Abstract
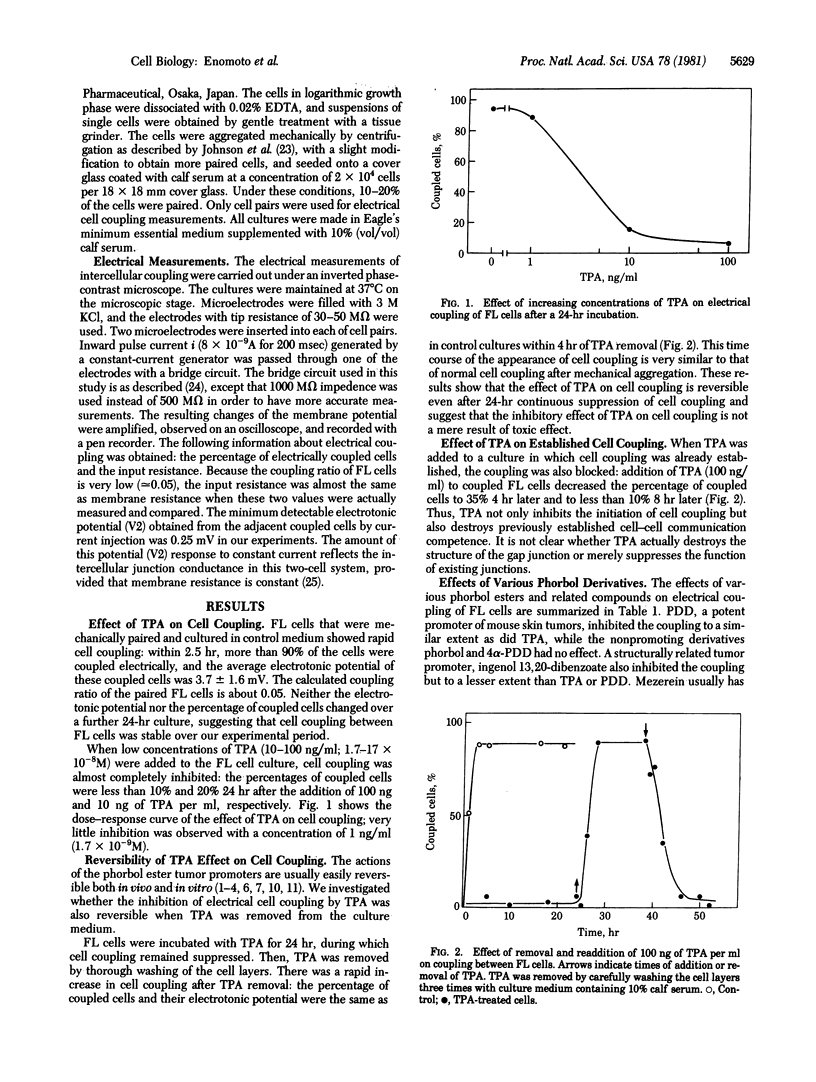
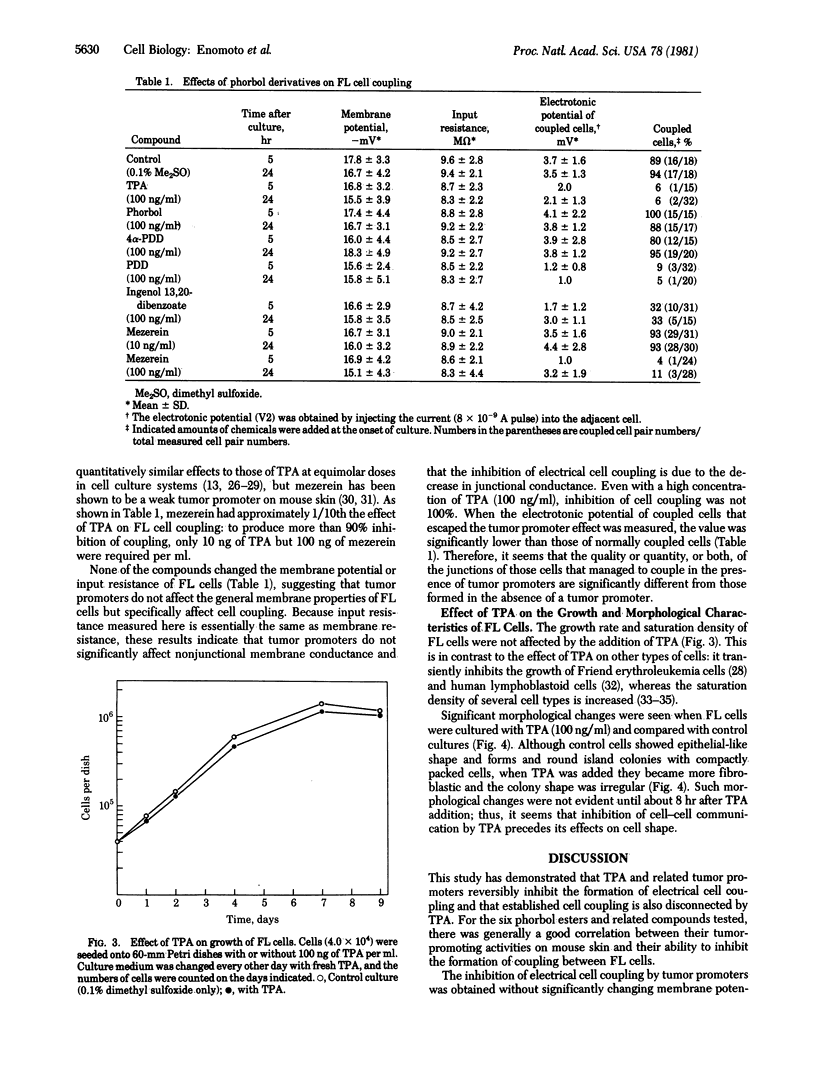
The effect of tumor promoters on electrical coupling between human FL cells was investigated with a microelectrode technique. When a low concentration (100 ng/ml) of 12-O-tetradecanoylphorbol 13-acetate (TPA) was added to culture medium, only 6% of the cells showed electrical coupling after 5 hr, whereas in control medium more than 90% of the cells were coupled. In the presence of TPA, cell coupling remained suppressed for at least another 19 hr. When TPA was washed out from the culture medium, the cells commenced electrical coupling: 90% of the cells were coupled within 4 hr of the removal of TPA, a rate very similar to that of nontreated control cells. Therefore, TPA-mediated inhibition of cell coupling is reversible. When TPA was added to a culture in which more than 90% of the cells had already established electrical coupling, the percentage of coupled cells decreased to 6% within 8 hr, indicating that TPA can also diminish already established cell coupling. Inhibition of cell coupling also was achieved with other mouse skin tumor promoters--phorbol 12,13-didecanoate, ingenol dibenzoate, and mezerein--whereas nonpromoting derivatives--phorbol and 4 alpha-phorbol 12,13-didecanoate-showed no effect. None of these compounds changed the membrane potential, membrane resistance, or growth rate of FL cells. Thus, it appears that TPA and structurally-related tumor promoters specifically disturb the formation or function, or both, of cell-cell junctions, without significantly affecting the general properties of the surface membrane or the growth of FL cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boutwell R. K. The function and mechanism of promoters of carcinogenesis. CRC Crit Rev Toxicol. 1974 Jan;2(4):419–443. doi: 10.3109/10408447309025704. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J. Calcium-dependent stimulation of BALB/c 3T3 mouse cell DNA synthesis by a tumor-promoting phorbol ester (PMA). J Cell Physiol. 1976 Jan;87(1):25–32. doi: 10.1002/jcp.1040870105. [DOI] [PubMed] [Google Scholar]
