Abstract
We have investigated the role of the isoflavones daidzein and genistein on the chemotropic behavior of germinating cysts of Phytophthora sojae. Hyphal germlings were shown to respond chemotropically to daidzein and genistein, suggesting that hyphal tips from zoospores that have encysted adjacent to the root may use specific host isoflavones to locate their host. Observations of the contact response of hyphal germlings were made on several different substrates in the presence and absence of isoflavones. Hyphal tips of germlings detected and penetrated pores in membranes and produced multiple appressoria on smooth, impenetrable surfaces. Hyphae that successfully penetrated the synthetic membrane were observed to grow away from the membrane surface. The presence of isoflavones in the medium surrounding the hyphal germlings did not appear to alter any of those habits. Daidzein and genistein did not inhibit germination or initial hyphal growth at concentrations up to 20 μm.
For host-specific pathogens or symbionts, the ability to recognize and move in the direction of a plant signal may be critical to the survival of the organism. Germination or chemotropism in response to a host-specific signal has now been described in several plant-fungus associations (Coley-Smith, 1990; Podila et al., 1993; Ruan et al., 1995). In bacteria, expression of nodulation genes in Rhizobium and Bradyrhizobium species is induced by flavones or isoflavones (Fisher and Long, 1992), and some species such as Rhizobium meliloti also respond chemotactically to the nodulation signals (Dharmatilake and Bauer, 1992). In addition, chemotaxis to plant exudates appears to be an important factor contributing to the virulence of Agrobacterium species in heterogenous soil mixtures (Hawes and Smith, 1989).
Zoospores of oömycetes also exhibit positive chemotaxis to plant-derived compounds (Zentmyer, 1961; Carlile, 1983; Horio et al., 1992; Morris and Ward, 1992; Sekizaki et al., 1993). Zoospores of the soybean pathogen Phytophthora sojae are highly sensitive to the isoflavones daidzein and genistein, which are exuded from the roots of soybeans into the rhizosphere (Morris and Ward, 1992; Tyler et al., 1996). Because six other species of Phytophthora and one species of Pythium displayed no sensitivity to these isoflavones, Morris and Ward (1992) suggested that the specific attraction to soybean isoflavones might be part of the mechanism that determines host range.
Although oömycetes have a predominantly filamentous hyphal growth pattern, the relationship between soil water and disease severity suggests that zoospores are the predominant means by which pathogenic oömycetes spread throughout the soil and infect plant roots, especially in flooded soils (Duniway, 1983; Erwin and Ribeiro, 1996). Despite the complex structure and small pore size of most soils, zoospores are capable of traveling substantial distances (25–35 mm) to initiate infection within a 24-h period (Duniway, 1976). However, the majority of zoospores released from the zoosporangium on the hyphae do not reach their host and eventually form cysts. Hardham and Gubler (1990) established that both adhesion and the initial direction from which the germ tube emerges are determined at the time of encystment. Factors that influence the direction of hyphal growth after germination are less clearly defined. Autoaggregation of zoospores in the absence of an available host is characteristic of some but not all oömycetes (Reid et al., 1995). Zentmyer (1961) demonstrated that Phytophthora cinnamoni cysts that were adjacent to the root of their host germinated rapidly and grew in the direction of the root, but the chemical signal was not identified. A chemotropic response of hyphae to nutrient sources has also been demonstrated in several saprophytic and parasitic oömycetes (Musgrave et al., 1977; Manavathu and Thomas, 1985; Jones et al., 1991). Thus, chemotropic responses of oömycetes hyphae might also contribute to their effectiveness as plant pathogens.
In this study we report on an in vitro system that we have developed to study the encystment and subsequent germination of zoospores in response to soybean isoflavones. We show that both thigmotropic (contact) and specific chemotropic determinants control the direction of growth in germinating hyphae.
MATERIALS AND METHODS
Preparation of Zoospores and Chemotropism Assays
Phytophthora sojae Kauf. & Gerd., strains P6497 and P7063 (Förster et al., 1994), were grown on vegetable juice agar, and zoospores were produced by repeated washing of plates with distilled water as described previously (Morris and Ward, 1992). These strains were used because their differential responses to daidzein and genistein have been well characterized (Tyler et al., 1996). In capillary assays, genistein is 10 and 100 times more potent than daidzein in P6497 and P7063, respectively. Chemotropism assays were performed in an assay chamber that was created by supporting a coverslip with two glass pieces on a glass slide. The weight of the coverslip was sufficient to hold a 250-μL drop of zoospores in place, and small soybean roots or 1-μL capillary tubes (Drummond microcaps) containing the isoflavones daidzein or genistein could be inserted directly into the chamber. Daidzein and genistein were obtained from Indofine Chemical (Somerville, NJ). Stock solutions of 40 μm in water were stored at −20°C.
