crowding agents under non-shaking conditions is the extent of the volume excluded. 12% dextran 70 corresponds to 1 × 1017 dextran molecules in the solution, whereas 12% Ficoll 400 corresponds to 1.8 × 1016 molecules. The Stokes radius of dextran 70 is 58 Å and that of Ficoll 400 is 100 Å. Therefore, 1 × 1017 dextran molecules will occupy 82 μl in a 100-μl reaction, whereas 1.8 × 1016 Ficoll molecules will occupy 75 μl. By excluding 1.1 times more volume than 12% Ficoll, 12% dextran increases further the effective concentration of Aβ, which may contribute to the less pronounced inhibition effect of dextran on Aβ fibrilization, under non-shaking conditions.
Very few macromolecular crowding-amyloid studies have been performed without shaking and most were done differently to ours; Tau244–441 assays were done with a fibrilization enhancer (heparin) and Ficoll 70, apolipoprotein C-II fibrilization was with dextran T10 and α-synuclein assays were with Ficoll 70 (26, 27, 50). The only comparable study to ours was done with α-synuclein, which showed that the excluded volume effect dominated when using 15% dextran 70. In our Aβ studies, viscosity dominated above 6% crowding agent. It has been previously postulated that the promoting effect of macromolecular crowding is expected to become more important with increasing size of the amyloid precursor but also with increasing volume occupancy of the local environment (26). α-Synuclein is much larger, 14.5 kDa, than the 4.4 kDa Aβ peptide, which may account for the different behaviors under similar crowding conditions. One could also hypothesize that these different behaviors may be contributed to by the different amyloidogenic folding pathways used by the two polypeptides. Unlike α-synuclein fibrils, which form through the stacking of linear β-sheets, Aβ has the propensity to form β-hairpins, which can associate side-to-side during β-sheet assembly (51–54). Previously, both dextran and Ficoll were shown to inhibit the folding of a 16 residue β-hairpin peptide, possibly because viscosity dominated the excluded volume effect by impeding relatively large-scale and non-local motions of side chains required for β-hairpin formation (34). In contrast the folding of a 34 residue α-helix was mostly unaffected by viscosity because only a series of local changes were necessary. Therefore, viscosity may not interfere with a more linear β-sheet formation (α-synuclein), which relies on a series of local changes, but may decrease the formation of a β-hairpin (Aβ), which is more dependent on larger scale movements. This may explain the dominance of viscosity when 6 to 12% crowding agents were used with Aβ.
As previously reported for other amyloidogenic polypeptides, Aβ fibrilization was accelerated by both shaking and macromolecular crowding (22, 25, 27). Moreover, both dextran and Ficoll reduced Aβ elongation rate, similarly to Ficoll slowing the elongation rate of α-synuclein (22). When shaken, the crowding polymers are not able to form a structured mesh. This would favor peptide chain movement and allow the excluded volume to dominate and to promote fibrilization. In addition, shaking can produce shear forces able to fragment fibrils, increasing the number of ends available for monomer addition. These shorter fibrils would be less affected by viscosity than their larger counterparts and the excluded volume effect would dominate and promote fibrilization, as we observed. Shorter fibrilar species were detected in the dextran and Ficoll shaken samples. Other parameters to be considered with shaking are that the adsorbed layer of amyloid species would be destroyed, fibrilization would then happen everywhere in the bulk solution, and the diffusion of the monomers in the bulk would be facilitated. Therefore, the nucleation and elongation rates would be quickened by shaking. In addition, the crowding agents would diminish the roaming volume of the monomers and the agent surface activity would become unimportant due to the destruction of the adsorbed layer. As a result, an increase in the amount of crowding agent would increase the excluded volume, which in turn would lead to an acceleration of the kinetics of fibrilization.