- Castagna M., Rochette-Egly C., Rosenfeld C. Tumour-promoting phorbol diester induces substrate-adhesion and growth inhibition in lymphoblastoid cells. Cancer Lett. 1979 Apr;6(4-5):227–234. doi: 10.1016/s0304-3835(79)80038-5. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Brien S., Donaldson C., Shimizu Y. Growth stimulation of human diploid fibro-blasts by the tumor promoter, 12-0-tetradecanoly-phorbol-13-acetate. Int J Cancer. 1974 May 15;13(5):721–730. doi: 10.1002/ijc.2910130516. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Rovera G. Tumor promoters: effects on proliferation and differentiation of cells in culture. Life Sci. 1978 Nov 13;23(20):1979–1988. doi: 10.1016/0024-3205(78)90229-1. [DOI] [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. Specific binding of phorbol ester tumor promoters. Proc Natl Acad Sci U S A. 1980 Jan;77(1):567–571. doi: 10.1073/pnas.77.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. The effect of phorbol diesters on chicken embryo fibroblasts. Cancer Res. 1977 Sep;37(9):3257–3265. [PubMed] [Google Scholar]
- Dunphy W. G., Delclos K. B., Blumberg P. M. Characterization of specific binding of [3H]phorbol 12,13-dibutyrate and [3H]phorbol 12-myristate 13-acetate to mouse brain. Cancer Res. 1980 Oct;40(10):3635–3641. [PubMed] [Google Scholar]
- FOGH J., LUND R. O. Continuous cultivation of epithelial cell strain (FL) from human amniotic membrane. Proc Soc Exp Biol Med. 1957 Mar;94(3):532–537. doi: 10.3181/00379727-94-23003. [DOI] [PubMed] [Google Scholar]
- Goshima K. Initiation of beating in quiescent myocardial cells by norepinephrine, by contact with beating cells and by electrical stimulation of adjacent FL cells. Exp Cell Res. 1974 Mar 15;84(1):223–234. doi: 10.1016/0014-4827(74)90400-5. [DOI] [PubMed] [Google Scholar]
- Hollstein M., McCann J., Angelosanto F. A., Nichols W. W. Short-term tests for carcinogens and mutagens. Mutat Res. 1979 Sep;65(3):133–226. doi: 10.1016/0165-1110(79)90014-9. [DOI] [PubMed] [Google Scholar]
- Johnson R., Hammer M., Sheridan J., Revel J. P. Gap junction formation between reaggregated Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4536–4540. doi: 10.1073/pnas.71.11.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasne C., Gentil A., Chouroulinkov I. Two-stage malignant transformation of rat fibroblasts in tissue culture. Nature. 1974 Feb 15;247(5441):490–491. doi: 10.1038/247490a0. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Cell surface membranes in close contact. Role of calcium and magnesium ions. J Colloid Interface Sci. 1967 Sep;25(1):34–46. doi: 10.1016/0021-9797(67)90007-0. