Background: Mammalian sperm lose sialic acids during capacitation through unknown mechanisms.
Results: Sialidases Neu1 and Neu3 are present on sperm. Their activity is required for capacitation and zona pellucida binding.
Conclusion: Sperm sialidases modulate sperm surface sialic acids en route to fertilization.
Significance: Understanding the mechanism of deciduous sialylation in sperm provides novel insights into sperm function and glycan-mediated fertility.
Keywords: Fertilization, Glycobiology, Sialic Acid, Signaling, Spermatozoa, Sialome, Capacitation, Infertility, Neuraminidase 1, Neuraminidase 3
Abstract
Sialic acids (Sias) mediate many biological functions, including molecular recognition during development, immune response, and fertilization. A Sia-rich glycocalyx coats the surface of sperm, allowing them to survive as allogeneic cells in the female reproductive tract despite female immunity. During capacitation, sperm lose a fraction of their Sias. We quantified shed Sia monosaccharides released from capacitated sperm and measured sperm sialidase activity. We report the presence of two sialidases (neuraminidases Neu1 and Neu3) on mammalian sperm. These are themselves shed from sperm during capacitation. Inhibiting sialidase activity interferes with sperm binding to the zona pellucida of the ovum. A survey of human sperm samples for the presence of sialidases NEU1 and NEU3 identified a lack of one or both sialidases in sperm of some male idiopathic infertility cases. The results contribute new insights into the dynamic remodeling of the sperm glycocalyx prior to fertilization.
Introduction
All cell surfaces in nature are covered by a glycocalyx, a dense “sugar coat” formed by a complex array of glycans, the oligo- and polysaccharides attached to glycoproteins and glycolipids (1). Sperm are coated with a thick (∼70 nm) glycocalyx rich in sialic acids (Sias)2 (2). Sias are nine-carbon backbone amino sugars found abundantly on mammalian cell surfaces and secretions (3, 4). These acidic sugars serve a wide variety of biological roles, ranging from structural roles based on their negative charge to involvement in molecular recognition during development, immune regulation, and carcinogenesis (1). Although there are >50 different forms of Sias in nature, the two most common Sias found in mammals are N-acetylneuraminic acid (Neu5Ac) and its derivative N-glycolylneuraminic acid (Neu5Gc) (1). Sias are found as the outermost monosaccharide, where they cap the majority of glycans at the sperm cell surface. The sperm sialome refers to the totality of Sias present on sperm. The sialome of each mature sperm cell consists of tens of millions of Sia molecules and is acquired during spermatogenesis and epididymal maturation and by incorporation of seminal fluid components into the sperm membrane during ejaculation (5, 6).
As foreign cells, sperm face important challenges once deposited inside the female reproductive tract (7). Humoral and cellular female immune factors lead to the rapid demise of most sperm after insemination when the leukocytic reaction in the female unleashes countless leukocytes in the uterus (8, 9). Antibodies and complement are also present in female reproductive tract secretions (10). Mammalian leukocytes express lineage-specific combinations of Sia-binding immune regulatory lectins (Siglecs (sialic acid-binding immunoglobulin superfamily lectins)). Most of these Siglecs exert inhibitory effects upon binding Sias, and properly engaging these Siglecs is likely to be important for sperm survival (11). Insufficient decoration with Sias will thus put sperm at increased risk of female immune discrimination. The complement system also relies in part on appropriate sialylation of cells to detect “self” and to inhibit activation based on factor H binding (12). Sia content of mammalian sperm has been shown to correlate positively with protection from phagocytosis but negatively with the capacity of sperm to bind to the zona pellucida of the ovum (13–15). In primates, sperm are also abundantly coated with sialylated β-defensin 126 in the distal epididymis, and this coating helps prevent the recognition of potent sperm antigens by female antibodies (16). It is also established that the mammalian process of sperm capacitation is accompanied by a reduction in sialylation (17, 18). Existing studies of Sia modulation in sperm have relied mostly on indirect measures of loss of sialoglycoconjugates, and mechanisms remain unknown. Sialidase activity has been reported in the sperm acrosome and female genital tract (19, 20). The aim of this study was to gain insight into the mechanism of sperm sialome loss during capacitation. We found evidence for shed Sia monosaccharides, quantified their release from sperm incubated under capacitating conditions, and measured sperm sialidase activity. We then probed mouse and human sperm for the presence of sialidases and used a sialidase inhibitor to test the role of sperm sialidases in capacitation and zona pellucida binding.
EXPERIMENTAL PROCEDURES
Ethics Statement
All samples used for this study were anonymized. Samples were from volunteer participants who gave written informed consent. The sample collection was approved by University of California San Diego Human Subject Protection Institutional Review Board 040613 or by University of Cincinnati Human Subject Protection Institutional Review Board 10-36.
Mouse Sperm
C57BL/6 mice were kept under University of California San Diego Institutional Animal Care and Use Committee Protocol S01227. Sperm were harvested from the cauda epididymides of males killed at 12–20 weeks of age. The cauda epididymis was squashed and kept on a shaker at room temperature for 10 min. Sperm were washed and subjected to a swim-up procedure in Biggers-Whitten-Whittingham (BWW) buffer following published procedures (21).
Human Sperm
Human samples were from volunteers <45 years of age after 3 days of abstinence under University of California San Diego Human Subject Protection Institutional Review Board 040613, from normal sperm donors at the Oregon Health & Science University, or from patients at Christ Hospital under University of Cincinnati Human Subject Protection Institutional Review Board 10-36. Sperm were washed twice with BWW buffer and subjected to a swim-up procedure in 100 μl of BWW buffer at 37 °C and 5% CO2 for 20 min following published procedures (21). Sperm parameters were determined by computer-assisted sperm analysis and followed the World Health Organization (2009) criteria for concentration, motility, and morphology.
