Background: Resveratrol has been proposed to have beneficial health effects due to its anti-inflammatory properties.
Results: Resveratrol suppressed IL-1β-induced activation of NF-κB and PI3K in a dose- and time-dependent manner.
Conclusion: Anti-inflammatory effects of resveratrol may be mediated at least in part through inhibition/deacetylation of PI3K and NF-κB.
Significance: Activated Sirt-1 plays an essential role in anti-inflammatory effects of resveratrol.
Keywords: Inflammation, NF-κ B (NF-KB), PI 3-Kinase (PI3K), Resveratrol, Sirt1, Scleraxis, Tenocyte
Abstract
Resveratrol, an activator of histone deacetylase Sirt-1, has been proposed to have beneficial health effects due to its antioxidant and anti-inflammatory properties. However, the mechanisms underlying the anti-inflammatory effects of resveratrol and the intracellular signaling pathways involved are poorly understood. An in vitro model of human tenocytes was used to examine the mechanism of resveratrol action on IL-1β-mediated inflammatory signaling. Resveratrol suppressed IL-1β-induced activation of NF-κB and PI3K in a dose- and time-dependent manner. Treatment with resveratrol enhanced the production of matrix components collagen types I and III, tenomodulin, and tenogenic transcription factor scleraxis, whereas it inhibited gene products involved in inflammation and apoptosis. IL-1β-induced NF-κB and PI3K activation was inhibited by resveratrol or the inhibitors of PI3K (wortmannin), c-Src (PP1), and Akt (SH-5) through inhibition of IκB kinase, IκBα phosphorylation, and inhibition of nuclear translocation of NF-κB, suggesting that PI3K signaling pathway may be one of the signaling pathways inhibited by resveratrol to abrogate NF-κB activation. Inhibition of PI3K by wortmannin attenuated IL-1β-induced Akt and p65 acetylation, suggesting that p65 is a downstream component of PI3K/Akt in these responses. The modulatory effects of resveratrol on IL-1β-induced activation of NF-κB and PI3K were found to be mediated at least in part by the association between Sirt-1 and scleraxis and deacetylation of NF-κB and PI3K. Overall, these results demonstrate that activated Sirt-1 plays an essential role in the anti-inflammatory effects of resveratrol and this may be mediated at least in part through inhibition/deacetylation of PI3K and NF-κB.
Introduction
Tendon overuse injuries and tendinopathies are a growing problem in sports medicine and in orthopedic clinics. These tendon conditions are accompanied by inflammation through up-regulation of proinflammatory cytokines, COX-2, matrix metalloproteinase (MMP)2 expression, and prostaglandin E2 (PGE2) production. This could initiate the degradation and remodeling of tendons in response to mechanical loading (1, 2).
Many of these catabolic enzymes induced by proinflammatory mediators are a result of the activation of the NF-κB transcription factor pathway, which is associated with the activation of many cellular genes (3). NF-κB is normally located in the cytoplasm in an inactive state in a complex by association with an inhibiting IκBα subunit. In response to phosphorylation, IκBα dissociates from the complex, and the p65 and p50 subunits freely translocate to the cell nucleus and bind to NF-κB recognition sites in the promotor regions of various NF-κB-regulated genes (4). Several studies have shown an alternative signaling pathway that has been described in various cell types. Here, Src/Akt directly phosphorylates IκB kinases (IKKs) leading to the activation of NF-κB independent of mitogen-activated protein kinase kinase-1 and NF-κB-inducing kinase (5). Interestingly, IL-1β has been found to activate the phosphatidylinositol 3-kinase (PI3K)/Src/Akt pathway, potentially providing an alternative pathway to activate NF-κB and increase phosphorylation of p300 to stimulate histone acetyltransferase activity. PI3K/Akt belong to the family of serine/threonine protein kinases that is involved in cell proliferation and survival, as demonstrated by the regulation of several downstream cellular targets, such as Bad, caspase-9, glycogen synthase kinase 3 (GSK3), and NF-κB. Several reports have suggested that cytokine-induced activation of the PI3K signaling pathway is associated to NF-κB-dependent signaling pathway in different cell types (6, 7).
For the treatment of tendon injuries or tendinitis non-steroidal anti-inflammatory drugs are commonly prescribed to minimize inflammation and subsequent damage to tissue integrity (8). However, the use of non-steroidal anti-inflammatory drugs is associated with numerous side effects including inhibitory effects on proteoglycan synthesis and cell proliferation (9). Therefore, the search is still on for safer and more selective pharmaco-therapies for tendinopathies.
Resveratrol, trans-3,5,4′-trihydroxy-stibene, a polyphenolic phytoestrogen and natural phytoalexin with antifungal properties in plants, has been shown to have anti-inflammatory, free radical scavenger capacity, anti-carcinogenic, immunomodulatory, and cardio-protective effects and was also found to suppress angiogenesis, prevent diabetes mellitus, and prolong life span (10–12). In previous studies on tenocytes we have demonstrated that another natural polyphenol, curcumin, modulates IL-1β-induced NF-κB activation by suppression of the regulatory subunit of phosphoinositide-3-kinase (PI3K/p85) signaling pathway. Furthermore, phosphorylation of Akt, an downstream intermediate of the PI3K pathway, was also decreased (13). These results indicate that suppression of the PI3K activity associated with anti-inflammation might represent a novel target mechanism for the anti-inflammatory effect of natural polyphenols in human tissues.
The exact intracellular mechanisms of resveratrol are still not fully understood. However, it has been shown that resveratrol effectively activates Sirt-1, a NAD-dependent nuclear class III histone deacetylase (14). Sirt-1 has been linked with regulating cell differentiation, proliferation, survival, and organism longevity (15). Several studies have associated Sirt-1 with gene silencing, DNA repair, ribosomal DNA recombination, and aging (16, 17). Besides lysine residues of histone proteins, it also deacetylates lysine residues of other nuclear proteins associated with apoptosis and inflammation, such as p53 (18) and NF-κB subunit p65 (19), thereby regulating transcriptional activity of target proteins. Deacetylation of NF-κB by Sirt-1 leads to reduced DNA binding ability, increased IκBα association, or loss of transactivation potential (20).
In this work we demonstrate that the anti-inflammatory effects of resveratrol in tenocytes may be mediated, at least in part, through inhibition of PI3K and NF-κB activation and the associated kinases and that the deacetylase Sirt-1 might play a critical role in this process.
EXPERIMENTAL PROCEDURES
Antibodies
Polyclonal anti-collagen type I, polyclonal anti-collagen type III, polyclonal anti-decorin antibody, and alkaline phosphatase linked sheep anti-mouse and sheep anti-rabbit secondary antibodies for immunoblotting were purchased from Millipore (Schwalbach, Germany). Acetylated lysine (Ac-Lys-103) antibody was purchased from Cell Signaling Technology (Danvers, MA). Polyclonal anti-active caspase-3 was obtained from R&D Systems. Monoclonal anti-β-actin and protein A/G-Sepharose beads were purchased from Sigma. Polyclonal anti-tenomodulin (sc-49325) and anti-PI3K p85 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal anti-scleraxis (SCXA) (ab58655), polyclonal anti-Sirt-1 were obtained from Abcam PLC (Cambridge, UK). Antibodies against phosphospecific IκBα (Ser-32/36) and against phosphospecific p65 (NF-κB)/(Ser-536) were obtained from Cell Technology (Beverly, MA). Anti-IκB kinase anti-IKK-α and anti-IKK-β antibodies were obtained from Imgenex (Hamburg, Germany). All antibodies were used at concentrations and dilutions recommended by the manufacturer.
Growth Media, Chemicals, and Cytokines
Growth medium (Ham's F-12/Dulbecco's modified Eagle's medium (50/50) containing 10% fetal calf serum (FCS), 25 mg/ml ascorbic acid, 50 IU/ml streptomycin, 50 IU/ml penicillin, 2.5 mg/ml amphotericin B, essential amino acids, and l-glutamine) was obtained from Seromed (Munich, Germany). Trypsin/EDTA (EC 3.4.21.4) and nicotinamide were purchased from Sigma. Epon was obtained from Plano (Marburg, Germany). Wortmannin and PP1 were purchased from Biomol (Plymouth Meeting, PA). SH-5 was from Calbiochem). Resveratrol with purity greater than 98% was purchased from Sigma). A 100 mm stock solution of resveratrol (molecular weight 228.2) was prepared in ethanol and further diluted in cell culture medium to prepare working concentrations. The maximum final content of ethanol in cultures was less than 0.1%. This concentration was also used as a control.
Tenocyte Isolation and Culture
Human tendon explants (healthy finger tendon of one male middle-aged donor) were received during tendon-rupture surgery with full informed consent and local ethics committee approval. The peritendineum was carefully removed, and tendon filaments were separated and cut into small pieces. After 1–2 weeks of culturing in growth medium, tenocytes continuously migrated from this explant and adhered to Petri dishes. Tendon cells were trypsinized, expanded in monolayers, and multiplied to gain a sufficient number of cells (21). Tenocytes used for experiments did not exceed passage 5.
Experimental Design
Tenocytes monolayer cultures were washed three times with serum-starved medium (3% FCS) and incubated for 1 h. Serum-starved human tenocytes were either left untreated, treated with 10 ng/ml IL-1β alone for the indicated time periods, or pretreated with 5 μm resveratrol for 4 h followed by co-treatment with 10 ng/ml IL-1β and 5 μm resveratrol for 24 h or for the indicated time periods. In a second approach, monolayer cultured tenocytes treated as above were transferred to high density cultures and cultured under identical conditions with serum-starved medium for 10 days to examine the effects of IL-1β and/or resveratrol on tenocytes differentiation potential in a three-dimensional environment. Three-dimensional high density cultures were prepared as previously described (22). Briefly, 1 × 106 cells were pipetted onto a nitrocellulose filter resting on a steel net bridge. Cell culture medium reached the filter medium interface, and the cells were nurtured through diffusion. After 1 day, the cells in the culture aggregated and formed a pellet on the filter. For investigation of NF-κB translocation and IκBα phosphorylation, tenocyte cultures were treated either with 10 ng/ml IL-1β or co-treated with 10 ng/ml IL-1β and resveratrol for 0, 5, 10, 20, 40, and 60 min, and nuclear and cytoplasmic extracts were prepared. These experiments were performed in triplicate, and the results are provided as mean values from three independent experiments.
Pharmacological Inhibition Experiments
Tenocytes (1 × 105 cells/Petri dish) were cultivated in growth medium for 24 h. PI3K inhibitor (wortmannin), Src inhibitor (PP1), and Akt inhibitor (SH-5) treatments were carried out in serum-starved medium. Tenocytes were pretreated with serum-starved medium containing different concentrations of resveratrol (0.1, 1, 5, 10 μm) for 4 h or wortmannin (10 nm), PP1 (1 μm), or SH-5 (1 μm) for 1 h or pretreatment with resveratrol (5 μm), wortmannin (10 nm), PP1 (1 μm), or SH-5 (1 μm) for 0, 5, 10, 20, 40, and 60 min and then exposed to 10 ng/ml IL-1β for 1 h. After these treatments, whole cell or nuclear extracts were prepared as described below and examined for NF-κB and NF-κB-related gene products.
