Background: TrxR1 is the only known reductant of thioredoxin.
Results: The glutaredoxin system showed the capacity of reducing thioredoxin 1. Depletion of both TrxR1 activity and glutathione in cells induced Trx1 oxidation and cell death.
Conclusion: The glutathione/glutaredoxin system is a backup of TrxR1 for reducing thioredoxin 1.
Significance: We demonstrated the critical role of the thioredoxin redox state in cell survival and a new role of glutathione.
Keywords: Cell Death, DNA, Glutathion, Metabolism, Oxidative Stress, Redox Regulation, Thioredoxin, Glutaredoxin, Redox State
Abstract
Thioredoxin reductase 1 (TrxR1) in cytosol is the only known reductant of oxidized thioredoxin 1 (Trx1) in vivo so far. We and others found that aurothioglucose (ATG), a well known active-site inhibitor of TrxR1, inhibited TrxR1 activity in HeLa cell cytosol but had no effect on the viability of the cells. Using a redox Western blot analysis, no change was observed in redox state of Trx1, which was mainly fully reduced with five sulfhydryl groups. In contrast, auranofin killed cells and oxidized Trx1, also targeting mitochondrial TrxR2 and Trx2. Combining ATG with ebselen gave a strong synergistic effect, leading to Trx1 oxidation, reactive oxygen species accumulation, and cell death. We hypothesized that there should exist a backup system to reduce Trx1 when only TrxR1 activity was lost. Our results showed that physiological concentrations of glutathione, NADPH, and glutathione reductase reduced Trx1 in vitro and that the reaction was strongly stimulated by glutaredoxin1. Simultaneous depletion of TrxR activity by ATG and glutathione by buthionine sulfoximine led to overoxidation of Trx1 and loss of HeLa cell viability. In conclusion, the glutaredoxin system and glutathione have a backup role to keep Trx1 reduced in cells with loss of TrxR1 activity. Monitoring the redox state of Trx1 shows that cell death occurs when Trx1 is oxidized, followed by general protein oxidation catalyzed by the disulfide form of thioredoxin.
Introduction
Cellular redox states play a critical role in regulating many signaling pathways for various biochemical and physiological events, including activation, differentiation, proliferation, and apoptosis (1). Imbalance in redox regulation leads to increased oxidative stress in cells, resulting in an impairment of cellular function, lipid peroxidation (2), degradation of proteins (3), double strand breakage of DNA (4), and cell death (5). The redox balance in cells is maintained by reactive oxygen species (ROS)2 production and antioxidant enzymes involving NADPH, thioredoxin, and thioredoxin (Trx) reductase (TrxR). Trx1 and TrxR1 are localized to cell cytosol/nucleus, whereas Trx2 and TrxR2 are mitochondrial proteins. The active site disulfide of oxidized Trx is reduced by TrxR to a dithiol in reduced Trx, which is the major cell protein disulfide reductase of a cell (6). Reduced Trx is essential for regulating DNA binding of the transcription factor, transferring electrons to ribonucleotide reductase for DNA synthesis and to peroxiredoxins for removal of peroxides (1, 6, 7).
The seleno-organic compound ebselen (2-phenyl-l,2-benzisoselenazol-3(2H)-one) is a lipid soluble compound that exhibits glutathione peroxidase-like activity in vitro (8). Ebselen has been shown to have antioxidant, anti-inflammatory, anti-atherosclerotic, and cytoprotective effects in a large number of studies, both in in vitro and in vivo models (8, 9). Unlike other inorganic selenium compounds and selenomethionine, ebselen is relatively non-toxic to mammals, most likely because its selenium atom is not liberated during biotransformation and therefore not involved in the selenium metabolism of cells (8). Our laboratory has shown that ebselen is an excellent substrate for human thioredoxin reductase, strongly stimulating its hydroperoxide reductase activity and a superfast thioredoxin oxidant (9). Together with a Trx system, ebselen can efficiently reduce hydrogen peroxide, peroxinitrite, and tocopherol-quinone (9–11). Therefore, ebselen has been widely used as an antioxidant and ROS scavenger in experimental models. However, it also has been found that a high concentration (50–100 μm) of ebselen induces depletion of intracellular thiols and apoptosis in human hepatoma cells (12), for which the mechanism remains unclear.
It has been shown that Trx1 and TrxR1 are often overexpressed in tumor cells and show involvement in drug resistance of cancer treatment (6, 13–18). Many anticancer compounds such as the alkylating agents cisplatin (19), cyclophosphamide (20), and arsenic trioxide (21) have been shown to be strong inhibitors of TrxR1. Recent studies have also revealed that several gold-containing compounds inhibited TrxR1 activity in the nanomolar range, such as aurothioglucose (ATG) (IC50 = 65 nm) and auranofin (AF) (IC50 = 20 nm) (22). ATG and AF have been widely used in clinical treatment of rheumatoid arthritis (16). Cells treated with AF underwent apoptosis with inhibition of TrxR1 activity and increased oxidative stress, such as accumulation of high level of ROS (18). In contrast, when cells were treated with ATG, there was no change in the redox state of the cells (23, 24). The mechanism by which the Trx1 redox state is maintained in TrxR1 activity-depleted cells is unclear so far. In addition, differently from AF, ATG exhibited very low cytotoxicity on cells (24, 25). But indeed, TrxR1 activity in vitro and in vivo was inhibited effectively by ATG (22, 26). That is, both ATG and AF inhibit TrxR1 activity in cells, but only AF exhibits cytotoxicity on cells. The mechanism to explain the difference is not clear so far. In previous studies, the relation between Trx system and glutaredoxin (Grx) system was reported. Trotter et al. (27) found that the redox state of the thioredoxin system is maintained independently of the Grx system in vivo. Tan et al. (28) found that the Trx system can function as an alternative system to reduce glutathione disulfide in Saccharomyces cerevisiae. It was also found that in mice, normal cell proliferation without TrxR1 occurred both embryonically and during liver regeneration (29).
In this study, the effects of AF and ATG in HeLa cells were explored and compared. Our result showed that the GSH system was a backup of TrxR1. Excessive oxidation of Trx was the key factor responsible for cell death, rather than the loss of TrxR1 activity. On this basis, we show that ATG and ebselen combined displayed synergistic cytotoxicity on cells through Trx1 oxidation.
EXPERIMENTAL PROCEDURES
Antibodies
Human Trx1 antibody was from IMCO Ltd. (Stockholm, Sweden). Human TrxR1 and anti-human Trx2 antibodies were from Santa Cruz Biotechnology, Inc.
Cell Culture
Human cervical carcinoma HeLa cells were cultured in DMEM medium (Invitrogen) supplemented with 2 mm l-glutamine, 10% (v/v) fetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C in an incubator with 5% CO2.
Cell Viability Assay
HeLa cells were plated at a density of 8 × 103 cells/well in 96-microwell plates and allowed to grow overnight. Then the medium was changed to 200 μl of fresh medium containing the compound to be studied at the appropriate concentration, and incubation was conducted for another 24, 48, or 72 h. Then each well was treated with 50 μl of thiazolyl blue tetrazolium bromide (MTT) (Sigma) solution (2 mg/ml in PBS), and after 4 h of incubation, 150 μl of dimethyl sulfoxide:glycine-NaCl buffer (pH 10.5, 4:1) was added to each well, and plates were shaken for 1 h. Then the cell viability were determined by measuring the absorbance at 550 nm. Mean absorbance for each compound was expressed as a percentage of the control.
Detection of the Trx Redox State in Cells
The redox state of Trx was detected using modified redox Western blot analysis developed from Refs. 30, 31. To prepare mobility standards, cell lysates were denatured with urea and fully reduced with DTT. Then varying molar ratios of iodoacetic acid (IAA) to iodoacetamide (IAM) were incubated with the reduced proteins containing n cysteines, producing n+1 protein isoforms with an introduced number of acidic carboxymethyl thiol adducts (-SA−) and neutral amidomethyl thiol adducts (-SM). During urea-PAGE, the ionized -SA− adducts resulted in faster protein migration toward the anode. Therefore, the n+1 isoforms were separated and used as a mobility standard for representing the number of -SA−. To determine the redox state of thioredoxin in chemical compound-treated cells, HeLa cells were washed twice with PBS and lysed in 300 μl of urea lysis buffer (50 mm Tris-HCl (pH 8.3)/1 mm EDTA/8 M urea) containing 30 mm IAA. Then free thiols were alkylated by IAA at 37 °C for 30 min. After removing the cell debris by centrifugation, the cell lysates were precipitated by ice-cold acetone-HCl. The precipitate was washed with ice-cold acetone-HCl two more times and resuspended in 100 μl of urea lysis buffer containing 3.5 mm DTT. After incubation at 37 °C for 30 min, 5 μl of 200 mm IAM was added to each sample and incubated for 15 min at 37 °C. Then protein concentration was determined by DC protein assay, and equal amounts of protein were separated by urea-PAGE gel and blotted to a nitrocellulose membrane (Bio-Rad). Membranes were probed with the appropriate primary antibody, biotinylated secondary antibody (Dako, Denmark), and streptavidin-alkaline phosphatase anti-biotin tertiary antibody (MABTECH AB, Sweden) and then visualized by using 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate (Sigma). The intensity of each band was quantitated with a Bio-Rad ChemDoc XRS scanning image analysis apparatus and Quantity One software (Bio-Rad).
