Background: Transcriptional regulation plays an important role in pluripotency maintenance.
Results: Knockdown of the nuclear receptor coactivator 3 (Ncoa3) compromises the pluripotency of mouse embryonic stem cells.
Conclusion: Ncoa3 binds to and activates the Nanog promoter, thus promoting self-renewal of mouse embryonic stem cells.
Significance: Uncovering the novel role and mechanism of Ncoa3 in pluripotency maintenance.
Keywords: Embryonic Stem Cell, Epigenetics, Gene Regulation, Signal Transduction, Transcription Coactivators, GSK3, Nanog, Ncoa3, Pluripotency
Abstract
Nuclear receptors, including Esrrb, Dax1, and Nr5a2, have been shown to be involved in pluripotency maintenance. Yet, the role of their coactivators in mouse embryonic stem cells remains unexplored. Here, we demonstrated that the nuclear receptor coactivator 3 (Ncoa3) is essential for pluripotency maintenance. Knockdown of Ncoa3 not only compromises the expression of pluripotency markers but also impairs in vitro and in vivo differentiation potential of mouse ESCs. Ncoa3 binds to the Nanog promoter and recruits the histone acetyltransferase CREB binding protein (CBP) and the histone arginine methyltransferase CARM1 to activate Nanog expression. Moreover, glycogen synthase kinase 3 GSK3 signaling down-regulates the Ncoa3 protein level to suppress Nanog expression. Thus, Ncoa3 not only contributes to self-renewal by activating Nanog but also facilitates ESC differentiation as a break point to disrupt the core transcriptional circuitry of pluripotency.
Introduction
Embryonic stem cells (ESCs)2, derived from the inner cell mass (ICM) of blastocysts, are able to self-renew indefinitely while maintaining the differentiation potential into all types of cells in the body (1, 2). Transcription factors play important roles in pluripotency maintenance. It is well established that transcription factors Oct4, Sox2, and Nanog, act as core pluripotency regulators that form a transcriptional circuitry to promote the expression of pluripotency-associated genes and to suppress differentiation-related genes (3–7). In addition, nuclear receptors (NRs), including Esrrb, Dax1 (also known as Nr0b1), and Nr5a2, have been shown to regulate the expression of pluripotency factors and to be critical for pluripotency maintenance. Esrrb interacts with Nanog and Oct4 and activates Nanog and Oct4 in ESCs (8–10). Moreover, overexpression of Esrrb allows ESCs to self-renew in the absence of leukemia inhibitory factor (LIF) (9). Dax1 enhances Oct4 gene expression but inhibits the transcriptional activity of Oct4 protein (11–13). Interestingly, either knockdown or overexpression of Dax1 leads to ESC differentiation, suggesting a critical dose-dependent activity of Dax1 in ESC maintenance (11, 14). Nr5a2 is required for maintaining proper levels of Oct4, Nanog, and Tbx3 in ESCs as well as for Oct4 expression at the epiblast stage of embryonic development (12, 15–17). The importance of NRs in pluripotency has been demonstrated further by their reprogramming activities in induced pluripotent stem cell derivation. Esrrb, together with Oct4 and Sox2, allows efficient derivation of iPS cells from mouse embryonic fibroblasts (MEFs) (8). Nr5a2 can replace Oct4 in reprogramming mouse somatic cells to the pluripotent state (15).
NR-mediated gene transcription usually requires nuclear receptor coactivators that are directly recruited by NRs to the promoter/enhancer regions of target genes. The three members of the p160 steroid receptor coactivator (SRC) family (Src1, Src2, and Src3, also known as Ncoa1, Ncoa2, and Ncoa3) are the first identified nuclear receptor coactivators (18–21) and have been implicated in somatic growth, reproductive function, uterine growth, blastocyst implantation, and mammary gland development as well as many types of cancers (22–28). After binding to target genes through the interaction with NRs or other transcription factors, SRCs recruit histone acetyltransferases and methyltransferases for chromatin remodeling and, consequently, activate gene expression. Three structural domains of SRCs are critical for their transcriptional activity. The amino-terminal basic helix-loop-helix-Per/ARNT/Sim (bHLH-PAS) domain is the most conserved region, interacting with several transcription factors such as myogenin, MEF2c, and transcriptional enhancer proteins (29–31). The central nuclear receptor interacting domain (NRID), containing three LXXLL motifs, mediates the interaction with NRs (32–34). The carboxyl terminus is composed of two transcriptional activating domains (AD1 and AD2). AD1 binds CREB binding protein (CBP) and the histone acetyltransferase p300, and AD2 interacts with coactivator-associated arginine methyltransferase 1 (CARM1) and protein arginine N-methyltransferase 1 (PRMT1) (35–37). Moreover, in response to several upstream signaling pathways, the transcriptional activities of SRCs are regulated by various posttranslational modifications, including phosphorylation, ubiquitylation, SUMOylation, acetylation, and methylation (38–45).
Despite the importance of NRs in pluripotency maintenance, the function of nuclear receptor coactivator in ESCs has not been fully investigated. Here, we studied the functions of the p160 family coactivators in mouse ESCs and showed that Ncoa3 is required for the expression of pluripotency genes in mouse ESCs. We further demonstrated that Ncoa3 is essential but not sufficient for pluripotency maintenance. Ncoa3 binds to the Nanog promoter and recruits CBP and CARM1, which in turn increase the levels of histone acetylation and histone arginine methylation at the Nanog promoter and activate Nanog expression. Moreover, Ncoa3 is essential for GSK3 signaling to repress Nanog. Taken together, Ncoa3 serves as a bridge to link transcription factors and epigenetic regulators as well as GSK3 signaling to regulate mouse ESC self-renewal and differentiation.
EXPERIMENTAL PROCEDURES
Cell Culture
V6.5 mouse ESCs were cultured in growth medium consisting of 85% DMEM (high-glucose, Invitrogen), 15% FBS (Hyclone), 2 mm l-glutamine, 5000 units/ml penicillin and streptomycin, 0.1 mm nonessential amino acids (Invitrogen), 0.1 mm 2-mercaptoethanol (Sigma), and 1000 units/ml LIF (ESGRO, Chemicon). MEFs and NIH3T3 were cultured in growth medium consisting of 90% high-glucose DMEM, 10% FBS, 2 mm l-glutamine, and 5000 units/ml penicillin and streptomycin.
Embryo Culture
Female ICR mice (4–6 weeks) were induced to superovulate by intraperitoneal injections of 5 IU of pregnant mare serum gonadotropin (Calbiochem) and, 48 h later, 5 IU human chorionic gonadotropin (Sigma). Then females were paired with ICR males overnight and checked for vaginal plugs the following morning. Two-cell embryos were flushed from oviducts at 42–48 h post-human chorionic gonadotropin and cultured in groups of 20–30 in a 50-μl droplet of potassium simplex optimization medium with amino acids (Millipore) covered by mineral oil (Sigma, for embryo culture) in a 37 °C incubator with 6.5% CO2.
Immunofluorescence
Embryos at desired stages were fixed in 4% paraformaldehyde for 20 min and then permeabilized with 0.2% Triton X-100 for 30 min. After blocking with 5% goat serum for 2 h, embryos were incubated with anti-Ncoa3 (Cell Signaling Technology) and anti-Cdx2 (Biogenex) antibodies overnight at 4 °C. Then embryos were washed and incubated with Alexa Fluor 488 anti-mouse and Alexa Fluor 594 anti-rabbit secondary antibodies (Molecular Probes). Hoechst 33342 (Sigma) was used for nucleus staining. Confocal images were captured with a Leica TCS SP5 confocal microscope.
Quantitative RT-PCR
Total RNA was extracted from cells using the RNeasy mini kit (Qiagen). cDNA synthesis was performed using the TransScript II First-Strand cDNA Synthesis SuperMix kit (Transgen) according to the instructions of the manufacturer. PCR reactions were performed with SYBR Green Real-time PCR MasterMix (TOYOBO) in a Bio-Rad iQ5 system. PCR cycling conditions were 95 °C for 2 min, 40 cycles of 95 °C for 15 s, 58 °C for 15 s, and 72 °C for 30s, and then a melting curve of the amplified DNA was acquired. Quantification of target genes was normalized with β-actin. Primer sequences are shown in supplemental Table S1.
Western Blot Analysis
Cells were lysed in lysis buffer (Beyotime), and protein concentration was measured using a BCA protein assay kit (Beyotime) to ensure equal loading. The samples were resolved by SDS-PAGE, followed by transfer onto a PVDF membrane (Millipore). Membranes were probed with anti-SRC3 (Cell Signaling Technology), anti-β-tubulin (Huada, Beijing), anti-Nanog, anti-CARM1 (Bethyl Laboratories), anti-SRC1, anti-SRC2, anti-Oct3/4, anti-CBP (Santa Cruz Biotechnology), and anti-Esrrb (R&D Systems). Bound primary antibodies were recognized by HRP-linked secondary antibodies (GE Healthcare). Immunoreactivity was detected by ECL Plus (Beyotime) and Kodak light film. Digital images of films were taken with Bio-Rad Molecular Imager Gel Doc XR. The intensity of bands was quantified with the Quantity One analysis software (Bio-Rad).
Alkaline Phosphatase Staining
Ncoa3 knockdown or control ESCs cultured on feeder were fixed in 3.7% formaldehyde solution for 30 min at room temperature. Cells were washed once with PBS solution and incubated with alkaline phosphatase substrate kit III (Vector) for 20 min at room temperature.