Two age-associated effects are inhibition of protein degradation (e.g. proteasomal clearance) and reduction in cell volume (e.g. loss of cellular and tissue water) (55–57). This would be the equivalent to increase volume occupancy by macromolecules, which may promote disease depending on the amyloid species being favored and/or stabilized. The presence of short fibrilar species in the non-shaken dextran solutions could have implications for the kinetics of Aβ fibrilogenesis as smaller species could act as seeds promoting nucleation. Shorter fibrils would also be less affected by viscosity, further contributing to dextran being better at promoting Aβ fibrilization. These short fibrils could possibly have arisen from the fragmentation of longer fibrils, as has been observed for Tau and the prion protein in presence of Ficoll (27). Small amyloid species are generally considered more toxic than long fibrils. Thus a crowded environment may affect the kinetics and the types of fibrilar species formed, which in turn may affect AD pathogenesis.
In this study, we demonstrate that the threshold between beneficial volume exclusion and inhibitory viscosity during macromolecular crowding is strictly dependent on the amyloid system studied, the properties of the crowding agent and the experimental conditions used. These are important considerations to take into account when investigating amyloid formation in vitro and may represent an under-appreciated effect of crowding in such assays. A physiological in vivo crowded environment may affect the types of amyloid species forming and the sites of their accumulation, which in turn may affect the pathogenesis of AD and amyloid diseases in general. In vitro shaking conditions may mimic in vivo membrane turn-over, in which the catalytic interface is destroyed. Aβ is generated in lipid rafts, which do not turn-over as fast as their surrounding membrane environment (58, 59). Therefore, macromolecular crowding may have a different outcome depending whether amyloid species accumulate in the vicinity of slowly or rapidly turning-over membranes. Thus, systematic studies on the effect of macromolecular crowding are necessary to obtain a more physiologically relevant understanding of amyloid systems. Such studies could also have major implications in the design of assays for discovering novel therapeutic agents to target amyloid formation.
Acknowledgment
We thank Dr. Robert Jacobs (Chemistry Research Laboratory, University of Oxford) for assistance and help with the surface tension measurements.
This work was supported by a research grant from Synaptica Ltd (to L. J.).
- AD
- Alzheimer's disease
- AWI
- air-water interface
- Aβ
- amyloid-β peptide
- CCD camera
- charge-coupled device camera
- DMSO
- dimethyl sulfoxide
- HHI
- hydrophobic-hydrophilic interface
- PBS
- phosphate buffer saline
- ThT
- thioflavin T.
REFERENCES
- 1. Stefani M., Dobson C. M. (2003) Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. 81, 678–699 [DOI] [PubMed] [Google Scholar]
- 2. Haass C., Selkoe D. J. (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 8, 101–112 [DOI] [PubMed] [Google Scholar]
- 3. Selkoe D. J. (2004) Cell biology of protein misfolding: the examples of Alzheimer's and Parkinson's diseases. Nat. Cell Biol. 6, 1054–1061 [DOI] [PubMed] [Google Scholar]
- 4. Harper J. D., Lansbury P. T., Jr. (1997) Models of amyloid seeding in Alzheimer's disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu. Rev. Biochem. 66, 385–407 [DOI] [PubMed] [Google Scholar]
- 5. Lee C. F. (2009) Self-assembly of protein amyloids: A competition between amorphous and ordered aggregation Phys. Rev. E. 80, 031922 [DOI] [PubMed] [Google Scholar]
- 6. Chiti F., Dobson C. M. (2006) Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 [DOI] [PubMed] [Google Scholar]