Reduction of Trx1-S2 (One Disulfide Isoform of Trx1) by GSH or Grx1 in Vitro
Trx1-S2 was prepared as follows. 0.6 mm human Trx1 (IMCO Ltd.) in TE buffer (50 mm Tris-HCl and 1 mm EDTA (pH 7.5)) was reduced by 3.5 mm DTT, and then the sample was desalted on a Sephadex G-25 gel filtration column to remove excess DTT. 90 μm reduced Trx1 was incubated with 45 μm insulin at room temperature for 30 min, and then the sample was spun at 16000 × g for 5 min to remove precipitated insulin. The redox state of Trx1-S2 was confirmed by redox urea-PAGE.
The reduction of Trx1-S2 by GSH or human Grx1 (IMCO Ltd.) was determined by monitoring the absorbance at 340 nm because of NADPH consumption. In a cuvette, the assay system was comprised of 60 nm glutathione reductase, 0.2 mm NADPH, and indicated amounts of GSH, with or without Grx1, in TE buffer. The reaction was initiated by the addition of 50 μm Trx1-S2.
Measurement of TrxR Activity in Vitro
Recombinant rat TrxR1 was a gift from Dr. Elias Arnér, Department of Medical Biochemistry and Biophysics, Karolinska Institutet, and was purified as described (32). Wild-type and mutant C62S/C73S Trx1 were from IMCO Ltd. The wild-type Trx1 was in an oxidized form after storage. Mutant C69S Trx1 was provided by Dr. Douglas A. Mitchell and Prof. Michael A. Marletta, Department of Chemistry, University of California, Berkeley. The activity of TrxR1 was determined by insulin disulfide reduction assay. The reaction solution consisted of 50 mm Tris-HCl and 1 mm EDTA (pH 7.5) (TE buffer). To perform the enzyme assay, 20 nm TrxR1, 0.2 mm NADPH, the indicated amounts of ATG (Sigma), the indicated amounts of ebselen (Daiichi Pharmaceutical Co., Tokyo, Japan) and 3 μm Trx1 were added into the reaction solution, and then reactions were initiated by the addition of 0.16 mm insulin. The activity of TrxR1 was determined by measuring the initial velocity from a linear decrease in absorbance at 340 nm because of NADPH consumption.
Effect of ATG and Ebselen on the Trx System in Vitro
Recombinant rat TrxR1 (4.5 μm) was incubated with NADPH (2.5 mm) in TE buffer for 10 min. Then 10 μm of ATG and/or 50 μm of ebselen were added into the reaction system for 20 min. Finally, 48 μm of Trx1 was added into the reaction system and incubated for 30 min. Proteins were separated using 4–12% NuPAGE Bis-Tris gel (Invitrogen) and transferred to a nitrocellulose membrane (Bio-Rad) by Western blotting. Then the membrane was probed with the appropriate primary antibody and HRP-conjugated secondary antibody and visualized using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences).
Determination of TrxR Activity in Cell Lysates
HeLa cells were plated at a density of 1.5 × 106 cells in 100-mm Petri dishes in DMEM. After overnight incubation at 37 °C, the cells were washed with PBS and treated with different concentrations of compound. After the treatment, the cells were washed with PBS twice and lysed in lysis buffer (25 mm Tris-HCl (pH 7.5)/100 mm NaCl/2.5 mm EDTA/2.5 mm EGTA/20 mm NaF/1 mm Na3VO4/20 mm sodium β-glycerophosphate/10 mm sodium pyrophosphate/0.5% (v/v) Triton X-100/protease inhibitor cocktails (Roche)). The protein concentration was determined by DC protein assay from Bio-Rad. TrxR activity in cell lysates was determined in 96-well plates with a fluorescent insulin assay3. Approximately 10 μg of cell lysates was mixed thoroughly with 80 μl of 50 mm Tris-HCl (pH 7.5)/1 mm EDTA/2.5 μm human Trx1 in a 96-well plate. The reaction solutions without human Trx1 were used as background. After the reaction solutions were incubated at 37 °C for 30 min, 20 μl of 30 μm insulin modified by FITC was added to each well. The emission at 525 nm was recorded after 485 nm excitation using a VICTOR3 multilabel plate reader (PerkinElmer Life Sciences). TrxR1 activity was determined by measuring the initial velocity from a linear fluorescence increase because of reduction of FITC-insulin.
Measurement of ROS Production
Intracellular ROS production was determined fluorometrically using the cell-permeable probe carboxy-H2DCFDA (Molecular Probes/Invitrogen). Carboxy-H2DCFDA was non-fluorescent in reduced form, but after cellular oxidation by intracellular ROS it became fluorescent. This nonpolar compound is converted to the membrane-impermeable polar derivative 2′-7′-dichlorofluorescein after removal of acetate groups by cellular esterases (33). HeLa cells were plated at a density of 2 × 104 cells/well in 96-microwell plates and allowed to grow overnight. Then the medium was changed to 200 μl of fresh medium containing the compound to be studied at the appropriate concentration, and incubation was conducted for another hour. After the removal of media from wells, each well was washed three times with PBS and incubated with 10 μm carboxy-H2DCFDA for 30 min at 37 °C. Then carboxy-H2DCFDA was removed, and each well was washed three times with PBS. The fluorescence intensity was measured immediately at 485 nm excitation and 515 nm emission using an EnSpire Alpha multilabel plate reader (PerkinElmer Life Sciences).
RESULTS
ATG and AF Showed Distinct Effects on HeLa Cell Viability
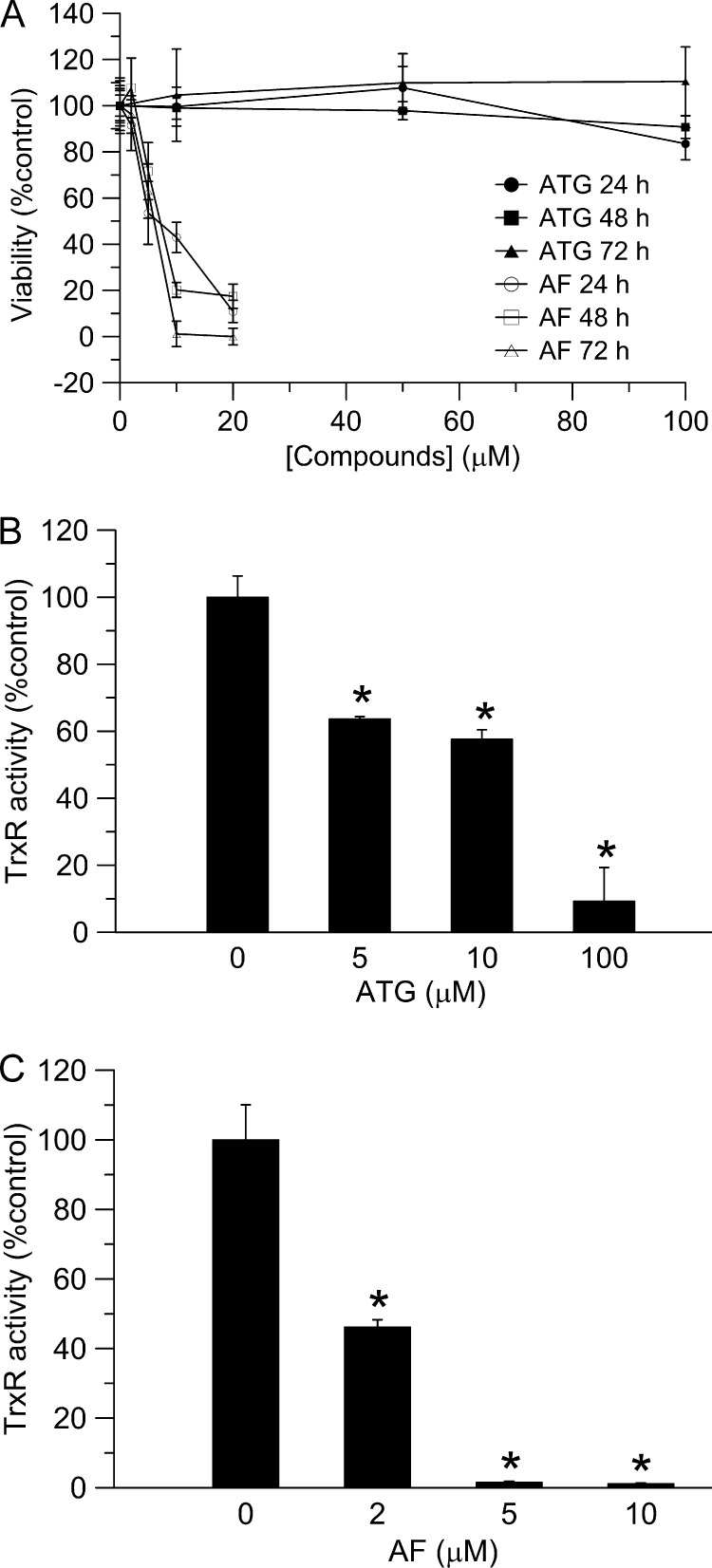
As shown in Fig. 1A, two potent TrxR inhibitors, ATG and AF, exerted distinct effects on HeLa cell viability. AF inhibited cell proliferation at a very low concentration. After 72 h, 10 μm of AF resulted in complete cell death. In contrast, cell viability was unaffected by ATG treatment, even at 100 μm after 72 h. To confirm that both ATG and AF targeted TrxR in HeLa cells and exerted their inhibitory ability effectively, cellular TrxR activity was measured after 24 h using a fluorescent insulin assay that was more sensitive than the classic insulin assay. ATG (100 μm) inhibited TrxR activity by more than 90% in HeLa cells (Fig. 1, B and C), and 10 μm of AF resulted in a 99% decrease in TrxR activity after 24 h. These data indicated that TrxR was inhibited by both ATG and AF. However, a decrease in TrxR activity did not correlate with the viability of HeLa cells treated by the inhibitors, suggesting that cell death cannot be assumed solely on the basis of loss of TrxR activity.