Isolation of Tenocyte Cytoplasmic and Nuclear Extracts
Isolation of cytoplasmic and nuclear extracts was performed as previously described in detail (23). Briefly, tenocytes were trypsinized and washed twice in 1 ml of ice-cold PBS. The cell pellet was resuspended in 400 μl of hypotonic lysis buffer containing protease inhibitors and incubated on ice for 15 min. 12.5 μl of 10% Nonidet P-40 were added, and the cell suspension was vigorously mixed for 15 s. The extracts were centrifuged for 1.5 min at 14.000 × g. The supernatants (cytoplasmic extracts) were frozen at −80 °C. 25 μl ice-cold nuclear extraction buffer were added to the pellets and incubated for 30 min with intermittent mixing. Extracts were centrifuged, and the supernatant (nuclear extracts) was transferred to the prechilled tubes for storage at −80 °C.
Electron Microscopy
Transmission electron microscopy was performed as previously described (24). Briefly, tenocyte cultures were fixed for 1 h in Karnovsky's fixative and post-fixed in 1% OsO4 solution. After dehydration, pellets were embedded in Epon, and ultrathin cuts were made on a Reichert-Ultracut E and contrasted with a mixture of 2% uranyl acetate/lead citrate. A transmission electron microscope (Zeiss, Jena, Germany) was used to examine the cultures.
Immunoprecipitation and Immunoblotting
A detailed description of the technique used for the following experiments has been previously published (23). Briefly, tenocyte monolayer cultures were rinsed in PBS, and the proteins were extracted with lysis buffer (50 mm Tris/HCl (pH 7.2), 150 mm NaCl, l% (v/v) Triton X-100, 1 mm sodium orthovanadate, 50 mm sodium pyrophosphate, 100 mm sodium fluoride, 0.01% (v/v) aprotinin, pepstatin A (4 μg/ml), leupeptin (10 μg/ml), and 1 mm phenylmethylsulfonyl fluoride (PMSF) for 30 min on ice. After adjusting the total protein concentration, samples were separated by SDS-PAGE (5%, 7.5% or 12% gels) under reducing conditions. For immunoprecipitation, the extracts were precleared by incubating them first with 25 μl of either normal rabbit IgG serum or normal mouse IgG serum and Staphylococcus aureus cells, then with primary antibodies diluted in wash buffer (0.1% Tween 20, 150 mm NaCl, 50 mm Tris-HCl (pH 7.2), 1 mm CaCl2, 1 mm MgCl2, and 1 mm PMSF) for 2 h at 4 °C, and finally with S. aureus cells for 1 h at 4 °C. Control immunoprecipitation experiments were performed by incubating the samples with non-immune rabbit anti-mouse IgG alone. S. aureus cells were washed 5 times with wash buffer and once with 50 mm Tris-HCl (pH 7.2) and then boiled in SDS-PAGE sample buffer. Separated proteins were transferred to nitrocellulose membranes and incubated in blocking buffer (5% (w/v) skimmed milk powder in PBS, 0.1% Tween 20) for 1 h at ambient temperature. Membranes were incubated overnight with the first antibody diluted in blocking buffer at 4 °C on a shaker, washed 3 times with blocking buffer, and then incubated with the secondary antibody conjugated with alkaline phosphatase for 90 min at ambient temperature. Membranes were rinsed and then washed 3 times in 0.1 m Tris (pH 9.5) containing 0.05 m MgCl2 and 0.1 m NaCl. Specific antigen-antibody complexes were rendered visible using nitro blue tetrazolium and 5-bromo-4-chloro-3-indoylphosphate (p-toluidine salt; Pierce) as the substrates for alkaline phosphatase. Total protein concentration was determined according to the bicinchoninic acid system (Pierce) using bovine serum albumin as a standard. Specific binding was quantified by densitometry using “quantity one” (Bio-Rad).
Immunoprecipitation of p65/PI3K and p65/PI3K Acetylation Assay
To examine the effect of resveratrol on IL-1β-induced acetylation of p65/PI3K, serum-starved tenocytes were pretreated with 5 μm resveratrol for 4 h and then exposed to 10 ng/ml IL-1β for 0, 5, 10, 20, 40, or 60 min or treated with IL-1β alone for the indicated times. The cells were washed and lysed to prepare whole cell lysates. Whole cell extracts were precleared by incubating with 25 μl of either normal rabbit IgG serum or normal mouse IgG serum and protein A/G-Sepharose beads. The precleared whole cell extract was incubated with primary antibodies (anti-p65 or anti-PI3K antibodies) appropriately diluted in wash buffer (0.1% Tween 20, 150 mm NaCl, 50 mm Tris-HCl (pH 7.2), 1 mm CaCl2, 1 mm MgCl2, and 1 mm PMSF) for 2 h at 4 °C and finally with protein A/G-Sepharose beads for 1 h at 4 °C. After incubation, immunocomplexes were washed with lysis buffer, boiled with SDS sample buffer for 5 min, resolved on SDS-PAGE, and subjected to Western blot analysis using an anti-acetyl-lysine antibody.
Immune Complex Kinase Assay
An immune complex kinase assay was performed as previously described in detail (25). Briefly, to test the effect of PI3K inhibitor (wortmannin) on IL-1β-induced IKK activation, an immune complex kinase assay was performed. The IKK complex was immunoprecipitated from whole tenocyte lysates with antibodies against IKK-α and IKK-β and subsequently incubated with protein A/G-agarose beads (Pierce). After 2 h of incubation, the beads were washed with lysis buffer and resuspended in a kinase assay solution containing 50 mm HEPES (pH 7.4), 20 mm MgCl2, 2 mm dithiothreitol, 10 μm unlabeled ATP, and 2 mg of IKK substrate GST-IκBα (amino acids 1–54) and incubated at 30 °C for 30 min. This was followed by boiling in SDS-PAGE sample buffer for 5 min. Proteins were separated using SDS-PAGE under reducing conditions as described above. Phosphorylation of GST-IκBα was assessed using a specific antibody against phospho-specific IκBα (Ser-32/36). To demonstrate the total amounts of IKK-α and IKK-β in each sample, whole-cell proteins were separated using SDS-PAGE under reducing conditions as described above. Detection of IKK-α and IKK-β was performed by immunoblotting with either anti-IKK-α or anti-IKK-β antibodies.
Apoptotic Assay
To quantify apoptosis and cells with mitochondrial changes (MC), ultrathin sections of the samples were prepared and evaluated with an electron microscope (TEM 10, Zeiss). The number of cells exhibiting typical morphological features of apoptotic cell death was determined by scoring 100 cells from 20 different microscopic fields per culture, and this was expressed as an indicator of tenocytes culture degradation.
Statistical Analysis
Numerical data are expressed as the mean values (±S.D.) for a representative experiment performed in triplicate. The means were compared using Student's t test assuming equal variances. Differences were considered to be statistically significant if the p value was less than 0.05.
RESULTS
In this study we evaluated the effect of resveratrol on IL-1β-induced signal transduction in human tenocytes. The concentrations of resveratrol applied in our study and the time of exposure had no effect on cell viability.
Resveratrol Suppresses IL-1β-induced Mitochondrial Changes and Apoptosis in Human Tenocytes in Monolayer and High Density Cultures
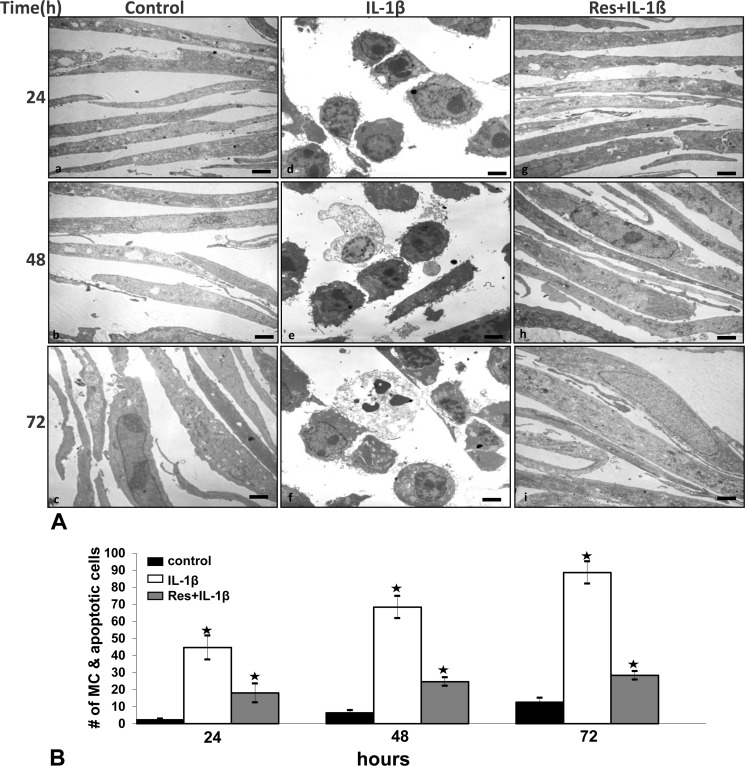
To see whether resveratrol can modulate the cytotoxic effects of IL-1β in tenocytes, primary human tenocytes were exposed to 10 ng/ml IL-1β alone or pretreated with 5 μm resveratrol for 4 h and then treated with 10 ng/ml IL-1β for 24, 48, and 72 h. The effect of resveratrol on IL-1β-induced apoptosis was examined at the ultrastructural level using transmission electron microscopy. Control primary human tenocytes (untreated cells) showed a typically flattened shape with small cytoplasmic processes, a large mostly euchromatic nucleus with nucleoli, and a well structured cytoplasm (Fig. 1A, a–c). Treatment of tenocytes with 10 ng/ml IL-1β for 24 h led to degenerative morphological changes such as swelling of rough endoplasmic reticulum, clustering of swollen mitochondria, and degeneration of other cell organelles (Fig. 1Ad). After longer treatment times (48–72 h), more severe features of cellular degeneration such as condensed heterochromatin in the cell nuclei and multiple vacuoles were observed. The flattened monolayer tenocytes became more and more rounded, lost their microvilli-like processes, and became apoptotic (Fig. 1A, e–f). In contrast, co-treatment with IL-1β and resveratrol significantly reduced the cytotoxic and apoptotic effects of IL-1β on the tenocytes (Fig. 1A, g–i). Cells treated with resveratrol alone (not shown) revealed a similar morphology as in control cultures.
FIGURE 1.
Effect of resveratrol on IL-1β-induced mitochondrial changes and apoptosis in human tenocytes in monolayer cultures. A, shown is a morphological evaluation of human tenocytes in monolayer culture by transmission electron microscopy. Tenocytes were either left untreated (a–c) or were treated with IL-1β (10 ng/ml) for 24, 48, and 72 h (d–f) or pretreated with resveratrol (5 μm) for 4 h and then stimulated with IL-1β (10 ng/ml) for 24, 48, and 72 h (g–i). B, to quantify apoptosis and MC in these cell cultures, 100 cells from 20 microscopic fields were counted. The examination was performed in triplicate, and the results are provided as the mean values with S.D. from three independent experiments. ×5000; bar = 1μm. Values were compared with the control, and statistically significant values with p < 0.05 were designated by an asterisk (*).
