Background: SAGA is a multiprotein complex that possesses histone acetyltransferase activity.
Results: Fission yeast strains with deletions in the SAGA acetyltransferase subunit exhibited increased leucine uptake in a manner dependent on an amino acid permease gene.
Conclusion: SAGA regulates amino acid uptake in fission yeast.
Significance: Regulation of nutrient uptake by SAGA provides a potential link between cellular metabolism and chromatin regulation.
Keywords: Amino Acid Transport, Molecular Cell Biology, Protein Acylation, Yeast Genetics, Yeast Metabolism, Schizosaccharomyces pombe, Lysine Acetyltransferase, Nutrient Regulation
Abstract
Metabolic responses of unicellular organisms are mostly acute, transient, and cell-autonomous. Regulation of nutrient uptake in yeast is one such rapid response. High quality nitrogen sources such as NH4+ inhibit uptake of poor nitrogen sources, such as amino acids. Both transcriptional and posttranscriptional mechanisms operate in nutrient uptake regulation; however, many components of this system remain uncharacterized in the fission yeast, Schizosaccharomyces pombe. Here, we demonstrate that the Spt-Ada-Gcn acetyltransferase (SAGA) complex modulates leucine uptake. Initially, we noticed that a branched-chain amino acid auxotroph exhibits a peculiar adaptive growth phenotype on solid minimal media containing certain nitrogen sources. In fact, the growth of many auxotrophic strains is inhibited by excess NH4Cl, possibly through nitrogen-mediated uptake inhibition of the corresponding nutrients. Surprisingly, DNA microarray analysis revealed that the transcriptional reprogramming during the adaptation of the branched-chain amino acid auxotroph was highly correlated with reprogramming observed in deletions of the SAGA histone acetyltransferase module genes. Deletion of gcn5+ increased leucine uptake in the prototrophic background and rendered the leucine auxotroph resistant to NH4Cl. Deletion of tra1+ caused the opposite phenotypes. The increase in leucine uptake in the gcn5Δ mutant was dependent on an amino acid permease gene, SPCC965.11c+. The closest budding yeast homolog of this permease is a relatively nonspecific amino acid permease AGP3, which functions in poor nutrient conditions. Our analysis identified the regulation of nutrient uptake as a physiological function for the SAGA complex, providing a potential link between cellular metabolism and chromatin regulation.
Introduction
Living organisms possess a variety of mechanisms that allow them to adjust to environmental changes. For unicellular organisms such as yeasts, the responses to changes in nutritional conditions are critical as these cells live in direct contact with the surrounding environment. Regulation of nutrient uptake is especially important in this regard. Yeasts possess intricate mechanisms for the regulation of the import of nutrients such as amino acids. In budding yeast, the uptake of nitrogenous compounds via permeases is negatively regulated by high quality nitrogen sources, by transcriptional (1) and/or posttranscriptional mechanisms (2). In an example of the latter mechanism, the HECT (homologous to the E6-AP carboxyl terminus)-type E3 ubiquitin-protein ligase Rsp5 promotes ubiquitination, endocytosis, and vacuolar degradation of a uracil permease, Fur4, and a general amino acid permease, Gap1 (3).
In the fission yeast Schizosaccharomyces pombe, Pub1, a homolog of Rsp5, negatively regulates leucine uptake in response to NH4+ (4). The sty1/spc1+ gene, which encodes a stress-activated MAPK, and its upstream regulator, mcs4+, function synergistically with pub1+ (4, 5). Over the past decade, the involvement of the TOR3 (target of rapamycin) pathways in nutrient uptake has been well documented. The tor1Δ mutant is defective in leucine uptake when ammonium compounds provide the nitrogen source (6). Gene expression of three amino acid permeases is down-regulated in the tor1Δ mutant. Tsc1 and Tsc2, which negatively regulate the TORC1 complex containing Tor2 (7), play an important role in nutrient uptake by regulating the expression and trafficking of permeases (8, 9). Disruption of tsc2+ results in mislocalization of the cationic amino acid transporter Cat1 (10), and the tor1Δ tsc2Δ double mutation exacerbates the defective leucine uptake of either single mutant (11). However, it seems likely that a number of regulatory components of nutrient uptake remain unidentified in fission yeast.
Spt-Ada-Gcn acetyltransferase (SAGA) is a multiprotein complex that primarily modifies chromatin (12). In fission yeast, the SAGA complex consists of 19 subunits, all of which are conserved in budding yeast (13). SAGA contains the histone acetyltransferase (HAT) catalytic subunit, Gcn5 (12, 14). Deletion of gcn5+ in fission yeast results in a global decrease in acetylation of histone H3 residues Lys-9, Lys-14, and Lys-18, but changes the expressions of only a relatively small subset of genes (1–2%) by more than 2-fold (13, 15). SAGA is known to control sexual differentiation in S. pombe (13). The gcn5Δ fission yeast also exhibits sensitivities to high temperature and high concentrations of KCl (15, 16). However, other physiological roles for SAGA are not yet known in fission yeast.
Here we report a new function of the fission yeast SAGA complex. During a systematic analysis of fission yeast metabolism-related genes, we noticed that the branched amino acid auxotroph, eca39Δ, exhibits a peculiar adaptive growth phenotype. The eca39+ gene codes for the branched-chain amino acid transaminase, which catalyzes the final step of biosynthesis of all three branched-chain amino acids, i.e. isoleucine, leucine, and valine (EC 2.6.1.42) (17). Because known metabolic responses are generally rapid and transient, this unusual adaptive phenomenon drew our attention. Transcriptome, genetic, and biochemical analyses of this phenomenon led us to the unexpected revelation that the SAGA HAT complex modulates leucine uptake.
EXPERIMENTAL PROCEDURES
Media and Strains
Rich YE medium contains 0.5% yeast extract and 3% glucose unless noted otherwise. Edinburgh minimal medium (EMM2) is described in Ref. 18. It should be noted that EMM2 contains 93.5 mm (5 g/liter) NH4Cl as the nitrogen source, whereas EMM2-N refers to EMM2 minus NH4Cl. To prepare solid media, agar, glucose, and 2.2× EMM2 liquid medium were autoclaved separately to prevent them from reacting with each other. The pH of each 2.2× EMM2 medium with or without a nitrogen source was adjusted to 5.5. The commercially available haploid gene deletion strains (19) were purchased from Bioneer Corp. Other strains are constructed using standard techniques as described previously (20). All strains are listed in supplemental Table 1.