FIGURE 1.
Effects of ATG and AF on cell viability and TrxR activity in HeLa cells. A, HeLa cells were treated with different concentrations of ATG or AF for 24, 48, and 72 h. Cell viability was determined with the MTT assay. Error bars show mean ± S.D. B and C, HeLa cells were treated with different concentrations of ATG (B) or AF (C) for 24 h. After treatment, TrxR activities were determined with the fluorescent insulin assay. Error bars show mean ± S.D. n = 2; *, p < 0.05, Student's t test, ATG or AF treated cells versus control.
Different Redox Responses in HeLa cells Exposed to ATG and AF
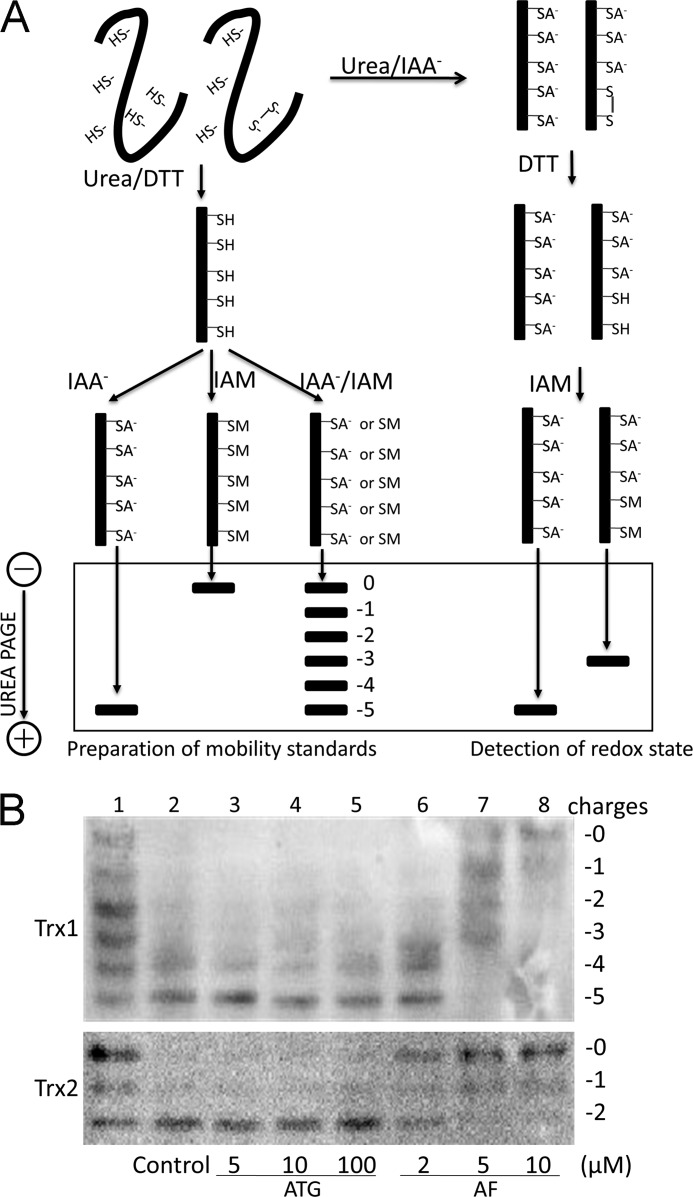
So far, TrxR is the only known enzyme reducing Trx. Therefore, loss of TrxR activity is supposed to result in a cellular redox shift because of oxidation of Trx. To determine the redox state in HeLa cells treated with ATG and AF, we analyzed the redox state of Trx1 and Trx2 in cells using a redox state Western blot analysis (Fig. 2A) and quantified the intensity of each band using Quantity One software (Bio-Rad). As shown in Fig. 2B, in cells without treatment, the redox state of Trx1 was mostly fully reduced (lane 2), in line with previous reports (23, 34, 35). In contrast, as shown in lane 6, in cells treated with 2 μm AF, 40% of the total Trx1 was in the fully reduced form, 36% of the total Trx1 migrated with the −4 mobility, representing a mixed disulfide intermediate with one active site cysteine as a free thiol and the other involved in a disulfide bond to a substrate. A total of 24% of Trx1 migrated with the −3 mobility, representing the one disulfide form of the protein. Because the active site cysteines are more easily oxidized (34), the one disulfide form of Trx1 represented oxidation of the active site. At this concentration of AF, there was a 56% inhibition of TrxR activity in cells (Fig. 1C). Incubation of HeLa cells with 5 μm AF resulted in 98% inhibition of TrxR activity. At the same concentration, there was no fully reduced Trx1 left in cells. 29% of total Trx1 was in the disulfide form, suggesting that all of the active site cysteines were oxidized. Indeed, at this concentration, AF resulted in loss of the Trx1 ability to reduce oxidized substrates. In addition, highly oxidized Trx1 species were observed. 31% of the total Trx1 migrated with the −1 mobility, representing two disulfide bond form. 11% of the total Trx1 migrated with the 0 mobility, representing a fully oxidized form of Trx1, suggesting that Trx1 began to be dimerized because of oxidative stress. At 10 μm AF, more severe oxidation of Trx1 was observed. Most Trx1 was in dimeric form. Importantly, increased level of oxidized Trx2 in HeLa cells exposed to AF also accompanied decreased TrxR activity (Fig. 1C). In addition, it seemed that the oxidation level of Trx2 was synchronous with that of Trx1 accompanying increased AF concentration. However, the redox state of Trx1 and Trx2 in HeLa cells exposed to ATG were unaffected, regardless of the decreased level of total TrxR activity. Exposure of HeLa cells to 100 μm ATG resulted in 90% inhibition of TrxR activity, but no significant oxidation of Trx1 or Trx2 was observed. These results suggested that the viability of HeLa cells was correlated to the redox shift of Trx1 and Trx2 but not TrxR activity. In addition, it seemed that there was no causative relation between loss of TrxR activity and oxidation of Trx, consistent with a previous study (23).
FIGURE 2.