Quantification of cellular degeneration and apoptosis was achieved by counting the number of cells with degenerative morphological changes (swollen mitochondria, apoptosis) in the samples evaluated by transmission electron microscopy (Fig. 1B). At 72 h of IL-1β, treatment increased the number of cells with MC and apoptotic features to 88%. In contrast, pretreatment with resveratrol significantly reduced the number of cells showing signs of MC and apoptosis to 28%. This demonstrates that resveratrol inhibits the cytotoxic and apoptotic effects induced by IL-1β in tenocytes (Fig. 1B).
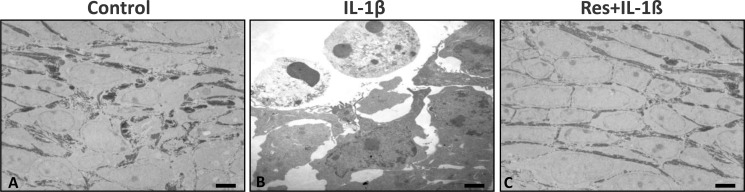
We previously reported that high density culture can promote tenocytes differentiation because it supports cell-cell interactions (26). We, therefore, also examined whether resveratrol can modulate IL-1β-induced dedifferentiation of tenocytes in high density culture. Over a time period of 10 days, high density cultures were stimulated with IL-1β alone (10 ng/ml) or exposed to resveratrol (5 μm) for 4 h before treatment with IL-1β. The ultrastructural morphology of the cells and the formation of extracellular matrix macromolecules was evaluated using electron microscopy. Untreated tenocytes were viable and exhibited fibroblast-like to oval shapes with large mostly euchromatic nuclei, mitochondria, rough endoplasmic reticulum, and a well structured extracellular matrix (Fig. 2A). Treatment with IL-1β alone resulted in degenerative and apoptotic features including formation of dense materials in the nuclei, blebs at the cell surface, apoptotic bodies, and degeneration of extracellular matrix structure (Fig. 2B). Stimulation of tenocytes with resveratrol before treatment with IL-1β resulted in more viable cells exhibiting an oval shape, euchromatic nuclei with prominent nucleoli, a well organized cytoplasm, and dense and regular extracellular matrix (Fig. 2C). Taken together, morphological evaluation of monolayer and high density cultures revealed that resveratrol considerably reduced the degenerative and cytotoxic effects induced by IL-1β in human tenocytes.
FIGURE 2.
Effects of resveratrol and IL-1β on human tenocytes in high density cultures. Human tenocytes either served as a control (A) or were stimulated with IL-1β (10 ng/ml) alone (B) or pretreated with resveratrol (5 μm) for 4 h and then exposed to IL-1β (C) for 10 days in high density cultures. Morphological evaluation was performed by transmission electron microscopy. ×4000; bars, 1μm.
Resveratrol Inhibits IL-1β-induced NF-κB and PI3K Activation in Human Tenocytes
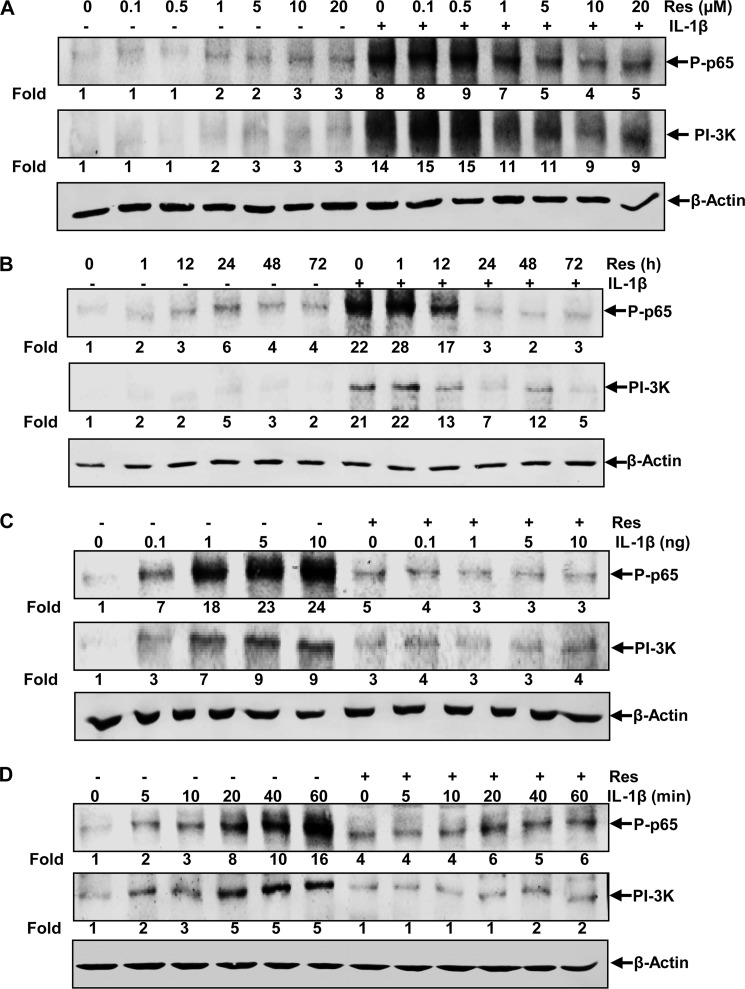
Tenocytes were pretreated for 4 h with different concentrations of resveratrol (0, 0.1, 0.5, 1, 5, 10, and 20 μm) and then stimulated with 10 ng/ml IL-1β for 30 min. Protein extracts were prepared and assayed for activated NF-κB subunit p65 and PI3K by Western blot analysis. As shown in Fig. 3A, IL-1β induced activation of NF-κB and PI3K 8- and 14-fold, respectively, compared with control cultures, and resveratrol inhibited this activation in a dose-dependent manner with a maximum effect at 10 μm resveratrol, which reduced activation level to 4- and 9-fold, respectively. We next investigated the effect of different incubation times with resveratrol on NF-κB or PI3K activation by IL-1β. Tenocytes were incubated with 5 μm resveratrol for different times (0, 1, 12, 24, 48, and 72 h) and then stimulated with 10 ng/ml IL-1β for 30 min and probed for NF-κB and PI3K. The results in Fig. 3B show that resveratrol inhibited IL-1β-induced NF-κB and PI3K activation with increasing times of incubation. 22- and 21-fold activation of NF-κB and PI3K, respectively, by treatment with IL-1β alone was reduced to control levels by 24 h of pretreatment with resveratrol. To examine the effect of resveratrol on NF-κB and PI3K activation at different concentrations of IL-1β, both untreated and resveratrol-pretreated cells (5 μm for 4 h) were incubated with various concentrations of IL-1β (0, 0.1, 1, 5, and 10 ng/ml) for 30 min and then assayed for NF-κB and PI3K by Western blot analysis (Fig. 3C). Although the activation of NF-κB and PI3K by 1 ng/ml IL-1β was strong (18- and 7-fold, respectively), it was reduced to basal levels by pretreatment with resveratrol and even at 5 or 10 ng/ml IL-1β, NF-κB and PI3K activation was efficiently suppressed. We also investigated the effect of resveratrol on the kinetics of IL-1β-induced NF-κB and PI3K activation. Both untreated and resveratrol-pretreated cells (5 μm for 4 h) were incubated with IL-1β (10 ng/ml) for different times (0, 5, 10, 20, 40, and 60 min) and then probed for NF-κB and PI3K activation. In cells not treated with resveratrol, IL-1β activated NF-κB and PI3K in a time-dependent manner with a maximum activation at 60 min (16- and 5-fold, respectively, compared with control cultures). In resveratrol-pretreated cells, however, the activation of NF-κB and PI3K was almost completely inhibited in cells exposed to IL-1β for up to 10 min (Fig. 3D) and was still significantly reduced in tenocytes treated with IL-1β for 20, 40, or 60 min. These results show that resveratrol is a very potent inhibitor of NF-κB and PI3K activation in human tenocytes.
FIGURE 3.
Effects of resveratrol on IL-1β-induced NF-κB and PI3K activation in human tenocytes. Human tenocytes in monolayer cultures were pretreated with different concentrations of resveratrol (0, 0.1, 0.5, 1, 5, 10, and 20 μm) for 4 h and then treated with 10 ng/ml IL-1β for 30 min (A) or treated with 5 μm resveratrol for different times (0, 1, 12, 24, 48, and 72 h) and then stimulated with 10 ng/ml IL-1β for 30 min (B). In a different approach, untreated cells and cells pretreated with resveratrol (5 μm for 4 h) were exposed to various concentrations of IL-1β (0, 0.1, 1, 5, and 10 ng/ml) for 30 min (C) or were incubated with 10 ng/ml IL-1β for different times (0, 5, 10, 20, 40, and 60 min) (D). After completion of the experiments, whole cell lysates were prepared and analyzed by Western blotting with antibodies against phosphospecific-p65 and PI3K. Housekeeping protein β-actin served as a loading control in all experiments. Densitometric analyses were performed by setting control cultures as 1-fold.
Resveratrol Abrogates IL-1β-induced Down-regulation of Extracellular Matrix Components and of Tenocyte-specific Transcription Factor SCXA in Human Tenocytes
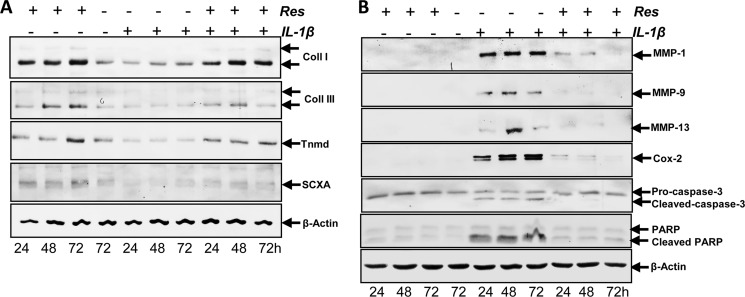
Serum-starved human tenocytes were cultured in monolayer for 24 h and then treated with 10 ng/ ml IL-1β or 5 μm resveratrol for 24, 48, and 72 h or were pretreated with 5 μm resveratrol for 4 h and then co-treated with 10 ng/ ml IL-1β and resveratrol for 24, 48, and 72 h or were left untreated. Western blotting was performed by probing whole cell lysates with antibodies against collagen types I and III, tenomodulin, and the tendon-specific transcription factor SCXA (Fig. 4A). Tenocytes stimulated with IL-1β alone showed a significant down-regulation of synthesis of collagen types I, III, tenomodulin, and SCXA expression (Fig. 4A). In contrast, pretreatment of tenocytes with resveratrol followed by stimulation with IL-1β resulted in an inhibition of cytokine-induced effects on the above-mentioned protein production (Fig. 4A).
FIGURE 4.