In Vitro Capacitation of Mouse and Human Sperm
Mouse sperm were incubated for 2 h in 5 mg/ml BSA (Sigma) and 20 mm NaHCO3 in BWW buffer, and human sperm were incubated for 4 h in 33 mg/ml human serum albumin (Sigma) and 20 mm NaHCO3 in BWW buffer at 37 °C and 5% CO2 following published methods (22, 23).
Western Blotting
Mouse and human sperm membrane proteins were extracted following a published protocol (24). For detection of Neu1, Neu3, ERK, phospho-ERK1/2, and PY20, the following antibodies were used: anti-Neu1 and anti-Neu3 (1:5000; Santa Cruz Biotechnology); anti-mouse ERK, anti-phospho-ERK1/2, and anti-PY20 (1:10,000; Cell Signaling Technology); and HRP-conjugated goat anti-mouse IgG and HRP-conjugated goat anti-rabbit IgG (1:10,000; Invitrogen). For detection of Neu1 dot blots, proteins were transferred using the Minifold I dot-blot system.
Detection of Lectin and Antibody Binding by Flow Cytometry
For lectin binding, sperm were fixed with 3% freshly thawed paraformaldehyde for 20 min at room temperature and blocked with 1% BSA in TBS/Tween. Biotinylated Sambucus nigra lectin, Maackia amurensis lectin II, and Erythrina crista-galli lectin (1:1000; Vector Labs) were used at room temperature for 30 min and detected with Alexa Fluor 555/488-conjugated streptavidin (1:1000; Invitrogen) at room temperature for 30 min. For detection of peanut agglutinin (PNA) on live sperm, propidium iodide (1:1000; Roche Applied Science) and FITC-PNA (1:1000; Vector Labs) were used at room temperature for 10 min.
For detection Neu1 and Neu3, sperm were fixed with methanol for 20 min at −20 °C and then blocked with 1% BSA in PBS at room temperature for 1 h. For Neu1, sperm were permeabilized with 0.2% Triton X-100 for 20 min at room temperature. Anti-Neu1 and anti-Neu3 antibodies (1:100 were used at 4 °C for 1 h. Goat anti-mouse/rabbit antibodies conjugated with Alexa Fluor 488 (1:200; Invitrogen) were used at 4 °C for 1 h. For detection of PY20, sperm were fixed with 3% paraformaldehyde at room temperature for 20 min. Anti-PY20 antibody conjugated with Alexa Fluor 647 and mouse IgG2b (1:1000; BioLegend) were used at 4 °C for 1 h. Stained sperm were analyzed by flow cytometry on a BD FACSCalibur. A smear of stained sperm was also made for microscopy and microphotography on an Applied Precision DeltaVision inverted deconvolution system.
Analysis of Levels of Bound and Free Sias
Sperm membrane-bound Sias were prepared by exposing washed sperm cells to double-distilled H2O for 15 min at 4 °C, followed by centrifugation at 10,000 × g for 15 min. Sia contents of sperm were analyzed by HPLC of Sia extracts of sperm membrane obtained after 2 m acetic acid hydrolysis at 80 °C for 3 h. Released Sias were filtered through Microcon 10 columns (Millipore), treated with mild base, and derivatized in 1,2-diamino-4,5-methylenedioxybenzene (Sigma) reagent for 2.5 h at 50 °C in the dark. HPLC was performed over a Varian C18 reverse phase column under isocratic conditions in 83% water, 7% methanol, and 8% acetonitrile at a flow rate of 0.9 ml/min over 50 min using a Hitachi HPLC system (25, 26). Sia standards were from bovine submaxillary mucin as well as commercially available Neu5Ac (Nacalai) and Neu5Gc (Inalco). Supernatants were separately analyzed by 1,2-diamino-4,5-methylenedioxybenzene derivatization and HPLC. Sia monosaccharides released into the medium were determined by 1,2-diamino-4,5-methylenedioxybenzene derivatization with trifluoroacetic acid (27) and then analyzed by HPLC.
Determination of Sialidase Activity
Assays were carried out in 100 μl of BWW buffer containing 25 μl of 0.2 mm sodium acetate (pH 5.5), 2.5 μl of 2 mm 4-methylumbelliferyl-α-d-N-acetylneuraminic acid (Sigma), and 2.5 μl of 10 mg/ml BSA (Sigma) and incubated at 37 °C and 5% CO2 for 2 h, after which the supernatant was collected. A reference standard was generated by hydrolyzing 1 mm 4-methylumbelliferyl-α-d-N-acetylneuraminic acid in 10 mm HCl at 80 °C for 10 min. The reaction was stopped by addition of 200 μl of 20 mm sodium bicarbonate (pH 10.6) and read using a SpectraMax M3 microplate reader at an excitation of 365 nm and an emission of 450 nm (28). At the same time, 2-deoxy-2,3-didehydro-d-N-acetylneuraminic acid (DANA) and free Neu5Ac were used at 1 mm to inhibit sialidase activity in each experimental group. Sialidase activity was assayed with and without protease inhibitor (protease inhibitor mixture set III, EDTA-free (1:100), Calbiochem) in the capacitation medium and incubated at 37 °C for 2 h.
In Vivo Capacitated Status of Mouse Sperm
A C57BL/6 male mouse was caged with two estrus females at 8:30 a.m. Females were inspected for the presence of a vaginal plug every 30 min thereafter. Mated females were killed 1.5 h post-mating, and the uterus was excised, clamped shut with forceps, and flushed with PBS (29).
Sperm-Egg Binding
Three-week-old FVB/N female mice were superovulated by sequential administration of pregnant mare serum gonadotropin and human chorionic gonadotropin (Sigma) (30). Oocytes were collected by flushing the oviduct and stripped of the cumulus cell by treatment with 1 mg/ml bovine hyaluronidase (Irvine Scientific) for 5 min. Fresh cauda epididymal sperm were incubated in the capacitation medium with or without DANA and added to the oocytes for co-incubation at 37 °C for 30 min. The eggs were individually aspirated into a new drop of medium, placed on glass, kept on ice for 5 min, stained with DAPI (1:50,000; Sigma) at room temperature for 2 min, and analyzed under a Zeiss Axiovert 200 microscope.