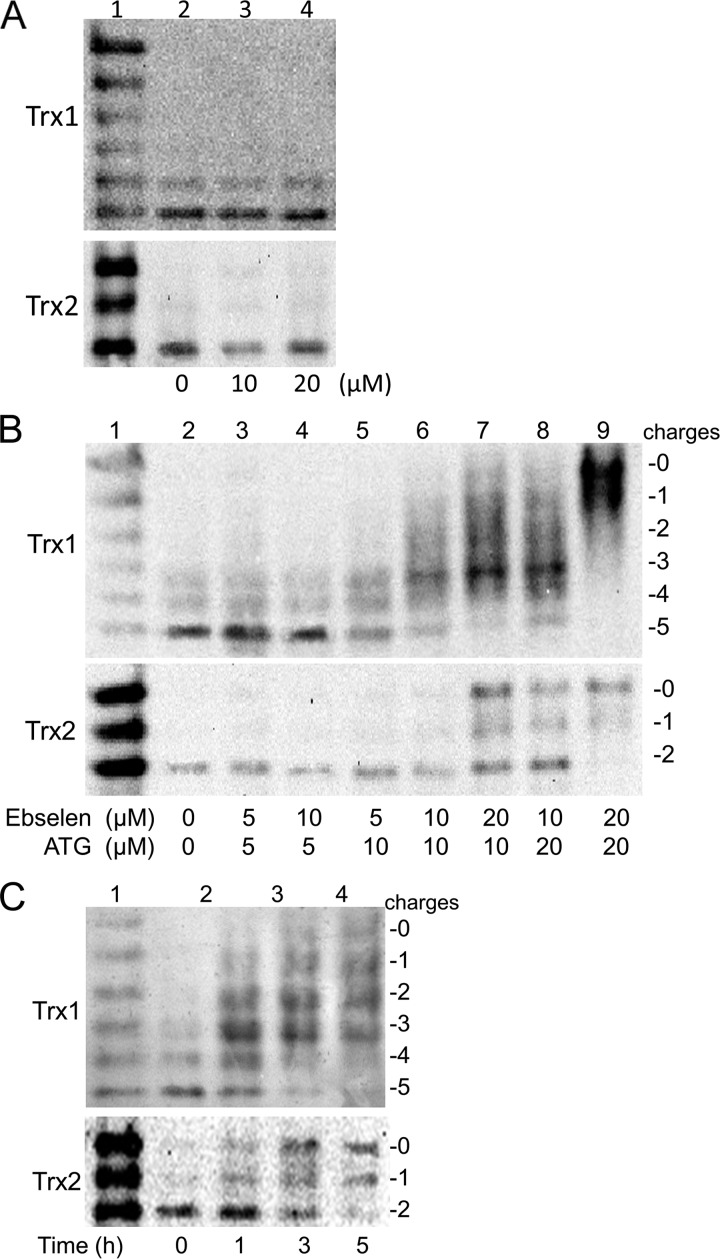
Redox state of Trx1/2 in HeLa cells exposed to ATG and AF. A, principle of redox Western blot analysis. To prepare mobility standards, cell lysates are denatured with urea and fully reduced with DTT. Then, varying molar ratios of IAA to IAM are incubated with the reduced thioredoxin containing five cysteines, producing six protein isoforms with introduced number of acidic carboxymethyl thiol adducts (-SA−) and neutral amidomethyl thiol adducts (-SM). During urea-PAGE, the ionized -SA− group resulted in faster protein migration toward the anode. Therefore, the six isoforms are separated and used as a mobility standard for representing the number of -SA−. To determine the redox state of thioredoxin in compound-treated cells, HeLa cells were lysed in urea lysis buffer containing IAA. After the free thiols of thioredoxin were alkylated by IAA, cell lysates were precipitated by ice-cold acetone-HCl. The precipitate was washed with ice-cold acetone-HCl two more times to remove excess IAA. Then the precipitate was resuspended in urea lysis buffer containing DTT to reduce thioredoxin containing an inter- or intramolecular disulfide bridge. The free thiols of thioredoxin were then alkylated by IAM. The alkylated thioredoxins in cell lysates were separated according to the charge amount. B, HeLa cells were treated by indicated concentrations of ATG (lanes 3-5) or AF (lanes 6-8) for 24 h, and then the redox states of Trx1 and Trx2 in HeLa cells were detected with redox state Western blot analysis under steady-state conditions. Lane 1, mobility standards. Lane 2, HeLa cells without treatment.
Exposure of Various Cells to Ebselen in Combination with ATG Resulted in Cell Death
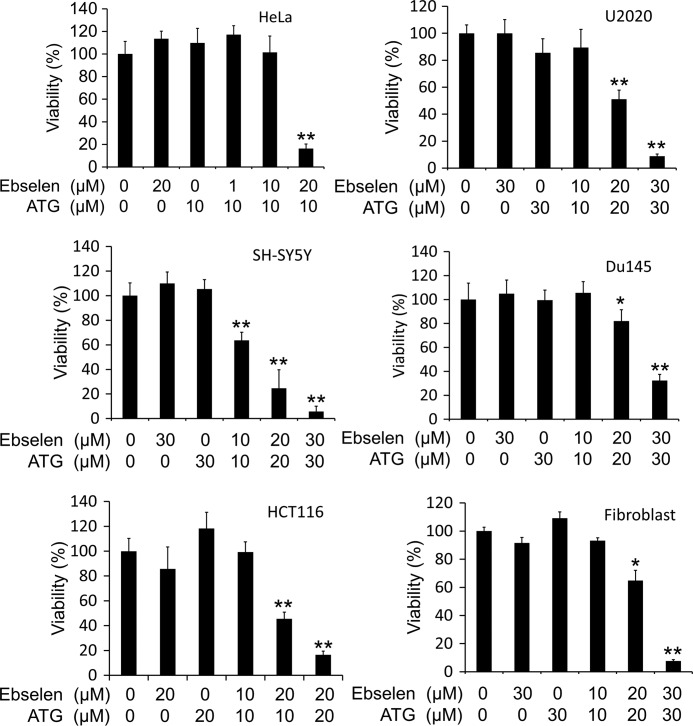
According to the above experiments, it seemed that the redox state of Trx rather than TrxR activity is directly linked to cell viability. Therefore, we speculated that the oxidation of Trx was the key factor responsible for cell death. To test this, ebselen, a superfast oxidant of Trx1 (9), was introduced to treat HeLa cells in combination with ATG, and then the viability of the cells was measured. As shown in Fig. 3, neither ebselen nor ATG affected the viability of HeLa cells at the indicated concentrations and time points. However, when HeLa cells encountered 24 h of combined treatment with ATG and ebselen, a marked synergism in decreasing viability on HeLa cells was observed. To investigate the generality of this phenomenon, the same viability assays were performed with five more cell lines (Fig. 3). The concentration-dependent decrease in viability of these five cell lines exposed to the combined treatment was similar to that of HeLa cells. These results indicate that the oxidation of Trx might be the molecular basis for the combined treatment induced cell death.
FIGURE 3.
Viabilities of human cancer cells exposed to ATG and ebselen. HeLa cells, SH-SY5Y cells, HCT116 cells, U2020 cells, Du145 cells, and fibroblast cells were treated with the indicated concentrations of ATG for 6 h, and then ebselen was added into the medium. After 24 h treatment, the cell viabilities were measured with an MTT assay. Error bars show mean ± S.D. n ≥ 3; *, p < 0.05; **, p < 0.01; Student's t test, treated cells versus control.
Synergic Effects of ATG and Ebselen on the Trx1 System in Vitro
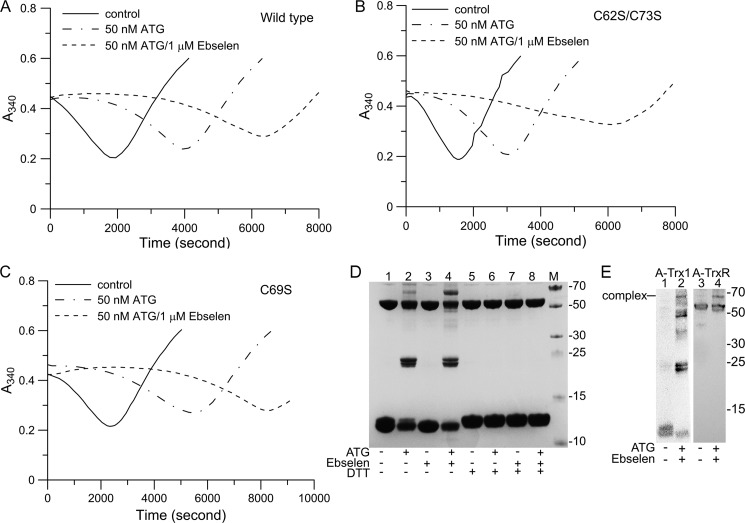
To confirm the synergic effects of ATG and ebselen on the Trx1 system, the activity of the Trx1 system was measured using an insulin disulfide reduction assay. As shown in Fig. 4A, Trx1 system activity was inhibited by 50 nm ATG. When 1 μm ebselen was introduced to the reaction system, there was a pronounced lag phase in the initial reaction phase. We also tested mutant C62S/C73S and C69S Trx1 activity (Fig. 4, B and C). There was no obvious difference between the mutant Trx1 and the wild-type Trx1 under the treatment of ATG and ebselen, indicating that these cysteines of Trx1 were not the target sites of ATG and ebselen. Because ebselen is a substrate of TrxR1 and Trx1, there was only a very small effect on TrxR1 activity using the insulin assay in the presence of ebselen (9). The major effect of ebselen on the Trx1 system is that ebselen rapidly reacted with Trx1 and then resulted in oxidation of Trx1. The oxidized Trx1 was able to be reactivated through reducing catalysis by TrxR1 and NADPH, presenting a lag phase in the reaction curve (36). Because Cys-32 and Cys-35 in the active site of Trx1 are responsible for the protein disulfide reductase activity, they are most important for cell viability.
FIGURE 4.
ATG and ebselen synergically affected the Trx system activity in vitro. Trx system activity was determined using an insulin assay in the presence or absence of ATG and/or ebselen. A, wild-type Trx1. B, C62S/C73S Trx1. C, C69S Trx1. To perform the enzyme assay, 20 nm TrxR1, 0.2 mm NADPH, the indicated amounts of ATG, the indicated amounts of ebselen, and 3 μm Trx1 were added into the reaction solution, and then reactions were initiated by the addition of 0.16 mm insulin. D, 4.5 μm pre-reduced TrxR1 was incubated with 10 μm ATG and/or 50 μm ebselen for 20 min. Then, 48 μm Trx1 was incubated with the mixture for 30 min. The reaction mixtures were then subjected to SDS-PAGE gel in the absence (lanes 1-4) or presence (lanes 5-8) of DTT. M, protein marker. E, the reaction mixtures were subjected to Western blot analysis using Trx1 antibody (lanes 1 and 2) and TrxR1 antibody (lanes 3 and 4).