Effects of resveratrol on IL-1β-induced matrix degradation, proinflammatory and apoptotic signaling, and down-regulation of tenocyte-specific transcription factor SCXA in human tenocytes. A, monolayer cultures of human tenocytes were either left untreated or were treated with 10 ng/ ml IL-1β or 5 μm resveratrol for 24, 48, and 72 h or were pretreated with 5 μm resveratrol for 4 h and then co-treated with 10 ng/ ml IL-1β and resveratrol for 24, 48, and 72 h. Whole cell lysates were subjected to Western blotting with antibodies against collagen (Coll) types I and III, tenomodulin (Tnmd), and the tendon-specific transcription factor SCXA. B, the same tenocyte cultures as in A were subsequently also probed for expression of Cox-2, MMP-1, MMP-9, MMP-13, cleavage of PARP, and caspase-3. Housekeeping protein β-actin served as a loading control.
Resveratrol Represses IL-1β-induced NF-κB-dependent Proinflammatory, Matrix-degrading, and Apoptotic Gene Products in Human Tenocytes
We examined whether resveratrol can modulate the expression of IL-1β-induced NF-κB-regulated gene products involved in the inflammatory and degradative processes in tenocytes. Primary human tenocytes with or without pretreatment with resveratrol were examined for IL-1β-induced gene products by Western blot analysis using specific antibodies (Fig. 4B). IL-1β induced the expression of Cox-2, MMP-1, MMP-9, MMP-13, and cleavage of PARP and caspase-3, but treatment with resveratrol inhibited the expression of the mentioned genes and cleavage of caspase-3 (Fig. 4B).
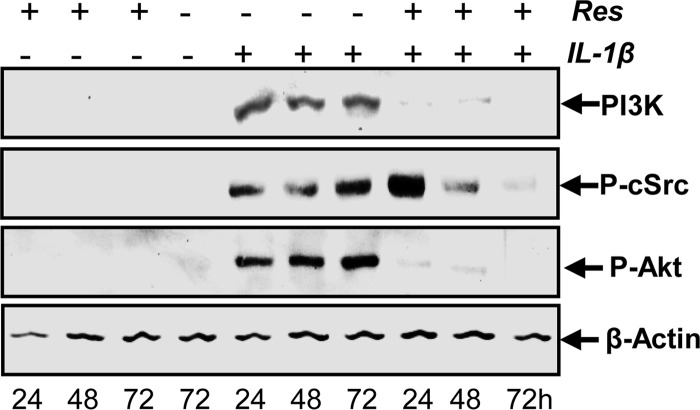
Resveratrol Inhibits IL-1β-induced Src and PI3K/Akt Activation in Tenocytes
To examine whether kinases upstream of PI3K like c-Src and Akt were also activated by IL-1β and whether this activation was blocked by resveratrol, cells were treated as described above for Fig. 4A, and expression of these kinases was determined by Western blotting with antibodies specific for PI3K, phosphorylated c-Src, and phosphorylated Akt. As shown in Fig. 5, IL-1β stimulated the expression of PI3K and the phosphorylation of c-Src and Akt in tenocytes. This was inhibited by pretreatment with resveratrol in a time-dependent manner. Given that resveratrol similarly inhibits IL-1β-induced activation of c-Src and PI3K/Akt as well as NF-κB activation, these results might suggest a link between these two pathways in tenocytes.
FIGURE 5.
Effects of resveratrol on IL-1β-induced Src and PI3K/Akt activation in human tenocytes. Serum-starved human tenocytes in monolayer cultures were either treated with 10 ng/ ml IL-1β or 5 μm resveratrol for 24, 48, and 72 h or pretreated with 5 μm resveratrol for 4 h and then co-treated with 10 ng/ml IL-1β and resveratrol for 24, 48, and 72 h or were left untreated. Whole cell lysates were prepared, and samples were examined by Western blot analysis with antibodies against PI3K, phosphorylated c-Src, and phosphorylated Akt. Housekeeping protein β-actin served as a loading control.
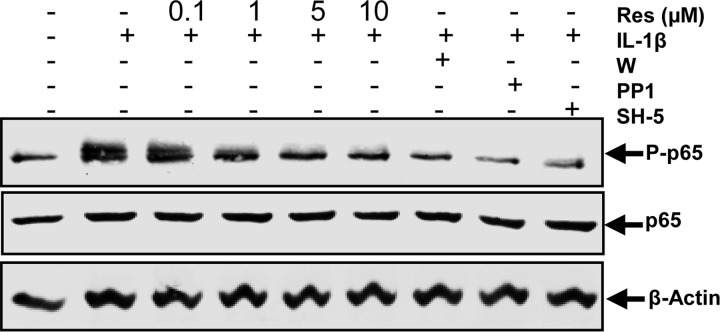
Resveratrol and c-Src, PI3K, and Akt Inhibitors Block IL-1β-induced Phosphorylation of NF-κB in Human Tenocytes
To further examine the role of the c-Src/PI3K/Akt signaling pathway in regulating IL-1β-mediated NF-κB activation, the level of activated NF-κB protein was analyzed using selective kinase inhibitors. Serum-starved human tenocytes were either left untreated or treated with 10 ng/ml IL-1β alone for 1 h or pretreated with resveratrol (0.1, 1, 5, 10 μm) for 4 h or inhibitors of c-Src (PP1, 1 μm), PI3K (wortmannin, 10 nm), or Akt (SH-5, 1 μm) for 1 h and then co-treated with 10 ng/ml IL-1β for 1 h. As shown in Fig. 6, the activation of NF-κB in tenocytes was significantly increased by incubation with IL-1β. However, the specific inhibitors for c-Src, PI3K, and Akt blocked IL-1β-stimulated NF-κB activation. In addition, IL-1β-stimulated NF-κB activation was also significantly inhibited by preincubation with resveratrol in a concentration-dependent manner, reaching a maximum effect at 5–10 μm. These results further demonstrate that resveratrol plays an important role in down-regulation of the IL-1β-induced NF-κB activation in tenocytes and that this might be mediated at least in part through inhibition of the c-Src/PI3K/Akt signaling pathway.
FIGURE 6.
Effects of resveratrol and c-Src, PI3K, and Akt inhibitors on IL-1β-induced phosphorylation of NF-κB in human tenocytes. Monolayer cultured serum-starved human tenocytes were either left untreated or treated with 10 ng/ml IL-1β alone for 1 h or pretreated with different concentrations of resveratrol (0.1, 1, 5, 10 μm) for 4 h or inhibitors of c-Src (PP1, 1 μm), PI3K (wortmannin (W), 10 nm), or Akt (SH-5, 1 μm) for 1 h and then co-treated with 10 ng/ml IL-1β for 1 h. Protein extracts from whole cell lysis were then probed by Western blotting with antibodies against NF-κB subunit p65 and phosphospecific-p65 as well as housekeeping protein β-actin.
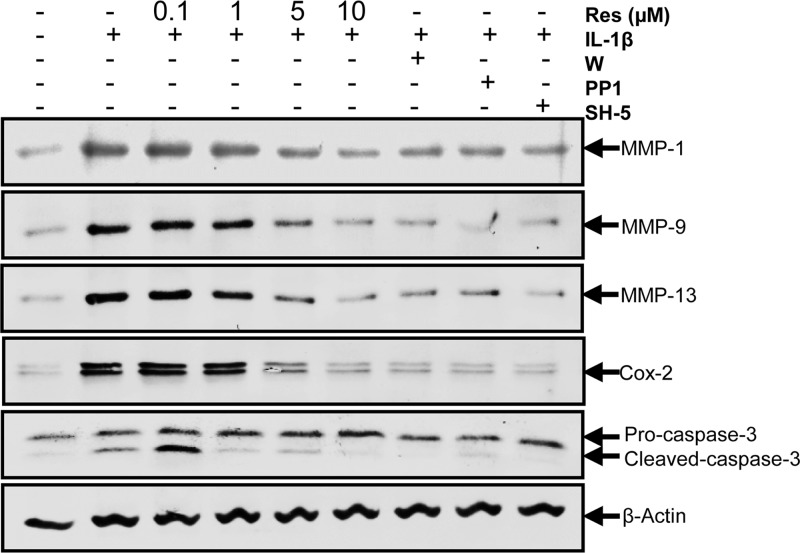
Resveratrol and c-Src, PI3K, AKT Inhibitors Repress IL-1β-induced NF-κB-dependent Proinflammatory, Matrix-degrading, and Apoptotic Gene Products in Human Tenocytes
To determine whether transactivation of c-Src and PI3K/Akt participated in inhibition of IL-1β-induced NF-κB-dependent proinflammatory, matrix-degrading, and apoptotic gene products by resveratrol, tenocytes were treated with inhibitors of c-Src (PP1), PI3K (wortmannin), Akt (SH-5), or different concentrations of resveratrol as mentioned for Fig. 6 and then stimulated with IL-1β for 1 h. As shown in Fig. 7, pretreatment with these inhibitors blocked IL-1β-stimulated MMP-1, MMP-9, MMP-13, and Cox-2 expression and cleavage of caspase-3. In addition, these IL-1β-stimulated gene products were also significantly inhibited by preincubation with resveratrol in a concentration-dependent manner. These results indicate that resveratrol might play an important role in down-regulation of the IL-1β-induced NF-κB-dependent proinflammatory, matrix-degrading, and apoptotic gene products in tenocytes and that this might be mediated at least in part through inhibition of the c-Src/PI3K/Akt signaling pathway.
FIGURE 7.
Effects of resveratrol and c-Src, PI3K, and Akt inhibitors on IL-1β-induced NF-κB-dependent proinflammatory, matrix-degrading, and apoptotic gene products in human tenocytes. Human tenocytes in monolayer cultures were incubated with serum-starved medium and were then treated with 10 ng/ml IL-1β alone for 1 h or pretreated with different concentrations of resveratrol (0.1, 1, 5, 10 μm) for 4 h or inhibitors of c-Src (PP1, 1 μm), PI3K (wortmannin (W), 10 nm) or Akt (SH-5, 1 μm) for 1 h and then co-treated with 10 ng/ml IL-1β for 1 h or were left untreated. Cells were lysed and subjected to Western blot analysis with antibodies specific for MMP-1, MMP-9, MMP-13, Cox-2, and cleaved caspase-3. Expression of β-actin was examined as a loading control.