Statistical Methods
Data sets were tested for normal distribution using a Kolmogorov-Smirnoff test and analyzed for statistical significance by one-way analysis of variance using Prism software. All values shown are means ± S.E.
RESULTS
Release of Sia Monosaccharides during Sperm Capacitation
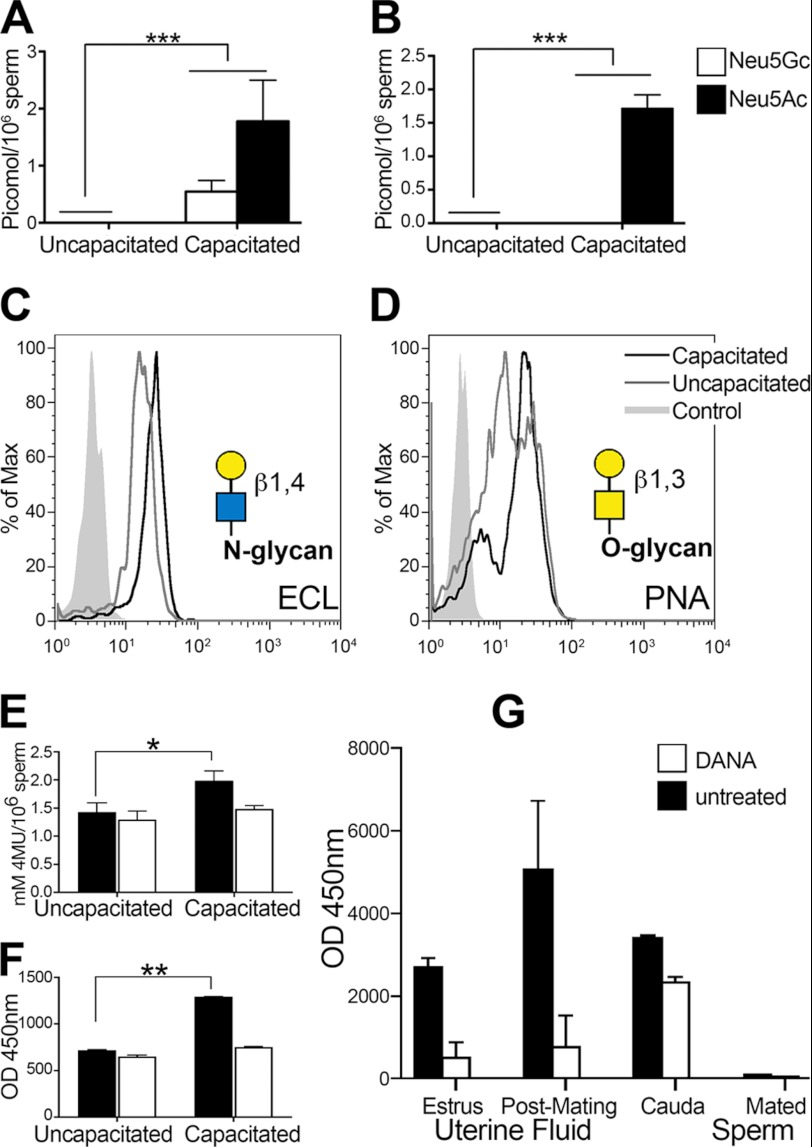
We detected free Sia monosaccharides in the supernatant of the capacitation medium (Fig. 1, A and B) by HPLC. Mice sperm released both Neu5Ac and Neu5Gc, whereas human sperm released only NeuAc. The presence of free Sias in the supernatant corresponded with a decrease in Sias on sperm after in vitro incubation under capacitating conditions. Furthermore, sialoglycoconjugates were also released from the sperm surface (supplemental Fig. 1, A and B). These experiments show that the sperm lost ∼20% of the sperm membrane Sias during capacitation and that ∼30% of the released Sias appeared to have been released in their monosaccharide form. Correspondingly, after incubation under capacitating conditions, the number of exposed underlying galactose residues increased (Fig. 1, C and D), as revealed by the increased binding of the galactose-specific E. crista-galli lectin and PNA, confirming the loss of Sias from both N- and O-linked glycans. E. crista-galli lectin is specific for galactose linked β1–4 to N-acetylglucosamine found mostly on N-glycans, whereas PNA is specific for galactose in β1–3-glycosidic linkage to N-acetylgalactosamine mostly found on O-glycans. The major cell surface glycosylphosphatidylinositol-anchored sialoglycopeptide is CD52, which sperm gain during epididymal maturation (31). We incubated epididymal mouse sperm with seminal vesicle fluid to allow acquisition of CD52. We measured the change in CD52 in sperm incubated under capacitating conditions using an antibody specific for the peptide of CD52 (supplemental Fig. 4A). We detected a minor loss, indicating that this key glycoprotein remains mostly on the sperm surface and thus must be one of the targets of the sperm sialidases.
FIGURE 1.
Release of Sia monosaccharides during sperm capacitation. A and B, released Sia (monosaccharides) from in vitro capacitated sperm. A, Neu5Gc and Neu5Ac from mouse sperm. B, Neu5Ac from human sperm. C and D, increase in exposed galactose as measured by lectins. C, E. crista-galli lectin (ECL; representative of five experiments). D, PNA lectin (representative of five experiments). Yellow circles, galactose; blue square, N-acetylglucosamine; yellow square, N-acetylgalactosamine. E and F, sialidase activity during sperm capacitation. E, sialidase activity of uncapacitated and in vitro capacitated mouse sperm with and without addition of the sialidase inhibitor DANA (representative of eight experiments). 4MU, 4-methylumbelliferyl. F, sialidase activity of uncapacitated and in vitro capacitated human sperm with and without addition of the sialidase inhibitor DANA (representative of eight experiments). G, sialidase activity in female mouse uterine fluid collected during estrous and 1.5 h after mating, capacitated mouse cauda epididymal sperm, and mouse sperm retrieved from the uterus 1.5 h after mating (representative of four experiments). *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001 versus the control.
