Background: Inheritable NOR polyagglutination is a rare phenomenon caused by the unusual Gal(α1–4)GalNAc glycolipid epitope.
Results: A point mutation, 631 C>G, in the gene encoding Gb3/CD77 synthase causes the enzyme to synthesize both Gal(α1–4)Gal- and Gal(α1–4) GalNAc- moieties.
Conclusion: The results pinpoint the cause of the NOR phenotype.
Significance: This is the first report of an altered acceptor specificity of a glycosyltransferase caused by a point mutation.
Keywords: Carbohydrate, Carbohydrate Glycoconjugate, Erythrocyte, Glycolipids, Glycosyltransferases, Gb3/CD77 Synthase, NOR Polyagglutination, Pk Transferase, Single Nucleotide Mutation
Abstract
Rare polyagglutinable NOR erythrocytes contain three unique globoside (Gb4Cer) derivatives, NOR1, NORint, and NOR2, in which Gal(α1–4), GalNAc(β1–3)Gal(α1–4), and Gal(α1–4)GalNAc(β1–3)Gal(α1–4), respectively, are linked to the terminal GalNAc residue of Gb4Cer. NOR1 and NOR2, which both terminate with a Gal(α1–4)GalNAc- sequence, react with anti-NOR antibodies commonly present in human sera. While searching for an enzyme responsible for the biosynthesis of Gal(α1–4)GalNAc, we identified a mutation in the A4GALT gene encoding Gb3/CD77 synthase (α1,4-galactosyltransferase). Fourteen NOR-positive donors were heterozygous for the C>G mutation at position 631 of the open reading frame of the A4GALT gene, whereas 495 NOR-negative donors were homozygous for C at this position. The enzyme encoded by the mutated gene contains glutamic acid instead of glutamine at position 211 (substitution Q211E). To determine whether this mutation could change the enzyme specificity, we transfected a teratocarcinoma cell line (2102Ep) with vectors encoding the consensus Gb3/CD77 synthase and Gb3/CD77 synthase with Glu at position 211. The cellular glycolipids produced by these cells were analyzed by flow cytometry, high-performance thin-layer chromatography, enzymatic degradation, and MALDI-TOF mass spectrometry. Cells transfected with either vector expressed the P1 blood group antigen, which was absent from untransfected cells. Cells transfected with the vector encoding the Gb3/CD77 synthase with Glu at position 211 expressed both P1 and NOR antigens. Collectively, these results suggest that the C631G mutation alters the acceptor specificity of Gb3/CD77 synthase, rendering it able to catalyze synthesis of the Gal(α1–4)Gal and Gal(α1–4)GalNAc moieties.
Introduction
Rare inheritable NOR polyagglutination derives its name from Norton, VA, where the first case was reported (1). A similar phenotype was then described in the Polish T.S. family (2). These two families are the only cases of NOR polyagglutination described so far. The erythrocytes of NOR-positive individuals from the Polish T.S. family were found to contain unique neutral glycosphingolipids formed by the elongation of globoside (3, 4): NOR1, Gal(α1–4)GalNAc(β1–3)Gal(α1–4)Gal(β1–4)GlcCer; NORint, GalNAc(β1–3)Gal(α1–4)GalNAc(β1–3)Gal(α1–4)Gal(β1–4)GlcCer; and NOR2, Gal(α1–4)GalNAc(β1–3)Gal(α1–4)GalNAc(β1–3)Gal(α1–4)Gal(β1–4)GlcCer. Identical NOR glycolipids were later identified in erythrocytes from a NOR-positive donor from the American family (5). The NOR glycolipids contain a unique Gal(α1–4)GalNAc- moiety that has not been described in any other mammal. The glycolipids that terminate with Gal(α1–4)GalNAc (NOR1 and NOR2) are recognized by anti-NOR antibodies (6, 7), which are commonly present in human sera. The NOR-positive individuals apparently do not show any morbid symptoms, but their erythrocytes are agglutinated by most human sera, creating a risk of complications during transfusion of NOR erythrocytes.
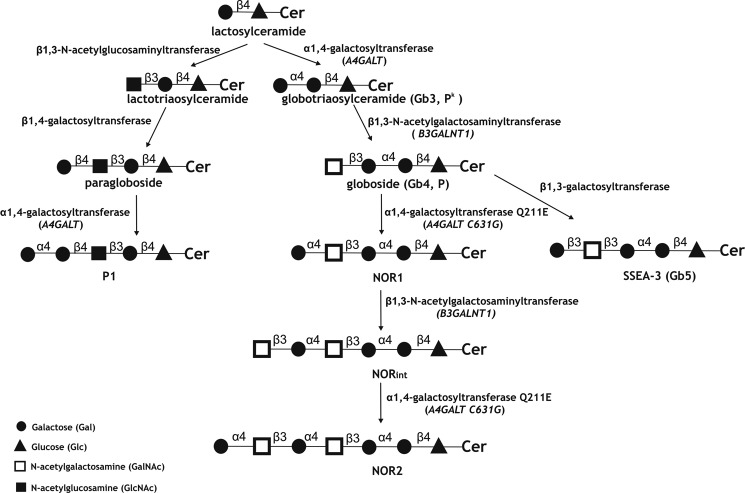
NOR antigens are synthesized by the consecutive transfer of Galα and GalNAcβ residues to Gb4Cer (globoside) (Fig. 1). The linkage of GalNAc is identical to that in Gb4Cer and is synthesized by Gb4 synthase (β1,3-GalNAc transferase), whereas the enzyme responsible for the transfer of Gal to GalNAc was previously unknown. The NOR glycolipids were believed to arise through a mutation in one of the known glycosyltransferases and the expression of an enzyme with altered specificity. The structure that is the most similar to Gal(α1–4)GalNAc is Gal(α1–4)Gal, which is present in glycolipids from the P1PK (formerly P) blood group system (8). Serologically, the P1PK blood group system consists of one antigen, P1, whose presence or absence determines the P1 (P1-positive) or P2 (P1-negative) blood groups, respectively. The P1 antigen is a pentaglycosylceramide of the neolacto series. Its structure of Gal(α1–4)Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)GlcCer was established relatively early (9), but the α1,4-galactosyltransferase responsible for its synthesis remained unknown for quite some time. Meanwhile, numerous studies focused on α1,4-galactosyltransferase (Gb3/CD77 synthase), which is encoded by the A4GALT locus and is responsible for the biosynthesis of Gb3Cer (Gal(α1–4)Gal(β1–4)GlcCer, also known as Pk or CD77). Pk is the direct precursor of globoside (Gb4Cer, P antigen), an abundant glycolipid of human erythrocytes. Various critical mutations in the A4GALT gene may abolish the expression of active α1,4-galactosyltransferase, resulting in a rare p phenotype characterized by the absence of Pk, P, and the P1 antigen (10–13). These findings and other experimental data strongly suggested that the Pk and P1 antigens are synthesized by the same enzyme (14). Recently, a C/T polymorphism within exon 2a of the A4GALT gene was shown to predict the P1 or P2 genotypes and give rise to a novel open reading frame encoding a 28-amino acid peptide (15). However, no report has yet described a possible role for this peptide or a molecular link between the described mutation and P1/P2 status. The A4GALT transcript level was found to be higher in the P1 phenotype compared with P2 and showed a correlation with the P1/P2 genotype (14, 15). It suggested that expression of the P1 antigen requires a high level of enzyme-encoding mRNA because of its lower activity toward neolactotetraosylceramide compared with LacCer (Gal(β1–4)GlcCer) as an acceptor substrate (Fig. 1) (14).
FIGURE 1.