Serial Dilution Assay
Yeasts pregrown on a YE plate for 1–2 nights were suspended in water at A600 = 0.4 (∼5000 cells/μl). 3 μl each of 1×, 5×, 25×, 125×, and 625× dilutions was spotted on each plate. The plates were incubated at 30 °C.
DNA Microarray Experiments
Fission yeasts were inoculated from solid media into liquid YE containing 2 mm each of isoleucine, leucine, and valine (ILV), cultured at 30 °C, and harvested at A600 = ∼1.0. Total RNA was isolated by the hot phenol method (21) followed by cleanup using the RNeasy mini kit (Qiagen). The cDNA synthesis and biotin-labeled target synthesis were conducted using a 3′ IVT Express kit (Affymetrix) according to the manufacturer's standard protocol. The GeneChip Yeast 2.0 microarray (Affymetrix) was hybridized with the probes and scanned on the GeneChip Scanner 3000 7G. The DNA microarray data were normalized and processed using R (from the R Project for Statistical Computing) and Bioconductor, based on the robust multiarray average (RMA) algorithm (22).
Bioinformatic Analysis
For the pairwise calculation of the Pearson's correlation coefficients between the whole-genome differential expression patterns of the eca39Δ mutants and those of published datasets, ∼120 DNA microarray datasets for fission yeast single mutants/treatments (but not double mutants or composite treatments) publicly available as of April 2010 were downloaded from ArrayExpress, Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo), and the pombeTV TranscriptomeViewer. In most cases, we used summarized but not raw data and took the log mean values among replicates/spots whenever available/appropriate. The Gene Ontology (GO) biological processes of the differentially expressed genes were determined using AmiGO GO Slimmer. The statistical significance of enrichment was assessed by Fisher's exact test with Bonferroni's correction for multiple testing.
Measurements of Intracellular Amino Acids
200 μl each of intracellular amino acid pools was prepared from five A600 of each fission yeast cells as described by others (9). Amino acid analysis was carried out by injecting 70 μl each of amino acid pools into an L-8900A Hitachi high speed amino acid analyzer equipped with a #2622sc (PF) column (4.6 inner diameter × 60 mm) for physiological fluids analysis. A lithium citrate buffer (L8900-PF) was used. Amino acids were made to react by on-line post-column reaction with ninhydrin.
Leucine Uptake Assay
Leucine uptake was assayed by incubating fission yeasts with 3H-labeled leucine as described by others (4, 6, 8, 9), with some modifications. Prototrophic fission yeast strains were grown to log phase in EMM2 containing 1 g/liter NH4Cl. The cells were adjusted to A600 = 0.5 in a total of 0.24 ml. The uptake assay was started by adding 0.6 ml of EMM2 containing 1 g/liter NH4Cl, 1 μCi of l-[4,5-3H]leucine (PerkinElmer Life Sciences), and 50 or 500 μm cold leucine. The cells were incubated at 30 °C for 2–5 min, at which point >1000-fold excess cold leucine was added. The cells were vacuum-collected on Whatman GF/C filters, washed three times, and then solubilized in Soluene-350 (PerkinElmer Life Sciences) at 37 °C for 1 h. The counts per minute (cpm) of 3H-labeled leucine were determined using the Beckman liquid scintillation counter, LC6500. To convert the cpm data to the leucine concentrations, known concentrations of 3H-labeled leucine were serially diluted and also subjected to liquid scintillation. The concentrations were normalized to the cell densities of the strains, which were measured using an improved Neubauer hemocytometer.
RESULTS
The eca39Δ Mutant Exhibits an Adaptive Growth Phenotype on Minimal Medium Supplemented with Branched-chain Amino Acids
Although a branched-chain amino acid auxotroph mutant, eca39Δ, was viable on rich medium (YE), this mutant was unable to grow on minimal medium (EMM2) containing NH4Cl as the nitrogen source, even when supplemented with adequate amounts of ILV (Fig. 1A). We sought media conditions that allow the mutant cells to grow and noticed that the nitrogen sources have a crucial effect. When we changed the nitrogen source in EMM2 from NH4Cl to glutamate, this mutant could not grow by itself for the first few days, just as on NH4Cl. Later, however, on or around the 5th day, a proportion of the eca39Δ cells started growing in the vicinity of a prototrophic fission yeast strain, and the growth initiation spread to other spotted parts of the identical mutant by the 11th day (Fig. 1B). Once the cells began growing on the 5th day, they grew steadily for the next few days, suggesting that the log-phase growth rate of the mutant cell was not severely retarded, but that the initial growth switch had been shut off in the absence of a nearby prototroph. Furthermore, most if not all of the serially diluted mutant cells grew by the 11th day, suggesting that the growing cells did not represent rare genetic suppressors. To confirm this idea, these late-growing eca39Δ cells were suspended in water and respotted on a fresh minimal plate containing glutamate. The respotted eca39Δ cells could not grow initially, but within a week, a proportion of the eca39Δ cells started growing in the vicinity of a prototrophic fission yeast strain, repeating the aforementioned late growth (data not shown). Thus, the late growth mostly reflects an adaptive reversible process not caused by a stable suppressor mutation. The identical eca39Δ cells spotted near a prototrophic budding yeast strain did not exhibit this adaptive growth, although they did so when growing near prototrophic fission yeast (Fig. 1B). Next, we added various types of nitrogen sources at a fixed concentration (15 mm) to EMM2 supplemented with ILV. On minimal medium containing 2 mm each ILV without other nitrogen sources (EMM2-N +ILV), the eca39Δ cells grew very slowly (supplemental Fig. 1, photo 20). Many nitrogen sources inhibited the growth of eca39Δ more strongly than EMM2-N +ILV alone (photos 2, 4, 6, 9, 11, 13, 16, and 18). The initial growth inhibition was followed by the adaptive growth stage, at least on certain nitrogen sources (photos 3, 5, 10, 12, 14, and 15). The strength of the growth inhibition appears to partially correlate with the number of (primary) amino groups in the amino acids used as the nitrogen source. For example, asparagine and glutamine were more inhibitory than aspartate and glutamate, which respectively each have one fewer amino group (photo 2 versus photo 3, as well as photo 4 versus photo 5). One possibility is that the growth of the eca39Δ mutant is repressed by extracellular nitrogen components.
FIGURE 1.