Embryoid Body Formation
Control and Ncoa3 knockdown ESCs were cultured in suspension in ESC medium without LIF in Petri dishes for 3 days and then plated on gelatin-coated tissue culture dishes. Cells were collected at indicated points.
Teratoma Formation
MEF cells, control, and Ncoa3 knockdown ESCs were harvested by trypsin treatment and resuspended in their culture medium accordingly. 1.5 × 106 cells in 150 μl were subcutaneously injected to the dorsolateral site. Every cell line was injected into three mice.
Luciferase Reporter Assay
The luciferase reporter containing the 6-kb Nanog promoter was constructed previously (46). A 250-bp Nanog promoter DNA fragment, immediately upstream of the Nanog transcription initiation site, was amplified by PCR and inserted into the pGL3 vector (Promega) to make the 250-bp Nanog reporter. V6.5 ESCs were seeded at a density of 1 × 105 cells per well in 24-well plates and transfected using Lipofectamine 2000 (Invitrogen) with the Nanog reporter plasmid (200 ng) and pRV-SV40 (8 ng, Promega) together with 600-ng control, Ncoa3 knockdown, or overexpression plasmids. At 24 h after transfection, luciferase activities were measured with the dual-luciferase reporter assay system (Promega) according to the instructions of the manufacturer. Each experiment was performed in triplicate and repeated three times.
Chromatin Immunoprecipitation
6 × 107 cells were cross-linked with 1% formaldehyde and sonicated to an average size of 0.5–1 kb. Chromatin extract from 1 × 107 cells was used for each immunoprecipitation. Anti-Ncoa3 (Santa Cruz Biotechnology), anti-CARM1 (Bethyl Laboratories), anti-CBP (Santa Cruz Biotechnology), anti-acetyl-histone H3 (Upstate), or anti-H3R17 dimethylation antibodies (Abcam) were used. Purified ChIP DNA and input DNA were analyzed by real-time PCR. Fold enrichments were calculated by comparing ChIP DNA to input DNA and normalized with the actin level defined as 1. Primer sequences are shown in supplemental Table S2.
Coimmunoprecipitation
V6.5 ESCs were collected, and cell extracts were prepared in lysis buffer (150 mm NaCl, 1% IGEPAL CA-630, 50 mm Tris (pH 8.0), 1 mm PMSF) with protease inhibitor (Roche). The cell extract was precleared using protein A-Sepharose (GE Healthcare) at 4 °C for 2 h. The supernatant was incubated with anti-Ncoa3 antibody (Cell Signaling Technology) or rabbit IgG (Santa Cruz Biotechnology) at 4 °C overnight. Then protein A-Sepharose was added and mixed for 4 h. After washing the pellet three times with lysis buffer, the bound proteins were released from the protein A-Sepharose by boiling for 5 min in 2× SDS loading buffer. A Western blot analysis was performed to detect the proteins present in the immunoprecipitation samples.
RNAi and Generation of Stable Ncoa3 Knockdown or Overexpression ESC Lines
Gene knockdown was performed with the pSuper-puro system (Oligoengine) following the instructions of the manufacturer. Briefly, for the expression of small hairpin RNA targeting a specific gene, a 60-nt oligo containing the specific 19-nt targeting sequence was cloned into the BglII/HindIII site of the pSuper-puro vector. A control plasmid targeting GFP was constructed at the same time. The 19-nt targeting sequences used in this study are listed in supplemental Table S3. The mouse Ncoa3 expression vector pSport-Ncoa3 was purchased from Thermo Fisher. The Ncoa3 coding region from pSport-Ncoa3 was cloned into the pCAGIPuro expression plasmid (a gift from Dr. Hitoshi Niwa) to make pCAGIPuro-Ncoa3. Empty pCAGIPuro, Ncoa3 overexpression, shGFP, shN3-1, or shN3-2 plasmids were transfected into V6.5 ESCs with Lipofectamine 2000. After 7- to 10-day puromycin selection, single clones were picked and tested for knockdown or overexpression efficiency by quantitative RT-PCR.
Microarray Analysis
V6.5 ESCs were transfected by shGFP control, shN3-1, or shN3-2 plasmids. At 48 h after transfection, cells were harvested for RNA purification. Hybridization and scanning of the chips (Affymetrix mouse genome 430 2.0 array) was performed as outlined in the Affymetrix technical manual by CapitalBio Corp., Beijing, China. The gene ontology analysis was performed with a molecule annotation system 3.0 (MAS 3.0)
Statistical Analysis
Data were analyzed by Student's t test. Statistically significant p values were indicated in figures as follows: ***, p < 0.001; **, p < 0.01; *, p < 0.05.
RESULTS
Ncoa3 Is Required for Pluripotency Maintenance
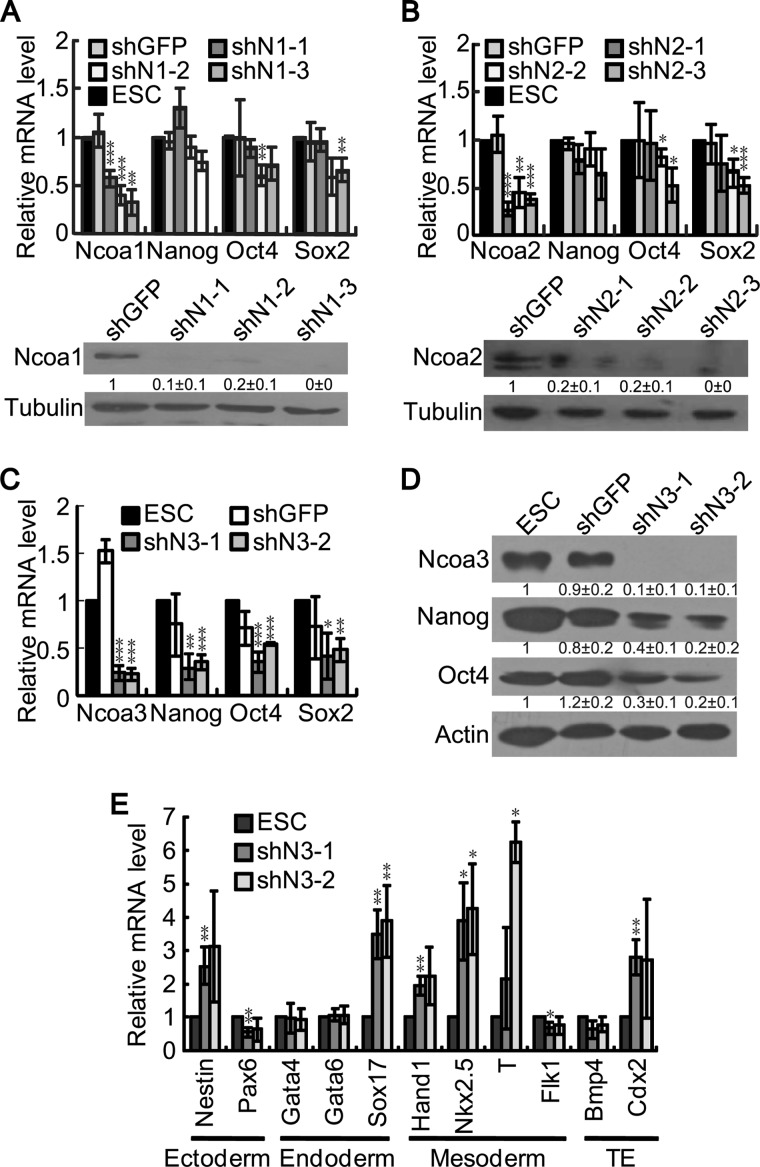
To explore the potential role of the p160 family coactivators in pluripotency maintenance, we performed shRNA knockdown experiments in mouse ESCs and quantified the expression levels of the pluripotency genes Nanog, Oct4, and Sox2. Despite knockdown efficiencies of the Ncoa1, Ncoa2, and Ncoa3 mRNAs varied to some extent, high efficient knockdown of Ncoa1, Ncoa2, and Ncoa3 proteins was achieved (Fig. 1, A–D). Knockdown of Ncoa1 or Ncoa2 only slightly reduced the expression of Oct4 and Sox2 but not Nanog. Moreover, not all three shRNA plasmids targeting Ncoa1 or Ncoa2 consistently affected Oct4 and Sox2 expression (Fig. 1, A and B). Therefore, Ncoa1 and Ncoa2 are not required for pluripotency maintenance in mouse ESCs. In contrast, down-regulation of Ncoa3 impaired Nanog, Oct4, and Sox2 expression, suggesting that Ncoa3 is required for pluripotency maintenance (Fig. 1C). Western blot analysis further confirmed that Ncoa3 depletion reduced the expression of Nanog and Oct4 proteins (Fig. 1D). In addition to decreased expression of pluripotency genes, differentiation markers, including Nestin for ectoderm; Sox17 for endoderm; Hand1, Nkx2.5, and T for mesoderm; and Cdx2 for trophectoderm, were activated in Ncoa3 knockdown cells, indicating a random differentiation of these cells (Fig. 1E). Taken together, knockdown of Ncoa3 in ESCs led to reduced expression of pluripotency markers and elevated expression of differentiation markers, suggesting a pivotal role of Ncoa3 in pluripotency maintenance.
FIGURE 1.