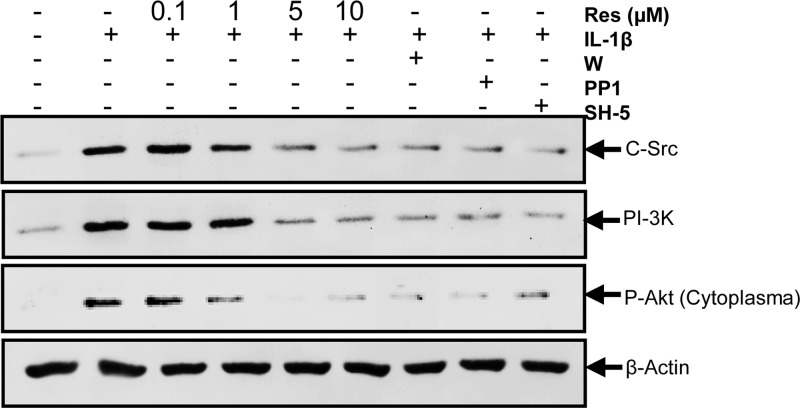
Resveratrol and c-Src, PI3K, AKT Inhibitors Reduce IL-1β-induced c-Src, PI3K, and Akt Activation in Human Tenocytes
To investigate whether resveratrol inhibited the expression of c-Src and PI3K/p85 or activation of Akt in a similar manner as specific inhibitors of the mentioned kinases, tenocytes were pretreated with resveratrol (0.1, 1, 5, 10 μm) for 4 h or inhibitors of c-Src (PP1, 1 μm), PI3K (wortmannin, 10 nm), or Akt (SH-5, 1 μm) for 1 h and then co-treated with 10 ng/ml IL-1β for 1 h. Control cultures were left untreated or treated with 10 ng/ml IL-1β alone for 1 h as a negative control. Activation of the kinases was determined by Western blotting with antibodies specific for c-Src, PI3K, and phosphorylated Akt. As shown in Fig. 8, IL-1β induced activation of c-Src, PI3K, and Akt in tenocytes. This was inhibited by pretreatment with inhibitors of c-Src (PP1), PI3K (wortmannin), and Akt (SH-5) and resveratrol in a concentration of 5 μm. Interestingly, specific inhibitors of the single kinases (wortmannin, PP1, SH-5) down-regulated the expression of all mentioned kinases, and resveratrol also affected all kinases in the same manner. These results suggest that there might be a functional interaction between the kinases of PI3K signaling pathway and that resveratrol is a potent inhibitor of IL-1β-induced c-Src/PI3K/Akt activation in tenocytes.
FIGURE 8.
Effects of resveratrol and c-Src, PI3K, and Akt inhibitors on IL-1β-induced c-Src, PI3K, and Akt activation in human tenocytes. Monolayer cultured serum-starved human tenocytes were either left untreated or treated with 10 ng/ml IL-1β alone for 1 h or pretreated with different concentrations of resveratrol (0.1, 1, 5, 10 μm) for 4 h or inhibitors of c-Src (PP1, 1 μm), PI3K (wortmannin (W), 10 nm) or Akt (SH-5, 1 μm) for 1 h and then co-treated with 10 ng/ml IL-1β for 1 h. Cell lysates were then analyzed by immunoblotting using antibodies raised against c-Src, PI3K, phosphospecific-Akt, and β-actin.
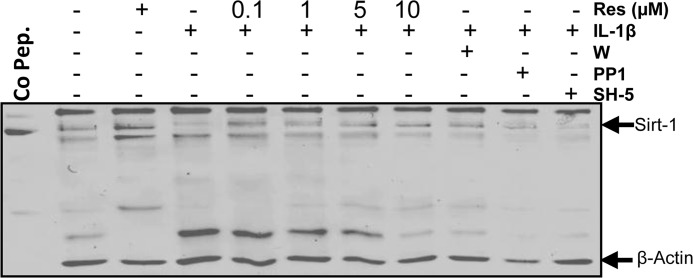
Resveratrol and c-Src, PI3K, AKT Inhibitors Reverse IL-1β-induced Sirt-1 Inhibition in Human Tenocytes
It has been reported that resveratrol is a potent pharmacological agonist of sirtuin activity in different cells (14), and it is also known that resveratrol inhibits the transcription factor NF-κB (27). To further determine the cellular mechanisms of resveratrol-induced NF-κB inhibition and the role of Sirt-1 in the c-Src/PI3K/Akt signaling pathway, the expression of Sirt-1 protein was analyzed using selective kinase inhibitors. Serum-starved human tenocytes were either left untreated, treated with 5 μm resveratrol or 10 ng/ml IL-1β alone for 1 h or pretreated with resveratrol (0.1, 1, 5, 10 μm) for 4 h or inhibitors of c-Src (PP1, 1 μm), PI3K (wortmannin, 10 nm) or Akt (SH-5, 1 μm) for 1 h, and then co-treated with 10 ng/ml IL-1β for 1 h. As shown in Fig. 9, the expression of Sirt-1 in tenocytes was significantly decreased by incubation with IL-1β. However, the specific inhibitors for c-Src, PI3K, and Akt reversed this negative effect of IL-1β similar to preincubation with different concentrations of resveratrol. At 5–10 μm resveratrol, Sirt-1 expression was comparable with control levels. These results indicate that c-Src/PI3K/Akt signaling pathway is involved at least in part in IL-1β-induced inhibition of Sirt-1 expression during inflammation in tenocytes and that resveratrol effectively counteracts in this process.
FIGURE 9.
Effects of resveratrol and c-Src, PI3K, and Akt inhibitors on IL-1β-induced Sirt-1 inhibition in human tenocytes. Human tenocytes cultured in monolayer were either left untreated, treated with 5 μm resveratrol or 10 ng/ml IL-1β alone for 1 h, or pretreated with different concentrations of resveratrol (0.1, 1, 5, 10 μm) for 4 h or inhibitors of c-Src (PP1, 1 μm), PI3K (wortmannin (W), 10 nm), or Akt (SH-5, 1 μm) for 1 h and then co-treated with 10 ng/ml IL-1β for 1 h. Whole cell lysates were prepared and analyzed by Western blotting using antibodies against Sirt-1 and β-actin. Sirt-1 control peptide (Co Pep.) was used as a control for antibody specificity.
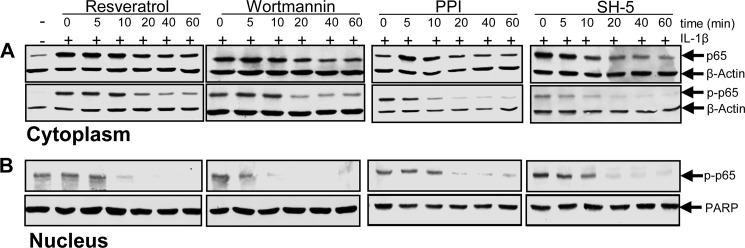
PI3K Signaling Is Involved in IL-1β-induced Activation of NF-κB Pathway in Human Tenocytes
To examine a possible regulatory interaction between the inhibition of the PI3K pathway and the suppression of NF-κB activation in response to resveratrol treatment, serum-starved human tenocytes were either left untreated or treated with 10 ng/ml IL-1β alone for 1 h or pre-stimulated with 5 μm resveratrol or with inhibitors of c-Src (PP1, 1 μm), PI3K (wortmannin, 10 nm), and Akt (SH-5, 1 μm) for 5, 10, 20, 40, or 60 min and then co-treated with 10 ng/ml IL-1β for 1 h. Nuclear and cytoplasmic cell fractions were prepared and analyzed by Western blotting. As shown in Fig. 10, IL-1β enhanced p65 expression and phosphorylation in the nuclear and cytoplasmic fraction. Pretreatment with resveratrol substantially inhibited the transactivating activity of NF-κB in a time-dependent manner (Fig. 10). Furthermore, the NF-κB activation and translocation to the nucleus was also inhibited by treatment with inhibitors of PI3K (wortmannin), c-Src (PP1), and Akt (SH-5), suggesting that the PI3K/Src/Akt signaling pathway is involved in the transactivation of NF-κB and translocation in the nucleus.
FIGURE 10.
Effects of resveratrol and c-Src, PI3K, Akt inhibitors on IL-1β-induced NF-κB p65 activation and nuclear translocation in human tenocytes. Serum-starved human tenocytes cultured in monolayer were either left untreated or treated with 10 ng/ml IL-1β alone for 1 h or prestimulated with 5 μm resveratrol or with inhibitors of c-Src (PP1, 1 μm), PI3K (wortmannin (W), 10 nm), and Akt (SH-5, 1 μm) for 5, 10, 20, 40, or 60 min and then co-treated with 10 ng/ml IL-1β for 1 h. Nuclear and cytoplasmic cell fractions were prepared and submitted to Western blot analysis using antibodies against p65, phosphospecific-p65, and β-actin or nuclear protein poly(ADP-ribose) polymerase (PARP) as a loading control.
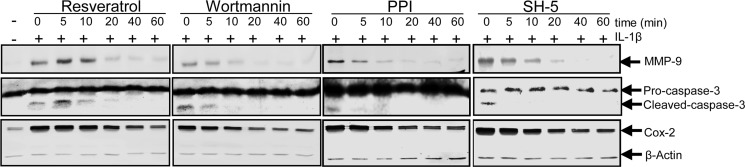
Suppression of IL-1β-induced Activation of NF-κB-regulated Proinflammatory and Apoptotic Gene Products by Resveratrol Might Involve c-Src/PI3K/AKT Signaling Pathway in Human Tenocytes
Furthermore, we wanted to examine whether the Src/PI3K/Akt pathway might be involved in the inhibitory effect of resveratrol on IL-1β-stimulated NF-κB-regulated gene products involved in inflammation and apoptosis (MMP-9, caspase-3, and Cox-2) in tenocytes. Therefore, serum-starved human tenocytes were either left untreated or treated with 10 ng/ml IL-1β alone for 1 h or pre-stimulated with 5 μm resveratrol or with inhibitors of c-Src (PP1, 1 μm), PI3K (wortmannin, 10 nm), and Akt (SH-5, 1 μm) for 5, 10, 20, 40, or 60 min and then co-treated with 10 ng/ml IL-1β for 1 h. Cytoplasmic cell protein fractions were prepared and analyzed by Western blotting. As shown in Fig. 11, IL-1β induced the expression of MMP-9 and Cox-2 and the cleavage of caspase-3. This was blocked by pretreatment with the inhibitors of PI3K (wortmannin), c-Src (PP1), and Akt (SH-5) in the same way as with resveratrol in a time-dependent manner, suggesting that there might be a link between PI3K/Src/Akt signaling pathway and the inhibitory effect of resveratrol on IL-1β-stimulated NF-κB-regulated gene products.
FIGURE 11.
Effects of resveratrol and c-Src, PI3K, and Akt inhibitors on IL-1β-induced activation of NF-κB-regulated proinflammatory and apoptotic gene products in human tenocytes. Serum-starved human tenocytes in monolayer culture were either left untreated or treated with 10 ng/ml IL-1β alone for 1 h or prestimulated with 5 μm resveratrol or with inhibitors of c-Src (PP1, 1 μm), PI3K (wortmannin, 10 nm), and Akt (SH-5, 1 μm) for 5, 10, 20, 40, or 60 min and then co-treated with 10 ng/ml IL-1β for 1 h. Cytoplasmic cell fractions were prepared, and expression of MMP-9 and Cox-2 as well as cleavage of caspase-3 was determined by Western blot analysis. Housekeeping protein β-actin was used as a loading control.