Growth of the eca39Δ mutant on minimal medium. A, a prototrophic fission yeast (SpHT219) and an eca39Δ mutant fission yeast (SpHT257-1) were serially diluted and spotted on the minimal medium containing 15 mm NH4Cl and on rich medium supplemented with isoleucine, leucine, and valine. B, the growth of the eca39Δ mutant on minimal medium containing glutamate as the nitrogen source. A prototrophic budding yeast (YHT842) was also spotted. The photo was taken on the 5th, 8th, and 11th days. C, growth of single auxotrophic mutants on minimal medium supplemented with uracil, adenine, leucine, phenylalanine, arginine, and histidine, with or without NH4Cl and KCl. Strains used are: SpHT219, -388, -224, -81, -377, -379, and -380. D, growth of single auxotrophic mutants on rich medium (2% glucose), with or without NH4Cl or KCl. E, the extended growth of the region marked with the black square in D, with or without NH4Cl or KCl, for one more day. The photos are enlarged to clearly show that those eca39Δ and leu1-32 cell populations that are facing toward the prototrophic or ade6-M216 cells (arrows) are growing.
Leucine Auxotrophs Are Sensitive to Excess NH4Cl
To examine whether this growth phenotype is specific to eca39Δ or associated with other biosynthetic mutants, we compared the growth of several known auxotrophic mutants on media containing excess NH4Cl. In both minimal and rich media, all strains except the adenine auxotroph ade6 failed to grow at 374 mm (20 g/liter) NH4Cl (Fig. 1, C and D). This growth inhibition is not due to general high salt or osmotic stress but is specific to NH4Cl as growth of these mutants was not severely impaired by an equivalent concentration of KCl. An h− leucine auxotroph, leu1-32, exhibited defective growth on both minimal and rich media containing 5 g/liter NH4Cl. Importantly, the growth of the eca39Δ and leu1 mutants was inhibited by NH4Cl to a similar degree as on rich medium (Fig. 1D). This result suggests that the growth inhibition of eca39Δ is at least in part associated with a defect in leucine biosynthesis. Growth of auxotrophs for uracil (ura4-D18), phenylalanine (pha2Δ), arginine (arg4Δ), and histidine (his2Δ) was also inhibited by 5 g/liter NH4Cl, but the extent of inhibition varied between minimal and rich medium. Importantly, both the leu1-32 and the eca39Δ mutants grew adaptively from the vicinity of other grown-up strains at 4 days, yielding a semicircular colony (Fig. 1E). These pieces of evidence imply that the growth inhibition and the adaptive growth are not limited to eca39Δ, but are also seen in some other auxotrophs; leucine biosynthetic mutants are the most sensitive to NH4+ among the mutants tested. Because leucine uptake is known to be inhibited by extracellular NH4+ (4), we hypothesized that the growth defects of the eca39Δ and leu1 mutants are caused at least in part by the inhibition of leucine uptake by nitrogen sources.
The eca39Δ Mutant Exhibits a Transcriptional Profile Consistent with Altered Metabolism
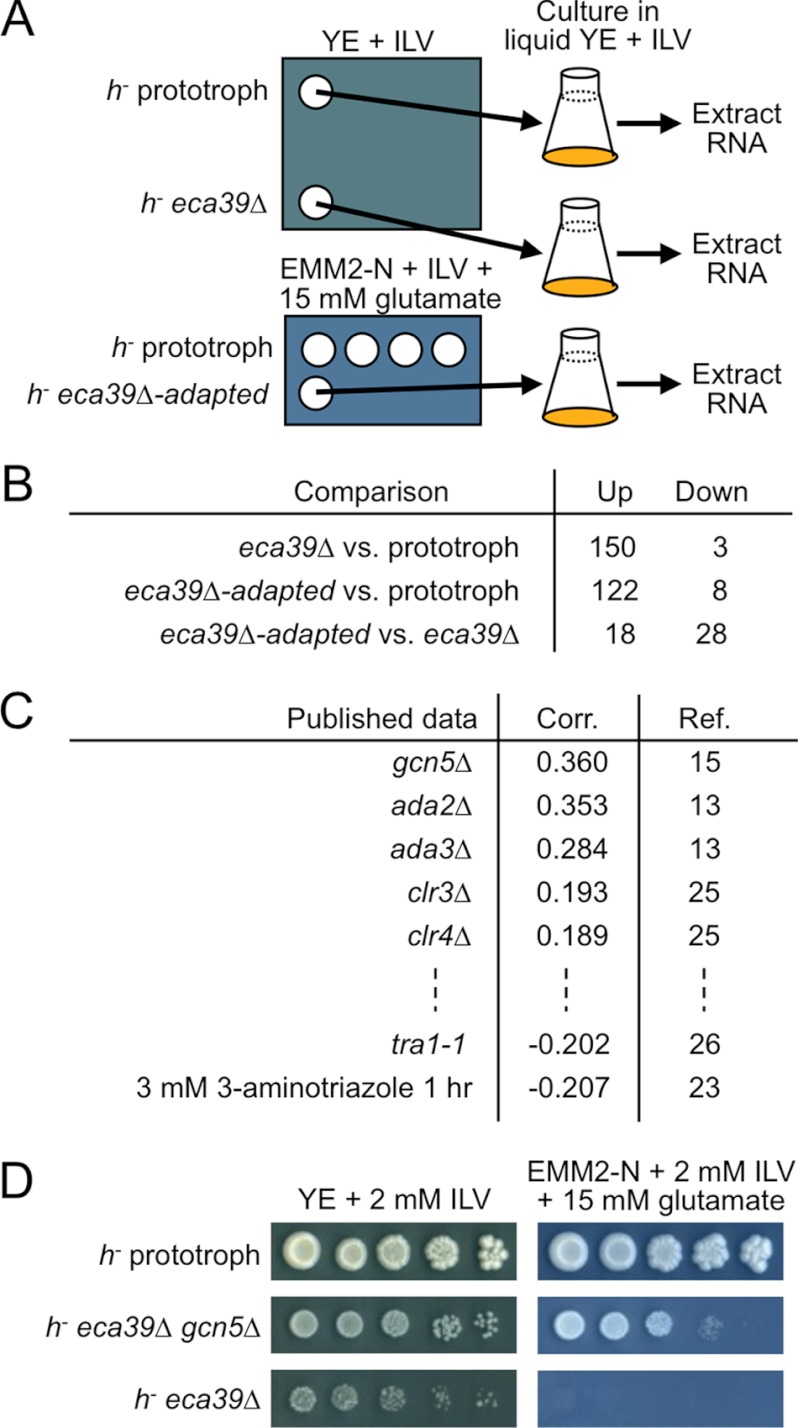
To gain an insight on the adaptive growth of the eca39Δ mutant, we conducted a DNA microarray analysis of the “normal” (preadapted) and adapted eca39Δ mutants. The prototroph and the eca39Δ mutant grown on rich medium, as well as the eca39Δ mutant adaptively grown out near the prototroph on minimal medium, were cultured in rich liquid medium for 3–4 divisions, and then total RNA was extracted from all samples (Fig. 2A).