To demonstrate the chemotropic properties of hyphal germlings, a 1-μL capillary tube containing 20 μm daidzein or genistein was introduced into a chemotaxis chamber and left undisturbed for 3 h. Hyphal germlings surrounding the mouth of the capillary tube were photographed under phase-contrast optics using a BH-2 microscope (Olympus). To test the ability of uniformly dispersed cysts to germinate and reorient in response to host roots or an isoflavone gradient, P6497 zoospores were induced to encyst by adding 10 mm CaCl2 combined with vortexing for 15 s. The zoospores were then pipetted into the chemotaxis chamber and left undisturbed for 1 h. A soybean root or a capillary tube containing 20 μm genistein was then inserted into the chemotaxis chamber. A 50-μL drop of genistein was also applied to the open end of the capillary tube, which was then left undisturbed for 3 h.
Quantitative Analysis of Hyphal Chemotropism
The angle of hyphal growth relative to the root surfaces was determined by defining a plane parallel to the surface of the root, and measuring the angle of the hyphal tip as a deviation (in degrees) from a direction directly toward this plane. Thus, hyphal tips growing directly toward the “source” were given a value of 0°, and hyphal tips growing directly away from the source were assigned a value of 180°. The angle of hyphal growth relative to the capillary tube was determined by drawing a line from the mouth of the capillary tube to the hyphal tip. The angle was measured as a deviation from that line, so that hyphal tips that pointed directly at the tube were given a value of 0°. The distance of each hyphal tip from the source was determined by measuring the distance in the photographs and converting this value to actual distances using a photograph of a stage micrometer taken at the same magnification. A total of 231 and 279 data points were calculated from 13 and 15 photographs of capillary tubes and roots, respectively. For each data set, all points were ordered by distance, and the mean of the cosine was calculated for a 200-μm window sliding from 0 μm to the end at 20-μm intervals. A 95% confidence interval was calculated for the points in each data window using a t distribution. The number of points in each window ranged from 30 to 74.
Assessment of Chemotropic and Contact Responses of Hyphal Germlings
Cell-culture plates and inserts (Falcon, Fisher Scientific) were used to test the chemotropic and thixotropic (contact) responses of hyphae on a solid surface. In a typical experiment, 1 mL of swimming zoospores was placed in one well of the cell-culture plate. The isoflavone attractant was dissolved in a 0.2% agarose solution, which was added to the cell-culture insert cup. The bottom layer of the cup consisted of a porous PET membrane with 3-μm pores. When the insert cup was placed in the well containing the zoospores, the isoflavones diffused through the pores, producing a concentration gradient that caused zoospores to swim toward the membrane. After 40 min the insert was removed from the chamber and the membrane surface carefully rinsed to remove any zoospores that had not encysted on the membrane surface. The insert was then placed in a second well containing only water or water plus isoflavonoids. To compare how gravity might influence the chemotropic response of hyphae, the zoospores were also placed in the cell-culture insert, and the agarose containing the isoflavone attractant was added directly to the cell-culture well.
To test the response of hyphae to a soft, smooth surface, a drop of Vitrogen 100 (Collagen Corp., Palo Alto, CA) was applied to one side of the PET membrane on the cell-insert cup and cross-linked by incubating in the presence of NH4OH vapor in a desiccation chamber for 12 h. The proteinaceous surface was rinsed briefly in distilled water and a 200-μL drop of 1 μm genistein was applied to the inner chamber of the cup. The cell-culture insert was then placed in a sample well containing swimming zoospores. After 4 h the cell-culture insert was removed and germinating hyphae on the membrane were fixed and prepared for scanning electron microscopy as described below.
To test the response of hyphae to a smooth, impermeable surface, a piece of clear plastic wrap (Dow Chemical, Indianapolis, IN) was glued to the lower end of the cell-culture insert. The cell-culture insert was inverted and a 30-μL drop of 20 μm daidzein or genistein was placed on the membrane surface. The cell-culture insert was quickly turned right-side up again and placed in a well containing 1 mL of swimming zoospores (1 × 106). Encysted zoospores were left undisturbed for 40 min because initial experiments confirmed the earlier observations by Donaldson and Deacon (1992) that further mechanical perturbation of newly formed cysts delayed germination of hyphae. The insert was then removed, rinsed free of swimming zoospores, and transferred to a new well containing water or water plus isoflavones. After 4 h cells were fixed and prepared for electron microscopy.