Sialidase Activity during in Vitro and in Vivo Capacitation
The release of Sia monosaccharides during in vitro capacitation of sperm, in the absence of any female enzymatic factors, indicates the existence of a sperm cell-autonomous enzymatic mechanism for desialylation. To detect sialidase activity on sperm during capacitation, we used 4-methylumbelliferylsialic acid substrate, which generates a fluorescent product when cleaved by any sialidase. Sialidase activity increased under in vitro capacitating conditions in mouse and human sperm and could be specifically inhibited by addition of the sialidase inhibitor DANA (Fig. 1, E and F). Furthermore, analysis of sialidase activity in vivo also revealed a marked increase in uterine fluid but not in sperm retrieved from the uterus at that time (Fig. 1G).
Sialidases Neu1 and Neu3 Are Involved in Capacitation
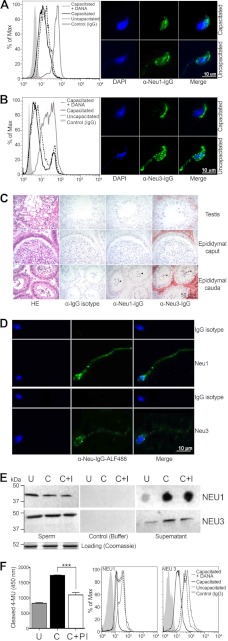
There are four known vertebrate sialidases/neuraminidases (32, 33). Two (Neu1 and Neu3) have been reported at the cell membrane. We stained mouse sperm with antibodies specific for Neu1, a lysosomal neuraminidase known to be active at the cell membrane, where it can associate with cathepsin A and β-galactosidase (34), and for Neu3, the neuraminidase known to act specifically on gangliosides (35). Flow cytometry revealed a reduction in the levels of Neu1 and Neu3 after in vitro capacitation. Both flow cytometry and fluorescence microscopy showed that anti-Neu1 and anti-Neu3 antibodies bound to uncapacitated mouse epididymal sperm in the head region and, to a lesser degree, after in vitro capacitation (Fig. 2, A and B). Neu1 and Neu3 were detected on the surface of mouse sperm in the cauda epididymis and on ejaculated human sperm (Fig. 2D). In contrast, neither sialidase was detected on mouse sperm in the testes or caput of the epididymis (Fig. 2C), suggesting a delayed expression of Neu1/3 transcripts. Both sialidases appeared to be released during in vitro incubation under capacitating conditions as measured by dot blotting as well by Western blotting of sperm membranes (Fig. 2E). Protease inhibition prevented much of this shedding and also reduced sialidase activity (Fig. 3D), indicating that shedding of sialidases depends at least in part on protease activity. The loss of α2–3- and α2–6-Sias, as well as exposure of the underlying β1–4-Gal, was only partly inhibited by treatment with DANA (supplemental Fig. 3, A–C). We also found Neu1 in the oviductal epithelium and Neu3 in the uterine luminal/glandular and oviductal epithelia (supplemental Fig. 2). Surprisingly, inhibition of sperm sialidases by DANA also resulted in increased shedding of the enzymes from the sperm (Fig. 2, C and D), indicating that merely engaging the binding pocket of the sialidases triggers their shedding.
FIGURE 2.
Neu1 and Neu3 on sperm and change during capacitation. A, fluorescent staining of Neu1 on mouse sperm head before and after capacitation and counterstained with DAPI and an unspecific IgG control. The change was quantified using FACS and could be partially inhibited by DANA. B, fluorescent staining of Neu3 on mouse sperm head before and after capacitation and counterstained with DAPI and an unspecific IgG control. The change was quantified using FACS and could be partially inhibited by DANA. A and B are representative of 12 experiments each. C, Neu1/3 on mouse sperm in the testes and the proximal and distal epididymis. Arrowheads show positive staining only in mature sperm of the cauda (distal epididymis). HE, hematoxylin and eosin. D, NEU1/3 on human ejaculated sperm. Expression was highest in the hind part of the sperm head. The counterstain is DAPI (blue). ALF488, Alexa Fluor 488. E, release of Neu1 and Neu3 from sperm into the supernatant and the effect of the sialidase inhibitor DANA. U, uncapacitated; C, capacitated; C+I, capacitated with the inhibitor DANA. F, sialidase activity in and release from sperm are both affected by protein inhibitor (PI). ***, p ≤ 0.001 versus the control.
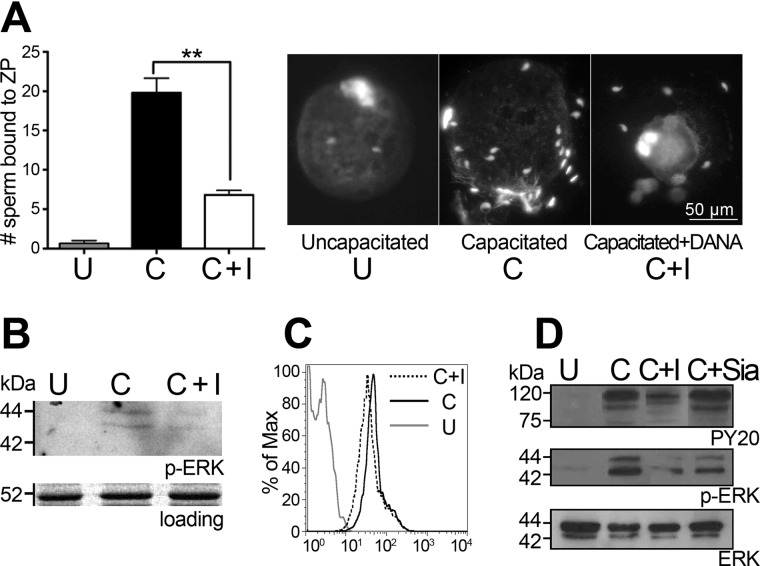
FIGURE 3.