FIGURE 2.
Transcriptional profiling reveals SAGA-dependent reprogramming upon the adaptation of the eca39Δ mutant. A, scheme of the DNA microarray analysis using the prototroph (SpHT219) and eca39Δ (SpHT257-1). B, the numbers of genes that are ≥2-fold differentially expressed among the prototroph (SpHT219), eca39Δ (SpHT257-1), and the adapted eca39Δ (SpHT257-1) are shown. Individual gene expression data are given in supplemental Table 2. C, comparison of the changes in the genome-wide expression pattern that occurred upon adaptation of the eca39Δ mutant (eca39Δ-adapted versus eca39Δ) with the changes in ∼120 publicly available DNA microarray datasets. The data with highest/lowest Pearson's correlation coefficients (Corr.) are shown. Very similar datasets have been omitted. The complete list is given in supplemental Table 3. Reference (Ref.) numbers are indicated. D, suppression of the growth defect of the eca39Δ mutant on minimal medium upon deletion of gcn5+. SpHT219, -433, and -257-1 were spotted separately (to avoid induction of the adaptive growth of SpHT257-1). The YE and minimal plates were incubated for 5 and 7 days, respectively.
150 and 3 genes were ≥2-fold up- and down-regulated, respectively, in the eca39Δ mutant when compared with the prototroph (Fig. 2B; supplemental Table 2). To determine whether deletion of the eca39+ gene leads to unanticipated defects, we classified the 150 up-regulated genes according to their biological processes, using GO. The genes involved in “stress response,” “cellular amino acid metabolism,” and “transmembrane transport,” but no other categories, were significantly enriched (supplemental Fig. 2A). In another bioinformatic approach, we calculated the Pearson's correlation coefficients between the genome-wide differential gene expression patterns of the eca39Δ (relative to the prototroph) and each of ∼120 publicly available DNA microarray datasets (supplemental Fig. 2B). High correlations with eca39Δ were observed in datasets involving metabolic and stress responses, such as 3-aminotriazole treatment (23), which causes histidine depletion, and hydrogen peroxide treatment, which causes oxidative stress (24). Thus, the predominant defects in eca39Δ are metabolism- and stress-related, and no additional defects are obvious.
Transcriptional Reprogramming during the Adaptation of eca39Δ Resembles Deletion of the SAGA HAT Modules
We then compared the transcriptional profiles before and after the adaptation of the eca39Δ mutant. 18 and 28 genes were ≥2-fold up- and down-regulated, respectively, in the adaptively grown state of eca39Δ, suggesting that the adaptive response involves RNA level regulation (Fig. 2B; supplemental Table 2). It should be emphasized that the change described here is not relative to the prototroph but to the original (preadapted) state. To relate the nature of this adaptation to other known phenomena/phenotypes of fission yeast, we calculated correlation coefficients relative to the aforementioned ∼120 publicly available datasets. Unexpectedly, the overall changes in the adapted state relative to the original state were highly correlated with those of several histone-modifying enzyme mutants (Fig. 2C; supplemental Table 3) (13, 15, 23, 25, 26). In particular, the deletions of the SAGA HAT module subunits, including ada2+ and ada3+ (12, 27) as well as the catalytic subunit gcn5+, were most highly correlated. In fission yeast, SAGA is known to control sexual differentiation through transcriptional regulation of ste11+ and mei2+ (13). Consistent with this notion, the genes up-regulated in the adapted state were enriched in the GO biological process category “conjugation with cellular fusion” (supplemental Fig. 2C) and include ste11+-, mei2+-, and ste11+-regulated genes (supplemental Table 2). On the other hand, 10 out of the 28 genes down-regulated in the adapted state are classified in the GO category “stress response” (supplemental Fig. 2C). It should be noted that none of SAGA subunit genes were ≥1.3-fold differentially expressed upon the adaptation (supplemental Fig. 2D), implying that the down-regulation of SAGA activity involves an unknown posttranscriptional mechanism.
We reasoned that if the down-regulation of the HAT activity does indeed play a crucial role, a deletion of SAGA acetylation subunit genes in the eca39Δ background would rescue the growth defect on minimal medium. Remarkably, this turned out to be the case. The deletion of gcn5+ partially suppressed the growth defect of eca39Δ on EMM2-N +glutamate +ILV plates (Fig. 2D). This result demonstrates a causal relationship, beyond a mere correlation, between the down-regulation of SAGA HAT activity and adaptive growth.
Deletion of the SAGA HAT Module Subunit in a Leucine Auxotrophic Background Causes Resistance to NH4Cl
If the growth defect of eca39Δ is mediated through leucine auxotrophy (Fig. 1D) and the adaptive growth is caused by the inactivation of the SAGA HAT module (Fig. 2), leu1− gcn5Δ double mutants should grow better than leu1− mutants in the presence of excess NH4Cl. This idea could be tested using a haploid commercially available gene deletion library constructed in the leu1-32 ura4-D18 ade6 background (19). Therefore, we examined the growth of these mutants on minimal media containing excess NH4Cl and the necessary supplements.
First, we asked whether the h+ leu1 ura4 ade6 auxotrophic parental strains without a gene knock-out exhibit the adaptive growth phenotype when spotted near a prototroph on minimal medium containing a high concentration (20 g/liter) of NH4Cl plus supplements. Indeed, on the 6th and 7th days, strains spotted near the prototroph grew better than those spotted far away (Fig. 3A). Thus, the phenomenon in the eca39Δ background can to some extent be recapitulated in the leucine auxotrophic background, which is consistent with Fig. 1E.
FIGURE 3.
The gcn5Δ mutant is resistant to excess NH4Cl in a leucine auxotrophic background. A, adaptive growth (indicated by arrows) of the h+ leu1-32 ura4-18 ade6-M216 strain (Parent 1) grown near the h+ prototroph on minimal medium containing 20 g/liter NH4Cl and adenine, leucine, and uracil. Strains used are: SpHT220 and -227. B, serial dilution assay of 10 SAGA subunit mutants constructed in the h+ leu1 ura4 ade6 background on minimal medium containing adenine, leucine, and uracil as well as increasing concentrations of NH4Cl and KCl. The plates were incubated for 4–5 days. Strains used (from top to bottom) are: SpHT309, -227, -344, -299, -300, -311, -310, -313, -298, -312, -343, and -392. Parent 2: h+ leu1 ura4 ade6-M210. C, serial dilution assay of several mutants of the h+ leu1 ura4 ade6 background implicated in histone acetylation or heterochromatin. Strains used are: SpHT309, -227, -300, -346, -396, -348, -349, -352, and -358. D, serial dilution assay of wild type and gcn5Δ constructed in the h− prototrophic background. Strains used re: SpHT219 and -374.