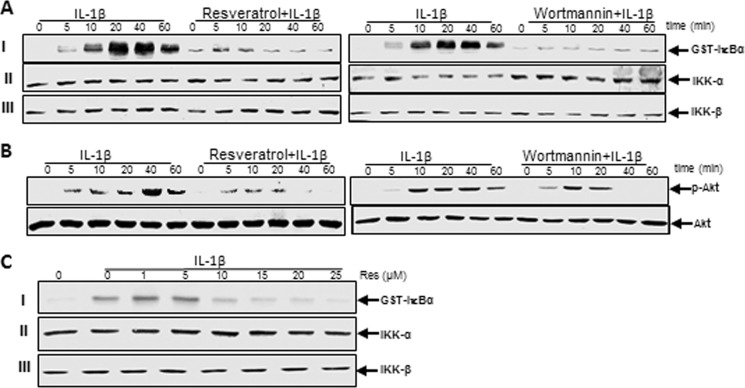
Suppression of IL-1β-induced Activation of IKK by Resveratrol Might Involve PI3K Signaling Pathway in Human Tenocytes
The activated NF-κB translocates to the nucleus after phosphorylation, ubiquitination, and proteolytic degradation of IκBα (28). To further ensure that PI3K signaling is required for IL-1β-induced activation of NF-κB, we evaluated the effect of resveratrol or PI3K inhibitor wortmannin on IL-1β-induced IKK activation. Tenocytes were either treated with IL-1β alone for 0, 5, 10, 20, 40, or 60 min or were pretreated with resveratrol (5 μm) or wortmannin (10 nm) for 1 h and then co-treated with IL-1β for the indicated times. The results from the immune complex kinase assay showed that IL-1β induced the activation of IKK in a time-dependent manner, whereas pretreatment of cells with resveratrol or PI3K inhibitor wortmannin followed by stimulation with IL-1β blocked the cytokine-induced effects on the activation of IKK to a similar extent (Fig. 12A, I ). This might suggest that resveratrol-mediated down-regulation of the IL-1β-induced NF-κB activation in tenocytes could be at least in part through inhibition of the PI3K signaling pathway. IL-1β, resveratrol, or wortmannin had no direct effect on the expression of IKK-α or IKK-β proteins (Fig. 12A, II and III).
FIGURE 12.
Effects of resveratrol and PI3K inhibitor wortmannin on IL-1β-induced activation of IKK and Akt in human tenocytes. Serum-starved human tenocytes in monolayer culture were either treated with IL-1β alone for 0, 5, 10, 20, 40, or 60 min or were pretreated with resveratrol (5 μm) or wortmannin (10 nm) for 1 h and then co-treated with IL-1β for 0, 5, 10, 20, 40, or 60 min. A, to determine the activation level of IKK, whole cell lysates were immunoprecipitated with an antibody against IKK and underwent immune complex kinase assay as described under “Experimental Procedures.” Extracts were then fractionated on SDS-PAGE and examined by Western blot analysis using anti-IKK-α, anti-IKK-β, and anti-phosphospecific-IκBα antibodies. B, cell lysates from the same cells were also examined for phosphorylation of Akt by immunoblotting with antibodies against Akt and phosphospecific-Akt. C, shown is the direct effect of resveratrol treatment on IL-1β-induced IκB kinase activation. Serum-starved human tenocytes were treated with 10 ng/ml IL-1β. The cell extracts were prepared and immunoprecipitated with anti-IKK-α antibodies. The immunocomplex kinase assay was performed in the absence or presence of resveratrol at the indicated concentrations. Data shown are representative of three independent experiments.
Suppression of IL-1β-induced Activation of Akt by Resveratrol Might Involve PI3K Signaling Pathway in Human Tenocytes
To gain a mechanistic insight into the mode of action of resveratrol in IL-1β-stimulated tenocytes, we studied the effects of resveratrol on PI3K activation using the Akt/IKK assay. Akt is an upstream protein kinase B to PI3K, and its activation is mainly induced by the phosphorylation of Ser or Thr residues (29). It has been reported that the PI3K signaling pathway is inhibited by other phytochemicals, like curcumin, in MCF7 cells (30). To examine whether Akt phosphorylation was necessary for the induction of NF-κB activation, we investigated the effect of resveratrol or PI3K inhibitor (wortmannin) on Akt activation. The activation of Akt was assayed using an antibody specific for the phosphorylated form of Akt. The cells were pretreated with resveratrol (5 μm) or PI3K inhibitor wortmannin (10 nm) for 1 h and then co-treated with IL-1β for 0, 5, 10, 20, 40, or 60 min or were treated with IL-1β alone for the indicated times. The whole cell extracts were prepared followed by Western blot analysis using anti-Akt or anti-phosphospecific Akt antibodies. Fig. 12B shows that IL-1β stimulated Akt phosphorylation in a time-dependent manner and that pretreatment with resveratrol or wortmannin significantly attenuated this also in a time-dependent manner. These results indicate that PI3K is involved in IL-1β-induced Akt phosphorylation in tenocytes and that resveratrol modulates inflammatory signaling at least in part through inhibition of Akt phosphorylation/activation. Next, to test further whether resveratrol inhibits IKK activity directly by binding IKK or indirectly by inhibition of its activation, human tenocytes were exposed with IL-1β or left untreated. The cell extracts from untreated cells and IL-1β-stimulated cells were incubated with anti-IKK-α antibody. After precipitation with protein A/G-Sephadex beads, the immunocomplexes were exposed with various concentrations of resveratrol. As shown in Fig. 12C, the immunocomplex kinase assay revealed that resveratrol directly suppressed the activity of IKK (Fig. 12C, I). Resveratrol or IL-1β had no direct effect on the expression of IKK-α or IKK-β (Fig. 12C, II and III).
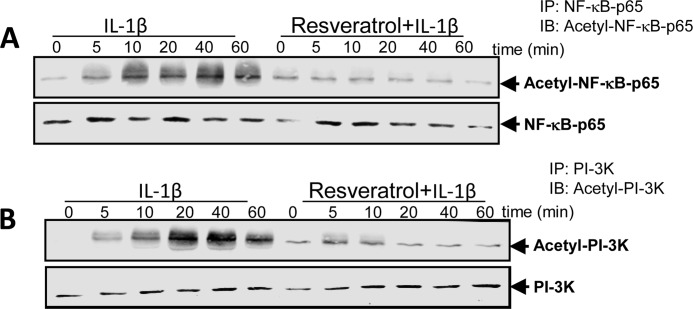
Resveratrol Suppresses IL-1β-induced Acetylation of NF-κB and PI3K Pathway
Acetylation of p65 is very important in IκBα-mediated activation of NF-κB transcriptional activity (20). Therefore, we examined if IL-1β also activates PI3K by acetylation and if resveratrol affects IL-1β-induced acetylation of NF-κB and PI3K. Human tenocytes were treated with 10 ng/ml IL-1β alone for 0, 5, 10, 20, 40, or 60 min or were pretreated with 5 μm resveratrol for 1 h and then exposed to IL-1β. Whole cell extracts were prepared and immunoprecipitated with anti-p65 or anti-PI3K antibody and then subjected to Western blot analysis using anti-acetyl-lysine antibody. As shown in Fig. 13, A and B, IL-1β induced acetylation of p65 and PI3K in a time-dependent manner. In contrast, pretreatment of cells with resveratrol followed by co-treatment with IL-1β suppressed IL-1β-induced acetylation of p65 and PI3K (Fig. 13, A and B), suggesting that resveratrol effectively inhibits activation of both NF-κB and PI3K signaling pathways by modulating acetylation.
FIGURE 13.
Effects of resveratrol on IL-1β-induced acetylation of NF-κB and PI3K in human tenocytes. Human tenocytes in monolayer culture were either treated with IL-1β alone for 0, 5, 10, 20, 40, or 60 min or were pretreated with resveratrol (5 μm) for 1 h and then co-treated with IL-1β for 0, 5, 10, 20, 40, or 60 min. After completion of the experiments, cell extracts were immunoprecipitated (IP) with anti-p65 antibody (A) or anti-PI3K antibody (B) and then analyzed by Western blotting (IB) using antibodies against acetyl-lysine, p65, and PI3K.
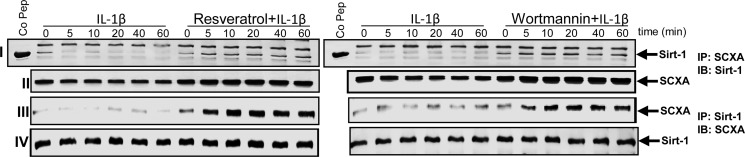
Interaction of Sirt-1 Protein with the Tenogenic Transcription Factor SCXA Is Enhanced by Resveratrol or Wortmannin in Human Tenocytes
It is known that resveratrol is a potent activator of histone-deacetylase Sirt-1 (14). To further examine if inhibition of NF-κB and PI3K acetylation by resveratrol as demonstrated in Fig. 13, A and B, was mediated via Sirt-1 activation and if the resveratrol stimulatory effects in the cells involved the tenogenic transcription factor SCXA through interaction with Sirt-1, we performed co-immunoprecipitation studies. Additionally, we assessed the involvement of PI3K signaling in this interaction. To this end, serum-starved human tenocytes in monolayer culture (1 × 105 cells/dish) were either treated with IL-1β (10 ng/ml) alone for 0, 5, 10, 20, 40, or 60 min or were pretreated with 5 μm resveratrol or 10 nm wortmannin for 1 h and then co-treated with 10 ng/ml IL-1β for 0, 5, 10, 20, 40, or 60 min. Whole cell lysates were immunoprecipitated with anti-Sirt-1 and then immunoblotted with anti-SCXA antibodies or vice versa. Interestingly, immunoprecipitates from resveratrol- or wortmannin-pretreated, but not from IL-1β-stimulated cultures, revealed co-immunoprecipitation of Sirt-1 protein with the tenogenic transcription factor SCXA (Fig. 14, I and III). The same immunoprecipitates were reblotted with SCXA or Sirt-1 antibody and showed a complex formation between Sirt-1 and SCXA (Fig. 14, II and IV). Taken together, these results indicate that resveratrol activates Sirt-1 and induces Sirt-SCXA complex formation, which activates the tenogenic differentiation pathway. Furthermore, a similar picture was found through PI3K inhibition by wortmannin, indicating that down-regulation of PI3K pathway might be part of the mechanisms of Sirt-1 activation by resveratrol.
FIGURE 14.
Effects of resveratrol and PI3K-inhibitor wortmannin on IL-1β-induced suppression of Sirt-1/SCXA interaction in human tenocytes. Serum-starved human tenocytes in monolayer culture were either treated with IL-1β alone for 0, 5, 10, 20, 40, or 60 min or pretreated with resveratrol (5 μm) or wortmannin (10 nm) for 1 h and then co-treated with IL-1β for 0, 5, 10, 20, 40, or 60 min. Whole cell lysates were immunoprecipitated with an antibody against Sirt-1 and then immunoblotted with an antibody against SCXA or vice versa. The same immunoprecipitates were then reblotted with SCXA or Sirt-1 antibody. Sirt-1 control peptide (Co Pep) was used as a control for antibody specificity.
DISCUSSION
In this study we examined the hypothesis that resveratrol suppresses NF-κB activation by involvement of the PI3K signaling pathway. In recent years several studies have reported about resveratrol and its multiple pharmacological activities and possible applications, including antioxidant, anti-inflammatory, antibacterial, and antiviral activities (31) as well as the use of resveratrol as a cancer chemopreventive and chemotherapeutic agent (32, 33). Furthermore, resveratrol has been implicated in the regulation of a variety of cellular responses such as cell cycle arrest, differentiation, and apoptosis in various cancer cell lines (34, 35). Resveratrol is a potent activator of sirtuin activity (14) and an inhibitor of NF-κB transcription factor (27, 32, 36).