SDS-PAGE and Western blot analysis also showed that the Trx1 system was inhibited by ATG and ebselen in the presence of NADPH, as expected. Because of loss of TrxR1 activity, Trx1 was not reduced by NADPH and TrxR1, and oxidized Trx1, as added, was still present, as shown in Fig. 4, D and E (24, 36, and 48 kDa). Interestingly, a 67-kDa band was found under the synergic effects of ATG and ebselen, as shown in Fig. 4D, lane 4. To identify this band, identical samples were subjected to two Western blot analyses using Trx1 and TrxR1 antibody, respectively. The results showed that this 67-kDa band was recognized by both Trx1 and TrxR1 antibodies, indicating the formation of a TrxR·Trx complex. (Fig. 4E). This TrxR·Trx complex could be removed by DTT, suggesting that a disulfide bridge formed between TrxR and Trx.
Combined Treatment of HeLa Cells with Ebselen and ATG Induced Oxidation of Trx
The redox state of Trx in HeLa cells treated with either ebselen, ATG, or a combination of the two compounds was determined to give further insight into the molecular basis. As shown in Fig. 5A, the redox states of both Trx1 and Trx2 in HeLa cells were unaffected by ebselen treatment after 24 h, which was not surprising, considering the antioxidant effects of ebselen.
FIGURE 5.
The Redox state of Trx1/2 in HeLa cells exposed to ATG and ebselen. A, HeLa cells were treated with the indicated concentration of ebselen (lanes 2-4) for 24 h, and then the redox states of Trx1 and Trx2 in HeLa cells were detected with a redox state Western blot analysis. Lane 1, mobility standards. B, HeLa cells were treated by the indicated concentrations of ATG for 6 h, and then ebselen was added into the medium (lanes 2-9). After 24 h, the redox state of Trx1 and Trx2 in HeLa cells were detected with a redox state Western blot analysis. Lane 1, mobility standards. C, HeLa cells were treated with 20 μm ATG for 6 h (lane 3-5), and then ebselen was added into the medium (lane 3-5). At the indicated time point, the redox states of Trx1 and Trx2 in HeLa cells were detected with a redox state Western blot analysis. Lane 1, mobility standards. Lane 2, HeLa cells without treatment.
ATG, which blocked TrxR activity without affecting the redox state of Trx, was introduced to treat HeLa cells in combination with ebselen. As shown in Fig. 5B, both Trx1 and Trx2 were oxidized in HeLa cells exposed to the combined drugs in a dose-dependent manner. Incubation of HeLa cells with 10 μm ATG and 10 μm ebselen resulted in a significant oxidation of Trx1. Only 13% of total Trx1 was in the fully reduced form. In contrast, at the same concentration, there was only a slight increase in the oxidized form of Trx2. This result suggested that Trx1 was more sensitive to the combined treatment with ATG and ebselen. Following 10 μm ATG and 20 μm ebselen treatment, only 6% of total Trx1 was in the fully reduced form. The majority of Trx1 migrated with the −3 mobility, representing the disulfide form of Trx1. At this concentration, the amount of the fully reduced form of Trx2 was almost the same as that of the fully oxidized form of Trx2. When the concentrations of both ATG and ebselen were increased to 20 μm, both Trx1 and Trx2 were completely oxidized. To further explore the sensitivity of Trx1 and Trx2 to ATG and ebselen treatment, we detected the time course of the redox state shift of Trx1 and Trx2 in HeLa cells exposed to 20 μm ATG and ebselen. As shown in Fig. 5C, both Trx1 and Trx2 in the cells were oxidized time-dependently. However, apparently, Trx1 was more sensitive to the combined drug treatment. Fig. 5C, lane 3, which was from cells treated for 1 h, showed that 65% of total Trx2 was still in fully reduced form, whereas only 12% of total Trx1 was in the fully reduced form. Even after 3 h (Fig. 5C, lane 4), there was still 35% of total Trx2 remaining fully reduced, whereas there was almost no fully reduced form of Trx1 left in cells.
Redox Imbalance because of Oxidation of Trx Resulted in Accumulation of ROS and Damage of TrxR Activity
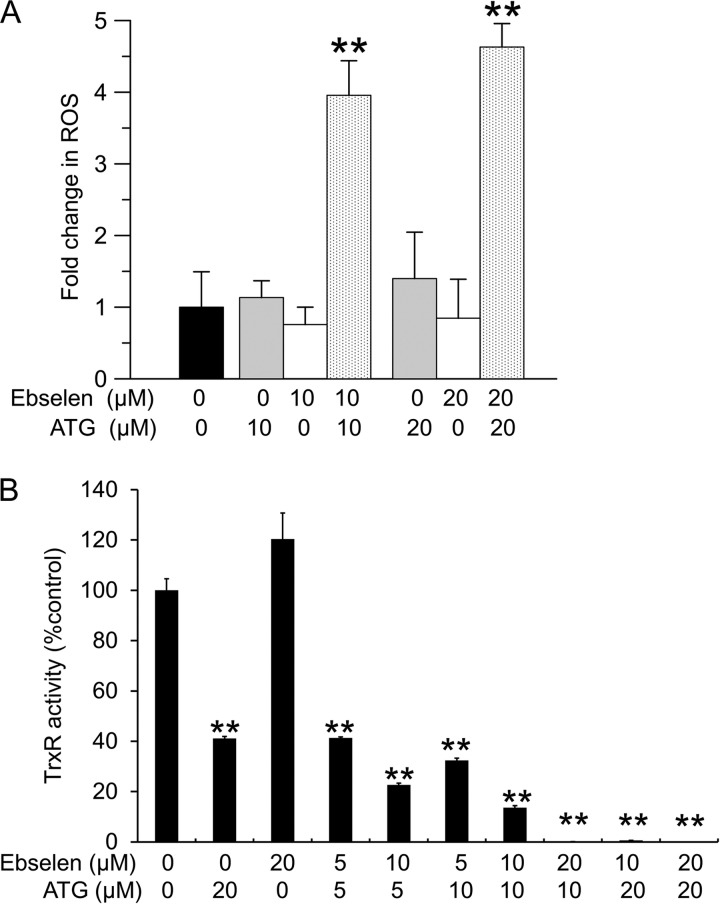
In general, inhibition of the thioredoxin system will lead to increased levels of ROS (37). Therefore, the production of ROS was measured in HeLa cells treated with ATG and ebselen using a carboxy-H2DCFDA probe. As shown in Fig. 6A, there was a slight increase in fluorescence induced by ATG and a slight decrease in fluorescence induced by ebselen, but these effects did not reach statistical significance. However, the combined presence of ATG and ebselen significantly enhanced the production of ROS in HeLa cells.
FIGURE 6.
Measurement of ROS generation and TrxR activity in HeLa cells exposed to ATG and ebselen. A, HeLa cells were treated with the indicated concentrations of ATG or ebselen or ATG plus ebselen for 30 min. After washing with PBS and treatment with 10 μm carboxy-H2DCFDA for 30 min, cells were washed again to remove excess H2DCFDA. Fluorescence was analyzed as described under “Experimental Procedures.” Error bars show mean ± S.D. n ≥ 3. **, p < 0.01, Student's t test, treated cells versus control. B, HeLa cells were treated with different concentrations of ATG or ebselen or ATG plus ebselen for 24 h. After treatment, TrxR activities were determined with a fluorescent insulin assay. Error bars show mean ± S.D. n = 2. **, p < 0.01, Student's t test, treated cells versus control.
TrxR activity in HeLa cells treated by ATG and ebselen after 24 h was measured using the fluorescent insulin assay. Because ebselen was a substrate for TrxR, ebselen did not inhibit TrxR activity in HeLa cells as expected (Fig. 6B). However, the combined treatment of HeLa cells with ATG and ebselen led to stronger loss of TrxR activity than ATG alone, consistent with in vitro results using recombinant TrxR1 and Trx1 (Fig. 4A). These results suggested that ATG and ebselen exerted synergic effects on the Trx1 system, resulting in further inhibition of TrxR1 activity, formation of the TrxR1·Trx1 complex, oxidation of Trx1, and elevation of ROS production.