Next, we tested the effects of deletions of SAGA subunit genes on NH4Cl resistance (Fig. 3B). In the leu1 ade6 ura4 background, deletion of the SAGA HAT module subunit genes, ada2+, ada3+, and gcn5+, led to resistance to higher concentrations of NH4Cl, but not KCl. These results are consistent with the idea that the adaptive phenotype of eca39Δ is due at least in part to leucine auxotrophy. The deletion of gcn5+ caused the strongest resistance, suggesting that down-regulation of HAT activity plays a major role in this phenomenon.
Milder resistance was observed upon deletion of sgf73+ gene, which encodes a deubiquitinase module subunit involved in mRNA export (28). However, deletion of the deubiquitinase catalytic subunit, ubp8+ (29), did not markedly alter the resistance, suggesting that the deubiquitinase activity plays no active role in this phenomenon. Deletion of the sgf29+ gene, which encodes a double Tudor domain protein that can bind to trimethylated H3K4 (30), also led to mild resistance to NH4Cl. On the other hand, deletion of spt8+, a TATA-binding protein (TBP)-interacting subunit (31), increased sensitivity to NH4Cl. The most striking sensitivity was seen upon deletion of tra1+, which recruits the SAGA complex to transcriptional activators (32). This sensitivity is in concordance with the DNA microarray analysis, which revealed that changes in the gene expression pattern upon the adaptation of eca39Δ are negatively correlated with those observed in a tra1 mutant (Fig. 2C; supplemental Table 3) (26). The involvement of spt8+ and tra1+ suggests that NH4Cl resistance is under the control of the whole SAGA complex, although smaller complexes such as the Ada2-Ada3-Gcn5 complex or Gcn5 alone have also been described (27).
We also tested known chromatin-related genes other than the SAGA complex (Fig. 3C). Unlike gcn5Δ, deletions of two other HAT genes, mst2+ or elp3+, did not cause clear resistance. Deletion of two histone deacetylase genes, clr3+ or hos2+, resulted in mild sensitivity to NH4Cl, raising a possibility that these histone deacetylases counteract the SAGA HAT activity. Deletion of classical heterochromatin silencing pathway genes, clr4+ or swi6+, did not change the NH4Cl resistance, although many amino acid transporter genes are located in subtelomeric regions of the fission yeast genome (33). Thus, the NH4Cl resistance conferred by mutations in the SAGA complex may be due not to gene silencing by heterochromatin formation but rather to effects on specific targets of the SAGA HAT complex.
SAGA Modulates Leucine Uptake
If the adaptive growth of the auxotrophic mutants on medium containing excess nitrogen source is due to derepression of leucine import, then deletion of gcn5+ in the prototrophic (leu1+) background should not confer NH4Cl resistance. Consistent with this premise, an h− gcn5Δ strain lacking auxotrophic mutations did not exhibit NH4Cl resistance (Fig. 3D).
To determine whether gcn5Δ mutants exhibit derepression of NH4Cl-mediated inhibition of leucine uptake, we next measured the intracellular amino acid concentrations in the h+ leu1 ade6 ura4 strain as well as the gcn5Δ mutant constructed in this auxotrophic background, cultured in the minimal medium containing 2 mm each of adenine, leucine, and uracil and a high (10 g/liter) concentration of NH4Cl. Strikingly, the leucine concentration in the leu1 ade6 ura4 strain was 6.4-fold lower than in the isogenic gcn5Δ mutant in this condition (Fig. 4A). Because these leu1-32 strains cannot synthesize leucine, this difference should be attributed to the uptake of leucine present in the medium. The amino acids analyzed, other than leucine, were not added to the medium, and thus, should reflect the difference between the internally synthesized pools in gcn5+ and gcn5Δ. In fact, none of other amino acids was as highly increased in gcn5Δ as leucine. Furthermore, the NH4+ concentration was not lower in gcn5Δ than in gcn5+, suggesting that the NH4Cl tolerance in the gcn5Δ mutant was not caused by a reversal of the increased NH4Cl concentration. These observations suggest that leucine uptake inhibition by nitrogen sources is compromised in gcn5Δ mutants.
FIGURE 4.
SAGA modulates leucine uptake. A, the intracellular concentrations of 20 amino acids plus ornithine (Orn), citrulline (Cit), and ammonia in an h+ ade6 leu1 ura4 strain and a gcn5Δ mutant constructed in this background. Means and standard deviations are shown. The result is obtained from two independent cultures of the strains. These strains (SpHT227 and -300) were grown in liquid minimal medium containing 10 g/liter NH4Cl and 2 mm each adenine, leucine, and uracil. B, [3H]leucine uptake assay of h− prototroph (SpHT219) and h− gcn5Δ (SpHT374) conducted in the liquid minimal medium containing 1 g/liter NH4Cl and 10 μm cold leucine. The result is based on three cultures of the strains. Mean values and standard deviations are shown. The difference was statistically significant (p value < 0.01 by t test). C, [3H]leucine uptake assay of h− prototroph (SpHT219) and h− tra1Δ (SpHT448) conducted in liquid minimal medium containing 1 g/liter NH4Cl and 100 μm cold leucine. The result is based on three cultures of the strains. Mean values and standard deviations are shown. The difference was statistically significant (p value < 0.02). D, genetic interactions between gcn5+ and tra1+ and between gcn5+ and tsc2+. The gcn5Δ tra1Δ and gcn5Δ tsc2Δ double mutants and the single mutants constructed in the h+ leu1 ura4 ade6 background were subjected to the dilution assay. Strains used are: SpHT309, -343, -453, -340, -454, and -416.
We therefore directly assayed [3H]leucine uptake in gcn5Δ and tra1Δ mutants constructed in the prototrophic background, cultured in the liquid minimal medium. The gcn5Δ mutant exhibited an ∼2.5-fold increase in leucine uptake when compared with the prototroph, whereas the tra1Δ mutant exhibited an ∼2-fold decrease (Fig. 4, B and C), supporting our hypothesis. The leucine uptake of the prototroph is greater in Fig. 4C than in Fig. 4B because the total leucine concentration is higher than in Fig. 4B. To determine whether gcn5+ acts downstream of tra1+ or vice versa, we generated a gcn5Δ tra1Δ double mutant in the leu1 ade6 ura4 background and assayed the growth on minimal medium. At 5 g/liter NH4Cl, the double mutant grew better than tra1Δ but more poorly than the gcn5Δ mutant, suggesting that no clear epistatic relationship exists (Fig. 4D). It is known that tsc2Δ mutants are defective in leucine transport (8, 9). Consistent with this knowledge, the tsc2Δ mutant in the leu1 ade6 ura4 background was sensitive to NH4Cl (Fig. 4D). We created a gcn5Δ tsc2Δ double mutant in this background to study genetic interaction between the two genes. The growth of the double mutant at 2.5–5 g/liter NH4Cl was better than that of tsc2Δ but poorer than gcn5Δ.