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 1979 Feb 4;560(1):1–65. doi: 10.1016/0304-419x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R., Kanno Y., Socolar S. J. Quantum jumps of conductance during formation of membrane channels at cell-cell junction. Nature. 1978 Jul 13;274(5667):133–136. doi: 10.1038/274133a0. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeability of membrane junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):441–472. doi: 10.1111/j.1749-6632.1966.tb50175.x. [DOI] [PubMed] [Google Scholar]
- Mastro A. M., Mueller G. C. Inhibition of the mixed lymphocyte proliferative response by phorbol esters. Biochim Biophys Acta. 1978 Jan 26;517(1):246–254. doi: 10.1016/0005-2787(78)90052-7. [DOI] [PubMed] [Google Scholar]
- Mondal S., Brankow D. W., Heidelberger C. Two-stage chemical oncogenesis in cultures of C3H/10T1/2 cells. Cancer Res. 1976 Jul;36(7 Pt 1):2254–2260. [PubMed] [Google Scholar]
- Mondal S., Heidelberger C. Inhibition of induced differentiation of C3H/10T 1/2 clone 8 mouse embryo cells by tumor promoters. Cancer Res. 1980 Feb;40(2):334–338. [PubMed] [Google Scholar]
- Mufson R. A., Fischer S. M., Verma A. K., Gleason G. L., Slaga T. J., Boutwell R. K. Effects of 12-O-tetradecanoylphorbol-13-acetate and mezerein on epidermal ornithine decarboxylase activity, isoproterenol-stimulated levels of cyclic adenosine 3':5'-monophosphate, and induction of mouse skin tumors in vivo. Cancer Res. 1979 Dec;39(12):4791–4795. [PubMed] [Google Scholar]
- Murray A. W., Fitzgerald D. J. Tumor promoters inhibit metabolic cooperation in cocultures of epidermal and 3T3 cells. Biochem Biophys Res Commun. 1979 Nov 28;91(2):395–401. doi: 10.1016/0006-291x(79)91535-3. [DOI] [PubMed] [Google Scholar]
- Schimmel S. D., Hallam T. Rapid alteration in Ca++ content and fluxes in phorbol 12-myristate 13-acetate treated myoblasts. Biochem Biophys Res Commun. 1980 Jan 29;92(2):624–630. doi: 10.1016/0006-291x(80)90379-4. [DOI] [PubMed] [Google Scholar]
- Scribner J. D., Süss R. Tumor initiation and promotion. Int Rev Exp Pathol. 1978;18:137–198. [PubMed] [Google Scholar]
- Sivak A., Van Duuren B. L. Phenotypic expression of transformation: induction in cell culture by a phorbol ester. Science. 1967 Sep 22;157(3795):1443–1444. doi: 10.1126/science.157.3795.1443. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Fischer S. M., Nelson K., Gleason G. L. Studies on the mechanism of skin tumor promotion: evidence for several stages in promotion. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3659–3663. doi: 10.1073/pnas.77.6.