Schematic representation of the biosynthesis of the Pk, P, P1, and NOR antigens. The symbols are as recommended by Varki et al. (33). Cer, ceramide. The biosynthesis-related glycosyltransferases are named.
These data and the structural similarities between Gal(α1–4)GalNAc and Gal(α1–4)Gal prompted us to study the Gb3/CD77 synthase in NOR-positive individuals. The results presented in this paper provide evidence that the NOR antigen emerges as the result of a single nucleotide mutation in the A4GALT gene encoding the Gb3/CD77 synthase.
EXPERIMENTAL PROCEDURES
Blood Samples and DNA Preparation
Blood samples representing NOR-positive and NOR-negative donors were obtained from the Regional Center of Transfusion Medicine and Blood Bank (Wrocław, Poland). The blood sample from an American NOR-positive donor was kindly supplied by Dr. John J. Moulds (Shreveport, LA). Genomic DNA was isolated from peripheral blood leukocytes using an Invisorb Spin Blood Midi kit (Invitek, Berlin, Germany) according to the instructions of the manufacturer.
PCR Amplification and DNA Sequencing
The coding region of the A4GALT gene was amplified by PCR using primers PkFor and PkRev. All primers are listed in supplemental Table 1. PCR was performed using an MJ Mini gradient PCR apparatus (Bio-Rad) in 20-μl reaction mixes containing 200 ng of genomic DNA, 0.2 mm dNTPs, Taq buffer with KCl (1:10 dilution), 1.5 mm MgCl2, 0.2 mm forward and reverse primers, and 1 unit of Taq polymerase (Fermentas, Vilnius, Lithuania). The PCR conditions are shown in supplemental Table 2. The resulting DNA fragments were purified with a gel extraction kit (Gel-Out kit; A&A Biotechnology, Gdynia, Poland), and the amplified products (1233 bp) were sequenced using primers PkSeqFor and PkSeqRev.
TaqMan SNP Genotyping Assay
A Custom TaqMan SNP genotyping assay (Applied Biosystems, Carlsbad, CA) was used to analyze the frequency of the A4GALT C631G mutation in the group of 470 individuals of Polish ethnicity. Real-time PCR was performed using an Applied Biosystems 7500 Fast apparatus (Applied Biosystems) with TaqMan probes (TMvic and TMfam) and primers TM For and TM Rev. The utilized 25-μl reaction mixtures contained 16 ng of genomic DNA, 12.5 μl of TaqMan Genotyping MasterMix, and 0.625 μl Custom TaqMan SNP genotyping assay for the C631G mutation. Data were analyzed using the 7500 sequence detection software version 1.3.1 (Applied Biosystems).
Construction of Expression Vectors
The coding region of the A4GALT gene was PCR-amplified from the genomic DNA of NOR-positive and NOR-negative donors and cloned into the pGEM-T easy vector (Promega, Madison, WI). Using this construct as the template, the A4GALT coding region was amplified with primers PkXho For and PkNot Rev, which introduced restriction sites into the resulting product. The complete sequence was cloned into the pGEM-T easy vector, which was then digested with XhoI/NotI (Fermentas). The resulting fragment was cloned into the pCAG vector (kindly provided by Dr. Peter W. Andrews, University of Sheffield, Sheffield, UK). The desired sequence was confirmed by nucleotide sequencing using primers PkSeqFor and PkSeqRev.
Mutagenesis
Site-directed mutagenesis was performed using overlap-extension PCR as described in Ref. 16. In the first reaction, two fragments of A4GALT gene were created, each containing the overlapping site with an introduced mutation. In the second reaction, the PCR products were duplexed to generate new template DNA. During the overlap extension phase, each fused product was amplified using primers complementary to the pCAG vector (PCAG For and PCAG Rev). The resulting full-length gene fragments were then ligated into the pCAG vector and confirmed by sequencing. The primers used for creation of the mutated A4GALT gene are as follows: for the consensus Gb3/CD77 synthase, PkWTmut1For and pCAGRev (I fragment) and PkWTmut2For and pCAGFor (II fragment); and for the Gb3/CD77 synthase with Glu at position 211, PkQ211Emut1For and pCAGRev (I fragment) and PkQ211Emut2For and pCAGFor (II fragment). Their sequences and applications are listed in supplemental Table S1. The schematic representation of different forms of the A4GALT gene is shown in supplemental Fig. S1.
Cell Culture and Transfection
2102Ep teratocarcinoma cells (kindly provided by Dr. Peter W. Andrews, University of Sheffield) (17) were grown in DMEM containing 4.5 g/liter glucose (Invitrogen), 10% fetal calf serum (Invitrogen), and 2 mm GlutaMAX (Invitrogen) in a 37 °C humidified 5% CO2 atmosphere. Cultures were passaged by treatment with 0.25% trypsin/1 mm EDTA. For experiments, cells were harvested, centrifuged, resuspended in fresh medium, and seeded to fresh tissue culture plates. One day before transfection, cells were seeded to six-well plates (2 × 105 cells per well). Monolayers were ∼60% confluent on the day of transfection. The medium was replaced with fresh DMEM, and 4 h later the cells were transfected with 9.5 μg of plasmid DNA using polyethylenimine (Polysciences, Warrington, PA). Briefly, plasmid DNA was diluted to the final concentration 15.8 μg/ml with buffer containing 0.15 m NaCl and 20 mm HEPES (pH 7.5) and then mixed with 35 μg of polyethylenimine. The transfection mixture was incubated for 20 min at room temperature and then added to each well. For stable transfection assays, the cells were subjected to drug selection 48 h after transfection using 0.44 μg/ml puromycin (Sigma-Aldrich, St. Louis, MO).
Antibodies
The mouse monoclonal anti-NOR antibody, nor118, was obtained as described (7) and used as a properly diluted culture supernatant. It is highly specific for Gal(α1–4)GalNAc and reacts exclusively with NOR glycolipids in human erythrocytes (7). For some experiments, the anti-NOR antibody was purified and biotinylated as described by Duk et al. (18). The other utilized monoclonal antibodies were commercial products. The anti-P1 antibody was purchased from Ce-Immunodiagostika (Eschelbronn, Germany), and the rabbit polyclonal anti-Gb4 antibody, GL-4, was obtained from Matreya (Pleasant Gap, PA).
Flow Cytometry
2102Ep cells were evaluated with a FACSCalibur (BD Biosciences) using monoclonal anti-NOR (1:20 dilution) or anti-P1 (1:10 dilution) antibodies. Cells were washed (all washes and dilutions were done with PBS), suspended in 100 μl of diluted primary antibody, incubated for 40 min on ice, washed again, and then incubated with 50 μl (diluted 1:50) of FITC-labeled F(ab′)2 fragments of polyclonal rabbit anti-mouse immunoglobulin (DakoCytomation, Glostrup, Denmark) for 40 min on ice in the dark. The cells were washed, resuspended in PBS (5 × 105 cells per 0.75 ml), and analyzed by flow cytometry. The number of events analyzed was 10,000/gated cell population, and analyses were carried out using the WinMDI 2.8 computer software (BD Biosciences).
For two-dimensional flow cytometry, the cells were coated with both anti-P1 and anti-NOR. The cells were first coated with anti-P1 and rabbit anti-mouse Ig F(ab′)2 fragments, as described above. The washed cells were incubated with 100 μl of non-immune mouse serum (1:10 dilution) for 30 min, washed twice, and incubated with 100 μl of biotinylated anti-NOR antibody (1:500 dilution). The cells were then washed and incubated with streptavidin-conjugated phycoerythrin (BD Biosciences) for 40 min. Finally, washed cells were resuspended (5 × 105 cells per 0.75 ml) and analyzed as described above.