SAGA Regulates Leucine Uptake through an Amino Acid Permease
To identify the gene(s) that are responsible for the nutrient uptake control by SAGA, we examined the effects of the deletions of the gcn5+-regulated genes (13, 15) that are also differentially expressed during the adaptation of the eca39Δ mutant. If the genes repressed in the gcn5Δ mutant were crucial for the NH4Cl resistance in the leu1− ade6− ura4− background, the deletion of such genes would cause the resistance. To test this, we obtained eight commercially available mutants defective in genes whose expression is dependent on Gcn5. However, none of these mutants constructed in the leu1 ade6 ura4 background were as clearly resistant as the gcn5Δ mutant (supplemental Fig. 3).
Amino acid uptake regulation in general is likely to involve amino acid transporter(s). The fission yeast genome encodes 21 putative amino acid permease proteins. 16 of them are available from a commercial deletion mutant collection. We could verify, by PCR, correct deletion of 13 of these 16 mutants. The NH4Cl resistance of these 13 mutants in the leu1 ade6 ura4 background was examined (Fig. 5A). The spcc965.11cΔ, spbc15c4.04cΔ, meu22Δ, and cat1Δ mutants exhibited greater sensitivity than did the parental strain to NH4Cl, whereas the other mutants retained the parental level of sensitivity (Fig. 5A). We combined the gcn5Δ allele with each of these 13 deletions to generate the double mutants in the same auxotrophic background. Strikingly, the spcc965.11cΔ gcn5Δ double mutant exhibited the NH4Cl sensitivity, indicating that gcn5 deletion failed to confer resistance to NH4Cl in the spcc965.11cΔ background (Fig. 5B, the third strain). On the other hand, the spbc15c4.04cΔ gcn5Δ, meu22Δ gcn5Δ, cat1Δ gcn5Δ, or any other double mutants did not exhibit the suppression of the resistance seen in the single gcn5Δ mutant (Fig. 5B). We finally examined whether the deletion of SPCC965.11c+ can suppress the increase in leucine uptake of the gcn5Δ mutant. The spcc965.11cΔ gcn5Δ double mutant constructed in the h− prototrophic background exhibited complete reversal of the increased uptake seen in the single gcn5Δ mutant (Fig. 5C). These pieces of evidence suggest that SPCC965.11c+ encodes the downstream effector permease of the leucine uptake regulation orchestrated by SAGA. Because the closest budding yeast homolog of this permease is Agp3p (data not shown), we hereafter refer to this permease protein as Agp3.
FIGURE 5.
Identification of an amino acid permease gene required for the increase in leucine uptake in the gcn5Δ mutant. A, serial dilution assay of 13 putative amino acid permease gene deletion mutants constructed in the h+ leu1 ura4 ade6 background on minimal medium containing adenine, leucine, and uracil as well as NH4Cl and/or KCl. The plates were incubated for 4 days. Strains used (from top to bottom) are: SpHT309, -343, -486, -479, -478, -480, -481, -482, -483, -484,-485, -300, -309, -502, -487, -488, and -489. B, serial dilution assay of 13 double mutants bearing the gcn5Δ allele as well as deletion in each of the putative amino acid permease genes constructed in the h+ leu1 ura4 ade6 background. The plates were incubated for 4 days. Strains used (from top to bottom) are: SpHT309, -486, -498, -491, -490, -492, -493, -494, -495, -496, -497, -300, -503, -499, -500, -501, and -300. C, [3H]leucine uptake assay of h− prototroph, gcn5Δ, spcc965.11cΔ, and gcn5Δ spcc965.11cΔ conducted in the liquid minimal medium containing 1 g/liter NH4Cl and 10 μm leucine. Strains used are: SpHT219, -374, -507, and -508. The result is based on six cultures of the strains. Mean values and standard deviations are shown. The overall difference was statistically significant (p < 0.01 by one-way analysis of variance). The means were significantly different between the gcn5Δ mutant and each of the three other strains (p < 0.01 by Tukey's post hoc test).
DISCUSSION
In this study, we discovered an adaptive growth phenotype of branched-chain amino acid and leucine auxotrophs on solid media, which at least in part reflects a nitrogen-dependent inhibition and subsequent restart of leucine uptake regulated by the SAGA HAT complex. It is unknown what triggers the adaptive growth of the auxotrophs near the prototrophic strain. Local depletion of nitrogen may contribute the initiation. Given the absence of the adaptive growth near the prototrophic budding yeast (Fig. 1B), however, transmission of an unknown fission yeast-specific signal is also possible. We also established a simple spot assay to screen a commercially available leucine auxotrophic deletion library for the genes required for leucine uptake. Although the growth defect of leucine auxotrophs at high pH has been previously linked to defective leucine uptake (4), spotting lower numbers of cells on solid media, as opposed to patching or liquid culture, enabled us to identify the growth sensitivity of several amino acid auxotrophs to high levels of NH4Cl (Fig. 1, C and D).
The changes in the transcriptome upon adaptive growth of eca39Δ are highly correlated to those observed in strains bearing mutations in SAGA components. Nevertheless, no individual SAGA subunits were differentially expressed, suggesting that the change(s) in SAGA function occur posttranscriptionally. Furthermore, we found that the SAGA HAT module and some other subunit genes act in opposite directions, in terms of the NH4Cl resistance and amino acid uptake. The opposing actions of gcn5+ and spt8+, as well as the lack of change in the RNA level of each SAGA subunit, are reminiscent of the induction of meiotic genes upon nutrient depletion (13), implying a mechanistic similarity between the two phenomena. It should be noted, however, that the NH4Cl resistance of gcn5Δ in the leu1 ade6 ura4 background was not suppressed by the gcn5Δ mei2Δ double mutation, (data not shown) and that tra1+ counteracts gcn5+, unlike that of mating/meiosis (32).