Down-regulation of Ncoa3 compromises the expression of pluripotency markers. A–C, V6.5 ESCs were transfected by pSuper-puro empty vector, shGFP control, or shRNA plasmids targeting Ncoa1 (A), Ncoa2 (B), or Ncoa3 (C). Cells were harvested 48 h after transfection and subjected to RNA purification and cDNA synthesis as well as protein extraction. The knockdown efficiency on Ncoa1, Ncoa2, and Ncoa3 and the expression levels of the pluripotency genes Nanog, Oct4, and Sox2 were examined by real-time PCR with normalization to β-actin. A Western blot analysis was performed to detect the expression of Ncoa1 and Ncoa2. Tubulin served as a loading control. Quantification results of the Western blot analysis are shown below corresponding bands as mean ± S.D. (n = 3). D, V6.5 ESCs were transfected as described in C. The expression of Ncoa3, Nanog, and Oct4 proteins were examined by Western blot analysis. β-Actin served as a loading control. Quantification results of the Western blot analysis were shown below corresponding bands as mean ± S.D. (n = 3). E, V6.5 ESCs were transfected as described in C. Quantitative RT-PCR was carried out to determine the expression levels of differential markers upon Ncoa3 knockdown; TE, trophectoderm. Averages and S.D. from three independent experiments are plotted. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
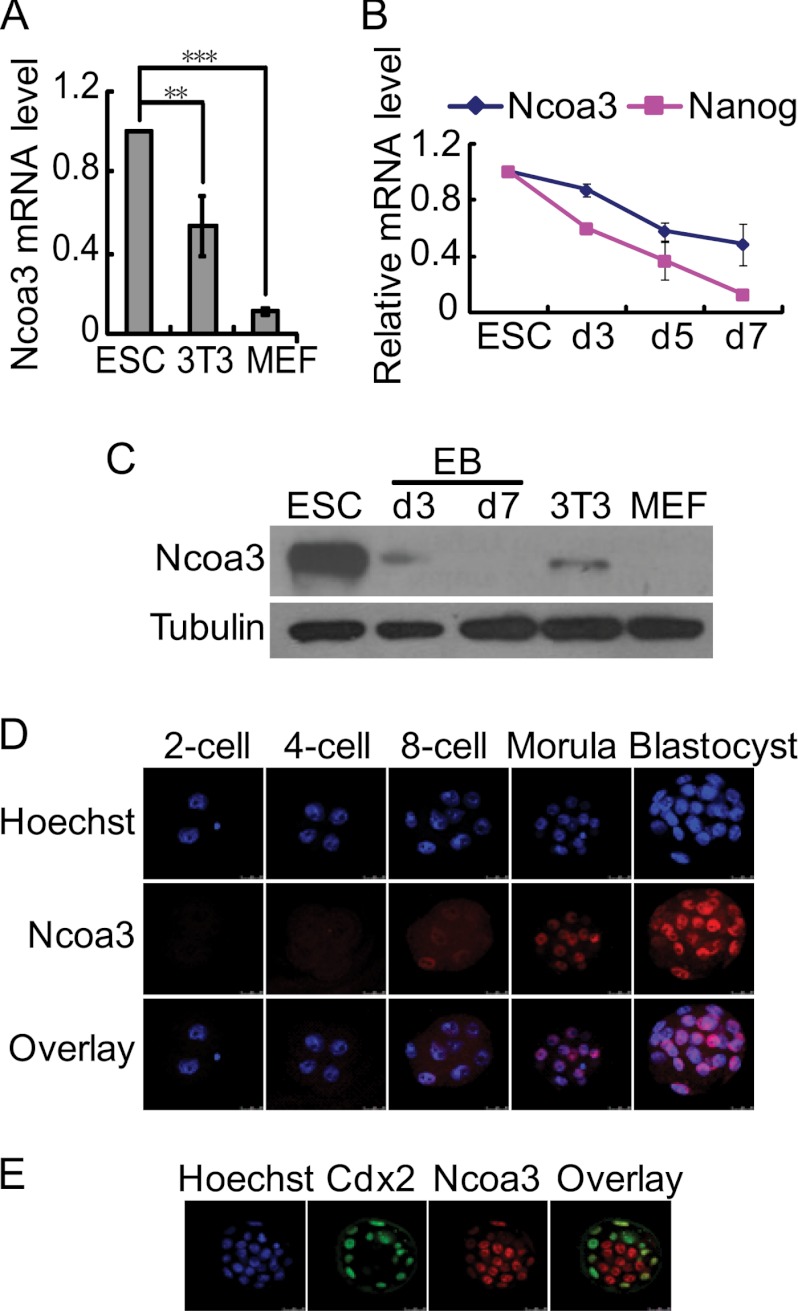
We then examined the expression of Ncoa3 in ESCs and differentiated cells as well as developing embryos. The Ncoa3 mRNA level was expressed at a higher level in ESCs than in MEFs and NIH3T3 cells (Fig. 2A). As ESCs differentiated into embryoid bodies (EBs), Ncoa3 mRNA expression dropped gradually (Fig. 2B). Ncoa3 protein was detected by Western blot analysis in undifferentiated ESCs at a high level and in NIH3T3 cells and day 3 EB cells at much lower levels but not in MEFs or day 7 EB cells (Fig. 2C). A more dramatic change of Ncoa3 at protein level than at mRNA level suggested that Ncoa3 is not only regulated at transcriptional level but also at posttranscriptional steps. During early embryogenesis, Ncoa3 protein was first detected in some blastomeres at the eight-cell embryo stage and was present in all cells in morula and blastocysts (Fig. 2D). However, in blastocysts, Ncoa3 was apparently expressed at a higher level in ICM cells than in trophectodermal cells (Fig. 2E). These data were consistent with the role of Ncoa3 in pluripotency maintenance.
FIGURE 2.
Ncoa3 expression is associated with pluripotent cells. A, mRNA levels of endogenous Ncoa3 in V6.5 ESCs, NIH3T3, and MEFs. **, p < 0.01; ***, p < 0.001. B, the expression pattern of Nanog and Ncoa3 mRNA in ESCs and day 3, day 5, and day 7 EBs. Means ± S.D. from three independent experiments are plotted. C, Western blot analysis of Ncoa3 in V6.5 ESCs, NIH3T3, and MEFs as well as day 3 and day 7 EBs. β-tubulin served as a loading control. D, expression dynamics of Ncoa3 during early embryogenesis. Mouse embryos from the two-cell to the blastocyst stages were immunostained with anti-Ncoa3 antibody. E, reciprocal expression of Ncoa3 and Cdx2 in blastocysts. Blastocysts were immunostained for Cdx2 (green) and Ncoa3 (red). Hoechst 33342 staining (blue) marked the nucleus. The overlay picture of Cdx2 and Ncoa3 is displayed. Confocal images are shown.
Depletion of Ncoa3 Compromises the in Vitro and in Vivo Differentiation Potential of Mouse ESCs
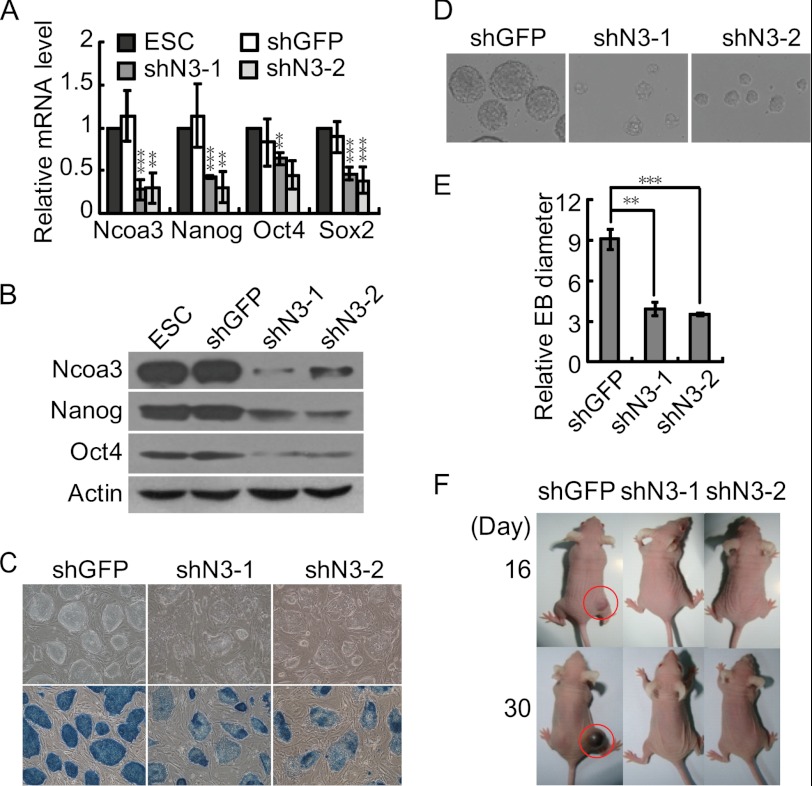
To further investigate whether Ncoa3 is essential for pluripotency maintenance in ESCs, multiple stable Ncoa3 knockdown ESC lines were established with two shRNA plasmids targeting Ncoa3 (shN3-1 and shN3-2). Consistent with transient transfection result, the expression levels of pluripotency markers Nanog, Oct4, and Sox2 decreased in stable Ncoa3 knockdown ESC lines (Fig. 3, A and B). Meanwhile, these Ncoa3 knockdown ESC lines displayed flattened colony morphology as well as reduced alkaline phosphatase staining, suggesting a compromised pluripotent state (Fig. 3C). We then focused on two stable Ncoa3 knockdown cell lines derived with different plasmids, shN3-1 and shN3-2, to evaluate their in vitro and in vivo differentiation potential. EBs formed by Ncoa3 knockdown cells were smaller than EBs from control ESCs (Fig. 3, D and E). To understand how knockdown of Ncoa3 affects EB size, we first analyzed the expression of differentiation markers in ESCs and day 2 EB cells. In contrast to transient knockdown of Ncoa3 in ESCs (Fig. 1E), most differentiation markers, except for T, were not up-regulated in stable Ncoa3 knockdown ESC lines (supplemental Fig. S1). However, many differentiation markers, including Nestin, Sox17, Nkx2.5, Flk1, Bmp4, and Cdx2, were expressed higher in day 2 Ncoa3 knockdown EBs compared with control shGFP EBs (supplemental Fig. S1). These data implied that during the selection and maintenance of stable knockdown ESCs, ESCs could compensate for the loss of Ncoa3 and suppress the expression of differentiation markers. Yet, because of the reduced expression of Ncoa3 as well as pluripotency genes, stable Ncoa3 knockdown ESCs have a stronger tendency to differentiate. The accelerated differentiation may be one reason for the reduced EB size. In addition, EB size could be affected by both cell death and proliferation. We then examined the rate of apoptosis and necrosis in both ESCs and day 2 EBs by annexin V and propidium iodide staining. The results showed that knockdown of Ncoa3 did not increase cell death in both ESCs and EBs (supplemental Fig. S2). Microarray and gene ontology term analysis revealed that knockdown of Ncoa3 affects the expression of many genes involved in cell proliferation regulation (Fig. 5A). Indeed, some of these genes, such as Nanog and Tbx3, are also involved in pluripotency maintenance. Thus, compromised pluripotency, accelerated differentiation, and reduced cell proliferation are tightly connected. All these factors contribute to the smaller size of Ncoa3 knockdown EBs.