The Grx System Is a Backup of TrxR1 for Reduction of Trx1
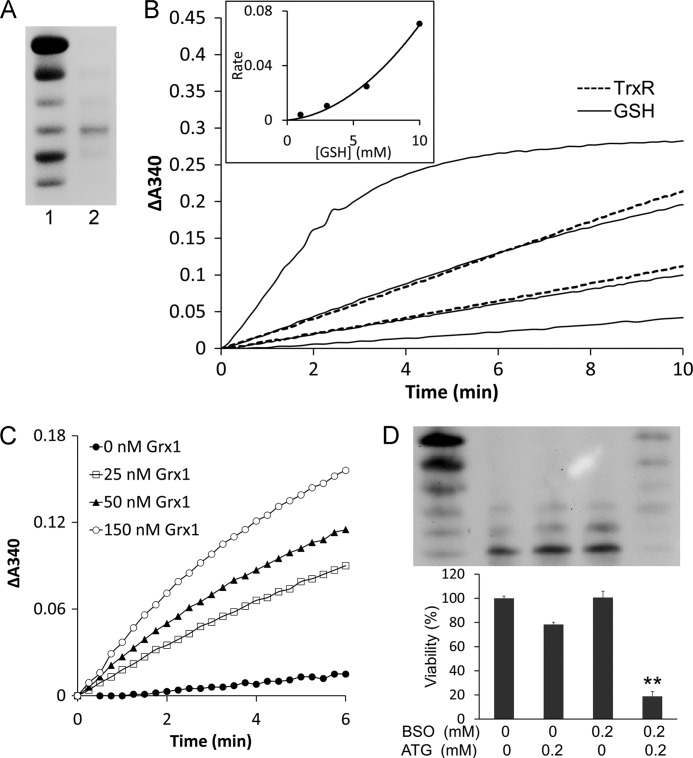
As the redox state of Trx1 in HeLa cells was still reduced even after TrxR1 activity was inhibited, there might be other systems in cells that could reduce oxidized Trx1. The Grx system is an important redox system in cells besides the Trx system. Therefore, we tested whether Trx1 could be reduced by the Grx system. First, we prepared the one disulfide isoform of Trx1 (Trx1-S2) which was confirmed by redox urea-PAGE gel (Fig. 7A). Because the intracellular GSH concentration is in the millimolar range, we determined the reduction efficiency of 0–10 mm GSH by monitoring NADPH oxidation, as shown in Fig. 7B. It showed that the reduction efficiency of 3–6 mm GSH was comparable with 5–10 nm TrxR1. The rates for reduction of Trx1-S2 in Fig. 7B were replotted against concentration of GSH and shown in Fig. 7B, inset, exhibiting a parabolic dependence on GSH concentration, and in line with that, two molecules of GSH reduced one molecule of Trx1-S2. We also found that Grx1 dramatically catalyzed the reduction of Trx1-S2, as shown in Fig. 7C.
FIGURE 7.
The glutathione system was a backup of TrxR1for reduction of Trx1. A, Trx1-S2 was prepared as described under “Experimental Procedures” and confirmed by redox urea-PAGE. Lane 1, mobility standard. Lane 2, Trx1-S2. B, 60 nm glutathione reductase, 0.2 mm NADPH, and 50 μm Trx1-S2 were added to cuvettes for the GSH reduction assay in the presence of 1, 3, 6, and 10 mm GSH (four solid lines from bottom to top). 0.2 mm NADPH and 50 μm Trx1-S2 were added to cuvettes for the TrxR1 reduction assay in the presence of 5 and 10 nm TrxR1 (two dotted lines from bottom to top). The absorbance at 340 nm was followed against an identical blank without Trx1-S2. A second-order polynomial regression line was fit in the inset. C, 60 nm glutathione reductase, 0.2 mm NADPH, 1 mm GSH, indicated amounts of Grx1 and 50 μm Trx1-S2 was added to cuvettes. The absorbance at 340 nm was followed against an identical blank without Trx1-S2. D, HeLa cells were treated with the indicated concentrations of ATG and/or BSO. After 48-h treatment, the redox state of Trx1 was detected using a redox Western blot analysis. The cell viability was measured with an MTT assay. Error bars show mean ± S.D. n = 4. **, p < 0.01, Student's t test, treated cells versus control.
To study the effect of targeting GSH in vivo, we treated HeLa cells simultaneously with ATG and BSO. ATG was able to inhibit cytosolic TrxR1 activity, and BSO would deplete GSH level in cells. As expected, an MTT assay (Fig. 7D) showed that after 48 h neither ATG nor BSO alone showed a significant cytotoxicity, whereas the combined drugs dramatically induced HeLa cell death. Using a redox Western blot analysis, we also found that Trx1 was strongly oxidized after 48 h of combined treatment with ATG and BSO (Fig. 7D).
DISCUSSION
Because TrxR is the only known enzyme able to reduce Trx, its inhibition should lead to oxidation of Trx, resulting in redox imbalance and even cell death. We used two well known TrxR inhibitors, namely AF and ATG, which gave strictly different outcomes in terms of cell-death of the HeLa cells. AF showed a strong proapoptotic effect and has been used in many anticancer and antirheumatic studies. The reason why AF is so toxic may lie in the greater liposolubility that facilitates the penetration of AF into both cytoplasm and mitochondria. Therefore, it inhibits both cytosolic and mitochondrial TrxR, consistent with our activity data. It was reported that AF exhibited signs of oxidative stress, such as a large accumulation of hydrogen peroxide and induced mitochondrial malfunction in cells (18). Another report showed that AF attenuated hydrogen peroxide removal rates in mitochondria by 80% (38). So these reports strongly indicated that mitochondria were the targets of AF for its anticancer effects. We showed oxidation of both Trx1 and Trx2 (Fig. 2) accompanying cell death. In contrast, when cells were treated with ATG alone, there was about 10% TrxR activity left, which might be the contribution of mitochondrial TrxR2. It is likely that ATG will not reach the mitochondria. There was no increase in the production of ROS, as shown in this study and reported previously as well (23). Trx stayed reduced, and cell death did not occur. Thus, although ATG inhibits TrxR activity in vitro and in vivo very effectively (22, 26), ATG shows a low cytotoxicity. In this study, we found that ATG did not induce cell death even when more than 90% of TrxR activity was inhibited (Fig. 1). This finding is consistent with the previous studies suggesting that the reduced redox state of Trx1 was maintained even in cells lacking 90% of their TrxR1 activity, either through ATG treatment or siRNA knockdown (23), for which the mechanism was unclear.
The value of the redox Western blot analysis is illustrated here because the reactivity of all five Cys residues is probed under denaturing conditions. Oxidation of Trx rather than inhibition of TrxR activity was clearly directly correlated to cell death. In a previous study it was found that genetic elimination of TrxR1 had no measurable effect on mouse hepatocyte proliferation, suggesting that TrxR1 activity might not be essential for cell proliferation (39). The cytosol of living cells is highly reducing because of GSH and protein thiols. Trx1 is essential for maintaining the redox state of cytosolic proteins (18, 40). Furthermore, the Trx system is involved in regulating the activity of transcription factors containing critical cysteines in their DNA-binding domains, such as NF-κB, AP-1, p53, and the glucocorticoid receptor in many cellular signaling pathways (40–42). Reduced Trx1 can bind to ASK1 (apoptosis signal-regulating kinase 1) and inhibit its activity, whereas the oxidization of Trx1 results in the activation of ASK1 and the induction of ASK1-dependent apoptosis (41, 42). In addition, reduced Trx is the electron donor to peroxiredoxins and ribonucleotide reductase, which is responsible for the reduction of ribonucleotides to deoxyribonucleotides for DNA synthesis (41, 43). Once Trx1 is oxidized and generates the two-disulfide isoform of Trx1, this overoxidized Trx1 was not a substrate of TrxR1 anymore and was inactive as a protein disulfide reductase (34, 44). Because reduced Trx plays a critical role in cellular proliferation and viability (Scheme 1), excessive oxidation of Trx will lead to cell death (41). Recently, a study showed that oxidation of the yeast mitochondrial thioredoxin promotes cell death (45).
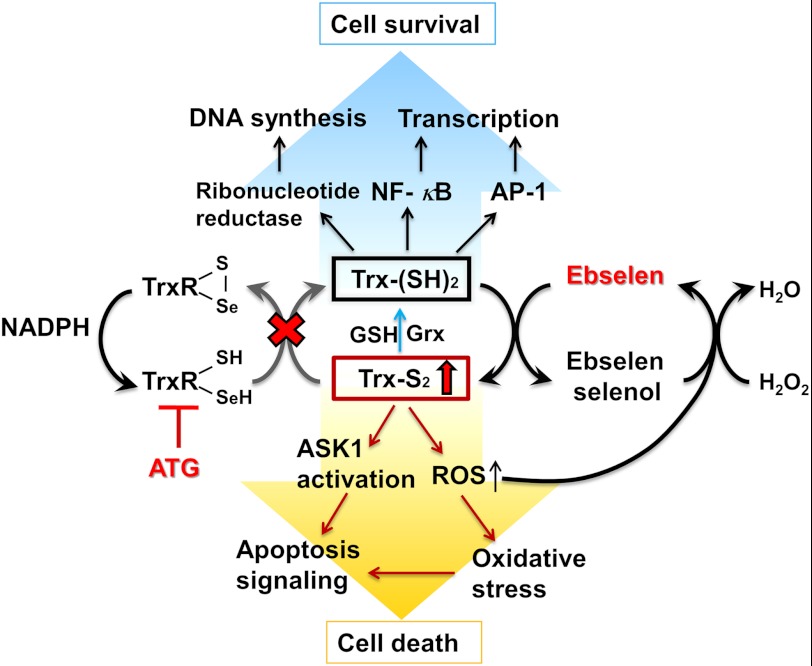
SCHEME 1.