Extraction and Purification of Glycosphingolipids
The isolation and fractionation of glycolipids and the orcinol staining were performed using the standard procedures applied previously to erythrocytes (2, 3). Briefly, glycolipids were extracted from 2102Ep cells with chloroform/methanol, and the neutral glycolipids were separated from the phospholipids and gangliosides, purified in peracetylated form, de-O-acetylated, and desalted. To isolate individual glycolipids, the neutral glycolipids were fractionated on Kieselgel H type 60 columns (Merck, Darmstadt, Germany), and the fractions were analyzed by high-performance thin-layer chromatography (HPTLC)2 using an anti-NOR antibody. Fractions containing the desired glycolipid were pooled, and the glycolipid was purified by preparative HPTLC followed by elution from the appropriate plate region, as described by (3).
Enzymatic Degradation
The glycolipid preparations were treated with coffee bean α-galactosidase (1.4 units in a total volume of 125 μl, 68 h at 37 °C) or with β-galactosidase from bovine testes (0.2 units in a total volume of 290 μl, 72 h at 37 °C), both of which were obtained from Sigma. The enzymatic treatment and sample processing were as described by Duk et al. (4). Control glycolipid samples were subjected to the same procedures in the absence of enzymes.
HPTLC Antibody Assays
Glycolipid samples were solubilized in chloroform/methanol (2:1, v/v), applied to HPTLC plates (Kieselgel 60, Merck), and developed with chloroform/methanol/water (55:45:10, v/v/v). The dried plates were immersed in 0.05% polyisobutylmethacrylate (Aldrich, Steinheim, Germany) in hexane for 1 min, dried, sprayed with TBS (0.05 m Tris buffer, 0.15 m NaCl (pH 7.4)), and blocked in 5% BSA for 1 h. For antibody assays, the plates were successively overlain with 1) mouse monoclonal or rabbit polyclonal antibody diluted in TBS/1% BSA (TBS-BSA) for 1–1.5 h; 2) biotinylated goat anti-mouse Ig antibody or goat anti-rabbit Ig antibody conjugated with alkaline phosphatase (Dako, Glostrup, Denmark), diluted 1:5000 with TBS-BSA; 3) ExtrAvidin-alkaline phosphatase conjugate (Sigma) diluted 1:1000 with TBS/BSA/0.2% Tween20 for 1 h; and 4) the substrate solution (nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate, Sigma). Other details were as described previously (3, 4). Each HPTLC experiment was repeated three times.
MALDI-TOF Mass Spectrometry
MALDI-TOF mass spectrometry was carried out on an Autoflex III TOF/TOFTM instrument (BrukerDaltonics, Bremen, Germany). Samples were dissolved in chloroform:methanol (2:1, v/v). Norharmane (9H-Pyrido[3,4-b]indole, Sigma) was used as a matrix (10 mg/ml, chloroform:methanol, 2:1, v/v). Spectra were scanned in the range of m/z 700–1600 in the reflectron-positive mode. External calibration was applied using the peptide calibration standard (BrukerDaltonics). For MS/MS analyses, the window range for the precursor ion selection was set at 9 Da.
ESI-MSn Analysis
MSn analyses of glycolipids were performed in the positive-ion mode on an AmaZon SL spectrometer (BrukerDaltonics) equipped with a standard ESI ion source (nebulizer pressure, 8 psi; drying gas flow rate, 5 liters/min; drying gas temperature, 200 °C). Full scan and manual MSn spectra were acquired in the enhanced resolution mode (8100 m/z/sec) between m/z 100–1500 using positive ionization. The TLC-purified sample was dissolved in 1 ml of chloroform/methanol (2:1) and analyzed by direct infusion at a rate of 3 μl/min. External calibration in the positive-ion mode was applied using the ESI-L low concentration tuning mix (Agilent, Santa Clara, CA). Each MALDI-TOF and ESI-MSn experiment was repeated at least 10 times.
RESULTS
Identification of a Mutation Correlated with the NOR Phenotype
Because the NOR phenotype is related to formation of a new glycosidic linkage in glycosphingolipid synthesis, we speculated that it could be due to a mutation in a functional glycosyltransferase. We examined Gb3/CD77 synthase (α1,4-galactosyltransferase, Pk transferase, A4GALT, GenBankTM AB041418) because its specificity is the most similar to that of NOR-transferase and we found a mutation related to the NOR phenotype in the A4GALT gene. All available NOR-positive donors (13 members of the Polish T.S. family and one member of the American family) were heterozygous for a C>G mutation at nucleotide position 631 of the A4GALT open reading frame. In contrast, all tested NOR-negative members of the T.S. family (37 individuals) were homozygous for C at this position. The C631G mutation is predicted to replace a glutamine with glutamic acid at position 211 of the encoded enzyme and is hereafter called the Q211E substitution. Our sequencing results were further supported by TaqMan genotyping of 470 NOR-negative Polish individuals, all of whom were homozygous for C at position 631 (supplemental Fig. S2).
Sequencing the open reading frame of A4GALT from NOR-positive individuals allowed us to identify three other sites at which DNA from NOR-positive individuals could differ from the human genome sequence found in the NCBI (GenBankTM AB041418). A substitution of adenine by guanine at position 109 of the open reading frame led to a methionine-to-valine substitution at position 37. This was not NOR-related because it was found in all sequenced clones of the A4GALT gene and was described previously (10). Two other substitutions, C>G at position 903 and G>A at position 987, were found only in NOR individuals but were silent mutations and have also been described (10).
Agglutination assays with an anti-P1 antibody indicated P1 phenotypes in all NOR-positive donors. To determine the relationship between NOR and P1/P2 genotype status, members of the T.S. family were genotyped for the C>T mutation within pseudoexon 2a of A4GALT, which allows prediction of P1/P2 phenotypes (15). Of nine tested NOR-positive individuals, five were P1P1 homozygotes and four were P1P2 heterozygotes. The NOR-negative members of the T.S. family included 8 P1P1 homozygotes, 9 P1P2 heterozygotes, and 13 P2P2 homozygotes. Thus, all tested NOR-positive individuals had at least one P1 allele.