Effect of Isoflavones on Cyst Germination
A cell-culture insert with affixed clear plastic wrap membrane was placed in a sample well containing swimming zoospores. Stock solutions of daidzein and genistein were added to the sample well to adjust the final isoflavone concentrations to 1 or 20 μm. CaCl2 (10 mm final concentration) was used to induce encystment of all zoospores (Griffith et al., 1988). After 2 h the cells on the plastic membrane were fixed with 2% glutaraldehyde in 0.05 m Na2HPO4, pH 7.2. Germination, hyphal germling length, and the percentage of hyphae with appressoria were counted by surveying several microscopic fields. Each experiment was repeated at least four times with both strains.
Fixation and Microscopy
For scanning electron microscopy, germinating cysts were fixed on membranes for 1 h in 2% glutaraldehyde in 0.05 m Na2HPO4, pH 7.2, and then rinsed in buffer. Some samples were postfixed for 2 h in 1% OsO4 in 0.05 m Na2HPO4 buffer, pH.7.2, and rinsed twice with buffer. After dehydration in a graded ethanol series, the zoospores were critical-point dried, mounted on aluminum stubs, and coated with 10 nm of gold-palladium using a sputter coater (Polaron, Milton Keynes, UK). The zoospores were viewed with a S-2700 scanning electron microscope (Hitachi, Tokyo, Japan).
Hyphae that had penetrated the PET membrane were photographed by light microscopy 16 h after encystment by adjusting the plane of focus to distances progressively closer to the membrane surface. Visualization of the hyphal threads was improved by prior addition of 0.1% 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazollium bromide in 1 mm KH2PO4, pH 7.0.
RESULTS
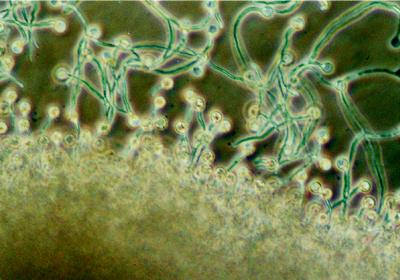
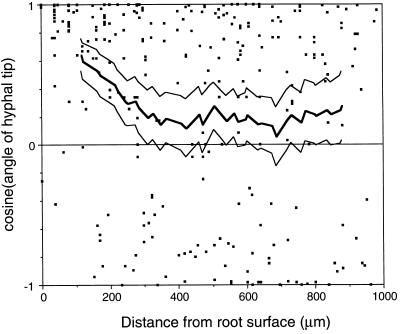
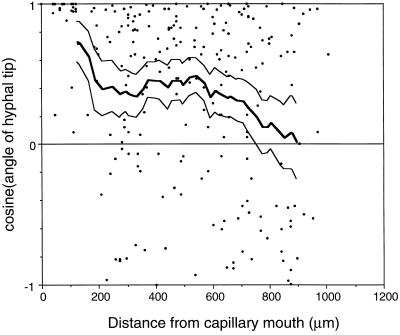
When a capillary tube containing 20 μm daidzein or genistein was introduced to the chemotaxis chamber and left undisturbed, zoospores rapidly plugged the capillary tube and others encysted around the mouth of the tube. Some zoospores that had encysted farthest from the capillary tube opening germinated and grew away from the capillary tube before turning in the direction of the isoflavone source (Fig. 1). Similarly, when a soybean root was introduced to swimming zoospores, encystment occurred both on and adjacent to the root tip. Cysts adjacent to the root germinated and grew toward the root surface (not shown). To confirm that the chemotropic signal was an isoflavone and was not derived from adjacent germinating cysts, zoospores were induced to encyst randomly, then a soybean root tip or a capillary tube containing genistein was introduced into the chemotaxis chamber after cyst germination had occurred. Visual inspection of hyphae indicated that both encystment and the initial direction of hyphal growth were random. The tropic response of the hyphae toward the root was significant at distances up to about 300 μm from the root surface (Fig. 2). The tropic response of hyphae toward genistein was significant at distances up to 750 μm from the mouth of the capillary tube (Fig. 3). Hyphae behaved in a similar manner in reorientation experiments using daidzein (not shown).
Figure 1.
Chemotropism of hyphae toward a capillary tube containing genistein (magnification ×165). Zoospores encysted around the capillary tube and the majority of cysts produced hyphae that grew in the direction of the source. Hyphae farthest from the source changed direction to grow toward the capillary tube.
Figure 2.