Function of neuraminidase during capacitation. A, zona pellucida (ZP) binding by uncapacitated sperm (U), capacitated sperm (C), and sperm capacitated in the presence of the sialidase inhibitor DANA (C+I). B, Western blot for phospho-ERK of mouse sperm. C, FACS histogram of mouse sperm stained for PY20. D, Western blot for phospho-ERK and PY20 of human sperm (C+Sia) capacitated in the presence of free Neu5Ac. **, p ≤ 0.01 versus the control.
Function of Sialidases Neu1 and Neu3 during Capacitation
We observed inhibition of binding to the ovum zona pellucida by sperm that had been capacitated in the presence of DANA (Fig. 3A). The effect of sperm sialidase activity on mouse and human sperm capacitation status and on the MAPK/ERK signaling pathway was analyzed by assessing tyrosine phosphorylation using antibodies against phosphotyrosine (PY20) and phosphorylated ERK. Inhibition of sperm sialidases by DANA led to reductions in tyrosine phosphorylation as well as ERK1/2 phosphorylation (Fig. 3, B and C) in mice and humans, suggesting that functional sperm sialidases mediate sperm glycocalyx changes associated with sperm cell signaling.
NEU1/3 in Human Idiopathic Subfertility Patients
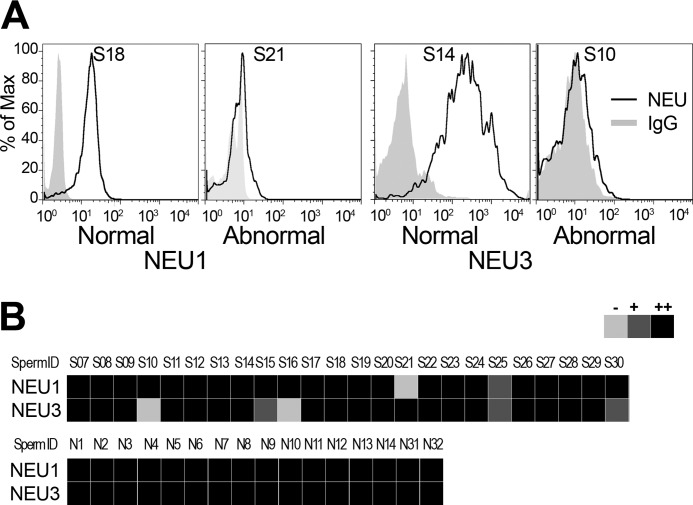
We established a flow cytometry-based assay for sperm NEU1/3 and analyzed human sperm samples collected at an infertility clinic. We found that out of 22 samples, four had very low levels of NEU3, one lacked NEU1, and one had low levels of both NEU1 and NEU3 (Fig. 4, A and B). Of these six subjects, only two had abnormal sperm parameters, whereas the other four had normal sperm parameters (based on computer-assisted sperm analysis and World Health Organization (2009) criteria for concentration, motility, and morphology). All 16 normal control samples had both NEU1 and NEU3 on their sperm surfaces.
FIGURE 4.
NEU1/3 in human idiopathic subfertility patients. A, FACS analysis of human ejaculated sperm samples stained for NEU1 and NEU3 with normal and abnormal expression levels (each representative of three experiments). B, summary panel of 40 human sperm samples, including 24 fertility patients and 16 normal controls (three independent measures each).
Binding of Mouse Sperm by Siglecs
We used chimeric Siglec-6 Fc probes and a flow cytometry-based assay to test the binding of Siglec-6 to mouse sperm. Live mouse epididymal sperm bound strongly to Siglec-6 Fc probes (supplemental Fig. 4B), indicating that the sperm sialome is efficiently “probed” by this Siglec.
DISCUSSION
As foreign cells, mammalian sperm face numerous challenges during their transit through the female reproductive tract en route to fertilization (7). Surviving the onslaught of female immune factors is an absolute prerequisite for sperm to reach the upper oviduct for fertilization. Abundant sialylation likely facilitates tolerance by female innate pattern recognition molecules, both secreted molecules such as complement (factor H, which binds to Sia and prevents complement deposition (36)) and cell-bound molecules on female leukocytes such as Siglecs (11, 37). In addition, terminal Sia decoration may mask potentially antigenic sperm molecules until after sperm have undergone capacitation. Removal of Sias exposes the underlying functional groups as the sperm nears the egg and its vestment. Such a antigen-masking role has been demonstrated for the highly sialylated β-defensin 126 on primate sperm (16).
Changes in sperm sialylation levels en route to fertilization have been reported for several decades. It has been proposed that such a loss involves small molecular weight sialoglycopeptides or gangliosides bearing α2–3- and α2–6-linked Sias prior to fertilization (18, 38, 39). The shedding of glycosylphosphatidylinositol-anchored sialoglycopeptides is also a distinct possibility. In our study, we detected, for the first time, the free Neu5Ac and Neu5Gc monosaccharides in the supernatant from the in vitro capacitation medium, indicating that some of the sialome loss is mediated by sperm sialidase activity (Fig. 1). We have reported the presence of two sperm enzymes (Neu1 and Neu3) that appear to modulate the sperm sialome by cleaving Sia molecules from sialoglycoconjugates during capacitation. Although we found evidence for some loss of CD52, the majority of this glycocalyx molecule remained on sperm after incubation under capacitating conditions.