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Iden S. S. Phorbol myristate acetate-induced release of granule enzymes from human neutrophils: inhibition by the calcium antagonist, 8-(N,N-diethylamino)-octyl 3,4,5-trimethoxybenzoate hydrochloride. Biochem Biophys Res Commun. 1979 Nov 14;91(1):263–271. doi: 10.1016/0006-291x(79)90612-0. [DOI] [PubMed] [Google Scholar]
- Trosko J. E., Dawson B., Yotti L. P., Chang C. C. Saccharin may act as a tumour promoter by inhibiting metabolic cooperation between cells. Nature. 1980 May 8;285(5760):109–110. doi: 10.1038/285109a0. [DOI] [PubMed] [Google Scholar]
- Umeda M., Noda K., Ono T. Inhibition of metabolic cooperation in Chinese hamster cells by various chemical including tumor promoters. Gan. 1980 Oct;71(5):614–620. [PubMed] [Google Scholar]
- Van Duuren B. L. Tumor-promoting agents in two-stage carcinogenesis. Prog Exp Tumor Res. 1969;11:31–68. doi: 10.1159/000391388. [DOI] [PubMed] [Google Scholar]
- Wigler M., DeFeo D., Weinstein I. B. Induction of plasminogen activator in cultured cells by macrocyclic plant diterpene esters and other agents related to tumor promotion. Cancer Res. 1978 May;38(5):1434–1437. [PubMed] [Google Scholar]
- Witz G., Banerjee S. Divalent cation effects in the interaction of the tumor promoter phorbol myristate acetate with rat liver plasma membranes. Chem Biol Interact. 1979;28(1):127–131. doi: 10.1016/0009-2797(79)90119-4. [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Fibach E., Nudel U., Weinstein I. B., Rifkind R. A., Marks P. A. Tumor promoters inhibit spontaneous and induced differentiation of murine erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3451–3455. doi: 10.1073/pnas.74.8.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H., Martel N. Inhibitory effect of tumor promoting phorbol esters and mezerein on human mixed lymphocyte reaction. Cancer Lett. 1981 Mar;12(1-2):43–52. doi: 10.1016/0304-3835(81)90036-7. [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Weinstein I. B., Fibach E., Rifkind R. A., Marks P. A. Tumor promoter-induced adhesion of the DS19 clone of murine erythroleukemia cells. Cancer Res. 1979 Jun;39(6 Pt 1):1989–1994. [PubMed] [Google Scholar]
- Yotti L. P., Chang C. C., Trosko J. E. Elimination of metabolic cooperation in Chinese hamster cells by a tumor promoter. Science. 1979 Nov 30;206(4422):1089–1091. doi: 10.1126/science.493994. [DOI] [PubMed] [Google Scholar]