- 7. Soreghan B., Kosmoski J., Glabe C. (1994) Surfactant properties of Alzheimer's A beta peptides and the mechanism of amyloid aggregation. J. Biol. Chem. 269, 28551–28554 [PubMed] [Google Scholar]
- 8. Cottingham M. G., Bain C. D., Vaux D. J. (2004) Rapid method for measurement of surface tension in multiwell plates. Lab. Invest. 84, 523–529 [DOI] [PubMed] [Google Scholar]
- 9. Lopes D. H., Meister A., Gohlke A., Hauser A., Blume A., Winter R. (2007) Mechanism of islet amyloid polypeptide fibrillation at lipid interfaces studied by infrared reflection absorption spectroscopy. Biophys. J. 93, 3132–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parhi P., Golas A., Barnthip N., Noh H., Vogler E. A. (2009) Volumetric interpretation of protein adsorption: capacity scaling with adsorbate molecular weight and adsorbent surface energy. Biomaterials 30, 6814–6824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krishnan A., Siedlecki C. A., Vogler E. A. (2003) Traube-rule interpretation of protein adsorption at the liquid-vapor interface. Langmuir 19, 10342–10352 [Google Scholar]
- 12. Terzi E., Hölzemann G., Seelig J. (1997) Interaction of Alzheimer beta-amyloid peptide(1–40) with lipid membranes. Biochemistry 36, 14845–14852 [DOI] [PubMed] [Google Scholar]
- 13. Jean L., Lee C. F., Lee C., Shaw M., Vaux D. J. (2010) Competing discrete interfacial effects are critical for amyloidogenesis. FASEB J. 24, 309–317 [DOI] [PubMed] [Google Scholar]
- 14. Jean L., Lee C. F., Vaux D. J. (2012) Enrichment of amyloidogenesis at an air-water interface. Biophys. J. 102, 1154–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chi E. Y., Frey S. L., Winans A., Lam K. L., Kjaer K., Majewski J., Lee K. Y. (2010) Amyloid-beta fibrillogenesis seeded by interface-induced peptide misfolding and self-assembly. Biophys. J. 98, 2299–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang D., Dinh K. L., Ruthenburg T. C., Zhang Y., Su L., Land D. P., Zhou F. (2009) A kinetic model for beta-amyloid adsorption at the air/solution interface and its implication to the beta-amyloid aggregation process. J. Phys. Chem. B. 113, 3160–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morinaga A., Hasegawa K., Nomura R., Ookoshi T., Ozawa D., Goto Y., Yamada M., Naiki H. (2010) Critical role of interfaces and agitation on the nucleation of Aβ amyloid fibrils at low concentrations of Aβ monomers. Biochim. Biophys. Acta 1804, 986–995 [DOI] [PubMed] [Google Scholar]
- 18. Morris V. K., Ren Q., Macindoe I., Kwan A. H., Byrne N., Sunde M. (2011) Recruitment of Class I hydrophobins to the air:water interface initiates a multi-step process of functional amyloid formation. J. Biol. Chem. 286, 15955–15963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fulton A. B. (1982) How crowded is the cytoplasm? Cell 30, 345–347 [DOI] [PubMed] [Google Scholar]
- 20. Ellis R. J. (2001) Macromolecular crowding: an important but neglected aspect of the intracellular environment. Curr. Opin. Struct. Biol. 11, 114–119 [DOI] [PubMed] [Google Scholar]
- 21. Minton A. P. (2000) Implications of macromolecular crowding for protein assembly. Curr. Opin. Struct. Biol. 10, 34–39 [DOI] [PubMed] [Google Scholar]
- 22. Munishkina L. A., Cooper E. M., Uversky V. N., Fink A. L. (2004) The effect of macromolecular crowding on protein aggregation and amyloid fibril formation. J. Mol. Recognit. 17, 456–464 [DOI] [PubMed] [Google Scholar]
- 23. Minton A. P. (2000) Effect of a concentrated “inert” macromolecular cosolute on the stability of a globular protein with respect to denaturation by heat and by chaotropes: a statistical-thermodynamic model. Biophys. J. 78, 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou H. X., Rivas G., Minton A. P. (2008) Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annu. Rev. Biophys. 37, 375–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uversky V. N., Cooper E. M., Bower K. S., Li J., Fink A. L. (2002) Accelerated α-synuclein fibrillation in crowded milieu. FEBS Lett. 515, 99–103 [DOI] [PubMed] [Google Scholar]