Generation of Cells Expressing the NOR Antigen
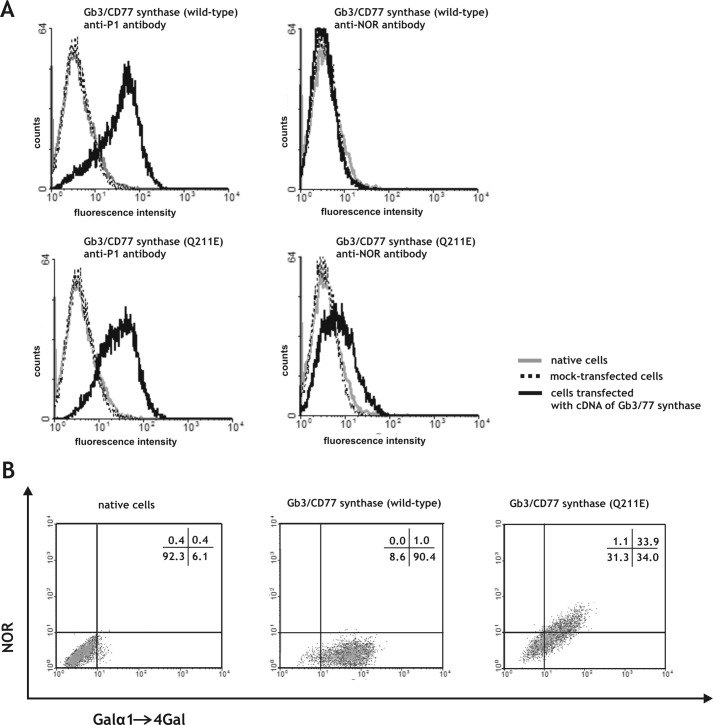
To further examine whether the C631G point mutation detected in the A4GALT gene is responsible for synthesis of NOR antigen, we transfected 2102Ep human teratocarcinoma cells (17, 19) with vectors encoding the consensus Gb3/CD77 synthase or Gb3/CD77 synthase with Glu at position 211 (hereafter called the Gb3/CD77 Q211E synthase). Teratocarcinoma cells were selected because they produce considerable amounts of globoside, which is a precursor of NOR (20, 21). The results of our transfection experiments were evaluated by flow cytometry using an anti-NOR antibody specific to the Gal(α1–4)GalNAc present in the NOR1 and NOR2 antigens and an anti-P1 antibody specific to the Gal(α1–4)Gal present in the Pk and P1 antigens (Fig. 2A). The latter antibody is called anti-P1 because it detects only the P1 glycolipid on the cell surface (15). Our results revealed that the anti-P1 antibody did not bind to untransfected cells, whereas cells transfected with the vector encoding the consensus or Q211E Gb3/CD77 synthase showed a strong binding, indicating the presence of the P1 antigen (Fig. 2A). Control cells and those transfected with the vector encoding the consensus Gb3/CD77 synthase failed to show any binding of the anti-NOR antibody. In contrast, cells transfected with the vector encoding the Gb3/CD77 Q211E synthase bound the anti-NOR antibody. These results allowed us to conclude that the NOR antigen appears as a result of the C631G mutation in the gene encoding Gb3/CD77 synthase.
FIGURE 2.
A, flow cytometric analysis of the binding of anti-P1 and anti-NOR antibodies to 2102Ep cells transfected with vectors encoding the consensus Gb3/CD77 synthase (containing a Gln residue at position 211) or Gb3/CD77 synthase containing a Glu at position 211 (Q211E). B, two-dimensional flow cytometric analysis of 2102Ep cells transfected with vectors encoding the consensus Gb3/CD77 or Gb3/CD77 Q211E synthase. The cells were coated with anti-P1 and anti-NOR antibodies. Percentages of cell number in each quadrant are indicated.
We next examined whether the amount of NOR antigen produced by cells transfected with the vector encoding the Gb3/CD77 Q211E synthase is comparable with the amount of P1 antigen. Two-dimensional flow cytometric analysis of cells coated with anti-NOR and anti-P1 antibodies showed that cells transfected with the vector encoding the Gb3/CD77 Q211E synthase constituted one population that expressed both antigens and that expression of NOR antigen is correlated with expression of the P1 antigen (Fig. 2B).
To confirm that biosynthesis of the NOR antigen is exclusively dependent on the C631G mutation and is independent of the additional silent mutations found at positions 903 and 987, we created two recombinant forms of A4GALT by introducing guanine at position 631 of the consensus A4GALT (called Pkmut) or cytosine at the same position of A4GALT found in NOR-positive individuals (called PkQ211Emut). A schematic representation of A4GALT mutants is shown in supplemental Fig. S1. Transfection of 2102Ep cells with vectors encoding Pkmut and PkQ211E, followed by flow cytometry, showed that both versions triggered the expression of detectable Gal(α1–4)Gal but that only Pkmut triggered expression of the NOR antigen (results not shown). This further supports our contention that the specificity of Gb3/CD77 synthase relies solely on the C631G mutation and is independent of the additional silent mutations found at positions 903 and 987 of the A4GALT gene in our NOR-positive donors.
HPTLC Analysis of Glycolipids from Transfected Cells
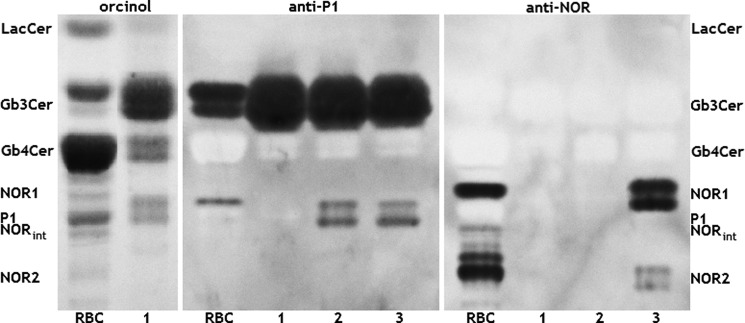
Neutral glycolipid fractions from NOR erythrocytes, untransfected 2102Ep cells, and cells transfected with vectors encoding the consensus Gb3/CD77 synthase or Gb3/CD77 Q211E synthase were isolated and analyzed by thin-layer chromatography. Orcinol staining showed that although Gb4Cer is a major neutral glycolipid component of NOR-positive and negative erythrocytes, the major glycolipid component of 2102Ep cells was Gb3Cer, followed by a distinct but lower content of Gb4Cer. Moreover, 2102Ep cells showed only trace amounts of LacCer, which was distinctly visible in the erythrocyte glycolipids and contained a significant amount of GlcCer, which was not detected in erythrocytes. The orcinol staining pattern of neutral glycolipids did not change significantly following transfection with vectors encoding the consensus Gb3/CD77 synthase or Gb3/CD77 Q211E synthase (Fig. 3).
FIGURE 3.
HPTLC analysis of neutral glycolipids extracted from red blood cells and 2102Ep cells. The assayed samples comprised total neutral glycolipid fractions isolated from NOR-positive erythrocytes (RBC), untransfected 2102Ep cells (lane 1), and 2102Ep cells transfected with vectors encoding the consensus (lane 2) or Gb3/CD77 Q211E synthase (lane 3). The glycolipids were detected by orcinol staining (left panel) or by overlaying with monoclonal antibodies against P1 (center panel) or NOR (right panel).
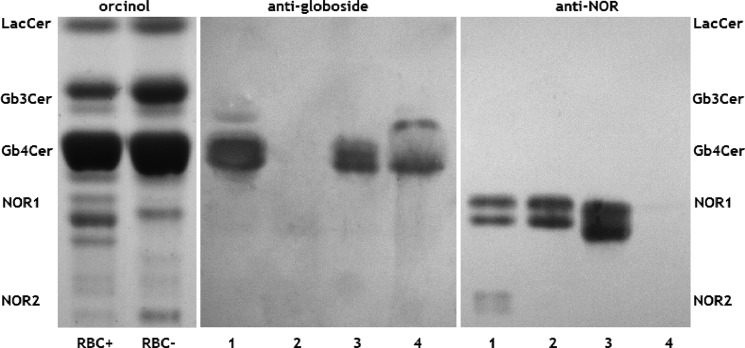
The anti-P1 antibody, which binds to the terminal Gal(α1–4)Gal epitope, detected the Gb3Cer (Pk) and P1 antigens in glycolipids from NOR erythrocytes and both types of transfected cells (Fig. 3). The doublet P1 bands seen in transfected cells were most likely caused by the presence of isoforms with different fatty acids, as is frequently found in glycolipids (22). Interestingly, only Gb3Cer was detected in untransfected 2102Ep cells, suggesting that the P1 antigen appeared because of the transfection of vectors encoding the consensus Gb3/CD77 synthase or Gb3/CD77 Q211E synthase. When we evaluated the same set of neutral glycolipids from 2102Ep cells using a monoclonal anti-NOR antibody, we did not detect binding to glycolipids isolated from native 2102Ep cells or those transfected with the vector encoding the consensus Gb3/CD77 synthase. However, the antibody detected two doublets in glycolipids from cells expressing the Gb3/CD77 Q211E synthase. Strong bands corresponded to the position of NOR1, whereas a weak double band corresponded to that of NOR2 (Fig. 3, right panel). The high specificity of the anti-NOR antibody (4) strongly suggested that these bands represented the NOR1 and NOR2 glycolipids. To obtain further evidence, the NOR1 glycolipid from 2102Ep cells transfected with the vector encoding the mutated Gb3/CD77 synthase was purified, treated with α- or β-galactosidase, and subjected to HPTLC analysis (Fig. 4). The untreated purified NOR sample did not contain globoside (Gb4Cer), but the anti-Gb4 antibody detected an immunoreactive band at the position of Gb4Cer following treatment with either α- or β-galactosidase. When we probed the same samples with anti-NOR antibody, bands corresponding to NOR1 were detected in the β-galactosidase-treated sample but disappeared completely after α-galactosidase treatment (Fig. 4). This shows that the glycolipid detected by the anti-NOR antibody is an α-galactosyl-Gb4Cer, which is consistent with the structure of the NOR1 glycolipid from NOR erythrocytes (3). The appearance of globoside after β-galactosidase treatment most likely reflects the presence of SSEA-3 (Gal(β1–3)Gb4Cer), which is abundant in teratocarcinoma cells and apparently comigrates with NOR1 in TLC (23).