FIGURE 3.
Ncoa3 depletion impairs ESC differentiation in vitro and in vivo. A, reduced expression of pluripotency genes in two stable Ncoa3 knockdown ESC lines. Stable shGFP control and Ncoa3 knockdown ESC lines were established by transfection of shGFP or shRNA plasmids targeting Ncoa3 (shN3-1 and shN3-2) into V6.5 ESCs, followed by puromycin selection. The expression levels of Ncoa3, Nanog, Oct4, and Sox2 in these cell lines were quantified with quantitative RT-PCR. Means ± S.D. from three independent experiments were plotted. B, Western blot analysis showed that the protein levels of Ncoa3, Nanog, and Oct4 in stable Ncoa3 knockdown ESC lines were decreased. β-Actin served as a loading control. C, alkaline phosphatase staining in stable shGFP and Ncoa3 knockdown ESCs. D, phase contrast images of day 2 EBs formed by stable shGFP and Ncoa3 knockdown ESCs. The size of day 2 Ncoa3 knockdown EBs was smaller than that of shGFP control EBs. E, 20 each of randomly selected shGFP and Ncoa3 knockdown EBs were scored, and their relative diameters from images taken under identical magnification were calculated. The means ± S.D. of relative diameters are presented. F, representative pictures of mice at day 16 and day 30 after injection of ESCs. Stable shGFP and Ncoa3 knockdown ESCs were injected subcutaneously into nude mice. Teratoma (red circles) formed by shGFP ESCs were observed as early as 16 days after injection. However, no teratoma was formed from shN3-1 or shN3-2 ESCs up to 30 days. **, p < 0.01; ***, p < 0.001.
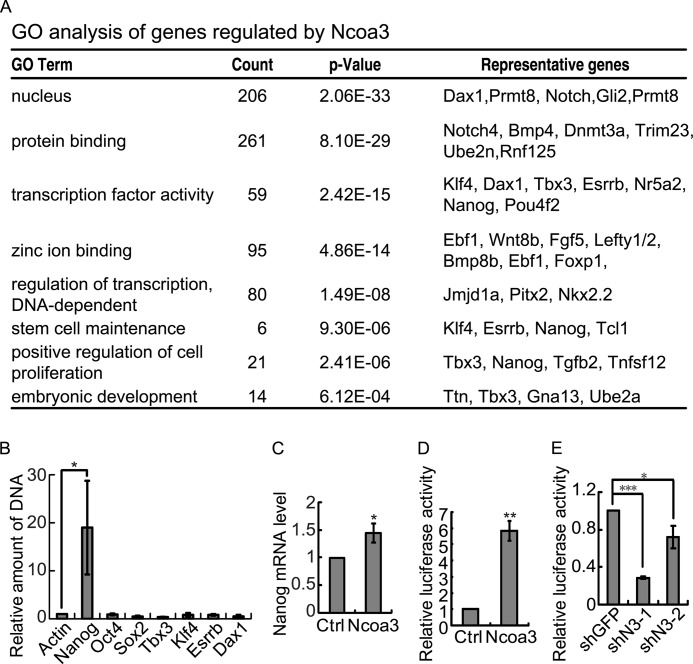
FIGURE 5.
Ncoa3 binds to and activates the Nanog promoter. A, GO term analysis of genes regulated by Ncoa3 knockdown in ESCs. B, chromatin immunoprecipitation in V6.5 ESCs with anti-Ncoa3 antibody demonstrated that Ncoa3 binds to the Nanog promoter but not the promoters of Sox2 or Oct4. C, overexpression of Ncoa3 enhanced endogenous Nanog expression. V6.5 ESCs were transfected by empty pCAGIPuro vector or pCAGIPuro-Ncoa3 overexpression plasmid. Twenty-four hours after transfection, cells were harvested. Nanog expression was measured by quantitative RT-PCR. D, luciferase assay showed that overexpression of Ncoa3 increased the Nanog promoter activity. Empty pCAGIPuro vector or pCAGIPuro-Ncoa3 overexpression plasmid, together with pRV-SV40 and the Nanog promoter reporter plasmids, were cotransfected into V6.5 ESCs. Luciferase activities were measured 24 h after transfection. Means ± S.D. from three independent experiments were plotted. E, knockdown of Ncoa3 suppresses the Nanog promoter activity. A luciferase assay was performed as described in B, except that shGFP control or two shNcoa3 plasmids (shN3-1 and shN3-2) were used. Means ± S.D. from three independent experiments were plotted. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
More importantly, when subcutaneously injected into immune-deficient mice, Ncoa3 knockdown ESCs did not support teratoma formation up to 30 days, whereas teratoma formed by control ESCs were observed as early as 16 days (Fig. 3F), indicating a compromised pluripotency of Ncoa3-deficient ESCs.
Ncoa3 Is Insufficient to Maintain ESC Self-renewal in the Absence of LIF
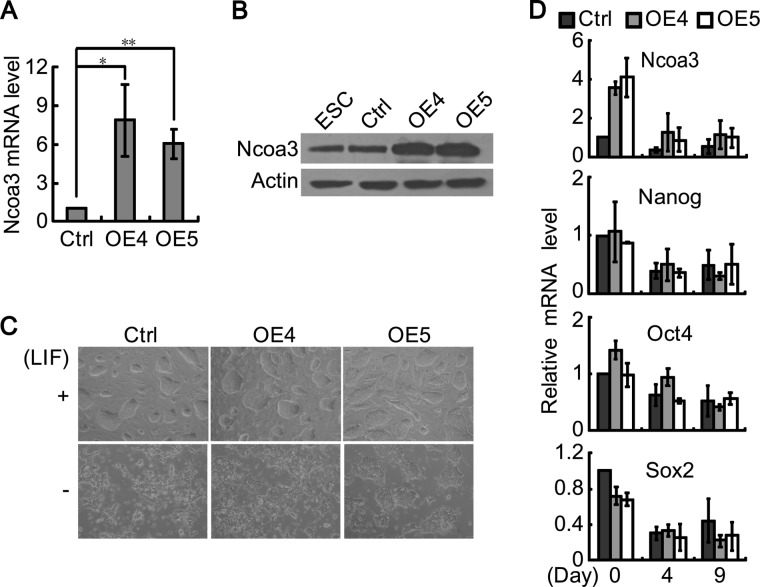
So far, we have demonstrated that Ncoa3 is critical for pluripotency maintenance in ESCs. We then asked whether forced expression of Ncoa3 is sufficient to maintain ESC self-renewal in the absence of LIF. Several stable Ncoa3 overexpression ESC lines were established. Elevated Ncoa3 expression was confirmed at both mRNA and protein levels in Ncoa3 overexpression ESCs (Fig. 4, A and B). Nevertheless, colony morphology and pluripotency markers expression were not affected by Ncoa3 overexpression (Fig. 4, C and D). When cultured on gelatin-coated dishes after LIF withdrawal, Ncoa3 overexpression ESCs differentiated similar to control ESCs containing empty vector, with flattened colony morphology and decreased expression of pluripotency markers (Fig. 4, C and 4D). Therefore, Ncoa3 is essential but not sufficient for pluripotency maintenance.
FIGURE 4.