Several lines of evidence have shown that resveratrol is a major inhibitor of proinflammatory agent IL-1β, thereby suppressing IL-1β-induced activation of NF-κB- and NF-κB-dependent gene products. Indeed, several studies have shown that IL-1β-induced cytotoxicity and caspase activation were also down-regulated by resveratrol (23, 35, 37, 38). Recently, we have also demonstrated that resveratrol prevented apoptosis in human tenocytes, and this was associated to changes in the expression of p53, Bax, and caspase-3 (51). However, the precise mechanisms regulating the inhibitory effect of resveratrol on IL-1β-induced NF-κB transcriptional activity have not yet been fully elucidated and understood.
Our laboratory has previously shown that another natural polyphenol (curcumin) can block IL-1β-induced expression of PI3K protein and phosphorylation of the PI3K upstream target Akt in the same tenocyte, suggesting that the effects of polyphenols like curcumin or resveratrol may be mediated by PI3K signaling pathway (13). Several studies have confirmed that PI3K might be involved in the IL-1β signaling pathway and is most likely related to NF-κB pathway through targeting IKK and IκB-α or phosphorylation of p65. This process is inhibited by the PI3K-specific inhibitor wortmannin. Furthermore, it was found that PI3K activates protein kinase B (Akt), one of the main downstream kinases in cells, and that Akt regulates downstream kinases involved in NF-κB activation (13, 29, 39).
This and previous studies from our laboratory have shown that resveratrol blocks the IL-1β-induced apoptosis in cultured human tenocytes by inhibiting of caspase-3 and cleavage of PARP (23, 40). In contrast, other studies have demonstrated that resveratrol promotes apoptotic cell death in several tumor cell lines through the induction of activated caspase-3, accumulation of p53 and p21, and cleavage of PARP (12, 38). There may be several reasons for our different observations. These include different cell types used and the knowledge that phytopharmaceuticals can act differently in different cell types. Varying between species, cell types, and stimulants, transcription factors such as NF-κB can have different impacts such as inhibition or stimulation of apoptosis, stimulating cell proliferation, inhibiting or stimulating inflammation and various cellular stress responses, and many others. The most important issues to consider are the effects of the concentration of resveratrol and the period of time cells are exposed to resveratrol.
Several studies have shown that different proinflammatory agents may activate NF-κB through specific mechanisms that consist of some overlapping and some non-overlapping steps (41). Therefore, we examined whether PI3K signaling pathway as an alternative mechanism for NF-κB activation by IL-1β is involved in IL-1β-induced inflammation and apoptosis in tenocytes. We found that resveratrol inhibited NF-κB activation and NF-κB-dependent gene products induced by proinflammatory agent IL-1β in a concentration- and dose-dependent fashion. Furthermore, we found that resveratrol not only suppressed NF-κB activation but also PI3K signaling pathway and the associated kinase Akt in the same concentration- and dose-dependent manner. These results indicate that activation of NF-κB by IL-1β might be linked to the PI3K/Akt cascade leading to inflammation and apoptosis, and resveratrol inhibits this. Indeed, several lines of evidence have shown that PI3K/Akt is involved in IL-1β-mediated signaling pathways (42, 43), and the PI3K pathway is associated to NF-κB-dependent signaling in different cell types (44, 45). Our results are consistent with these studies showing that PI3K/Akt is at least in part an important regulator for NF-κB activity in response to IL-1β-stimulation in tenocytes. As shown in this study, pretreatment with pharmacological inhibitors of c-Src (PP1), Akt (SH-5), or PI3K (wortmannin) or with resveratrol attenuated IL-1β-induced NF-κB activation and NF-κB-dependent gene products. Furthermore, the activation of NF-κB by IL-1β led to its translocation from the cytosol into the nucleus (Fig. 10), which was inhibited by pretreatment with PP1, SH-5, wortmannin, and resveratrol, suggesting that IL-1β-induced NF-κB activity involves, at least in part, the PI3K/Akt pathway. These results are consistent with earlier reports that phosphorylation of Akt may regulate NF-κB activation and translocation through targeting IKKs or phosphorylation of p65 (46) and stimulate histone acetyltransferase activity (35, 47). In this study IL-1β-induced Akt and p65 phosphorylation was inhibited by pretreatment with PP1, SH-5, wortmannin, and resveratrol in human tenocytes, and activation of IKK was also inhibited by wortmannin and resveratrol (Fig. 12A). These results point out that IL-1β-induced Akt and IKK activity might be linked, at least in part, to the PI3K/Akt pathway.
Our laboratory and others have demonstrated that inflammatory cytokines and RANKL stimulated p300 acetyltransferase, and this in turn activates NF-κB (35, 43). Previously, it was reported that phosphorylation and acetylation of NF-κB are required for its transcriptional activity, and acetylation of NF-κB by p300 acetyltransferase thereby plays an essential role in regulating NF-κB signaling (48). It was shown that acetylation of p65 at lysine 310 is regulated by prior phosphorylation of serines 276 and 536. The phosphorylated and acetylated RelA displays enhanced transcriptional activity (48). Resveratrol is a potent activator of Sirt-1 deacetylase activity (14). In this context our results indicate that resveratrol may suppress IL-1β-induced p65- and PI3K acetylation through inhibition of histone acetyltransferase activity. We demonstrated previously that resveratrol-stimulated Sirt-1 deacetylase promoted Sirt-1-p300 complex formation during osteogenesis, resulting in inactivation of p300 acetyltransferase and a reduction of the acetylation of NF-κB-p65 (35).
We also showed that the specific tenogenic transcription factor SCXA as a marker for tenogenic activity was activated by resveratrol. SCXA is the most specific marker of tenogenesis (49). Therefore, we examined the effect of resveratrol on the SCXA activity of tenocytes. In this study SCXA activation was observed in control cells and resveratrol-pretreated primary isolated tenocytes even after exposure to proinflammatory cytokine IL-1β. Resveratrol increased SCXA activity and stimulated the proliferation and differentiation of tenocytes. It is established that resveratrol is one of the most potent Sirt-1 activators, and many Sirt-1 substrates are transcription factors and key regulators known to participate in embryonic growth and in neoplasia (50). However, the relationship between Sirt-1 activity and tenogenesis is not yet fully elucidated and is still open to debate. In this study we were able to show, for the first time, that the Sirt-1 protein expressed in human tenocytes is stimulated by resveratrol and that it associates with SCXA in vitro as demonstrated by co-immunoprecipitation experiments.
Consistent with this observation, SCXA appears to be a direct substrate to Sirt-1 deacetylation and in this way Sirt-1 might contribute to the maintenance of tenocyte phenotype by direct regulation of important transcription factors such as SCXA. It is, therefore, plausible that SCXA, similar to a growing number of other transcription factors, is susceptible to modification by acetylation/deacetylation.
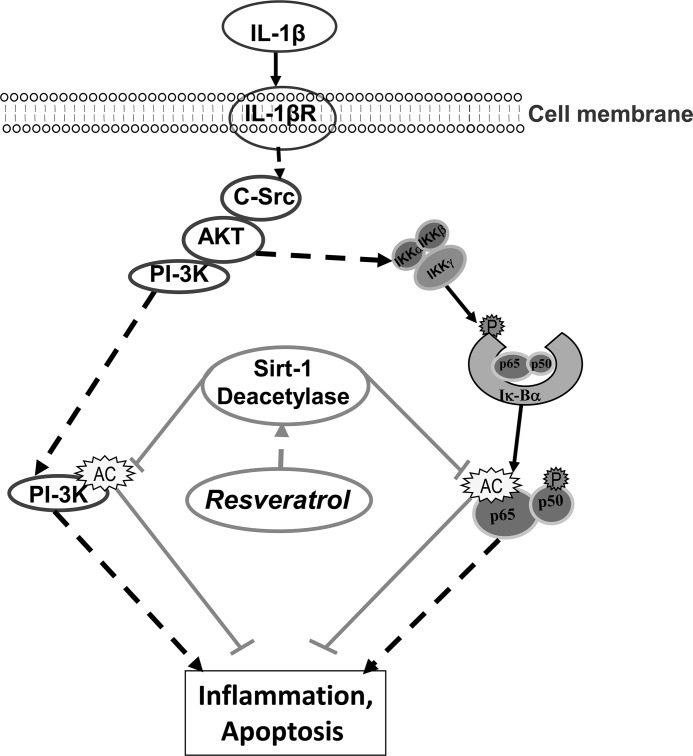
In conclusion, this study has provided important insights into the mechanisms through which IL-1β functions and modulates NF-κB and PI3K signaling as well as how their target genes regulate inflammation and apoptosis in a tendinitis model. We have further demonstrated that resveratrol can abrogate the inflammatory effects of proinflammatory cytokines, which are most likely mediated through the suppression of PI3K and NF-κB activation (Fig. 15). Further studies will be required to determine the full diversity of effects of resveratrol and its potential for the prevention and treatment of inflammatory diseases.
FIGURE 15.
Schematic diagram showing IL-1β-induced proinflammatory and apoptotic signaling pathways modulated by resveratrol.
Footnotes
- MMP
- matrix metalloproteinase
- IKK
- IκB kinase
- SCXA
- scleraxis
- MC
- mitochondrial changes
- Res
- resveratrol.