We could demonstrate sialidase activity as well as its selective inhibition with DANA, a known inhibitor of Neu1 and Neu3 (40). The shedding of sialoglycoproteins and gangliosides might result from alteration in sperm membrane fluidity with cholesterol efflux induced by BSA and NaHCO3 (41). Furthermore, Neu1 and Neu3 were shed from capacitated sperm as indicated by a reduction in antibody staining and their detection in the supernatant of the capacitation medium. It remains to be determined to what degree residual function of the shed sialidases could play a role, such as in the desialylation of highly sialylated and sperm-modulating glycodelin-A in the oviduct (42). Our finding that protease inhibition interfered with sialidase activity and shedding of sialidases from sperm in vitro indicates that a capacitation-associated enzymatic cleavage of Neu1 and Neu3 is required for function and shedding. Treatment with DANA strongly reduced the shedding of the two sialidases (Fig. 2, C and D). Neu1 and Neu3 were both found along the female reproductive tract as well (supplemental Fig. 2). The distribution of Neu1 in the uterus and Neu3 in the fallopian tube in female mice would indicate that Sias from glycoproteins and glycolipids (gangliosides) might be differentially targeted. To what extent the sialidases on sperm and those along the female reproductive tract might synergize or conflict for modifying sperm remains unclear.
Inhibition with DANA affected the function and capacitation status of sperm (Fig. 3) as determined by sperm-egg binding assay and by analysis of phosphorylation levels. These findings indicate that the partial loss of sperm Sias represents not just a side effect of capacitation but rather plays a key role in mediating the process of sperm capacitation.
We investigated the potential role of sperm sialidase NEU1 and NEU3 levels in human male idiopathic infertility (Fig. 4B). Male infertility is a common condition (43), with ∼75% involving idiopathic factors. It is conceivable that inappropriate sialidase expression on sperm (total absence or low levels) might underlie some of the unexplained male infertility. Our results indicate that such inadequate levels of sperm sialidase might underlie some forms of idiopathic male infertility. Furthermore, lack of appropriate protease function might also hinder sialidase shedding during capacitation.
The precise consequences of controlled and enzymatically mediated partial loss of Sias from the sperm surface on sperm function during capacitation remain unknown. The partial Sia loss may serve to uncover critical receptors and contribute to membrane reorganization. It may also potentially allow uncapped desialylated glycans to engage lectins of the female tract in a manner that contributes to clustering of the sperm surface glycoconjugates and, in the process, facilitate signaling (Fig. 5), analogous to that of T-cell and dendritic cell regulation (40, 44). Having identified two types of sialidases on sperm with a role in mediating capacitation in vitro opens the way to studies of Sia modulation and its significance for sperm function in vivo. The recent finding that human sperm bind to a sialylated ligand (sialyl-LewisX) on the zona pellucida (45) is very interesting, as it might indicate that Sia loss from sperm may also unmask key receptors for sialylated ligands on the egg zona pellucida.
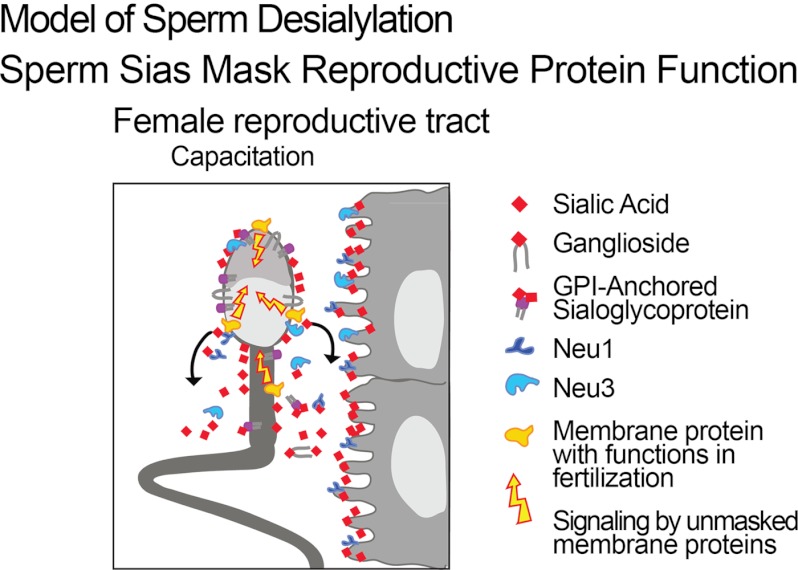
FIGURE 5.
Model of Sia loss during capacitation. Neu1 and Neu3 are responsible for cleaving terminal Sias and are themselves shed. Sialoglycoconjugates such as gangliosides and glycosylphosphatidylinositol (GPI)-anchored sialoglycoproteins are also shed. Unmasked glycoproteins come into action and allow cell signaling during capacitation.
Supplementary Material
Acknowledgments
We gratefully acknowledge the Neuroscience Microscopy Facility of the University of California, San Diego (supported by National Institutes of Health Grant P30 NS047101). We thank Stevan Springer for help with the figures.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 GM095882. This work was also supported by the G. Harold and Leila Y. Mathers Charitable Foundation.

This article contains supplemental “Experimental Procedures,” Figs. 1–4, and an additional reference.
- Sia
- sialic acid
- Neu5Ac
- N-acetylneuraminic acid
- Neu5Gc
- N-glycolylneuraminic acid
- BWW
- Biggers-Whitten-Whittingham
- PNA
- peanut agglutinin
- DANA
- 2-deoxy-2,3-didehydro-d-N-acetylneuraminic acid.