Chemotropic growth of hyphae toward soybean root tips. Zoospores were induced to encyst in a random orientation. Approximately 3 h after the introduction of the root tip into the chemotaxis chamber, the angles of hyphal tips were measured relative to a line drawn perpendicular to the root surface. ▪, Cosine of growth angle; thick line, cosines averaged over a 200-μm window at 20-μm increments; thin lines, upper and lower 95% significance limits for cosine means calculated using a t test. The number of data points in each window ranged from 38 to 74. Chemotropism was considered significant when the lower confidence limit was greater than 0.
Figure 3.
Chemotropic growth of hyphae toward capillary tubes containing 20 μm genistein. Zoospores were induced to encyst in a random orientation. Approximately 3 h after the introduction of the capillary into the chemotaxis chamber, the angles of hyphal tips were measured relative to a line drawn from the counterpoint of the mouth of the capillary. •, Cosine of growth angle; thick line, cosines averaged over a 200-μm window at 20-μm increments; thin line, upper and lower 95% significance limits for cosine means calculated using a t test. The number of data points in each window ranged from 30 to 66. Chemotropism was considered significant when the lower confidence limit was greater than 0.
To determine the effect of isoflavones on newly formed cysts, zoospores were induced to encyst on clear plastic wrap membranes glued to cell-culture inserts in the presence of varying levels of isoflavones. After 2 h, germination in both strains was 100% in the presence of 0, 1, or 20 μm levels of daidzein and genistein for both strains there were no significant differences in the length of the germinating hyphae or the percentage of hyphae that had formed appressoria (Table I). The wide variation in the length of the germlings appeared to be dependent on whether the germinating cyst immediately produced an appressorium or continued to grow along the membrane surface.
Table I.
Effect of isoflavones on initial growth of germinating cysts
| Treatment | Concentration | Strain
P7063
|
Strain P6497
|
||
|---|---|---|---|---|---|
| Hyphal length | Appressoria | Hyphal length | Appressoria | ||
| μm | μm | % | μm | % | |
| Control | 750 ± 230 | 50 | 720 ± 300 | 38 | |
| Daidzein | 1 | 515 ± 50 | 32 | 710 ± 200 | 36 |
| Daidzein | 20 | 610 ± 180 | 44 | 650 ± 150 | 51 |
| Genistein | 1 | 680 ± 260 | 52 | 740 ± 100 | 41 |
| Genistein | 20 | 540 ± 180 | 54 | 640 ± 130 | 46 |
Data from a typical experiment are shown. Germination in all treatment groups at 2 h was 100%. Differences in length of the hyphae and cyst or in the percentage of hyphae forming appressoria are not significant.
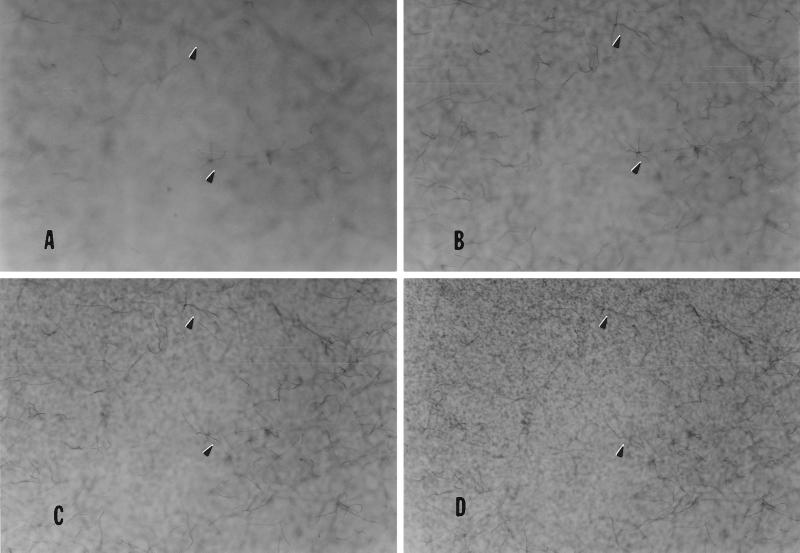
To determine how germinating hyphae responded to tactile and chemical signals, zoospores were induced to encyst on the porous PET membranes of cell-culture inserts by adding isoflavones to the adjacent or opposite side of the PET membrane. In the presence of isoflavones, hyphae germinated and grew along the membrane surface until they came in contact with a pore (Fig. 4). The hyphae grew through the pores and emerged on the other side of the membrane. Hyphae emerging from the pores grew away from the membrane rather than remaining in contact with the membrane surface (Fig. 5).
Figure 4.