- 26. Hatters D. M., Minton A. P., Howlett G. J. (2002) Macromolecular crowding accelerates amyloid formation by human apolipoprotein C-II. J. Biol. Chem. 277, 7824–7830 [DOI] [PubMed] [Google Scholar]
- 27. Zhou Z., Fan J. B., Zhu H. L., Shewmaker F., Yan X., Chen X., Chen J., Xiao G. F., Guo L., Liang Y. (2009) Crowded cell-like environment accelerates the nucleation step of amyloidogenic protein misfolding. J. Biol. Chem. 284, 30148–30158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bokvist M., Gröbner G. (2007) Misfolding of amyloidogenic proteins at membrane surfaces: the impact of macromolecular crowding. J. Am. Chem. Soc. 129, 14848–14849 [DOI] [PubMed] [Google Scholar]
- 29. Shen C. L., Murphy R. M. (1995) Solvent effects on self-assembly of beta-amyloid peptide. Biophys. J. 69, 640–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Snyder S. W., Ladror U. S., Wade W. S., Wang G. T., Barrett L. W., Matayoshi E. D., Huffaker H. J., Krafft G. A., Holzman T. F. (1994) Amyloid-beta aggregation: selective inhibition of aggregation in mixtures of amyloid with different chain lengths. Biophys. J. 67, 1216–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tripp B. C., Magda J. J., Andrade J. D. (1995) Adsorption of Globular-Proteins at the Air/Water Interface as Measured Via Dynamic Surface-Tension - Concentration-Dependence, Mass-Transfer Considerations, and Adsorption-Kinetics J. Colloid Interf. Sci. 173, 16–27 [Google Scholar]
- 32. Lin S. Y., Lu T. L., Hwang W. B. (1995) Adsorption-Kinetics of Decanol at the Air-Water-Interface. Langmuir 11, 555–562 [Google Scholar]
- 33. Rotenberg Y., Boruvka L., Neumann A. W. (1983) Determination of Surface-Tension and Contact-Angle from the Shapes of Axisymmetric Fluid Interfaces. J. Colloid Interf. Sci. 93, 169–183 [DOI] [PubMed] [Google Scholar]
- 34. Mukherjee S., Waegele M. M., Chowdhury P., Guo L., Gai F. (2009) Effect of macromolecular crowding on protein folding dynamics at the secondary structure level. J. Mol. Biol. 393, 227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Venturoli D., Rippe B. (2005) Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: effects of molecular size, shape, charge, and deformability. Am. J. Physiol. Renal Physiol 288, F605–F613 [DOI] [PubMed] [Google Scholar]
- 36. Wenner J. R., Bloomfield V. A. (1999) Crowding effects on EcoRV kinetics and binding. Biophys. J. 77, 3234–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luby-Phelps K., Castle P. E., Taylor D. L., Lanni F. (1987) Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proc. Natl. Acad. Sci. U.S.A. 84, 4910–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. LeVine H., 3rd (1993) Thioflavine T interaction with synthetic Alzheimer's disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 2, 404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giasson B. I., Murray I. V., Trojanowski J. Q., Lee V. M. (2001) A hydrophobic stretch of 12 amino acid residues in the middle of α-synuclein is essential for filament assembly. J. Biol. Chem. 276, 2380–2386 [DOI] [PubMed] [Google Scholar]
- 40. Conway K. A., Lee S. J., Rochet J. C., Ding T. T., Williamson R. E., Lansbury P. T., Jr. (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. U.S.A. 97, 571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Collins S. R., Douglass A., Vale R. D., Weissman J. S. (2004) Mechanism of prion propagation: amyloid growth occurs by monomer addition. PLoS Biol. 2, e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Petkova A. T., Yau W. M., Tycko R. (2006) Experimental constraints on quaternary structure in Alzheimer's beta-amyloid fibrils. Biochemistry 45, 498–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alvarez-Martinez M. T., Fontes P., Zomosa-Signoret V., Arnaud J. D., Hingant E., Pujo-Menjouet L., Liautard J. P. (2011) Dynamics of polymerization shed light on the mechanisms that lead to multiple amyloid structures of the prion protein. Biochim. Biophys. Acta 1814, 1305–1317 [DOI] [PubMed] [Google Scholar]