Ncoa3 is insufficient to maintain the self-renewal of ESCs. A, stable control and Ncoa3 overexpression (OE) ESC lines were established by transfection of pCAGIPuro or pCAGIPuro-Ncoa3 plasmids into V6.5 ESCs, followed by puromycin selection. The expression levels of Ncoa3 in these cells were quantified with quantitative RT-PCR. Means ± S.D. from three independent experiments were plotted. *, p < 0.05; **, p < 0.01. B, Western blot analysis of Ncoa3 in stable control and Ncoa3 overexpression ESC lines. β-Actin served as a loading control. C, phase contrast images of control and Ncoa3 overexpression ESCs grown with or without LIF at day 2. D, quantitative RT-PCR analyzed the expression levels of Ncoa3, Nanog, Oct4, and Sox2 in control and Ncoa3 overexpression ESCs cultured without LIF at days 0, 4, and 9. Means ± S.D. from three independent experiments were plotted.
Ncoa3 Binds to and Activates the Nanog Promoter
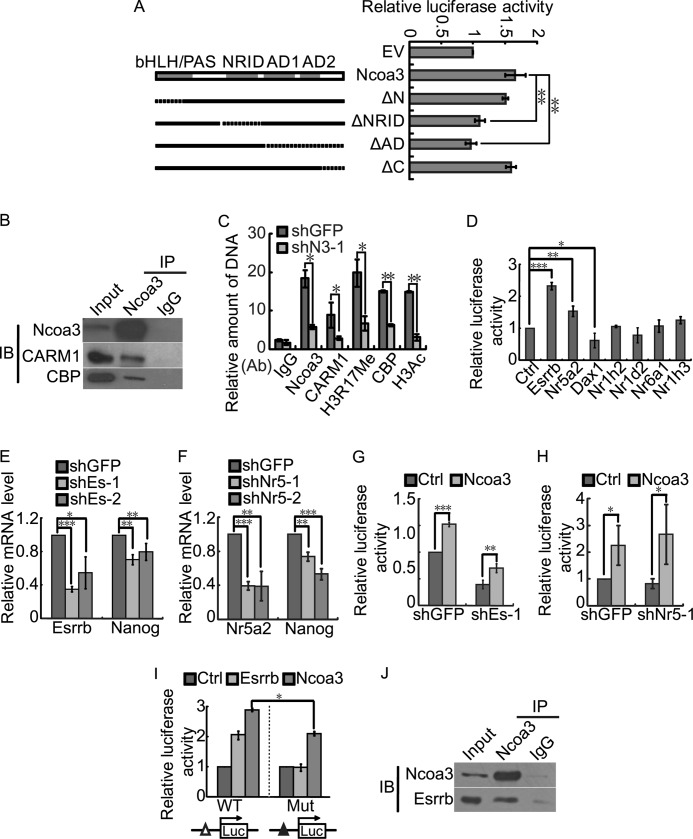
To further understand how Ncoa3 regulates pluripotency, microarray experiments were carried out to characterize the change of the global expression profile upon Ncoa3 knockdown in mouse ESCs. 311 genes were up-regulated, and 398 genes were down-regulated more than 1.5-fold by both shN3-1 and shN3-2 (supplemental Table S4). Gene ontology term analysis revealed that genes affected by Ncoa3 knockdown are enriched for genes encoding transcription factors and genes involved in stem cell maintenance (Fig. 5A). Among the 399 down-regulated genes, five genes, Esrrb, Klf4, Nanog, Dax1, and Tbx3, have been shown to be involved in pluripotency maintenance (4, 5, 8–10, 12, 13, 47, 48). To identify direct target gene(s) of Ncoa3 among these pluripotency genes, ChIP was carried out to examine the occupancy of Ncoa3 at these genes as well as core pluripotency genes, including Oct4 and Sox2. Indeed, the binding of Ncoa3 was only detected at the promoter of Nanog but not the promoters of other tested pluripotency genes (Fig. 5B). Transient overexpression of Ncoa3 enhanced the level of endogenous Nanog mRNA (Fig. 5C). Luciferase assays using a 6-kb Nanog promoter reporter also demonstrated that overexpression of Ncoa3 enhanced the activity of the Nanog reporter, whereas knockdown of Ncoa3 suppressed the Nanog promoter activity (Fig. 5, D and E).
Three known structural domains of Ncoa3, bHLH/PAS, NRID, and C-terminal activation domains, are critical for the activity of Ncoa3. The bHLH/PAS and NRID domains mediate the interaction with transcription factors and nuclear receptors, respectively (29–34). The C terminus activation domain is composed of two transcriptional activating domains, AD1 and AD2, which are responsible for the recruitment of the histone acetyltransferases CBP and p300 and the histone arginine methyltransferase CARM1 and PRMT1, respectively (35–37). To identify the crucial motifs of Ncoa3 for Nanog activation, we constructed four truncation mutants of Ncoa3, deleting the bHLH/PAS domain (ΔN), the NRID domain (ΔNRID), the AD1/AD2 domains (ΔAD), or the C-terminal end (ΔC) separately. Overexpression of the ΔNRID and the ΔAD mutants failed to activate the Nanog promoter, whereas the ΔN and the ΔC mutants activated the Nanog reporter activity to the same extent as WT Ncoa3 (Fig. 6A). Quantitative RT-PCR and Western blot analysis demonstrated similar or even higher expression levels of these WT and truncated Ncoa3 at both mRNA and protein levels (supplemental Fig. S3), thus ruling out the possibility that the inefficient activation of Nanog by the ΔNRID and the ΔAD mutants is due to reduced expression of these mutants. These data suggest that the binding of Ncoa3 at the Nanog promoter requires the interaction between the NRID domain and a certain nuclear receptor. In addition, the recruitment of histone acetyltransferase and/or histone arginine methyltransferase is likely essential for the activation of the Nanog promoter.
FIGURE 6.
Ncoa3 recruits histone acetyltransferase CBP and histone arginine methyltransferase CARM1 to activate Nanog. A, schematic illustration of Ncoa3 deletion mutants (left panel). V6.5 ESCs were cotransfected with the Nanog promoter reporter, pRV-SV40, and pSPORT plasmids expressing WT or Ncoa3 deletion mutants. Luciferase activities were measured 24 h after transfection (right panel). EV, empty vector; ΔN, deletion of the N-terminal region; ΔNRID, deletion of the nuclear receptor interacting domain; ΔAD, deletion of the activation domains; ΔC, deletion of the C-terminal of Ncoa3. Means ± S.D. from three independent experiments were plotted. B, immunoprecipitation was performed with V6.5 ESC protein extract and Ncoa3 antibody or rabbit IgG. The immunoprecipitation (IP) samples were then immunoblotted (IB) with Ncoa3, CARM1, and CBP antibodies. C, ChIP was performed in stable shGFP and Ncoa3 knockdown (shN3-1) ESCs with anti-Ncoa3, anti-CARM1, anti-CBP, anti-acetyl-Histone H3, or anti-H3R17 dimethylation antibodies (Ab). D, Esrrb and Nr5a2 activated the Nanog promoter. Empty vector, Esrrb, Nr5a2, Dax1, Nr1h2, Nr1d2, Nr6a1, or Nr1h3 overexpression plasmid, together with pRV-SV40 and the Nanog promoter reporter plasmids, were cotransfected into V6.5 ESCs. Luciferase activities were measured 48 h after transfection. E and F, V6.5 ESCs were transfected by shGFP control or shRNA plasmids targeting Esrrb (E, shEs-1, 2) or Nr5a2 (F, shNr5–1, 2). Cells were harvested 48 h after transfection. Knockdown efficiency and Nanog expression were examined by real-time PCR. G and H, V6.5 ESCs were transfected by pRV-SV40 and the Nanog promoter reporter plasmids, shGFP control, shEs-1 (G), or shNr5–1(H) plasmids, together with empty pCAGIPuro vector or pCAGIPuro-Ncoa3. Luciferase activities were measured at 48 h after transfection. I, A 250-bp Nanog promoter containing a known Esrrb binding site (△) was used. V6.5 ESCs were transfected by pRV-SV40 together with empty pCAGIPuro control, Esrrb, or Ncoa3 overexpression plasmids and the wild-type or mutant 250-bp Nanog promoter reporter plasmids. Luciferase activities were measured 48 h after transfection. Mutation of the Esrrb binding site (▴) not only abolished the activation effect of Esrrb on the Nanog reporter but also compromised the reporter activation by Ncoa3. J, immunoprecipitation was performed with V6.5 ESC protein extract and Ncoa3 antibody or rabbit IgG. The immunoprecipitation samples were then immunoblotted with Ncoa3 and Esrrb antibodies. Means ± S.D. from three independent experiments were plotted. *, p < 0.05; **, p < 0.01.
To elucidate the role of histone acetylation and histone arginine methylation in Nanog activation by Ncoa3, a coimmunoprecipitation assay was performed in V6.5 ESCs. Both CBP and CARM1 were detected in a Ncoa3 immunoprecipitated sample but not in an IgG control sample, confirming that Ncoa3 interacts with CBP and CARM1 in ESCs (Fig. 6B). Next, ChIP was carried out to determine the binding of CBP and CARM1 at the Nanog promoter in stable shGFP and Ncoa3 knockdown ESCs. Ncoa3 knockdown reduced the occupancy of Ncoa3, as well as CBP and CARM1, at the Nanog promoter, suggesting that Ncoa3 recruits both CBP and CARM1 to the Nanog promoter (Fig. 6C). Consequently, the levels of histone H3 acetylation and histone H3 arginine 17 dimethylation (H3R17 di-Me) at the Nanog promoter decreased in Ncoa3 knockdown ESCs. Taken together, Ncoa3 recruits the histone acetyltransferase CBP and the histone arginine methyltransferase CARM1 to acetylate histones and methylate histone arginine at the Nanog promoter, leading to the activation of Nanog.