Thioredoxin oxidation caused by ATG and ebselen induced cell death.
In the presence of ebselen, Trx1 can be rapidly oxidized through a very fast reaction with a rate constant that is orders of magnitude faster than the reaction of Trx with insulin disulfides (9). At the same time, depending on reductase activity of TrxR1, oxidized Trx1 is continuously reduced back to reduced form through the thiol-disulfide redox cycle, maintaining the fully reduced and functional form of Trx1 in cells, consistent with our observation using a redox state Western blot analysis (Fig. 5A). However, in the presence of ATG plus ebselen, a strong synergistic effect of cell death was observed. Accumulation of oxidized Trx1 in cells (Scheme 1), consistent with the result in Figs. 4 and 5, was observed. Compared with mitochondrial Trx2, cytosolic Trx1 is more sensitive to the combination treatment with ATG and ebselen, indicating that ATG and ebselen may first target the cytosolic Trx. The malfunction of the cytosolic Trx1 system should result in a high level of oxidative stress, such as accumulation of ROS, subsequently leading to oxidation of the mitochondrial Trx system.
Reduced Trx serves as an electron donor for Trx-linked peroxiredoxins, and inhibition of the thioredoxin system will lead to increased levels of ROS (7, 37), in line with our result in Fig. 6A. The reaction between ebselen and Trx1 starts with the formation of the ebselen selenol reduced by the Trx1 system, and then the ebselen selenol will quickly react with its substrates, such as hydrogen peroxide, reforming ebselen, thus finishing a catalytic cycle (9) (Scheme 1). Therefore, increased ROS will react with the ebselen selenol and, consequently, reform ebselen. Then the continuously reformed ebselen is involved in oxidation of the Trx1 in reduced form, resulting in more severe oxidation of Trx1 in quantity (Fig. 5). Because the two-disulfide form of Trx1 is inactive, this form of Trx1 is not able to reduce Prx1. Accordingly, a higher level of ROS is produced, and then more ebselen selenol is consumed. At last, a vicious circle is formed that results in severe oxidation of Trx and a high level of ROS in cells.
ROS include a number of chemically reactive molecules derived from oxygen, such as the hydroxyl radical, superoxide, and hydrogen peroxide (37, 46). The continuous formation of low levels of ROS is part of the normal O2 metabolism. Low amounts of ROS are involved in the intracellular signaling pathway, regulating both cell survival and cell death (47, 48). However, increased generation of ROS, or inadequacy of the antioxidative and protective systems in the cells, can result in oxidative stress and lead to necrosis or apoptosis (18, 37). The major effect of increased ROS is to threaten the integrity of various biomolecules, including lipids (2), DNA (49), and proteins (3). ROS have been shown to react with several amino acid residues, resulting in less active enzyme or nonfunctional proteins (50, 51). Our previous study has also found that, in the presence of xanthine and the xanthine oxidase system with ROS generation in vitro, TrxR activity was decreased compared with that in the absence of xanthine oxidase without ROS generation (52). Therefore, a high level of ROS can damage various proteins, including TrxR.
The fact that Trx1 stays reduced despite the loss of TrxR1 activity requires a reducing system in the cytosol. We show here that in the presence of NADPH and glutathione reductase, GSH will directly and effectively reduce hTrx1. Importantly, Grx1 strongly stimulated the reaction. The redox potential of human Trx1 is −230 mV (31), which is significantly higher than that of Escherichia coli Trx with −270 mV (37). Because in vivo redox measurements in yeast and mammalian cells of GSH confirm a very reduced state for the cytosolic GSH/GSSG pool of −290 to −300 mV (53–55), the reaction is thermodynamically possible. In addition, our results show that the disulfide form of Trx1 is an excellent substrate for Grx1, as shown in Fig. 7C. Also, recent in vivo data show that either glutathione or a single allele of the TrxR-dependent redox pathway can independently support hepatocyte DNA replication and proliferation (29).
In summary, our studies show that the glutaredoxin system with glutathione plays a backup role to keep Trx1 reduced in cells with loss of TrxR1 activity. We also found a novel combination of two anti-inflammatory drugs (ATG and ebselen) that showed strong synergistic effects on cell death and Trx oxidation. Because oxidation of Trx cannot be deduced from measuring TrxR or Trx activity alone in cells, the redox Western blot analysis method should be employed when attributing oxidation of Trx to a TrxR inhibitor. On the basis of the results of this study, a combined inhibition of TrxR and Trx, or a combined depletion of TrxR activity and GSH, should be sufficient for inducing cell death. Further studies should be performed to determine whether tumors in vivo can be specifically targeted by the synergistic effect of ATG and ebselen.
This work was supported by the Swedish Research Council Medicine, the Swedish Cancer Society, and the K.A. Wallenberg Foundation.
S. Montano, J. Lu, and A. Holmgren, manuscript in preparation.
- ROS
- reactive oxygen species
- Trx
- thioredoxin
- TrxR
- thioredoxin reductase
- ATG
- aurothioglucose
- AF
- auranofin
- Grx
- glutaredoxin
- IAA
- iodoacetic acid
- IAM
- iodoacetamide
- BSO
- buthionine sulfoximine.
REFERENCES
- 1. Kondo N., Nakamura H., Masutani H., Yodoi J. (2006) Redox regulation of human thioredoxin network. Antioxid. Redox Signal. 8, 1881–1890 [DOI] [PubMed] [Google Scholar]
- 2. Ylä-Herttuala S. (1999) Oxidized LDL and atherogenesis. Ann. N.Y. Acad. Sci. 874, 134–137 [DOI] [PubMed] [Google Scholar]
- 3. Stadtman E. R., Levine R. L. (2000) Protein oxidation. Ann. N.Y. Acad. Sci. 899, 191–208 [DOI] [PubMed] [Google Scholar]
- 4. Sallmyr A., Fan J., Rassool F. V. (2008) Genomic instability in myeloid malignancies. Increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Lett. 270, 1–9 [DOI] [PubMed] [Google Scholar]
- 5. Berg D., Youdim M. B., Riederer P. (2004) Redox imbalance. Cell Tissue Res. 318, 201–213 [DOI] [PubMed] [Google Scholar]
- 6. Arnér E. S., Holmgren A. (2006) The thioredoxin system in cancer. Semin. Cancer Biol. 16, 420–426 [DOI] [PubMed] [Google Scholar]
- 7. Ahsan M. K., Lekli I., Ray D., Yodoi J., Das D. K. (2009) Redox regulation of cell survival by the thioredoxin super-family. An implication of redox gene therapy in the heart. Antioxid. Redox Signal. 11, 2741–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sies H. (1993) Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic. Biol. Med. 14, 313–323 [DOI] [PubMed] [Google Scholar]
- 9. Zhao R., Masayasu H., Holmgren A. (2002) Ebselen. A substrate for human thioredoxin reductase strongly stimulating its hydroperoxide reductase activity and a superfast thioredoxin oxidant. Proc. Natl. Acad. Sci. 99, 8579–8584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao R., Holmgren A. (2002) A novel antioxidant mechanism of ebselen involving ebselen diselenide, a substrate of mammalian thioredoxin and thioredoxin reductase. J. Biol. Chem. 277, 39456–39462 [DOI] [PubMed] [Google Scholar]
- 11. Fang J., Zhong L., Zhao R., Holmgren A. (2005) Ebselen. A thioredoxin reductase-dependent catalyst for α-tocopherol quinone reduction. Toxicol. Appl. Pharmacol. 207, 103–109 [DOI] [PubMed] [Google Scholar]
- 12. Yang C. F., Shen H. M., Ong C. N. (2000) Ebselen induces apoptosis in HepG2 cells through rapid depletion of intracellular thiols. Arch. Biochem. Biophys. 374, 142–152 [DOI] [PubMed] [Google Scholar]
- 13. Yoo M. H., Xu X. M., Carlson B. A., Patterson A. D., Gladyshev V. N., Hatfield D. L. (2007) Targeting thioredoxin reductase 1 reduction in cancer cells inhibits self-sufficient growth and DNA replication. PLoS ONE 2, e1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoo M. H., Xu X. M., Carlson B. A., Gladyshev V. N., Hatfield D. L. (2006) Thioredoxin reductase 1 deficiency reverses tumor phenotype and tumorigenicity of lung carcinoma cells. J. Biol. Chem. 281, 13005–13008 [DOI] [PubMed] [Google Scholar]
- 15. Becker K., Gromer S., Schirmer R. H., Müller S. (2000) Thioredoxin reductase as a pathophysiological factor and drug target. Eur. J. Biochem. 267, 6118–6125 [DOI] [PubMed] [Google Scholar]