- 44. Hortschansky P., Schroeckh V., Christopeit T., Zandomeneghi G., Fändrich M. (2005) The aggregation kinetics of Alzheimer's beta-amyloid peptide is controlled by stochastic nucleation. Protein Sci. 14, 1753–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Atarashi R., Satoh K., Sano K., Fuse T., Yamaguchi N., Ishibashi D., Matsubara T., Nakagaki T., Yamanaka H., Shirabe S., Yamada M., Mizusawa H., Kitamoto T., Klug G., McGlade A., Collins S. J., Nishida N. (2011) Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat. Med. 17, 175–178 [DOI] [PubMed] [Google Scholar]
- 46. Pronchik J., He X., Giurleo J. T., Talaga D. S. (2010) In vitro formation of amyloid from α-synuclein is dominated by reactions at hydrophobic interfaces. J. Am. Chem. Soc. 132, 9797–9803 [DOI] [PubMed] [Google Scholar]
- 47. Zhou B. R., Zhou Z., Hu Q. L., Chen J., Liang Y. (2008) Mixed macromolecular crowding inhibits amyloid formation of hen egg white lysozyme. Biochim. Biophys. Acta 1784, 472–480 [DOI] [PubMed] [Google Scholar]
- 48. Sukenik S., Politi R., Ziserman L., Danino D., Friedler A., Harries D. (2011) Crowding alone cannot account for cosolute effect on amyloid aggregation. PLoS One 6, e15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Segur J. B., Oberstar H. E. (1951) Viscosity of glycerol and its aqueous solutions. Ind. Eng. Chem. 43, 2117–2120 [Google Scholar]
- 50. Shtilerman M. D., Ding T. T., Lansbury P. T., Jr. (2002) Molecular crowding accelerates fibrillization of α-synuclein: could an increase in the cytoplasmic protein concentration induce Parkinson's disease? Biochemistry 41, 3855–3860 [DOI] [PubMed] [Google Scholar]
- 51. Petkova A. T., Ishii Y., Balbach J. J., Antzutkin O. N., Leapman R. D., Delaglio F., Tycko R. (2002) A structural model for Alzheimer's beta -amyloid fibrils based on experimental constraints from solid state NMR. Proc. Natl. Acad. Sci. U.S.A. 99, 16742–16747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lührs T., Ritter C., Adrian M., Riek-Loher D., Bohrmann B., Döbeli H., Schubert D., Riek R. (2005) 3D structure of Alzheimer's amyloid-beta(1–42) fibrils. Proc. Natl. Acad. Sci. U.S.A. 102, 17342–17347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sgourakis N. G., Yan Y., McCallum S. A., Wang C., Garcia A. E. (2007) The Alzheimer's peptides Aβ40 and 42 adopt distinct conformations in water: a combined MD / NMR study. J. Mol. Biol. 368, 1448–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dupuis N. F., Wu C., Shea J. E., Bowers M. T. (2009) Human islet amyloid polypeptide monomers form ordered beta-hairpins: a possible direct amyloidogenic precursor. J. Am. Chem. Soc. 131, 18283–18292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Naber D., Korte U., Krack K. (1979) Content of water-soluble and total proteins in the aging human brain. Exp. Gerontol. 14, 59–63 [DOI] [PubMed] [Google Scholar]
- 56. Nagy I. Z., Nagy K., Lustyik G. (1982) Protein and water contents of aging brain. Exp. Brain Res. Suppl. 5, 118–122 [DOI] [PubMed] [Google Scholar]
- 57. Barber B. J., Babbitt R. A., Parameswaran S., Dutta S. (1995) Age-related changes in rat interstitial matrix hydration and serum proteins. J Gerontol. A. Biol. Sci. Med. Sci. 50, B282–287 [DOI] [PubMed] [Google Scholar]
- 58. Ehehalt R., Keller P., Haass C., Thiele C., Simons K. (2003) Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J. Cell Biol. 160, 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hur J. Y., Welander H., Behbahani H., Aoki M., Frånberg J., Winblad B., Frykman S., Tjernberg L. O. (2008) Active γ-secretase is localized to detergent-resistant membranes in human brain. FEBS J 275, 1174–1187 [DOI] [PubMed] [Google Scholar]