FIGURE 4.
Effect of α- and β-galactosidase on the NOR1 glycolipid from 2102Ep cells transfected with vectors encoding the Gb3/CD77 Q211E synthase. The following samples were fractionated by HPTLC: total neutral glycolipid fraction from NOR-positive 2102Ep cells (lane 1) and NOR1 glycolipid isolated by elution from the HPTLC plate and left untreated (lane 2) or treated with β-galactosidase (lane 3) or α-galactosidase (lane 4). The glycolipids were detected by overlaying the plates with an anti-globoside antibody (center panel) or an anti-NOR antibody (right panel). For comparison, orcinol-stained total neutral glycolipid fractions from NOR-positive red blood cells (RBC+) and NOR-negative red blood cells (RBC−) are shown in the left panel.
Mass Spectrometric Analysis of NOR Glycolipids
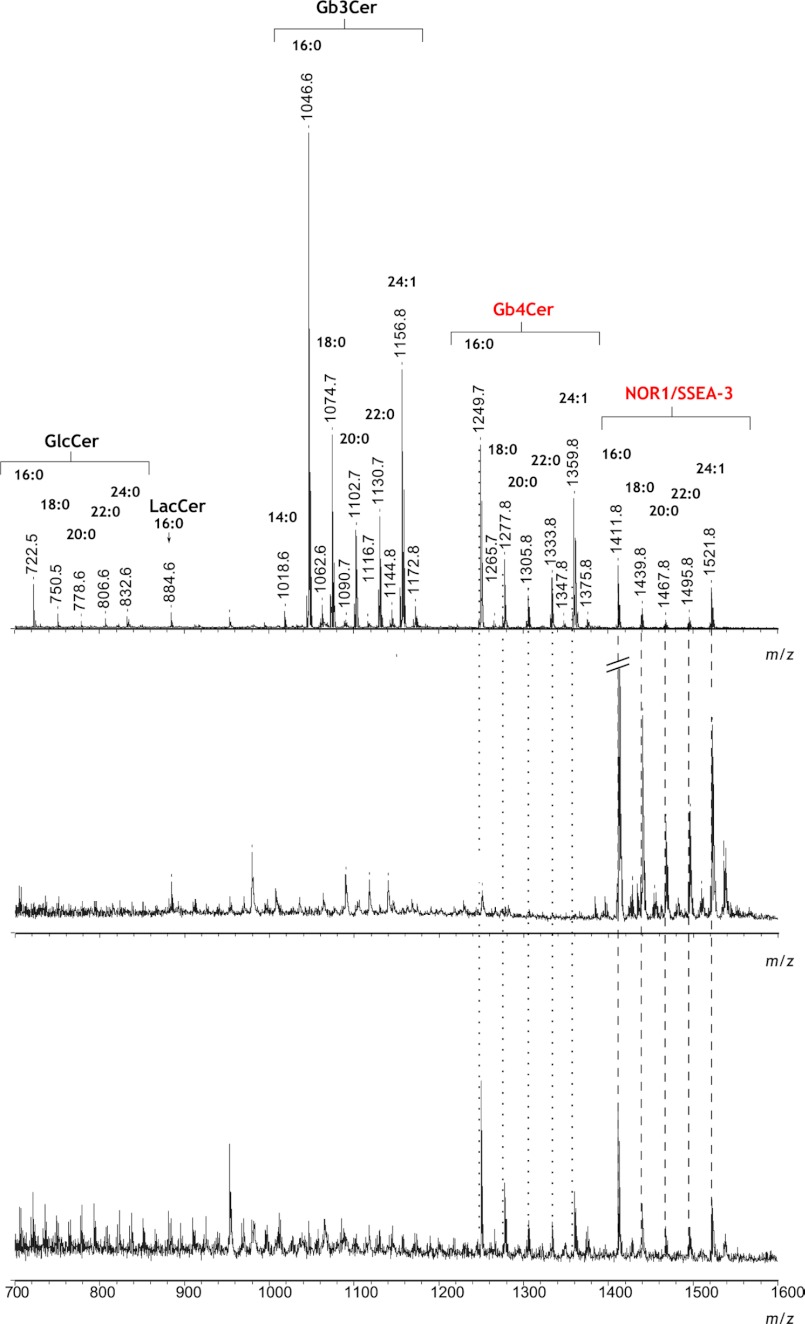
The neutral glycolipid fraction from 2102Ep cells transfected with the vector encoding the Gb3/CD77 Q211E synthase was analyzed by MALDI-TOF mass spectrometry. The reflectron-mode spectrum comprised several clusters of monosodiated molecular adduct ions [M+Na]+ corresponding to glucosylceramide (GlcCer) and certain globo-series glycosphingolipids, including pentaglycosylceramides (e.g. NOR1, SSEA-3). The mass differences between the ions (mostly ∼28 Da, which is the molecular mass of two methylene groups) apparently corresponded to isoforms with ceramides having acyl groups of different lengths (e.g. 16:0, 18:0, 20:0, 22:0) but the same long-chain base (d18:1 sphingosine) (Fig. 5, upper panel). The major ions at m/z 1046.6 and m/z 1156.8 corresponded to Gb3Cer with 16:0 (Gb3Cer(d18:1/16:0)) and 24:1 (Gb3Cer(d18:1/24:1)) acyl groups, respectively. Less abundant ions at m/z 1074.7, m/z 1102.7, and m/z 1130.7 corresponded to the Gb3Cer(d18:1/18:0), Gb3Cer(d18:1/20:0), and Gb3Cer(d18:1/22:0) isoforms, respectively. A similar set of isoforms (with respect to the lengths of the ceramide acyl groups) was observed for GlcCer, Gb4Cer, and NOR1/SSEA-3. The relative intensities of the peaks derived from certain glycosphingolipids reflected the patterns seen in our TLC experiments, with Gb3Cer being the most abundant component.
FIGURE 5.
Reflectron-positive mode MALDI-TOF mass spectra of glycolipids isolated from 2102Ep cells transfected with vectors encoding the Gb3/CD77 Q211E synthase. Shown are spectra from the total neutral glycolipid fraction (upper panel) and the NOR1 glycolipid isolated from the HPTLC plate, first as the native sample (center panel) and then following treatment with coffee bean α-galactosidase (bottom panel). All experiments were carried out as described under “Experimental Procedures.” The dotted lines indicate ions diagnostic for NOR1/SSEA-3 and Gb4Cer. Acyl group symbols (e.g. 16:0) indicate the glycolipid isoforms.