- 16. Bindoli A., Rigobello M. P., Scutari G., Gabbiani C., Casini A., Messori L. (2009) Thioredoxin reductase. A target for gold compounds acting as potential anticancer drugs. Coord. Chem. Rev. 253, 1692–1707 [Google Scholar]
- 17. Urig S., Becker K. (2006) On the potential of thioredoxin reductase inhibitors for cancer therapy. Semin. Cancer Biol. 16, 452–465 [DOI] [PubMed] [Google Scholar]
- 18. Tonissen K. F., Di Trapani G. (2009) Thioredoxin system inhibitors as mediators of apoptosis for cancer therapy. Mol. Nutr. Food Res. 53, 87–103 [DOI] [PubMed] [Google Scholar]
- 19. Sasada T., Nakamura H., Ueda S., Sato N., Kitaoka Y., Gon Y., Takabayashi A., Spyrou G., Holmgren A., Yodoi J. (1999) Possible involvement of thioredoxin reductase as well as thioredoxin in cellular sensitivity to cis-diamminedichloroplatinum (II). Free Radic. Biol. Med. 27, 504–514 [DOI] [PubMed] [Google Scholar]
- 20. Wang X., Zhang J., Xu T. (2007) Cyclophosphamide as a potent inhibitor of tumor thioredoxin reductase in vivo. Toxicol. Appl. Pharmacol. 218, 88–95 [DOI] [PubMed] [Google Scholar]
- 21. Lu J., Chew E. H., Holmgren A. (2007) Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc. Natl. Acad. Sci. 104, 12288–12293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gromer S., Arscott L. D., Williams C. H., Jr., Schirmer R. H., Becker K. (1998) Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem. 273, 20096–20101 [DOI] [PubMed] [Google Scholar]
- 23. Watson W. H., Heilman J. M., Hughes L. L., Spielberger J. C. (2008) Thioredoxin reductase-1 knock down does not result in thioredoxin-1 oxidation. Biochem. Biophys. Res. Commun. 368, 832–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Traber K. E., Okamoto H., Kurono C., Baba M., Saliou C., Soji T., Packer L., Okamoto T. (1999) Anti-rheumatic compound aurothioglucose inhibits tumor necrosis factor α-induced HIV-1 replication in latently infected OM10.1 and Ach2 cells. Int. Immunol. 11, 143–150 [DOI] [PubMed] [Google Scholar]
- 25. Pourahmad J., Ross S., O'Brien P. J. (2001) Lysosomal involvement in hepatocyte cytotoxicity induced by Cu2+ but not Cd2+. Free Radic. Biol. Med. 30, 89–97 [DOI] [PubMed] [Google Scholar]
- 26. Smith A. D., Guidry C. A., Morris V. C., Levander O. A. (1999) Aurothioglucose inhibits murine thioredoxin reductase activity in vivo. J. Nutr. 129, 194–198 [DOI] [PubMed] [Google Scholar]
- 27. Trotter E. W., Grant C. M. (2003) Non-reciprocal regulation of the redox state of the glutathione-glutaredoxin and thioredoxin systems. EMBO Rep. 4, 184–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tan S. X., Greetham D., Raeth S., Grant C. M., Dawes I. W., Perrone G. G. (2010) The thioredoxin-thioredoxin reductase system can function in vivo as an alternative system to reduce oxidized glutathione in Saccharomyces cerevisiae. J. Biol. Chem. 285, 6118–6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prigge J. R., Eriksson S., Iverson S. V., Meade T. A., Capecchi M. R., Arnér E. S., Schmidt E. E. (2012) Hepatocyte DNA replication in growing liver requires either glutathione or a single allele of txnrd1. Free Radic. Biol. Med. 52, 803–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takahashi N., Hirose M. (1990) Determination of sulfhydryl groups and disulfide bonds in a protein by polyacrylamide gel electrophoresis. Anal. Biochem. 188, 359–365 [DOI] [PubMed] [Google Scholar]
- 31. Bersani N. A., Merwin J. R., Lopez N. I., Pearson G. D., Merrill G. F. (2002) Protein electrophoretic mobility shift assay to monitor redox state of thioredoxin in cells. Methods Enzymol. 347, 317–326 [DOI] [PubMed] [Google Scholar]
- 32. Rengby O., Johansson L., Carlson L. A., Serini E., Vlamis-Gardikas A., Kårsnäs P., Arnér E. S. (2004) Assessment of production conditions for efficient use of Escherichia coli in high-yield heterologous recombinant selenoprotein synthesis. Appl. Environ. Microbiol. 70, 5159–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eruslanov E., Kusmartsev S. (2010) Identification of ROS using oxidized DCFDA and flow cytometry. Methods Mol. Biol. 594, 57–72 [DOI] [PubMed] [Google Scholar]
- 34. Watson W. H., Pohl J., Montfort W. R., Stuchlik O., Reed M. S., Powis G., Jones D. P. (2003) Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. J. Biol. Chem. 278, 33408–33415 [DOI] [PubMed] [Google Scholar]
- 35. Myers J. M., Myers C. R. (2009) The effects of hexavalent chromium on thioredoxin reductase and peroxiredoxins in human bronchial epithelial cells. Free Radic. Biol. Med. 47, 1477–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holmgren A. (1979) Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J. Biol. Chem. 254, 9627–9632 [PubMed] [Google Scholar]
- 37. Nordberg J., Arnér E. S. (2001) Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 31, 1287–1312 [DOI] [PubMed] [Google Scholar]
- 38. Drechsel D. A., Patel M. (2010) Respiration-dependent H2O2 removal in brain mitochondria via the thioredoxin/peroxiredoxin system. J. Biol. Chem. 285, 27850–27858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rollins M. F., van der Heide D. M., Weisend C. M., Kundert J. A., Comstock K. M., Suvorova E. S., Capecchi M. R., Merrill G. F., Schmidt E. E. (2010) Hepatocytes lacking thioredoxin reductase 1 have normal replicative potential during development and regeneration. J. Cell Sci. 123, 2402–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meyer Y., Buchanan B. B., Vignols F., Reichheld J. P. (2009) Thioredoxins and glutaredoxins: unifying elements in redox biology. Annu. Rev. Genet. 43, 335–367 [DOI] [PubMed] [Google Scholar]
- 41. Lillig C. H., Holmgren A. (2007) Thioredoxin and related molecules-from biology to health and disease. Antioxid. Redox Signal. 9, 25–47 [DOI] [PubMed] [Google Scholar]
- 42. Lu J., Holmgren A. (2009) Selenoproteins. J. Biol. Chem. 284, 723–727 [DOI] [PubMed] [Google Scholar]
- 43. Holmgren A. (1989) Thioredoxin and glutaredoxin systems. J. Biol. Chem. 264, 13963–13966 [PubMed] [Google Scholar]
- 44. Holmgren A. (1977) Bovine thioredoxin system. Purification of thioredoxin reductase from calf liver and thymus and studies of its function in disulfide reduction. J. Biol. Chem. 252, 4600–4606 [PubMed] [Google Scholar]
- 45. Greetham D., Kritsiligkou P., Watkins R. H., Carter X., Parkin J., Grant C. M. (2012) Oxidation of the yeast mitochondrial thioredoxin promotes cell death. Antioxid. Redox Signal. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Forman H. J., Maiorino M., Ursini F. (2010) Signaling Functions of reactive oxygen species. Biochemistry 49, 835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mustacich D., Powis G. (2000) Thioredoxin reductase. Biochem. J. 346, 1–8 [PMC free article] [PubMed] [Google Scholar]
- 48. Azad M. B., Chen Y., Gibson S. B. (2009) Regulation of autophagy by reactive oxygen species (ROS). Implications for cancer progression and treatment. Antioxid. Redox Signal. 11, 777–790 [DOI] [PubMed] [Google Scholar]
- 49. Marnett L. J. (2000) Oxyradicals and DNA damage. Carcinogenesis 21, 361–370 [DOI] [PubMed] [Google Scholar]
- 50. Butterfield D. A., Koppal T., Howard B., Subramaniam R., Hall N., Hensley K., Yatin S., Allen K., Aksenov M., Aksenova M., Carney J. (1998) Structural and functional changes in proteins induced by free radical-mediated oxidative stress and protective action of the antioxidants N-tert-butyl-α-phenylnitrone and vitamin E. Ann. N.Y. Acad. Sci. 854, 448–462 [DOI] [PubMed] [Google Scholar]
- 51. Stadtman E. R., Berlett B. S. (1998) Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab. Rev. 30, 225–243 [DOI] [PubMed] [Google Scholar]
- 52. Lu J., Papp L. V., Fang J., Rodriguez-Nieto S., Zhivotovsky B., Holmgren A. (2006) Inhibition of Mammalian thioredoxin reductase by some flavonoids. Implications for myricetin and quercetin anticancer activity. Cancer Res. 66, 4410–4418 [DOI] [PubMed] [Google Scholar]
- 53. Rothwarf D. M., Scheraga H. A. (1992) Equilibrium and kinetic constants for the thiol-disulfide interchange reaction between glutathione and dithiothreitol. Proc. Natl. Acad. Sci. 89, 7944–7948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Østergaard H., Tachibana C., Winther J. R. (2004) Monitoring disulfide bond formation in the eukaryotic cytosol. J. Cell Biol. 166, 337–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gutscher M., Pauleau A. L., Marty L., Brach T., Wabnitz G. H., Samstag Y., Meyer A. J., Dick T. P. (2008) Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 5, 553–559 [DOI] [PubMed] [Google Scholar]