REFERENCES
- 1. Varki A., Schauer R. (2009) Essentials of Glycobiology, pp. 199–218, Cold Spring Harbor Press, Plainview, NY: [PubMed] [Google Scholar]
- 2. Schröter S., Osterhoff C., McArdle W., Ivell R. (1999) The glycocalyx of the sperm surface. Hum. Reprod. Update 5, 302–313 [DOI] [PubMed] [Google Scholar]
- 3. Schauer R. (1982) Chemistry, metabolism, and biological functions of sialic acids. Adv. Carbohydr. Chem. Biochem. 40, 131–234 [DOI] [PubMed] [Google Scholar]
- 4. Schauer R. (2004) Sialic acids: fascinating sugars in higher animals and man. Zoology 107, 49–64 [DOI] [PubMed] [Google Scholar]
- 5. Bernal A., Torres J., Reyes A., Rosado A. (1980) Presence and regional distribution of sialyltransferase in the epididymis of the rat. Biol. Reprod. 23, 290–293 [DOI] [PubMed] [Google Scholar]
- 6. Fournier-Delpech S., Bayard F., Boulard C. (1973) Sperm maturation. An acid protein of the rat epididymis. Androgen dependence, relationship with sialic acid. C. R. Seances Soc. Biol. Fil. 167, 1989–1996 [PubMed] [Google Scholar]
- 7. Drobnis E. Z., Overstreet J. W. (1992) Natural history of mammalian spermatozoa in the female reproductive tract. Oxf. Rev. Reprod. Biol. 14, 1–45 [PubMed] [Google Scholar]
- 8. Pandya I. J., Cohen J. (1985) The leukocytic reaction of the human uterine cervix to spermatozoa. Fertil. Steril. 43, 417–421 [DOI] [PubMed] [Google Scholar]
- 9. Thompson L. A., Barratt C. L., Bolton A. E., Cooke I. D. (1992) The leukocytic reaction of the human uterine cervix. Am. J. Reprod. Immunol. 28, 85–89 [DOI] [PubMed] [Google Scholar]
- 10. Brandtzaeg P. (1997) Mucosal immunity in the female genital tract. J. Reprod. Immunol. 36, 23–50 [DOI] [PubMed] [Google Scholar]
- 11. Crocker P. R., Paulson J. C., Varki A. (2007) Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266 [DOI] [PubMed] [Google Scholar]
- 12. Meri S., Pangburn M. K. (1990) Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion-binding site on factor H. Proc. Natl. Acad. Sci. U.S.A. 87, 3982–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toshimori K., Araki S., Oura C., Eddy E. M. (1991) Loss of sperm surface sialic acid induces phagocytosis: an assay with a monoclonal antibody T21, which recognizes a 54K sialoglycoprotein. Arch. Androl. 27, 79–86 [DOI] [PubMed] [Google Scholar]
- 14. Lassalle B., Testart J. (1994) Human zona pellucida recognition associated with removal of sialic acid from human sperm surface. J. Reprod. Fertil. 101, 703–711 [DOI] [PubMed] [Google Scholar]
- 15. Velásquez J. G., Canovas S., Barajas P., Marcos J., Jiménez-Movilla M., Gallego R. G., Ballesta J., Avilés M., Coy P. (2007) Role of sialic acid in bovine sperm-zona pellucida binding. Mol. Reprod. Dev. 74, 617–628 [DOI] [PubMed] [Google Scholar]
- 16. Yudin A. I., Generao S. E., Tollner T. L., Treece C. A., Overstreet J. W., Cherr G. N. (2005) β-Defensin 126 on the cell surface protects sperm from immunorecognition and binding of anti-sperm antibodies. Biol. Reprod. 73, 1243–1252 [DOI] [PubMed] [Google Scholar]
- 17. Familiari G., Motta P. M. (1980) Morphological changes of mouse spermatozoa in uterus as revealed by scanning and transmission electron microscopy. Acta Biol. Acad. Sci. Hung. 31, 57–67 [PubMed] [Google Scholar]
- 18. Focarelli R., Giuffrida A., Rosati F. (1995) Changes in the sialylglycoconjugate distribution on the human sperm surface during in vitro capacitation: partial purification of a 20-kDa sialylglycoprotein of capacitated spermatozoa. Hum. Reprod. 10, 2755–2759 [DOI] [PubMed] [Google Scholar]
- 19. Srivastava P. N., Zaneveld L. J., Williams W. L. (1970) Mammalian sperm acrosomal neuraminidases. Biochem. Biophys. Res. Commun. 39, 575–582 [DOI] [PubMed] [Google Scholar]
- 20. Ganguly S., Sarkar D., Ghosh J. J. (1976) Sialic acid and sialidase activity in human endometrial tissue, uterine fluid, and plasma under different conditions of uterine dysfunction. Acta Endocrinol. 81, 574–579 [DOI] [PubMed] [Google Scholar]
- 21. Nixon B., MacIntyre D. A., Mitchell L. A., Gibbs G. M., O'Bryan M., Aitken R. J. (2006) The identification of mouse sperm surface-associated proteins and characterization of their ability to act as decapacitation factors. Biol. Reprod. 74, 275–287 [DOI] [PubMed] [Google Scholar]
- 22. Tash J. S., Bracho G. E. (1998) Identification of phosphoproteins coupled to initiation of motility in live epididymal mouse sperm. Biochem. Biophys. Res. Commun. 251, 557–563 [DOI] [PubMed] [Google Scholar]
- 23. Munné S., Estop A. M. (1993) Chromosome analysis of human spermatozoa stored in vitro. Hum. Reprod. 8, 581–586 [DOI] [PubMed] [Google Scholar]
- 24. Shetty J., Diekman A. B., Jayes F. C., Sherman N. E., Naaby-Hansen S., Flickinger C. J., Herr J. C. (2001) Differential extraction and enrichment of human sperm surface proteins in a proteome: identification of immunocontraceptive candidates. Electrophoresis 22, 3053–3066 [DOI] [PubMed] [Google Scholar]
- 25. Manzi A. E., Diaz S., Varki A. (1990) High-pressure liquid chromatography of sialic acids on a pellicular resin anion-exchange column with pulsed amperometric detection: a comparison with six other systems. Anal. Biochem. 188, 20–32 [DOI] [PubMed] [Google Scholar]