Another important regulatory mechanism is the TOR pathway; inactivation of the Tor2 kinase subunit of TORC1 complex mimics nitrogen starvation response and activates the sexual development pathway (34, 35). The tsc1+ and tsc2+ genes, which negatively regulate the TORC1 complex, are important for expression of mei2+ under nitrogen starvation (36). Despite these functional similarities with SAGA mutants, only a partial suppression of the gcn5Δ phenotype was detected upon simultaneous tsc2 mutation, suggesting that no simple upstream/downstream relationship exists between gcn5+ and tsc2+ (Fig. 4D).
We identified the Agp3 amino acid permease as a probable effector required for the increase in leucine uptake in the gcn5Δ mutant (Fig. 5). This permease may be dispensable for leucine uptake under normal conditions due to the existence of other permeases, but is crucial for adaptive increase in leucine uptake mediated by SAGA, which occurs when other permeases are probably inhibited by a high concentration of nitrogen. Interestingly, the budding yeast Agp3 is a low affinity, relatively nonspecific permease, which transports amino acids including leucine when nitrogen sources are limiting and/or other permeases are inactive (37). It is an open question whether Agp3 is a direct non-histone substrate for the SAGA acetyltransferase as we have not yet successfully purified this protein. It is also possible that SAGA acts on an upstream regulatory point rather than this permease itself.
Histone acetylation links gene expression and carbon metabolism in budding yeast (38). Interestingly, SAGA mediates the acetyl-CoA-dependent induction of histone acetylation and transcription of growth genes (39, 40). Our finding that SAGA regulates nutrient uptake in fission yeast may provide an additional link between cellular metabolism and acetylation signaling.
Supplementary Material
Acknowledgments
We are grateful to the RIKEN Brain Science Institute support unit, especially K. Fukumoto for DNA microarray analysis, T. Morishita for amino acid analysis, and other unit members for DNA sequencing analyses. We thank S. Sekido for technical assistance, J. Boeke and A. Matsuyama for providing strains, and T. Schneider-Poetsch and J. Piotrowski for proofreading part of the manuscript. We appreciate advice from current/former members of the RIKEN Chemical Genetics Laboratory.
This work was supported in part by a grant from CREST from the Japan Science and Technology Agency (to M. Y.) and by Grant-in-aid for Scientific Research on Innovative Areas 3307 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H. T.)

This article contains supplemental Tables 1–3 and Figs. S1–S3.
The DNA microarray data reported in this paper have been deposited in Gene Expression Omnibus (GEO) under accession number GSE29355.
- TOR
- target of rapamycin
- SAGA
- Spt-Ada-Gcn acetyltransferase
- HAT
- histone acetyltransferase
- EMM2
- Edinburgh minimal medium
- ILV
- isoleucine, leucine, and valine
- GO
- Gene Ontology.
REFERENCES
- 1. Godard P., Urrestarazu A., Vissers S., Kontos K., Bontempi G., van Helden J., André B. (2007) Effect of 21 different nitrogen sources on global gene expression in the yeast Saccharomyces cerevisiae. Mol. Cell Biol. 27, 3065–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Magasanik B., Kaiser C. A. (2002) Nitrogen regulation in Saccharomyces cerevisiae. Gene 290, 1–18 [DOI] [PubMed] [Google Scholar]
- 3. Hein C., Springael J. Y., Volland C., Haguenauer-Tsapis R., André B. (1995) NPl1, an essential yeast gene involved in induced degradation of Gap1 and Fur4 permeases, encodes the Rsp5 ubiquitin-protein ligase. Mol. Microbiol. 18, 77–87 [DOI] [PubMed] [Google Scholar]
- 4. Karagiannis J., Saleki R., Young P. G. (1999) The pub1 E3 ubiquitin ligase negatively regulates leucine uptake in response to NH4+ in fission yeast. Curr. Genet. 35, 593–601 [DOI] [PubMed] [Google Scholar]
- 5. Saleki R., Jia Z., Karagiannis J., Young P. G. (1997) Tolerance of low pH in Schizosaccharomyces pombe requires a functioning pub1 ubiquitin ligase. Mol. Gen. Genet. 254, 520–528 [DOI] [PubMed] [Google Scholar]
- 6. Weisman R., Roitburg I., Nahari T., Kupiec M. (2005) Regulation of leucine uptake by tor1+ in Schizosaccharomyces pombe is sensitive to rapamycin. Genetics 169, 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Otsubo Y., Yamamato M. (2008) TOR signaling in fission yeast. Crit. Rev. Biochem. Mol. Biol. 43, 277–283 [DOI] [PubMed] [Google Scholar]
- 8. Matsumoto S., Bandyopadhyay A., Kwiatkowski D. J., Maitra U., Matsumoto T. (2002) Role of the Tsc1-Tsc2 complex in signaling and transport across the cell membrane in the fission yeast Schizosaccharomyces pombe. Genetics 161, 1053–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Slegtenhorst M., Carr E., Stoyanova R., Kruger W. D., Henske E. P. (2004) Tsc1+ and tsc2+ regulate arginine uptake and metabolism in Schizosaccharomyces pombe. J. Biol. Chem. 279, 12706–12713 [DOI] [PubMed] [Google Scholar]
- 10. Aspuria P. J., Tamanoi F. (2008) The Tsc/Rheb signaling pathway controls basic amino acid uptake via the Cat1 permease in fission yeast. Mol. Genet. Genomics 279, 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weisman R., Roitburg I., Schonbrun M., Harari R., Kupiec M. (2007) Opposite effects of Tor1 and Tor2 on nitrogen starvation responses in fission yeast. Genetics 175, 1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koutelou E., Hirsch C. L., Dent S. Y. (2010) Multiple faces of the SAGA complex. Curr. Opin. Cell Biol. 22, 374–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Helmlinger D., Marguerat S., Villén J., Gygi S. P., Bähler J., Winston F. (2008) The S. pombe SAGA complex controls the switch from proliferation to sexual differentiation through the opposing roles of its subunits Gcn5 and Spt8. Genes Dev. 22, 3184–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuo M. H., Brownell J. E., Sobel R. E., Ranalli T. A., Cook R. G., Edmondson D. G., Roth S. Y., Allis C. D. (1996) Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines. Nature 383, 269–272 [DOI] [PubMed] [Google Scholar]