Hyphal germlings growing along and penetrating pores of a PET membrane. Zoospores were induced to encyst on the membrane by application of an agar plug containing isoflavones to the opposite side of the membrane. Germinating hyphae penetrated pores detected by the hyphal tip. The distance indicated is the distance between two hatch marks.
Figure 5.
Hyphae growing away from a membrane after having penetrated a pore (magnification ×27). Zoospores were induced to encyst on a PET membrane. Hyphae that had successfully penetrated through pores were visualized by staining with 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazollium bromide. The plane of focus in each picture (A–D) is progressively closer to the membrane surface and is slightly above the membrane in D. Arrows point to hyphae that are more visible in frames A, B, or C, indicating that they must be growing away from the membrane surface (D).
Several attempts were made to maintain an unequal distribution of isoflavones across the porous membrane to try to change the initial tactile responses of the hyphae. For these experiments we placed an agar plug containing isoflavones on the same or the opposite side of the membrane as the encysted zoospores. In no case were we able to induce the hyphae to emerge from the cyst and grow away from the membrane surface (not shown); nor were we able to inhibit the hyphae from growing through the membrane pores.
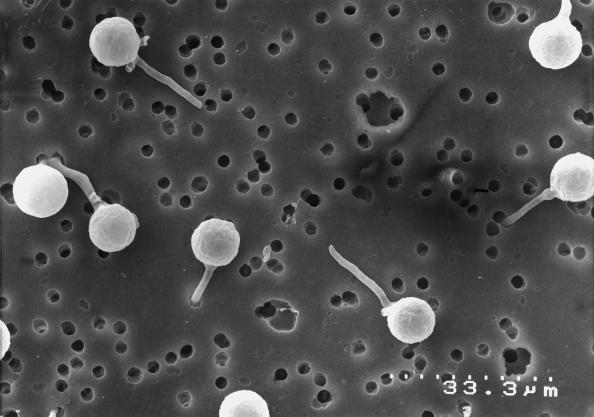
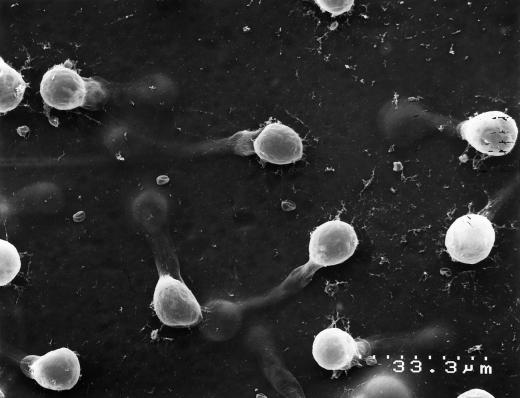
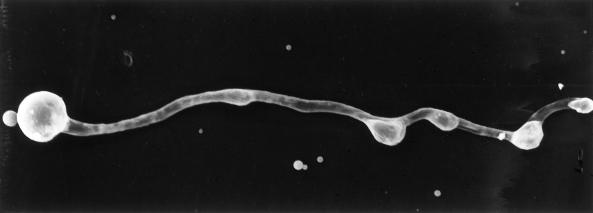
Zoospores that were induced to encyst on a smooth surface consisting of cross-linked collagen overlying the PET membrane germinated and immediately penetrated the collagen layer. The hyphae grew along the PET membrane a short distance before forming an appressorial structure, possibly over a membrane pore (Fig. 6). Zoospores that were induced to encyst on a smooth template of clear plastic wrap germinated and produced multiple appressoria (Fig. 7) in what appeared to be successive attempts to penetrate the membrane surface. None of the hyphae were observed to grow away from the template surface. In chemotropism assays the majority of the zoospores encysted on the coverslip and also tended to grow along the surface rather than away from it.
Figure 6.
Germination of cysts on a surface of cross-linked collagen overlying a PET membrane. Zoospores were induced to encyst on the collagen by application of an agar plug containing isoflavones to the opposite side of the membrane. Germinating hyphae penetrated the collagen layer and subsequently formed appressorial structures that are believed to be located over membrane pores. The distance indicated is the distance between two hatch marks.
Figure 7.
Multiple appressoria were produced by a germinating hyphae on clear plastic wrap (magnification ×1000). Zoospores were induced to encyst on a smooth surface. The hyphal germlings produced multiple appressoria as they grew along the surface.