- 26. Lewis A. L., Nizet V., Varki A. (2004) Discovery and characterization of sialic acid O-acetylation in group B streptococcus. Proc. Natl. Acad. Sci. U.S.A. 101, 11123–11128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng M. C., Lin S. L., Wu S. H., Inoue S., Inoue Y. (1998) High-performance capillary electrophoretic characterization of different types of oligo- and polysialic acid chains. Anal. Biochem. 260, 154–159 [DOI] [PubMed] [Google Scholar]
- 28. Moore M. L., Chi M. H., Zhou W., Goleniewska K., O'Neal J. F., Higginbotham J. N., Peebles R. S., Jr. (2007) Cutting edge: oseltamivir decreases T cell GM1 expression and inhibits clearance of respiratory syncytial virus: potential role of endogenous sialidase in antiviral immunity. J. Immunol. 178, 2651–2654 [DOI] [PubMed] [Google Scholar]
- 29. Yamauchi Y., Ajduk A., Riel J. M., Ward M. A. (2007) Ejaculated and epididymal mouse spermatozoa are different in their susceptibility to nuclease-dependent DNA damage and in their nuclease activity. Biol. Reprod. 77, 636–647 [DOI] [PubMed] [Google Scholar]
- 30. Singavarapu R., Buchinsky N., Cheon D. J., Orsulic S. (2010) Whole ovary immunohistochemistry for monitoring cell proliferation and ovulatory wound repair in the mouse. Reprod. Biol. Endocrinol. 8, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schröter S., Derr P., Conradt H. S., Nimtz M., Hale G., Kirchhoff C. (1999) Male-specific modification of human CD52. J. Biol. Chem. 274, 29862–29873 [DOI] [PubMed] [Google Scholar]
- 32. Monti E., Bonten E., D'Azzo A., Bresciani R., Venerando B., Borsani G., Schauer R., Tettamanti G. (2010) Sialidases in vertebrates: a family of enzymes tailored for several cell functions. Adv. Carbohydr. Chem. Biochem. 64, 403–479 [DOI] [PubMed] [Google Scholar]
- 33. Miyagi T., Takahashi K., Hata K., Shiozaki K., Yamaguchi K. (2012) Sialidase significance for cancer progression. Glycoconj. J., in press [DOI] [PubMed] [Google Scholar]
- 34. Coutinho M., Lacerda L., Macedo-Ribeiro S., Baptista E., Ribeiro H., Prata M., Alves S. (2012) Lysosomal multienzymatic complex-related diseases: a genetic study among Portuguese patients. Clin. Genet. 81, 370–393 [DOI] [PubMed] [Google Scholar]
- 35. Hasegawa T., Yamaguchi K., Wada T., Takeda A., Itoyama Y., Miyagi T. (2000) Molecular cloning of mouse ganglioside sialidase and its increased expression in Neuro2a cell differentiation. J. Biol. Chem. 275, 8007–8015 [DOI] [PubMed] [Google Scholar]
- 36. Ram S., Sharma A. K., Simpson S. D., Gulati S., McQuillen D. P., Pangburn M. K., Rice P. A. (1998) A novel sialic acid-binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J. Exp. Med. 187, 743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Varki A., Gagneux P. (2012) Multifarious roles of sialic acids in immunity. Ann. N.Y. Acad. Sci. 1253, 16–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Davis B. K., Byrne R., Bedigian K. (1980) Studies on the mechanism of capacitation: albumin-mediated changes in plasma membrane lipids during in vitro incubation of rat sperm cells. Proc. Natl. Acad. Sci. U.S.A. 77, 1546–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Focarelli R., Rosati F., Terrana B. (1990) Sialyglycoconjugate release during in vitro capacitation of human spermatozoa. J. Androl. 11, 97–104 [PubMed] [Google Scholar]
- 40. Stamatos N. M., Carubelli I., van de Vlekkert D., Bonten E. J., Papini N., Feng C., Venerando B., d'Azzo A., Cross A. S., Wang L. X., Gomatos P. J. (2010) LPS-induced cytokine production in human dendritic cells is regulated by sialidase activity. J. Leukocyte Biol. 88, 1227–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Osheroff J. E., Visconti P. E., Valenzuela J. P., Travis A. J., Alvarez J., Kopf G. S. (1999) Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol. Hum. Reprod. 5, 1017–1026 [DOI] [PubMed] [Google Scholar]
- 42. Chiu P. C., Chung M. K., Koistinen R., Koistinen H., Seppala M., Ho P. C., Ng E. H., Lee K. F., Yeung W. S. (2007) Cumulus oophorus-associated glycodelin-C displaces sperm bound glycodelin-A and -F and stimulates spermatozoa-zona pellucida binding. J. Biol. Chem. 282, 5378–5388 [DOI] [PubMed] [Google Scholar]
- 43. Akanji Tijani H., Bhattacharya S. (2010) The role of intrauterine insemination in male infertility. Hum. Fertil. 13, 226–232 [DOI] [PubMed] [Google Scholar]
- 44. Wang J., Lu Z. H., Gabius H. J., Rohowsky-Kochan C., Ledeen R. W., Wu G. (2009) Cross-linking of GM1 ganglioside by galectin-1 mediates regulatory T cell activity involving TRPC5 channel activation: possible role in suppressing experimental autoimmune encephalomyelitis. J. Immunol. 182, 4036–4045 [DOI] [PubMed] [Google Scholar]
- 45. Pang P. C., Chiu P. C., Lee C. L., Chang L. Y., Panico M., Morris H. R., Haslam S. M., Khoo K. H., Clark G. F., Yeung W. S., Dell A. (2011) Human sperm binding is mediated by the sialyl-LewisX oligosaccharide on the zona pellucida. Science 333, 1761–1764 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.