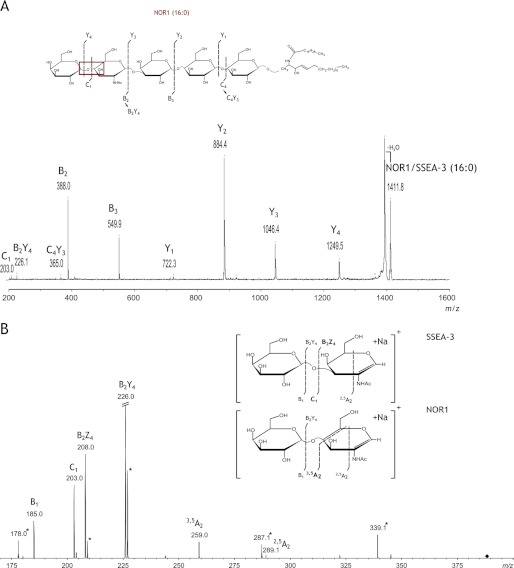
MALDI-TOF analysis of the NOR1 glycolipid purified from the TLC plate yielded a mass spectrum with ions corresponding to the isobaric structures of NOR1 and SSEA-3 (Fig. 5, center panel). The most abundant ions [M+Na]+ at m/z 1411.8, m/z 1439.8, and m/z 1521.8 corresponded to isoforms with ceramide acyl groups of 16:0, 18:0, and 24:1, respectively. Treatment with α-galactosidase decreased the relative intensities of these peaks and gave rise to respective molecular ions devoid of their terminal Gal residues, corresponding to the appropriate isoforms of Gb4Cer (Fig. 5, bottom panel). The residual ions observed for the α-galactosidase-treated sample were as described above for NOR1/SSEA-3 (m/z range 1411.8–1521.8), indicating their isobaric character. SSEA-3 (Gb5Cer) is known to differ from NOR1 only (3) by the type of glycosidic bond between the terminal Gal and GalNAc, which is Gal(β1–3)GalNAc(β1- for SSEA-3 but Gal(α1–4)GalNAc(β1- for NOR1 (Fig. 1). MALDI-TOF MS/MS analysis of the ion at m/z 1411.8 [M+Na]+ confirmed the structure of NOR1 (Fig. 6A). Most of observed fragment ions were sodiated [Bi+Na]+ and [Yi+Na]+ ions, according to the nomenclature of Domon and Costello (24), and indicated the sugar sequence of NOR1 and SSEA-3 as Hex-HexNAc-Hex-Hex-HexCer (Fig. 6A, inset). Multiple-stage electrospray ionization ion-trap mass spectrometry (ESI-IT-MSn) was used to confirm that the isobaric ion [M+Na]+ at m/z 1411.8 corresponded to both NOR1 and SSEA-3 in the TLC-purified sample. ESI-MS analysis of the TLC-purified NOR1/SSEA-3 sample yielded the same spectral data as our MALDI-TOF mass spectrometric analysis, except that NOR1/SSEA-3 isoforms with 16:0 were primarily represented by a doubly charged disodiated molecular ion [M+2Na]2+ at m/z 717.4 (data not shown). Fragmentation of that ion yielded doubly and singly charged di- and monosodiated daughter ions, respectively (data not shown). The monosodiated singly charged ions were identical to these shown in Fig. 6A. The monosodiated [B2+Na]+ ion at m/z 388.1 was chosen for further MS3 analysis, and cross-ring fragmentation produced a distinct monosodiated 3,5A2 ion at m/z 259.0, which is diagnostic for 1→4 linked sugar residues (Fig. 6B). Ions at m/z 203.0 and 208.0 indicated a terminal group 3-O-elimination, resulting in the emergence of the monosodiated C1 and B2Z4 ions that were reported previously as characteristic of 1→3-linked residues (3, 25). Together, these data confirmed that the NOR1 glycolipid from 2102Ep cells transfected with the vector encoding the mutated Gb3/CD77 synthase migrated together with the SSEA-3 glycolipid in the TLC analysis.
FIGURE 6.
Structural analysis of NOR1 and SSEA-3 glycolipids. A, MALDI-TOF MS/MS analysis of the most abundant ion, [M+Na]+, at m/z 1411.8, corresponding to NOR1 and SSEA-3 (Cer(d18:1/16:0) isoform), which are isobars and differ only by one glycosidic bond. The inset explains the fragmentation pattern and the sequence within the carbohydrate region of the glycolipids. B, ESI-IT MS3 analysis of the B2 ion at m/z 388.1 (MS2 717.4→MS3 388.1). The insets explain the fragmentation pattern and the sequence within the disaccharide. Ions diagnostic for 1→4 linked (3,5A2 at m/z 259.0) and 1→3 linked (C1 at m/z 203.0 and B2Z4 at m/z 208.0) sugar residues are shown in boldface. Ions were assigned according to the nomenclature of Domon and Costello (24). An asterisk indicates ions that were not interpreted because of difficulties with precise parent ion isolation.
DISCUSSION
The results presented in this paper explain the genetic background of inheritable NOR polyagglutination. We report for the first time that the NOR phenotype strictly correlates with a single nucleotide mutation, C631G, in the open reading frame of the A4GALT gene, which encodes the galactosyltransferase responsible for biosynthesis of the Gal(α1–4)Gal moiety in Pk and P1 glycolipids. This mutation replaces the glutamine at position 211 of the enzyme polypeptide chain with glutamic acid (substitution Q211E), which broadens acceptor specificity of the enzyme, causing the transferase to acquire the ability to catalyze the synthesis of the Gal(α1–4)GalNAc present in NOR-related glycolipids without losing its ability to transfer the Gal residue to the C4 of Gal. 2102Ep cells transfected with vectors encoding Gb3/CD77 synthase harboring the C631G mutation produced NOR glycolipids, as identified by TLC mobility, interaction with a highly specific monoclonal anti-NOR antibody, and mass spectrometric analysis. Furthermore, α-galactosidase treatment transformed the NOR glycolipids into Gb4Cer, which is analogous to the effect of α-galactosidase on NOR glycolipids from erythrocytes (3). In human erythrocytes, globoside is a major neutral glycolipid component, indicating that erythroid cells have a highly active β1,3-N-acetylgalactosaminyltransferase that transforms most of the Gb3Cer into Gb4Cer. In NOR-positive erythrocytes, the Gb3/CD77 synthase with Q211E substitution transfers αGal to the GalNAc of Gb4Cer, transforming a small portion of Gb4Cer into the NOR1 glycolipid. This becomes a new substrate for highly active β1,3-N-acetylgalactosaminyltransferase, resulting in synthesis of NORint, the most abundant of the NOR-related glycolipids (4). A small portion of NORint is then further elongated by NOR transferase, giving rise to the NOR2 glycolipid (Fig. 1). The content of NOR2 glycolipid in erythrocytes is much lower than that of NOR1, but the former is easily detectable because it reacts more strongly with antibodies, most likely because of its longer oligosaccharide chain and/or double Gal(α1–4)GalNAc moiety (7). In contrast to erythrocytes, 2102Ep teratocarcinoma cells have Gb3Cer as their most abundant glycolipid and show a much lower Gb4Cer content, suggesting that these cells have relatively low β3GalNAc-transferase activity. This may explain why transfected 2102Ep cells expressing NOR transferase mainly produced the NOR1 glycolipid and the products of its further elongation were only weakly detectable.