- 15. Nugent R. L., Johnsson A., Fleharty B., Gogol M., Xue-Franzén Y., Seidel C., Wright A. P., Forsburg S. L. (2010) Expression profiling of S. pombe acetyltransferase mutants identifies redundant pathways of gene regulation. BMC Genomics 11, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnsson A., Durand-Dubief M., Xue-Franzén Y., Rönnerblad M., Ekwall K., Wright A. (2009) HAT-HDAC interplay modulates global histone H3K14 acetylation in gene-coding regions during stress. EMBO Rep. 10, 1009–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eden A., Benvenisty N. (1998) Characterization of a branched-chain amino-acid aminotransferase from Schizosaccharomyces pombe. Yeast 14, 189–194 [DOI] [PubMed] [Google Scholar]
- 18. Moreno S., Klar A., Nurse P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823 [DOI] [PubMed] [Google Scholar]
- 19. Kim D. U., Hayles J., Kim D., Wood V., Park H. O., Won M., Yoo H. S., Duhig T., Nam M., Palmer G., Han S., Jeffery L., Baek S. T., Lee H., Shim Y. S., Lee M., Kim L., Heo K. S., Noh E. J., Lee A. R., Jang Y. J., Chung K. S., Choi S. J., Park J. Y., Park Y., Kim H. M., Park S. K., Park H. J., Kang E. J., Kim H. B., Kang H. S., Park H. M., Kim K., Song K., Song K. B., Nurse P., Hoe K. L. (2010) Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 28, 617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takahashi H., Suzuki T., Shirai A., Matsuyama A., Dohmae N., Yoshida M. (2011) Mitochondrial localization of fission yeast manganese superoxide dismutase is required for its lysine acetylation and for cellular stress resistance and respiratory growth. Biochem. Biophys. Res. Commun. 406, 42–46 [DOI] [PubMed] [Google Scholar]
- 21. Collart M. A., Oliviero S. (2001) In Current Protocols in Molecular Biology (Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K., eds) pp. 13.12.11–13.12.15, John Wiley and Sons, Inc., New York [Google Scholar]
- 22. Irizarry R. A., Hobbs B., Collin F., Beazer-Barclay Y. D., Antonellis K. J., Scherf U., Speed T. P. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 [DOI] [PubMed] [Google Scholar]
- 23. Udagawa T., Nemoto N., Wilkinson C. R., Narashimhan J., Jiang L., Watt S., Zook A., Jones N., Wek R. C., Bähler J., Asano K. (2008) Int6/eIF3e promotes general translation and Atf1 abundance to modulate Sty1 MAPK-dependent stress response in fission yeast. J. Biol. Chem. 283, 22063–22075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen D., Toone W. M., Mata J., Lyne R., Burns G., Kivinen K., Brazma A., Jones N., Bähler J. (2003) Global transcriptional responses of fission yeast to environmental stress. Mol. Biol. Cell 14, 214–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hansen K. R., Burns G., Mata J., Volpe T. A., Martienssen R. A., Bähler J., Thon G. (2005) Global effects on gene expression in fission yeast by silencing and RNA interference machineries. Mol. Cell Biol. 25, 590–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calonge T. M., Eshaghi M., Liu J., Ronai Z., O'Connell M. J. (2010) Transformation/transcription domain-associated protein (TRRAP)-mediated regulation of Wee1. Genetics 185, 81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grant P. A., Duggan L., Côté J., Roberts S. M., Brownell J. E., Candau R., Ohba R., Owen-Hughes T., Allis C. D., Winston F., Berger S. L., Workman J. L. (1997) Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11, 1640–1650 [DOI] [PubMed] [Google Scholar]
- 28. Köhler A., Schneider M., Cabal G. G., Nehrbass U., Hurt E. (2008) Yeast Ataxin-7 links histone deubiquitination with gene gating and mRNA export. Nat. Cell Biol. 10, 707–715 [DOI] [PubMed] [Google Scholar]
- 29. Henry K. W., Wyce A., Lo W. S., Duggan L. J., Emre N. C., Kao C. F., Pillus L., Shilatifard A., Osley M. A., Berger S. L. (2003) Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA-associated Ubp8. Genes Dev. 17, 2648–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vermeulen M., Eberl H. C., Matarese F., Marks H., Denissov S., Butter F., Lee K. K., Olsen J. V., Hyman A. A., Stunnenberg H. G., Mann M. (2010) Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell 142, 967–980 [DOI] [PubMed] [Google Scholar]
- 31. Sterner D. E., Grant P. A., Roberts S. M., Duggan L. J., Belotserkovskaya R., Pacella L. A., Winston F., Workman J. L., Berger S. L. (1999) Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol. Cell Biol. 19, 86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Helmlinger D., Marguerat S., Villén J., Swaney D. L., Gygi S. P., Bähler J., Winston F. (2011) Tra1 has specific regulatory roles, rather than global functions, within the SAGA co-activator complex. EMBO J. 30, 2843–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hunt C., Moore K., Xiang Z., Hurst S. M., McDougall R. C., Rajandream M. A., Barrell B. G., Gwilliam R., Wood V., Lyne M. H., Aves S. J. (2001) Subtelomeric sequence from the right arm of Schizosaccharomyces pombe chromosome I contains seven permease genes. Yeast 18, 355–361 [DOI] [PubMed] [Google Scholar]
- 34. Alvarez B., Moreno S. (2006) Fission yeast Tor2 promotes cell growth and represses cell differentiation. J. Cell Sci. 119, 4475–4485 [DOI] [PubMed] [Google Scholar]
- 35. Matsuo T., Otsubo Y., Urano J., Tamanoi F., Yamamoto M. (2007) Loss of the TOR kinase Tor2 mimics nitrogen starvation and activates the sexual development pathway in fission yeast. Mol. Cell Biol. 27, 3154–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakase Y., Fukuda K., Chikashige Y., Tsutsumi C., Morita D., Kawamoto S., Ohnuki M., Hiraoka Y., Matsumoto T. (2006) A defect in protein farnesylation suppresses a loss of Schizosaccharomyces pombe tsc2+, a homolog of the human gene predisposing to tuberous sclerosis complex. Genetics 173, 569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schreve J. L., Garrett J. M. (2004) Yeast Agp2p and Agp3p function as amino acid permeases in poor nutrient conditions. Biochem. Biophys. Res. Commun. 313, 745–751 [DOI] [PubMed] [Google Scholar]
- 38. Takahashi H., McCaffery J. M., Irizarry R. A., Boeke J. D. (2006) Nucleocytosolic acetyl-coenzyme a synthetase is required for histone acetylation and global transcription. Mol. Cell 23, 207–217 [DOI] [PubMed] [Google Scholar]
- 39. Friis R. M., Wu B. P., Reinke S. N., Hockman D. J., Sykes B. D., Schultz M. C. (2009) A glycolytic burst drives glucose induction of global histone acetylation by picNuA4 and SAGA. Nucleic Acids Res. 37, 3969–3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cai L., Sutter B. M., Li B., Tu B. P. (2011) Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol. Cell 42, 426–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.