DISCUSSION
Zoospores that encyst adjacent to the root tend to germinate and grow toward the root surface (Zentmyer, 1961; Musgrave et al., 1977; Manavathu and Thomas, 1985; Jones et al., 1991). Here we have shown that when P. sojae zoospores have been induced to encyst and germinate before the introduction of a soybean root, the hyphal tips closest to the root grew chemotropically toward the root surface. Because the hyphal tips from germinating zoospores are also chemotropically attracted to a capillary tube containing genistein, the release of isoflavones from the root tips (Graham, 1991) may function as a chemotropic signal for P. sojae hyphae. Thus, both P. sojae zoospores (Morris and Ward, 1992) and hyphal germlings respond to host signals. The chemotropic response of hyphae to daidzein and genistein is significant, because in our laboratory experiments, many zoospores encysted before reaching the root. In the soil zoospores that encyst adjacent to the root can produce hyphae that can use an isoflavone gradient to reorient in the direction of a growing root tip. Although genistein is more potent than daidzein in chemotaxis assays (Tyler et al., 1996) for these isolates, daidzein is the most abundant isoflavone excreted into the rhizosphere by root tips and may be the most important isoflavone used by P. sojae zoospores and germlings to locate soybean roots.
These experiments do not rule out the possibility that hyphal tips of P. sojae also respond to an attractant from adjacent masses of zoospores, such as those clustered around a root (Reid et al., 1995). However, because the zoospores encysted without clustering in the chemotaxis chamber, and the direction of initial hyphal growth was fixed before introduction of the root or capillary tube, we observed that an autoaggregation signal could not account for the tropic response of hyphae to roots or isoflavones. The isoflavones daidzein and genistein function as chemical signals directing several key steps in the early stages of the infection response: (a) They mediate chemotaxis of swimming zoospores toward the root tips (Morris and Ward, 1992), where most of the isoflavones are exuded by the root (Graham, 1991). (b) Sudden exposure of zoospores to elevated levels of isoflavones was effective in inducing the encystment of zoospores on artificial surfaces such as a capillary tube or plastic membrane (Morris and Ward, 1992; this paper). (c) Isoflavones induce chemotropic growth of germlings toward the roots (this paper).
At elevated concentrations, genistein (concentration that results in 50% inhibition of growth = 150 μm) but not daidzein appears to function as a phytoalexin (Rivera-Vargas et al., 1993), and Vedenyalpina et al. (1993) reported that at lower levels (11 μm), genistein altered the pattern of mycelial branching. Genistein acts to inhibit Tyr kinase activity (inhibitor concentration for 50% displacement = 3–50 μm; Akiyama et al., 1987), so inhibition at very high levels of genistein might be expected unless this pathogen has also evolved to become more tolerant. Isoflavones are stored in the endosperm tissue as malonylated and glucosylated compounds (Graham, 1991; Morris et al., 1991), but there is no evidence that the localized concentrations of these conjugates contribute to either cultivar-specific resistance of soybeans to P. sojae or nonspecific age-related resistance in mature tissues such as leaves (Morris et al., 1991). In chemotactic assays, zoospores are strongly attracted to the rhizosphere surrounding the root cap and the area of the root immediately behind the meristem. Graham (1991) estimated that in this region of the rhizosphere, concentrations of genistein and its conjugates would not exceed 2 μm. However, in results reported here, germination of cysts and initial hyphal elongation were not inhibited by 20 μm daidzein or genistein. Thus, it seems unlikely that in vivo levels of these isoflavones in the roots are sufficient to restrict the initial growth of the pathogen.
The ability of hyphal germlings to respond to surface topography has been described for both plant and animal pathogens (Allen et al., 1991; Read et al., 1992; Gow et al., 1994). Surface cues such as pores, grooves, and ridges are primary determinants of hyphal growth on both their hosts and on artificial templates. In experiments reported here, hyphae penetrated pores on the membrane surface or, in the absence of pores, formed appressoria to attempt the penetration of plastic membranes or glass surfaces. Germinating hyphae typically infect soybean hypocotyl tissue by penetrating between the anticlinal walls of epidermal cells (Stössel et al., 1980; Ward et al., 1989). The preference for this site may be attributable to the hyphae detecting a concentration gradient of plant metabolites at this site or its ability to recognize grooves and ridges on the host surface. Isoflavones appear not to be necessary to induce appressorial formation, because zoospores that encysted on clear plastic wrap germinated and produced appressoria in the presence and absence of isoflavones. Although the isoflavones stimulated hyphal germination and chemotropic growth, they would not induce the hyphal tip to grow away from the membrane surface. Thus, the thigmotropic response appears to be a more important determinant of the direction of hyphal growth in germinating cysts. Nevertheless, chemotropic orientation of hyphae on the coverslip of the chemotaxis chamber was still observed in response to an isoflavone gradient. The initial thigmotropic response of P. sojae hyphae to surfaces is consistent with previous accounts of the importance of surface features for both plant and animal fungal pathogens. In contrast to the growth habit of Candida albicans, in which the hyphae continued to be appressed to the membrane surface after emergence on the opposite side of the membrane (Gow et al., 1994), the hyphae of P. sojae grew away from the membrane surface into the aqueous medium. Because this behavior was not observed on smooth, impermeable templates despite the production of multiple appressoria, the process of successful penetration could trigger a new developmental response that might be similar to what happens during the normal course of infection (Ward et al., 1989).