Another difference between glycolipids present in erythrocytes and 2102Ep cells concerns their ceramide portions. The NOR1 glycolipid derived from erythrocytes contains predominantly 24:1 fatty acid (3), whereas the globo-series glycolipids (including NOR) from 2102Ep cells transfected with the vector encoding the Gb3/CD77 Q211E synthase formed a series of isoforms with fatty acyl chains of different lengths, dominated by fatty acids of 16:0 and 24:1. For this reason, NOR1 and other glycolipids of 2102Ep cells are seen as double bands in our TLC results (Fig. 3).
2102Ep cells transfected with the vector encoding the consensus Gb3/CD77 synthase and Gb3/CD77 Q211E synthase expressed the P1 glycolipid, which was not detected in untransfected cells (Figs. 2 and 3). This provides new evidence that Pk synthase is able to synthesize P1 and shows that this activity is apparently not affected by the Q211E substitution. However, it is interesting to speculate on why untransfected 2102Ep cells, which must have an intrinsic consensus Gb3/CD77 synthase (reflected in their expression of Gb3Cer), do not produce the P1 glycolipid. Perhaps our transfections yielded a much higher level of enzyme expression in comparison with that of the intrinsic form.
The Gal and GalNAc residues are structurally similar. The differences between them are sufficient to direct specific recognition of these residues by glycosyltransferases, but because of their structural similarities, the enzymes responsible for transferring these residues to the same acceptor may show a high degree of homology. A good example is the human ABO blood group system, wherein the A and B blood groups differ by the linkage of a terminal GalNAc(α1–3) or Gal(α1–3), respectively, to the same precursor. Specific blood group A and B transferases (GTA and GTB) are encoded by the same locus and differ by only four amino acid residues. Two of these residues, Leu/Met at position 266 and Gly/Ala at position 268, determine A and B specificity, respectively (26). Mutations leading to the substitution of one of these four amino acid residues (or some adjacent residues) of GTA for those of GTB, or vice versa, generate an enzyme that can use both UDP-Gal and UDP-GalNAc as donors, leading to the cis-AB phenotype (27). Another case is β1,4-galactosyltransferase which, after replacing Tyr-289 with Leu, Ile, or Asn, shows the same catalytic activity using either UDP-Gal or UDP-GalNAc as donors (28). The reverse substitution (I289Y) in a homolog of the enzyme, the β1,4-N-acetylgalactosaminyltransferase from Drosophila, generates a mutant enzyme that shows low catalytic activity using UDP-GalNAc and high catalytic activity for transferring Gal to GlcNAc (29). These examples show that substitution of one amino acid residue in a Gal- or GalNAc-transferring enzyme can alter or broaden its donor specificity.
To the best of our knowledge, Gb3/CD77 synthase is the first described enzyme in which a point mutation changes the acceptor specificity from Gal to Gal/GalNAc. The replacement of Gln-211 with Glu in this enzyme either directly broadens the specificity of its acceptor binding site or indirectly affects the acceptor specificity by modifying another binding site. It is now generally accepted that glycosyltransferases form enzymatically active homo- and heteromeric complexes (30, 31). The formation of such complexes may play an important role in the synthesis of particular glycoforms. For example, the dominant expression of glycosphingolipids from the globo- or ganglio-series depends on whether lactosylceramide synthase, a branch-point enzyme of the GSL biosynthesis pathway, forms a complex with Gb3/CD77 synthase or GM3 synthase (32). The formation of such a complex may be the main factor that renders an enzyme able to transfer a particular monosugar to two structurally related monosaccharides of the acceptor (e.g. Gal and GalNAc). Its restricted specificity in a cell may then depend on its heterodimerization with a transferase that determines the structure of the synthesized oligosaccharide chain.
Future studies are needed to examine the mechanism through which the acceptor specificity of Gb3/CD77 synthase is broadened by a single point mutation. The NOR phenotype is rare, but its biological significance is increased by the common presence of natural anti-NOR antibodies in human sera, and their ability to create complications during blood transfusion. It also remains formally possible that NOR glycolipids play roles in transplantation and maternofetal interactions, although this is not yet supported by experimental evidence because of the limited number of identified NOR-positive individuals, most of whom are women. However, these results explain the genetic background of the NOR phenotype and suggest new directions for studies on the structure/acceptor specificity relationship of glycosyltransferases.
Supplementary Material
Acknowledgments
We thank Dr. Peter W. Andrews (University of Sheffield, UK) for generously providing the 2102Ep cells and pCAG vector and Dr. Martin Olsson and Dr. Britt Thuresson (University of Lund, Sweden) for P1/P2 genotyping of the NOR donors. We also thank Prof. Olga Haus and Prof. Małgorzata Sąsiadek (Wrocław Medical University) for analyzing the karyotypes of the NOR donors; Dr. Elzbieta Klausa, Dr. Barbara Żukowska, and Teresa Karulek from the Regional Center of Transfusion Medicine and Blood Bank (Wrocław Poland), and Dr. Izabela Nowak from the Ludwik Hirszfeld Institute of Immunology and Experimental Therapy, Wrocław, Poland for blood samples. In addition, we thank the donors for participation.
This work was supported by National Science Center of Poland Grants N N302 662940 (to M. C.) and N N401 631640 (to A. S.).
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) GQ365679.

This article contains supplemental Figs. S1 and S2 and Tables S1 and S2.
- HPTLC
- high-performance thin-layer chromatography
- SSEA-3
- stage-specific embryonic antigen-3
- ESI
- electrospray ionization.