Ncoa3, as a nuclear receptor coactivator, activates gene transcription in cooperation with nuclear receptors. We have demonstrated that the ΔNRID mutant failed to activate the Nanog promoter (Fig. 6A). We set out to identify the nuclear receptor, which recruits Ncoa3 to the Nanog promoter, and activate Nanog expression. Seven nuclear receptors, including Esrrb, Nr5a2, Dax1, Nr1h2, Nr1d2, Nr6a1, and Nr1h3, which are highly expressed in ESCs and down-regulated upon differentiation (49), were tested for their ability to activate the Nanog promoter. Only Esrrb and Nr5a2 were able to activate the Nanog luciferase reporter (Fig. 6D). Consistently, knockdown of Esrrb and Nr5a2 reduced the expression of Nanog in ESCs (Fig. 6, E and F). However, Ncoa3 still activated the Nanog reporter when either Esrrb or Nr5a2 was knocked down (Fig. 6, G and H). With these data, we could not rule out the possibility that Esrrb or Nr5a2 recruit Ncoa3 to the Nanog promoter. The knockdown efficiency was only around 60% for both Esrrb and Nr5a2 (Fig. 6, E and F). The remaining Esrrb or Nr5a2 could still facilitate Ncoa3 to activate Nanog. To avoid this problem, a 250-bp Nanog promoter luciferase reporter harboring an Esrrb binding site was used (10). Mutation in the Esrrb binding site abolished the activation effect of Esrrb on the Nanog reporter. Ncoa3 activated both the WT and mutant 250-bp Nanog promoter reporter, but the mutation in the Esrrb binding site compromised the activation efficiency of Ncoa3 (Fig. 6I). A coimmunoprecipitation experiment also detected the interaction between Esrrb and Ncoa3 in ESCs (Fig. 6J). These data suggest that Esrrb is involved in recruiting Ncoa3 to the Nanog promoter but that other factor(s) independent of Esrrb might also recruit Ncoa3 to activate Nanog.
Ncoa3 Is Required for GSK3 Signaling to Repress Nanog
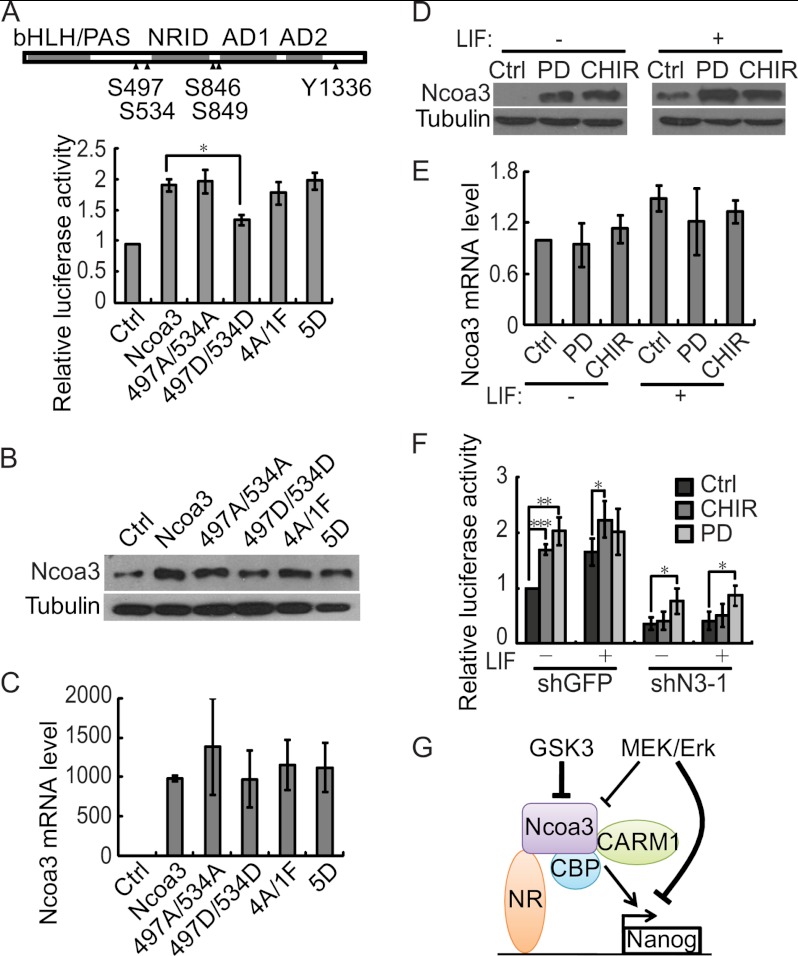
It has been demonstrated that the transcriptional activity of Ncoa3 is regulated by phosphorylation in response to various upstream signaling pathways (38, 41, 42, 45). We then asked whether the phosphorylation status of Ncoa3 affects Nanog activation. Point mutations were introduced to five known phosphorylated residues: serine 497, 534, 846, and 849 and tyrosine 1336 of Ncoa3. Mutation to alanine or phenylalanine prevents phosphorylation, whereas mutation to aspartate mimics the phosphorylated status. A luciferase assays showed that mutations to alanine and phenylalanine at these sites did not affect the activation of Nanog by Ncoa3. However, the Ncoa3 mutant harboring S497D and S534D was less efficient in activating the Nanog promoter compared with WT Ncoa3. Interestingly, when these five residues were all mutated into aspartate, the transcriptional activity of this Ncoa3 mutant (5D) on the Nanog promoter was similar to WT Ncoa3 (Fig. 7A). These data suggested that the transcriptional activity of Ncoa3 on the Nanog promoter is indeed regulated by its phosphorylation status. Western blot analysis showed that the Ncoa3 mutant harboring S497D and S534D was expressed at a lower level than other Ncoa3 mutants and WT Ncoa3, whereas no significant difference was detected at the mRNA level (Fig. 7, B and C), suggesting that the phosphorylation status of Ncoa3 regulates the protein stability, hence the transcriptional activity.
FIGURE 7.
The GSK3 pathway regulates the Nanog promoter through Ncoa3. A, the transcriptional activity of Ncoa3 is regulated by its phosphorylation status. A schematic illustration of Ncoa3 is shown. Five phosphorylation sites, Ser-497, 534, 846, and 849 and Tyr-1336, are marked (▴). 497A/534A represents the Ncoa3 mutant with S497A and S534A mutations. 497D/534D means that Ser-497 and Ser-534 were mutated to aspartate. In the 4A/1F mutant, Ser-497, Ser-534, Ser-846, and Ser-849 were replaced with alanine, and Tyr-1336 was mutated into phenylalanine. Ser-497, Ser-534, Ser-846, Ser-849, and Tyr-1336 were all replaced by aspartate in the 5D mutant. V6.5 ESCs were cotransfected with the Nanog promoter reporter, pRV-SV40, and pSPORT plasmids expressing WT or Ncoa3 mutants with mutations in phosphorylation sites. Luciferase activities were measured 24 h after transfection. Means ± S.D. from three independent experiments were plotted. B, Ncoa3 protein levels in V6.5 ESCs described in A were detected by Western blot analysis. C, Ncoa3 mRNA expression in V6.5 ESCs described in A was measured by quantitative RT-PCR. D, GSK3 and Mek/Erk signaling repress the expression of Ncoa3 protein. V6.5 ESCs were cultured in medium supplemented with CHIR99021 (3 μm) (CHIR) or PD0325901 (1 μm) (PD), with or without LIF, for 48 h. Cell lysates were analyzed by Western blot analysis. β-tubulin served as a loading control (Ctrl). E, Ncoa3 mRNA expression in V6.5 ESCs described in D was measured by quantitative RT-PCR. F, inhibition of GSK3 signaling does not enhance the activity of the Nanog promoter in stable Ncoa3 knockdown ESCs. Stable shGFP and Ncoa3 knockdown (shN3-1) ESCs were cotransfected with the Nanog promoter reporter and pRV-SV40 plasmids and cultured in medium containing CHIR99021 (3 μm) or PD0325901 (1 μm) with or without LIF. Luciferase activities were measured 24 h after transfection. Means ± S.D. from three independent experiments were plotted. G, a working model for Ncoa3 in pluripotency maintenance. Through interaction with the nuclear receptor, Ncoa3 binds to the promoter of Nanog and subsequently recruits the histone acetyltransferase CBP and the histone arginine methyltransferase CARM1 to modify the histones and activate the Nanog promoter. Upon differentiation, GSK3 and Mek/Erk signaling down-regulate Ncoa3 protein, leading to Nanog repression. In addition to Ncoa3, Mek/Erk might suppress Nanog through other mechanism(s). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
It has been shown that ERK2 phosphorylates NCOA3 in vitro and that GSK3 signaling regulates the stability of NCOA3 protein through phosphorylation of serine 505 (equivalent to Ser-497 in mouse Ncoa3) in human breast cancer cells (41, 50). Moreover, inhibition of Mek/Erk and GSK3 signaling allows ESCs to self-renew in the absence of LIF (51). Thus, we asked whether Mek/Erk and GSK3 signaling regulate ESC self-renewal through Ncoa3. ESCs cultured with the Mek inhibitor PD0325901 or the GSK3 inhibitor CHIR99021 expressed more Ncoa3 than control ESCs, no matter whether LIF was present or not (Fig. 7D). Despite the elevated Ncoa3 protein expression, the Ncoa3 mRNA level was not increased by PD0325901 or CHIR99021 treatment, indicating that Mek/Erk and GSK3 signaling regulate Ncoa3 expression at a posttranscriptional step (Fig. 7E). Residue(s) of Ncoa3 phosphorylated by Mek/Erk and GSK3 signaling in ESCs remains to be characterized.