In summary, we have shown that the chemotropic response of germinating hyphae toward roots may be explained by their ability to respond to soybean isoflavones. Isoflavones in the range of concentrations likely to be present in the rhizosphere are not toxic to zoospores or germinating hyphae. However, the tactile response of hyphae appears to be a more important determinant of early hyphal growth than the isoflavones, since germinating hyphae could not be induced to grow away from the substrate on which they had encysted.
ACKNOWLEDGMENTS
We thank Dan Schwab and Carol Heckman for their assistance with scanning microscopy.
Abbreviation:
- PET
polyethylene terephthalate
Footnotes
This work was supported by U.S. Department of Agriculture grant no. 94-37303-0700 to P.F.M. and B.M.T., and by National Science Foundation equipment grants BIR-9009697 and BIR-9249275.
LITERATURE CITED
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabee S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Allen EA, Hazen BE, Hoch HC, Kwon Y, Leinhos ME, Staples RC, Stumpf MA, Terhune BT. Appressorium formation in response to topographical signals by 27 rust species. Phytopathology. 1991;81:323–331. [Google Scholar]
- Carlile MJ (1983) Motility, taxis, and tropism in Phytophthora. In DC Erwin, S Bartnicki-Garcia, PH Tsao, eds, Phytophthora: Its Biology, Taxonomy, Ecology, and Pathology. American Phytopathological Society Press, St. Paul, MN, pp 95–107
- Coley-Smith JR. White rot disease of Allium: problems of soil-borne diseases in microcosm. Plant Pathol. 1990;39:214–222. [Google Scholar]
- Dharmatilake AJ, Bauer WD. Chemotaxis of Rhizobium meliloti towards nodulation gene-inducing compounds from alfalfa roots. Appl Environ Microbiol. 1992;58:1153–1158. doi: 10.1128/aem.58.4.1153-1158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson SP, Deacon JW. Role of calcium in adhesion and germination of zoospore cysts of Pythium: a model to explain infection of host plants. J Gen Microbiol. 1992;138:2051–2059. [Google Scholar]
- Duniway JM. Movement of zoospores of Phytophthora cryptogea in soils of various textures and matric potentials. Phytopathology. 1976;66:877–882. [Google Scholar]
- Duniway JM (1983) Role of physical factors in the development of Phytophthora diseases. In DC Erwin, S Bartnicki-Garcia, PH Tsao, eds, Phytophthora: Its Biology, Taxonomy, Ecology, and Pathology. American Phytopathological Society, St. Paul, MN, pp 175–187
- Erwin DC, Ribeiro OK (1996) Phytophthora Diseases Worldwide. American Phytopathological Society, St. Paul, MN, pp 146–184
- Fisher RF, Long SR. Rhizobium-plant signal exchange. Nature. 1992;357:655–659. doi: 10.1038/357655a0. [DOI] [PubMed] [Google Scholar]
- Förster H, Tyler BM, Coffey MD. Phytophthora sojae races have arisen by clonal evolution and by rare outcrosses. Mol Plant Microbe Interact. 1994;7:780–791. [Google Scholar]
- Gow NAR, Perera THS, Sherwood HJ, Gooday GW, Gregory DW, Marshall D. Investigation of touch-sensitive responses by hyphae of the human pathogenic fungus Candida albicans. Scanning Microsc. 1994;8:705–709. [PubMed] [Google Scholar]
- Graham TL. Flavonoid and isoflavonoid distribution in developing soybean seedling tissues and in seed and root exudates. Plant Physiol. 1991;95:594–603. doi: 10.1104/pp.95.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JM, Iser JR, Grant BR. Calcium control of differentiation of Phytophthora palmivora. Arch Microbiol. 1988;149:565–571. [Google Scholar]
- Hardham A, Gubler F. Polarity of attachment of zoospores of a root pathogen and prealignment of the emerging germ tube. Cell Biol Int Rep. 1990;14:947–955. [Google Scholar]