REFERENCES
- 1. Harris P. A., Roman G. K., Moulds J. J., Bird G. W., Shah N. G. (1982) An inherited RBC characteristic, NOR, resulting in erythrocyte polyagglutination. Vox Sang. 42, 134–140 [DOI] [PubMed] [Google Scholar]
- 2. Kuśnierz-Alejska G., Duk M., Storry J. R., Reid M. E., Wiecek B., Seyfried H., Lisowska E. (1999) NOR polyagglutination and Sta glycophorin in one family. Relation of NOR polyagglutination to terminal α-galactose residues and abnormal glycolipids. Transfusion 39, 32–38 [DOI] [PubMed] [Google Scholar]
- 3. Duk M., Reinhold B. B., Reinhold V. N., Kusnierz-Alejska G., Lisowska E. (2001) Structure of a neutral glycosphingolipid recognized by human antibodies in polyagglutinable erythrocytes of the rare NOR phenotype. J. Biol. Chem. 276, 40574–40582 [DOI] [PubMed] [Google Scholar]
- 4. Duk M., Singh S., Reinhold V. N., Krotkiewski H., Kurowska E., Lisowska E. (2007) Structures of unique globoside elongation products present in erythrocytes with a rare NOR phenotype. Glycobiology 17, 304–312 [DOI] [PubMed] [Google Scholar]
- 5. Duk M., Lisowska E., Moulds J. J. (2006) Polyagglutinable NOR red blood cells found in an American family and a Polish family have the same unique glycosphingolipids. Transfusion 46, 1264–1265 [DOI] [PubMed] [Google Scholar]
- 6. Duk M., Westerlind U., Norberg T., Pazynina G., Bovin N. N., Lisowska E. (2003) Specificity of human anti-NOR antibodies, a distinct species of “natural” anti-α-galactosyl antibodies. Glycobiology 13, 279–284 [DOI] [PubMed] [Google Scholar]
- 7. Duk M., Kusnierz-Alejska G., Korchagina E. Y., Bovin N. V., Bochenek S., Lisowska E. (2005) Anti-α-galactosyl antibodies recognizing epitopes terminating with α1,4-linked galactose. Human natural and mouse monoclonal anti-NOR and anti-P1 antibodies. Glycobiology 15, 109–118 [DOI] [PubMed] [Google Scholar]
- 8. Spitalnik P. F., Spitalnik S. L. (1995) The P blood group system. Biochemical, serological, and clinical aspects. Transfus. Med. Rev. 9, 110–122 [DOI] [PubMed] [Google Scholar]
- 9. Naiki M., Fong J., Ledeen R., Marcus D. M. (1975) Structure of the human erythrocyte blood group P1 glycosphingolipid. Biochemistry 14, 4831–4837 [DOI] [PubMed] [Google Scholar]
- 10. Steffensen R., Carlier K., Wiels J., Levery S. B., Stroud M., Cedergren B., Nilsson Sojka B., Bennett E. P., Jersild C., Clausen H. (2000) Cloning and expression of the histo-blood group Pk UDP-galactose:Galβ1–4Glcβ1-Cer α1,4-galactosyltransferase. Molecular genetic basis of the p phenotype. J. Biol. Chem. 275, 16723–16729 [DOI] [PubMed] [Google Scholar]
- 11. Furukawa K., Iwamura K., Uchikawa M., Sojka B. N., Wiels J., Okajima T., Urano T., Furukawa K. (2000) Molecular basis for the p phenotype. Identification of distinct and multiple mutations in the α1,4-galactosyltransferase in Swedish and Japanese individuals. J. Biol. Chem. 275, 37752–37756 [DOI] [PubMed] [Google Scholar]
- 12. Hellberg A., Ringressi A., Yahalom V., Säfwenberg J., Reid M. E., Olsson M. L. (2004) Genetic heterogeneity at the glycosyltransferase loci underlying the GLOB blood group system and collection. Br. J. Haematol. 125, 528–536 [DOI] [PubMed] [Google Scholar]
- 13. Hellberg A., Schmidt-Melbye A. C., Reid M. E., Olsson M. L. (2008) Expression of a novel missense mutation found in the A4GALT gene of Amish individuals with the p phenotype. Transfusion 48, 479–487 [DOI] [PubMed] [Google Scholar]
- 14. Iwamura K., Furukawa K., Uchikawa M., Sojka B. N., Kojima Y., Wiels J., Shiku H., Urano T., Furukawa K. (2003) The blood group P1 synthase gene is identical to the Gb3/CD77 synthase gene. A clue to the solution of the P1/P2/p puzzle. J. Biol. Chem. 278, 44429–44438 [DOI] [PubMed] [Google Scholar]
- 15. Thuresson B., Westman J. S., Olsson M. L. (2011) Identification of a novel A4GALT exon reveals the genetic basis of the P1/P2 histo-blood groups. Blood 117, 678–687 [DOI] [PubMed] [Google Scholar]
- 16. Czerwinski M., Kern J., Grodecka M., Paprocka M., Krop-Watorek A., Wasniowska K. (2007) Mutational analysis of the N-glycosylation sites of Duffy antigen/receptor for chemokines. Biochem. Biophys. Res. Commun. 356, 816–821 [DOI] [PubMed] [Google Scholar]
- 17. Liew C. G., Draper J. S., Walsh J., Moore H., Andrews P. W. (2007) Transient and stable transgene expression in human embryonic stem cells. Stem Cells 25, 1521–1528 [DOI] [PubMed] [Google Scholar]
- 18. Duk M., Lisowska E., Wu J. H., Wu A. M. (1994) The biotin/avidin-mediated microtiter plate lectin assay with the use of chemically modified glycoprotein ligand. Anal. Biochem. 221, 266–272 [DOI] [PubMed] [Google Scholar]
- 19. Chen C., Fenderson B. A., Andrews P. W., Hakomori S. (1989) Glycolipid glycosyltransferases in human embryonal carcinoma cells during retinoic acid induced differentiation. Biochemistry, 28, 2229–2238 [DOI] [PubMed] [Google Scholar]
- 20. Andrews P. W., Casper J., Damjanov I., Duggan-Keen M., Giwercman A., Hata J., von Keitz A., Looijenga L. H., Millán J. L., Oosterhuis J. W., Pera M., Sawada M., Schmoll H. J., Skakkebaek N. E., van Putten W., Stern P. (1996) Comparative analysis of cell surface antigens expressed by cell lines derived from human germ cell tumours. Int. J. Cancer. 66, 806–816 [DOI] [PubMed] [Google Scholar]
- 21. Wenk J., Andrews P. W., Casper J., Hata J., Pera M. F., von Keitz A., Damjanov I., Fenderson B. A. (1994) Glycolipids of germ cell tumors. Extended globo-series glycolipids are a hallmark of human embryonal carcinoma cells. Int. J. Cancer. 58, 108–115 [DOI] [PubMed] [Google Scholar]
- 22. Lingwood C. A., Manis A., Mahfoud R., Khan F., Binnington B., Mylvaganam M. (2010) New aspects of the regulation of glycosphingolipid receptor function. Chem. Phys. Lipids. 163, 27–35 [DOI] [PubMed] [Google Scholar]
- 23. Kannagi R., Cochran N. A., Ishigami F., Hakomori S., Andrews P. W., Knowles B. B., Solter D. (1983) Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 2, 2355–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Domon B., Costello C. E. (1988) A systematic nomenclature for carbohydrate fragmentations in FAB- MS/MS spectra of glycoconjugates. Glycoconjugate J. 5, 397–409 [Google Scholar]
- 25. Sheeley D. M., Reinhold V. N. (1998) Structural characterization of carbohydrate sequence, linkage, and branching in a quadrupole ion trap mass spectrometer. Neutral oligosaccharides and N-linked glycans. Anal. Chem. 70, 3053–3059 [DOI] [PubMed] [Google Scholar]
- 26. Storry J. R., Olsson M. L. (2009) The ABO blood group system revisited. A review and update. Immunohematology 25, 48–59 [PubMed] [Google Scholar]
- 27. Yazer M. H., Olsson M. L., Palcic M. M. (2006) The cis-AB blood group phenotype. Fundamental lessons in glycobiology. Transf. Med. Rev. 20, 207–217 [DOI] [PubMed] [Google Scholar]
- 28. Ramakrishnan B., Qasba P. K. (2002) Structure-based design of β1,4-galactosyltransferase I (β4Gal-T1) with equally efficient N-acetylgalactosaminyltransferase activity. Point mutation broadens β4Gal-T1 donor specificity. J. Biol. Chem. 277, 20833–20839 [DOI] [PubMed] [Google Scholar]
- 29. Ramakrishnan B., Qasba P. K. (2007) Role of a single amino acid in the evolution of glycans of invertebrates and vertebrates. J. Mol. Biol. 365, 570–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hassinen A., Rivinoja A., Kauppila A., Kellokumpu S. (2010) Golgi N-glycosyltransferases form both homo- and heterodimeric enzyme complexes in live cells. J. Biol. Chem. 285, 17771–17777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hassinen A., Pujol F. M., Kokkonen N., Pieters C., Kihlström M., Korhonen K., Kellokumpu S. (2011) Functional organization of Golgi N- and O-glycosylation pathways involves pH-dependent complex formation that is impaired in cancer cells. J. Biol. Chem. 286, 38329–38340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takematsu H., Yamamoto H., Naito-Matsui Y., Fujinawa R., Tanaka K., Okuno Y., Tanaka Y., Kyogashima M., Kannagi R., Kozutsumi Y. (2011) Quantitative transcriptomic profiling of branching in a glycosphingolipid biosynthetic pathway. J. Biol. Chem. 286, 27214–27224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Varki A., Cummings R., Esko J., Freeze H., Hart G., Marth J. (1999) Essentials of Glycobiology, Chapter 1, p. 6, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.