Inhibition of Mek/Erk and GSK3 signaling facilitate pluripotency maintenance in ESCs (51). Our data showed that Mek/Erk and GSK3 signaling suppress the expression of Ncoa3 protein, which can activate Nanog to maintain pluripotency in ESCs. We then asked whether Mek/Erk and GSK3 signaling act through Ncoa3 to repress Nanog expression. Luciferase reporter assays showed that inhibition of Mek/Erk or GSK3 signaling indeed enhanced the Nanog promoter activity in shGFP control ESCs, especially in the absence of LIF. However, when Ncoa3 was depleted, the Nanog promoter activity was not increased by CHIR99021 treatment, whereas PD0325901 still activated the Nanog promoter (Fig. 7F). These data suggest that GSK3 signaling represses Nanog expression mainly through down-regulation of Ncoa3 protein. Mek/Erk signaling may also inactivate the Nanog promoter. However, Ncoa3 is not the key downstream mediator of Mek/Erk to suppress Nanog expression.
DISCUSSION
Previous studies have demonstrated the importance of certain nuclear receptors, including Essrb, Nr5a2, and Dax1, in pluripotency maintenance (9, 11, 12, 14–16). However, it is not clear whether nuclear receptor coactivators are also required for pluripotency and ESC self-renewal. Here, we demonstrated that the nuclear receptor coactivator Ncoa3 is essential for pluripotency maintenance in mouse ESCs. Depletion of Ncoa3 in ESCs compromises the expression of the pluripotency genes Nanog, Oct4, and Sox2 as well as the in vitro and in vivo differentiation potential of ESCs. However, Ncoa3 appears to be dispensable for the pluripotency of ICM cells. Ncoa3 null mice are viable but associated with a pleiotropic phenotype showing growth retardation, delayed puberty, reduced female reproductive function, and blunted mammary gland development (22, 23). This discrepancy might be caused by redundant factors during embryogenesis. Alternatively, ESCs, as the in vitro counterpart of the ICM, are not completely identical to the ICM. For example, LIF is required for in vitro culture of ESCs, but knockout of LIF or the LIF receptor gp130 has no effect on the pluripotency of ICM cells (52, 53).
Ncoa3 might maintain pluripotency through activating Nanog expression. The NRID and AD domains of Ncoa3 are necessary for the transcriptional activation of Nanog by Ncoa3, suggesting that the interactions with nuclear receptor and histone modifying enzymes are required for the function of Ncoa3. Essrb and Nr5a2 have been shown to bind to and activate the Nanog promoter (8, 10, 15, 16). Here, we have demonstrated that Esrrb interacts with Ncoa3 in mouse ESCs, and contributes to the activation of Nanog by Ncoa3. Our data also suggest that other transcription factors might facilitate Ncoa3 to activate the Nanog promoter independently of Esrrb. More experiments are required to identify other transcription factor responsible for Ncoa3 recruitment to the Nanog promoter. Upon binding to the Nanog promoter, Ncoa3 recruits the histone acetyltransferase CBP and the histone arginine methyltransferase CARM1 to acetylate histones and methylate histone H3 arginine 17. Consequently, Nanog expression is activated (Fig. 7G).
ESCs have two critical properties, namely self-renewal and differentiation potential into all types of cells in the body. Self-renewal requires the maintenance of a pluripotency-associated transcriptional profile. In contrast, during differentiation, the pluripotency-associated transcriptional profile has to be altered in response to various environmental cues. It has been shown that β-catenin, together with Tcf3, activates the expression of Nr5a2, which, in turn, promotes the expression of Nanog, Oct4, and Tbx3. GSK3 signaling stimulates the degradation of β-catenin, thus repressing downstream Nr5a2, Nanog, Oct4, and Tbx3 (16). Our data suggest that Ncoa3 might be another downstream mediator for GSK3 signaling to down-regulate Nanog expression. Activated GSK3 signaling might phosphorylate Ncoa3 and accelerate Ncoa3 degradation, leading to suppression of Nanog. Thus, down-regulation of Ncoa3 by GSK3 signaling might facilitate disrupting the core transcriptional circuitry of pluripotency and allow ESCs to differentiate. Moreover, during ESC differentiation by LIF withdrawal, multiple signaling pathways might work on many downstream targets simultaneously to regulate transcriptional profile change. For example, Mek/Erk signaling could suppress Nanog independent of Ncoa3 (Fig. 7F). This explains why Ncoa3 overexpression is insufficient to maintain ESC self-renewal in the absence of LIF.
In summary, Ncoa3 plays a dual role in pluripotency maintenance. It activates the expression of Nanog to promote the self-renewal of ESCs. Meanwhile, Ncoa3 protein is subjected to various posttranslational modifications, which are regulated by signaling pathways such as GSK3 and Mek/Erk. These signaling pathways may stimulate the degradation of Ncoa3 or suppress the transcriptional activity of Ncoa3, subsequently reducing Nanog expression and allowing ESCs to differentiate. Given the reprogramming activities of potential Ncoa3-interacting nuclear receptor Esrrb and Nr5a2 (8, 15), Ncoa3 might cooperate with Esrrb or Nr5a3 to enhance reprogramming efficiency. Further investigation is required to demonstrate the function of Ncoa3 in iPS derivation.
Supplementary Material
This work was supported by National Natural Science Foundation of China Grant 90919009 and 31271547, by National Basic Research Program of China Grants 2009CB941000 and 2010CB833603, and by the Transgenic Program from Ministry of Agriculture of China Grants 2009ZX08006-010B and 2009ZX08006-011B.

This article contains supplemental Figs. S1–S3, Tables S1–S4, and Experimental Procedures.
- ESC
- embryonic stem cell
- ICM
- inner cell mass
- NR
- nuclear receptor
- LIF
- leukemia inhibitory factor
- MEF
- mouse embryonic fibroblasts
- SRC
- steroid receptor coactivator
- NRID
- nuclear receptor interacting domain
- CBP
- CREB binding protein
- EB
- embryoid body
- AD
- activation domain
- CREB
- cAMP-responsive binding protein.
REFERENCES
- 1. Evans M. J., Kaufman M. H. (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 [DOI] [PubMed] [Google Scholar]
- 2. Martin G. R. (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. U.S.A. 78, 7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nichols J., Zevnik B., Anastassiadis K., Niwa H., Klewe-Nebenius D., Chambers I., Schöler H., Smith A. (1998) Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95, 379–391 [DOI] [PubMed] [Google Scholar]
- 4. Mitsui K., Tokuzawa Y., Itoh H., Segawa K., Murakami M., Takahashi K., Maruyama M., Maeda M., Yamanaka S. (2003) The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113, 631–642 [DOI] [PubMed] [Google Scholar]
- 5. Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., Smith A. (2003) Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113, 643–655 [DOI] [PubMed] [Google Scholar]
- 6. Avilion A. A., Nicolis S. K., Pevny L. H., Perez L., Vivian N., Lovell-Badge R. (2003) Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 17, 126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyer L. A., Lee T. I., Cole M. F., Johnstone S. E., Levine S. S., Zucker J. P., Guenther M. G., Kumar R. M., Murray H. L., Jenner R. G., Gifford D. K., Melton D. A., Jaenisch R., Young R. A. (2005) Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng B., Jiang J., Kraus P., Ng J. H., Heng J. C., Chan Y. S., Yaw L. P., Zhang W., Loh Y. H., Han J., Vega V. B., Cacheux-Rataboul V., Lim B., Lufkin T., Ng H. H. (2009) Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nat. Cell Biol. 11, 197–203 [DOI] [PubMed] [Google Scholar]
- 9. Zhang X., Zhang J., Wang T., Esteban M. A., Pei D. (2008) Esrrb activates Oct4 transcription and sustains self-renewal and pluripotency in embryonic stem cells. J. Biol. Chem. 283, 35825–35833 [DOI] [PubMed] [Google Scholar]
- 10. van den Berg D. L., Zhang W., Yates A., Engelen E., Takacs K., Bezstarosti K., Demmers J., Chambers I., Poot R. A. (2008) Estrogen-related receptor β interacts with Oct4 to positively regulate Nanog gene expression. Mol. Cell. Biol. 28, 5986–5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun C., Nakatake Y., Akagi T., Ura H., Matsuda T., Nishiyama A., Koide H., Ko M. S., Niwa H., Yokota T. (2009) Dax1 binds to Oct3/4 and inhibits its transcriptional activity in embryonic stem cells. Mol. Cell. Biol. 29, 4574–4583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelly V. R., Xu B., Kuick R., Koenig R. J., Hammer G. D. (2010) Dax1 up-regulates Oct4 expression in mouse embryonic stem cells via LRH-1 and SRA. Mol. Endocrinol. 24, 2281–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang J., Rao S., Chu J., Shen X., Levasseur D. N., Theunissen T. W., Orkin S. H. (2006) A protein interaction network for pluripotency of embryonic stem cells. Nature 444, 364–368 [DOI] [PubMed] [Google Scholar]