- Hawes MC, Smith LY. Requirement for chemotaxis in pathogenicity of Agrobacterium tumefaciens on roots of soil-grown pea plants. J Bacteriol. 1989;71:5668–5671. doi: 10.1128/jb.171.10.5668-5671.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T, Kawabata Y, Takayama T, Tahara S, KawabataY, Fukushi Y, Nishimura H, Mizutani J. A potent attractant of zoospores of Aphanomyces cochlioides isolated from its host, Spinacia oleracea. Experientia. 1992;48:410–414. [Google Scholar]
- Jones SW, Donaldson SP, Deacon JW. Behaviour of zoospores and zoospore cysts in relation to root infection by Pythium aphanidermatum. New Phytol. 1991;117:289–301. [Google Scholar]
- Manavathu EK, Thomas DS. Chemotropism of Achlya ambisexualis to methionine and methionyl compounds. J Gen Microbiol. 1985;131:751–756. [Google Scholar]
- Morris PF, Savard ME, Ward EWB. Identification and accumulation of isoflavonoids and isoflavone glucosides in soybean leaves and hypocotyls in resistance responses to Phytophthora megasperma f sp glycinea. Physiol Mol Plant Pathol. 1991;39:229–234. [Google Scholar]
- Morris PF, Ward EWB. Chemoattraction of zoospores of the soybean pathogen Phytophthora sojae by isoflavones. Physiol Mol Plant Pathol. 1992;40:17–22. [Google Scholar]
- Musgrave A, Ero L, Scheffer R, Oehlers E. Chemotropism of Achlya bisexualis germ hyphae to casein hydrolysate and amino acids. J Gen Microbiol. 1977;101:65–70. [Google Scholar]
- Podila GK. Chemical signals from avocado surface wax trigger germination and appressorium formation in Colletotrichum gloeosporioides. Plant Physiol. 1993;103:267–272. doi: 10.1104/pp.103.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read ND, Kellock LJ, Knight H, Trewavas AJ. Contact sensing during infection by fungal pathogens. In: Callow JA, Green JR, editors. Perspectives in Plant Cell Recognition. London: Cambridge University Press; 1992. pp. 137–172. [Google Scholar]
- Reid B, Morris BM, Gow NAR. Calcium-dependent, genus specific, autoaggregation of zoospores of phytopathogenic fungi. Exp Mycol. 1995;19:202–213. [Google Scholar]
- Rivera-Vargas LI, Schmitthenner AF, Graham TL. Soybean flavonoid effects on and metabolism by Phytophthora sojae. Phytochemistry. 1993;32:851–857. [Google Scholar]
- Ruan Y, Kotriaiah V, Straney DC. Flavonoids stimulate spore germination in Fusarium solani pathogenic on legumes in a manner sensitive to inhibitors of cAMP-dependent protein kinase. Mol Plant Microbe Interact. 1995;8:929–938. [Google Scholar]
- Sekizaki H, Yokosawa R, Chinen C, Adachi H, Yamane Y. Studies on zoospore attracting activity. II. Synthesis of isoflavones and their attracting activity to Aphanomyces euteiches zoospores. Biol Pharm Bull. 1993;16:698–701. doi: 10.1248/bpb.16.698. [DOI] [PubMed] [Google Scholar]
- Stössel P, Lazarovits G, Ward EWB. Penetration and growth of compatible and incompatible races of Phytophthora megasperma var. sojae in soybean hypocotyl tissues differing in age. Can J Bot. 1980;58:2594–2601. [Google Scholar]
- Tyler BM, Wu MH, Wang JM, Cheung W, Morris PF. Chemotactic preferences and strain variation in the response of Phytophthora sojae zoospores to host isoflavones. Appl Environ Microbiol. 1996;62:2811–2817. doi: 10.1128/aem.62.8.2811-2817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedenyalpina EG, Safir GR, Niemira BA, Chase TE. Low concentrations of the isoflavone genistein influence in vitro asexual reproduction and growth of Phytophthora sojae. Phytopathology. 1993;86:144–148. [Google Scholar]
- Ward EWB, Cahill DM, Bhattacharyya MK. Early cytological differences between compatible and incompatible interactions of soybeans with Phytophthora megasperma f sp. glycinea. Physiol Mol Plant Pathol. 1989;34:267–273. [Google Scholar]
- Zentmyer GA. Chemotaxis of zoospores for root exudates. Science. 1961;133:1595–1596. doi: 10.1126/science.133.3464.1595. [DOI] [PubMed] [Google Scholar]