REFERENCES
- 1. Alvares O., Klebe R., Grant G., Cochran D. L. (1995) Growth factor effects on the expression of collagenase and TIMP-1 in periodontal ligament cells. J. Periodontol. 66, 552–558 [DOI] [PubMed] [Google Scholar]
- 2. Tewari D. S., Qian Y., Tewari M., Pieringer J., Thornton R. D., Taub R., Mochan E. O. (1994) Mechanistic features associated with induction of metalloproteinases in human gingival fibroblasts by interleukin-1. Arch. Oral. Biol. 39, 657–664 [DOI] [PubMed] [Google Scholar]
- 3. Baldwin A. S., Jr. (1996) The NF-κB and IκB proteins. New discoveries and insights. Annu. Rev. Immunol. 14, 649–683 [DOI] [PubMed] [Google Scholar]
- 4. Kumar A., Takada Y., Boriek A. M., Aggarwal B. B. (2004) Nuclear factor-κB. Its role in health and disease. J. Mol. Med. 82, 434–448 [DOI] [PubMed] [Google Scholar]
- 5. Malinin N. L., Boldin M. P., Kovalenko A. V., Wallach D. (1997) MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature 385, 540–544 [DOI] [PubMed] [Google Scholar]
- 6. Reddy S. A., Huang J. H., Liao W. S. (1997) Phosphatidylinositol 3-kinase in interleukin 1 signaling. Physical interaction with the interleukin 1 receptor and requirement in NFκB and AP-1 activation. J. Biol. Chem. 272, 29167–29173 [DOI] [PubMed] [Google Scholar]
- 7. Sun L., Wang S., Hu C., Zhang X. (2011) Down-regulation of PKHD1 induces cell apoptosis through PI3K and NF-κB pathways. Exp. Cell Res. 317, 932–940 [DOI] [PubMed] [Google Scholar]
- 8. Bernard-Beaubois K., Hecquet C., Houcine O., Hayem G., Adolphe M. (1997) Culture and characterization of juvenile rabbit tenocytes. Cell Biol. Toxicol. 13, 103–113 [DOI] [PubMed] [Google Scholar]
- 9. Riley G. P., Cox M., Harrall R. L., Clements S., Hazleman B. L. (2001) Inhibition of tendon cell proliferation and matrix glycosaminoglycan synthesis by non-steroidal anti-inflammatory drugs in vitro. J. Hand. Surg. Br. 26, 224–228 [DOI] [PubMed] [Google Scholar]
- 10. Frémont L. (2000) Biological effects of resveratrol. Life Sci. 66, 663–673 [DOI] [PubMed] [Google Scholar]
- 11. Pozo-Guisado E., Merino J. M., Mulero-Navarro S., Lorenzo-Benayas M. J., Centeno F., Alvarez-Barrientos A., Fernandez-Salguero P. M. (2005) Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with down-regulation of Bcl-2 and NF-κB. Int. J. Cancer 115, 74–84 [DOI] [PubMed] [Google Scholar]
- 12. Gupta S. C., Kannappan R., Reuter S., Kim J. H., Aggarwal B. B. (2011) Chemosensitization of tumors by resveratrol. Ann. N.Y. Acad. Sci. 1215, 150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buhrmann C., Mobasheri A., Busch F., Aldinger C., Stahlmann R., Montaseri A., Shakibaei M. (2011) Curcumin modulates nuclear factor κB (NF-κB)-mediated inflammation in human tenocytes in vitro. Role of the phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 286, 28556–28566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howitz K. T., Bitterman K. J., Cohen H. Y., Lamming D. W., Lavu S., Wood J. G., Zipkin R. E., Chung P., Kisielewski A., Zhang L. L., Scherer B., Sinclair D. A. (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 [DOI] [PubMed] [Google Scholar]
- 15. Sauve A. A., Wolberger C., Schramm V. L., Boeke J. D. (2006) The biochemistry of sirtuins. Annu. Rev. Biochem. 75, 435–465 [DOI] [PubMed] [Google Scholar]
- 16. Imai S., Armstrong C. M., Kaeberlein M., Guarente L. (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 [DOI] [PubMed] [Google Scholar]
- 17. Lin S. J., Defossez P. A., Guarente L. (2000) Requirement of NAD and SIR2 for lifespan extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128 [DOI] [PubMed] [Google Scholar]
- 18. Vaziri H., Dessain S. K., Ng Eaton E., Imai S. I., Frye R. A., Pandita T. K., Guarente L., Weinberg R. A. (2001) hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 [DOI] [PubMed] [Google Scholar]
- 19. Yeung F., Hoberg J. E., Ramsey C. S., Keller M. D., Jones D. R., Frye R. A., Mayo M. W. (2004) Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kiernan R., Brès V., Ng R. W., Coudart M. P., El Messaoudi S., Sardet C., Jin D. Y., Emiliani S., Benkirane M. (2003) Post-activation turn-off of NF-κ B-dependent transcription is regulated by acetylation of p65. J. Biol. Chem. 278, 2758–2766 [DOI] [PubMed] [Google Scholar]
- 21. Schulze-Tanzil G., Mobasheri A., Clegg P. D., Sendzik J., John T., Shakibaei M. (2004) Cultivation of human tenocytes in high density culture. Histochem. Cell Biol. 122, 219–228 [DOI] [PubMed] [Google Scholar]
- 22. Shakibaei M., Abou-Rebyeh H., Merker H. J. (1993) Integrins in ageing cartilage tissue in vitro. Histol. Histopathol. 8, 715–723 [PubMed] [Google Scholar]
- 23. Shakibaei M., Csaki C., Nebrich S., Mobasheri A. (2008) Resveratrol suppresses interleukin-1β-induced inflammatory signaling and apoptosis in human articular chondrocytes. Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. 76, 1426–1439 [DOI] [PubMed] [Google Scholar]
- 24. Shakibaei M., De Souza P., Merker H. J. (1997) Integrin expression and collagen type II implicated in maintenance of chondrocyte shape in monolayer culture. An immunomorphological study. Cell Biol. Int. 21, 115–125 [DOI] [PubMed] [Google Scholar]
- 25. Buhrmann C., Mobasheri A., Matis U., Shakibaei M. (2010) Curcumin-mediated suppression of nuclear factor κB promotes chondrogenic differentiation of mesenchymal stem cells in a high density co-culture microenvironment. Arthritis Res. Ther. 12, R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schneider P. R., Buhrmann C., Mobasheri A., Matis U., Shakibaei M. (2011) Three-dimensional high density co-culture with primary tenocytes induces tenogenic differentiation in mesenchymal stem cells. J. Orthop. Res. 29, 1351–1360 [DOI] [PubMed] [Google Scholar]
- 27. Manna S. K., Mukhopadhyay A., Aggarwal B. B. (2000) Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-κB, activator protein-1, and apoptosis. Potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 164, 6509–6519 [DOI] [PubMed] [Google Scholar]
- 28. Aggarwal B. B. (2004) Nuclear factor κB. The enemy within. Cancer Cell 6, 203–208 [DOI] [PubMed] [Google Scholar]
- 29. Ozes O. N., Mayo L. D., Gustin J. A., Pfeffer S. R., Pfeffer L. M., Donner D. B. (1999) NF-κB activation by tumor necrosis factor requires the Akt serine-threonine kinase. Nature 401, 82–85 [DOI] [PubMed] [Google Scholar]
- 30. Squires M. S., Hudson E. A., Howells L., Sale S., Houghton C. E., Jones J. L., Fox L. H., Dickens M., Prigent S. A., Manson M. M. (2003) Relevance of mitogen activated protein kinase (MAPK) and phosphotidylinositol-3-kinase/protein kinase B (PI3K/PKB) pathways to induction of apoptosis by curcumin in breast cells. Biochem. Pharmacol. 65, 361–376 [DOI] [PubMed] [Google Scholar]
- 31. Das S., Das D. K. (2007) Anti-inflammatory responses of resveratrol. Inflamm. Allergy Drug Targets 6, 168–173 [DOI] [PubMed] [Google Scholar]
- 32. Jang M., Cai L., Udeani G. O., Slowing K. V., Thomas C. F., Beecher C. W., Fong H. H., Farnsworth N. R., Kinghorn A. D., Mehta R. G., Moon R. C., Pezzuto J. M. (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275, 218–220 [DOI] [PubMed] [Google Scholar]
- 33. Gupta S. C., Kim J. H., Prasad S., Aggarwal B. B. (2010) Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 29, 405–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Joe A. K., Liu H., Suzui M., Vural M. E., Xiao D., Weinstein I. B. (2002) Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin. Cancer Res. 8, 893–903 [PubMed] [Google Scholar]
- 35. Shakibaei M., Buhrmann C., Mobasheri A. (2011) Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-κB ligand (RANKL) activation of NF-κB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 286, 11492–11505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gupta S. C., Sundaram C., Reuter S., Aggarwal B. B. (2010) Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 1799, 775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Csaki C., Mobasheri A., Shakibaei M. (2009) Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes. Inhibition of IL-1β-induced NF-κB-mediated inflammation and apoptosis. Arthritis Res. Ther. 11, R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Estrov Z., Shishodia S., Faderl S., Harris D., Van Q., Kantarjian H. M., Talpaz M., Aggarwal B. B. (2003) Resveratrol blocks interleukin-1β-induced activation of the nuclear transcription factor NF-κB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood 102, 987–995 [DOI] [PubMed] [Google Scholar]
- 39. Romashkova J. A., Makarov S. S. (1999) NF-κB is a target of AKT in anti-apoptotic PDGF signaling. Nature 401, 86–90 [DOI] [PubMed] [Google Scholar]
- 40. Csaki C., Keshishzadeh N., Fischer K., Shakibaei M. (2008) Regulation of inflammation signaling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. 75, 677–687 [DOI] [PubMed] [Google Scholar]
- 41. Imbert V., Rupec R. A., Livolsi A., Pahl H. L., Traenckner E. B., Mueller-Dieckmann C., Farahifar D., Rossi B., Auberger P., Baeuerle P. A., Peyron J. F. (1996) Tyrosine phosphorylation of IκBα activates NF-κB without proteolytic degradation of IκBα. Cell 86, 787–798 [DOI] [PubMed] [Google Scholar]
- 42. Cheng C. Y., Kuo C. T., Lin C. C., Hsieh H. L., Yang C. M. (2010) IL-1β induces expression of matrix metalloproteinase-9 and cell migration via a c-Src-dependent, growth factor receptor transactivation in A549 cells. Br. J. Pharmacol. 160, 1595–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee C. W., Lin C. C., Lin W. N., Liang K. C., Luo S. F., Wu C. B., Wang S. W., Yang C. M. (2007) TNF-α induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-κB/p300 binding in human tracheal smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 292, L799–L812 [DOI] [PubMed] [Google Scholar]
- 44. Mattioli B., Giordani L., Quaranta M. G., Viora M. (2009) Leptin exerts an anti-apoptotic effect on human dendritic cells via the PI3K-Akt signaling pathway. FEBS Lett. 583, 1102–1106 [DOI] [PubMed] [Google Scholar]
- 45. Tseng W. P., Su C. M., Tang C. H. (2010) FAK activation is required for TNF-α-induced IL-6 production in myoblasts. J. Cell Physiol. 223, 389–396 [DOI] [PubMed] [Google Scholar]
- 46. Reddy S. A., Huang J. H., Liao W. S. (2000) Phosphatidylinositol 3-kinase as a mediator of TNF-induced NF-κB activation. J. Immunol. 164, 1355–1363 [DOI] [PubMed] [Google Scholar]
- 47. Darieva Z., Lasunskaia E. B., Campos M. N., Kipnis T. L., Da Silva W. D. (2004) Activation of phosphatidylinositol 3-kinase and c-Jun-N-terminal kinase cascades enhances NF-κB-dependent gene transcription in BCG-stimulated macrophages through promotion of p65/p300 binding. J. Leukoc. Biol. 75, 689–697 [DOI] [PubMed] [Google Scholar]
- 48. Chen L. f., Fischle W., Verdin E., Greene W. C. (2001) Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293, 1653–1657 [DOI] [PubMed] [Google Scholar]
- 49. Schweitzer R., Chyung J. H., Murtaugh L. C., Brent A. E., Rosen V., Olson E. N., Lassar A., Tabin C. J. (2001) Analysis of the tendon cell fate using scleraxis, a specific marker for tendons and ligaments. Development 128, 3855–3866 [DOI] [PubMed] [Google Scholar]
- 50. Shishodia S., Aggarwal B. B. (2002) Nuclear factor-κB activation: a question of life or death. J. Biochem. Mol. Biol. 35, 28–40 [DOI] [PubMed] [Google Scholar]
- 51. Busch F., Mobasheri A., Shayan P., Stahlmann R., Shakibaei M. (2012) Sirt-1 is required for the inhibition of apoptosis and inflammatory responses in human tenocytes. J. Biol. Chem. 287, 25770–25781 [DOI] [PMC free article] [PubMed] [Google Scholar]