- 14. Khalfallah O., Rouleau M., Barbry P., Bardoni B., Lalli E. (2009) Dax-1 knockdown in mouse embryonic stem cells induces loss of pluripotency and multilineage differentiation. Stem Cells 27, 1529–1537 [DOI] [PubMed] [Google Scholar]
- 15. Heng J. C., Feng B., Han J., Jiang J., Kraus P., Ng J. H., Orlov Y. L., Huss M., Yang L., Lufkin T., Lim B., Ng H. H. (2010) The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell 6, 167–174 [DOI] [PubMed] [Google Scholar]
- 16. Wagner R. T., Xu X., Yi F., Merrill B. J., Cooney A. J. (2010) Canonical Wnt/β-catenin regulation of liver receptor homolog-1 mediates pluripotency gene expression. Stem Cells 28, 1794–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gu P., Goodwin B., Chung A. C., Xu X., Wheeler D. A., Price R. R., Galardi C., Peng L., Latour A. M., Koller B. H., Gossen J., Kliewer S. A., Cooney A. J. (2005) Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol. Cell. Biol. 25, 3492–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oñate S. A., Tsai S. Y., Tsai M. J., O'Malley B. W. (1995) Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270, 1354–1357 [DOI] [PubMed] [Google Scholar]
- 19. Hong H., Kohli K., Trivedi A., Johnson D. L., Stallcup M. R. (1996) GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc. Natl. Acad. Sci. U.S.A. 93, 4948–4952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Voegel J. J., Heine M. J., Zechel C., Chambon P., Gronemeyer H. (1996) TIF2, a 160-kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 15, 3667–3675 [PMC free article] [PubMed] [Google Scholar]
- 21. Guan X. Y., Xu J., Anzick S. L., Zhang H., Trent J. M., Meltzer P. S. (1996) Hybrid selection of transcribed sequences from microdissected DNA. Isolation of genes within amplified region at 20q11-q13.2 in breast cancer. Cancer Res. 56, 3446–3450 [PubMed] [Google Scholar]
- 22. Wang Z., Rose D. W., Hermanson O., Liu F., Herman T., Wu W., Szeto D., Gleiberman A., Krones A., Pratt K., Rosenfeld R., Glass C. K., Rosenfeld M. G. (2000) Regulation of somatic growth by the p160 coactivator p/CIP. Proc. Natl. Acad. Sci. U.S.A. 97, 13549–13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu J., Liao L., Ning G., Yoshida-Komiya H., Deng C., O'Malley B. W. (2000) The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc. Natl. Acad. Sci. U.S.A. 97, 6379–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu J., Qiu Y., DeMayo F. J., Tsai S. Y., Tsai M. J., O'Malley B. W. (1998) Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 279, 1922–1925 [DOI] [PubMed] [Google Scholar]
- 25. Gehin M., Mark M., Dennefeld C., Dierich A., Gronemeyer H., Chambon P. (2002) The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol. Cell. Biol. 22, 5923–5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu J., Wu R. C., O'Malley B. W. (2009) Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat. Rev. Cancer 9, 615–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. York B., O'Malley B. W. (2010) Steroid receptor coactivator (SRC) family. Masters of systems biology. J. Biol. Chem. 285, 38743–38750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen X., Liu Z., Xu J. (2010) The cooperative function of nuclear receptor coactivator 1 (NCOA1) and NCOA3 in placental development and embryo survival. Mol. Endocrinol. 24, 1917–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen S. L., Dowhan D. H., Hosking B. M., Muscat G. E. (2000) The steroid receptor coactivator, GRIP-1, is necessary for MEF-2C-dependent gene expression and skeletal muscle differentiation. Genes Dev. 14, 1209–1228 [PMC free article] [PubMed] [Google Scholar]
- 30. Belandia B., Parker M. G. (2000) Functional interaction between the p160 coactivator proteins and the transcriptional enhancer factor family of transcription factors. J. Biol. Chem. 275, 30801–30805 [DOI] [PubMed] [Google Scholar]
- 31. Kim J. H., Li H., Stallcup M. R. (2003) CoCoA, a nuclear receptor coactivator which acts through an N-terminal activation domain of p160 coactivators. Mol. Cell 12, 1537–1549 [DOI] [PubMed] [Google Scholar]
- 32. Darimont B. D., Wagner R. L., Apriletti J. W., Stallcup M. R., Kushner P. J., Baxter J. D., Fletterick R. J., Yamamoto K. R. (1998) Structure and specificity of nuclear receptor-coactivator interactions. Genes Dev. 12, 3343–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heery D. M., Kalkhoven E., Hoare S., Parker M. G. (1997) A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387, 733–736 [DOI] [PubMed] [Google Scholar]
- 34. Voegel J. J., Heine M. J., Tini M., Vivat V., Chambon P., Gronemeyer H. (1998) The coactivator TIF2 contains three nuclear receptor-binding motifs and mediates transactivation through CBP binding-dependent and -independent pathways. EMBO J. 17, 507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Torchia J., Rose D. W., Inostroza J., Kamei Y., Westin S., Glass C. K., Rosenfeld M. G. (1997) The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387, 677–684 [DOI] [PubMed] [Google Scholar]
- 36. Anafi M., Yang Y. F., Barlev N. A., Govindan M. V., Berger S. L., Butt T. R., Walfish P. G. (2000) GCN5 and ADA adaptor proteins regulate triiodothyronine/GRIP1 and SRC-1 coactivator-dependent gene activation by the human thyroid hormone receptor. Mol. Endocrinol. 14, 718–732 [DOI] [PubMed] [Google Scholar]
- 37. Koh S. S., Chen D., Lee Y. H., Stallcup M. R. (2001) Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J. Biol. Chem. 276, 1089–1098 [DOI] [PubMed] [Google Scholar]
- 38. Oh A. S., Lahusen J. T., Chien C. D., Fereshteh M. P., Zhang X., Dakshanamurthy S., Xu J., Kagan B. L., Wellstein A., Riegel A. T. (2008) Tyrosine phosphorylation of the nuclear receptor coactivator AIB1/SRC-3 is enhanced by Abl kinase and is required for its activity in cancer cells. Mol. Cell. Biol. 28, 6580–6593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Naeem H., Cheng D., Zhao Q., Underhill C., Tini M., Bedford M. T., Torchia J. (2007) The activity and stability of the transcriptional coactivator p/CIP/SRC-3 are regulated by CARM1-dependent methylation. Mol. Cell. Biol. 27, 120–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen D., Ma H., Hong H., Koh S. S., Huang S. M., Schurter B. T., Aswad D. W., Stallcup M. R. (1999) Regulation of transcription by a protein methyltransferase. Science 284, 2174–2177 [DOI] [PubMed] [Google Scholar]
- 41. Wu R. C., Feng Q., Lonard D. M., O'Malley B. W. (2007) SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell 129, 1125–1140 [DOI] [PubMed] [Google Scholar]
- 42. Wu R. C., Qin J., Yi P., Wong J., Tsai S. Y., Tsai M. J., O'Malley B. W. (2004) Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic responses to multiple cellular signaling pathways. Mol. Cell 15, 937–949 [DOI] [PubMed] [Google Scholar]
- 43. Li X., Lonard D. M., Jung S. Y., Malovannaya A., Feng Q., Qin J., Tsai S. Y., Tsai M. J., O'Malley B. W. (2006) The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGγ proteasome. Cell 124, 381–392 [DOI] [PubMed] [Google Scholar]
- 44. Chen H., Lin R. J., Xie W., Wilpitz D., Evans R. M. (1999) Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell 98, 675–686 [DOI] [PubMed] [Google Scholar]
- 45. Wu H., Sun L., Zhang Y., Chen Y., Shi B., Li R., Wang Y., Liang J., Fan D., Wu G., Wang D., Li S., Shang Y. (2006) Coordinated regulation of AIB1 transcriptional activity by SUMOylation and phosphorylation. J. Biol. Chem. 281, 21848–21856 [DOI] [PubMed] [Google Scholar]
- 46. Chen L., Yabuuchi A., Eminli S., Takeuchi A., Lu C. W., Hochedlinger K., Daley G. Q. (2009) Cross-regulation of the Nanog and Cdx2 promoters. Cell Res. 19, 1052–1061 [DOI] [PubMed] [Google Scholar]
- 47. Jiang J., Chan Y. S., Loh Y. H., Cai J., Tong G. Q., Lim C. A., Robson P., Zhong S., Ng H. H. (2008) A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat. Cell Biol. 10, 353–360 [DOI] [PubMed] [Google Scholar]
- 48. Lu R., Yang A., Jin Y. (2011) Dual functions of T-box 3 (Tbx3) in the control of self-renewal and extraembryonic endoderm differentiation in mouse embryonic stem cells. J. Biol. Chem. 286, 8425-8436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xie C. Q., Jeong Y., Fu M., Bookout A. L., Garcia-Barrio M. T., Sun T., Kim B. H., Xie Y., Root S., Zhang J., Xu R. H., Chen Y. E., Mangelsdorf D. J. (2009) Expression profiling of nuclear receptors in human and mouse embryonic stem cells. Mol Endocrinol 23, 724–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Font de Mora J., Brown M. (2000) AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol. Cell. Biol. 20, 5041–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ying Q. L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. (2008) The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stewart C. L., Kaspar P., Brunet L. J., Bhatt H., Gadi I., Köntgen F., Abbondanzo S. J. (1992) Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79 [DOI] [PubMed] [Google Scholar]
- 53. Nichols J., Chambers I., Taga T., Smith A. (2001) Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development 128, 2333–2339 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.