Background: RhoA/C and RhoB have homologous sequences, but opposing functions.
Results: IQGAP1 binds prenylated, active RhoA/C, but not RhoB; IQGAP1 increases RhoA/C GTP loading and is required for RhoA/C-induced proliferation and motility of breast cancer cells.
Conclusion: IQGAP1 is a regulator and pro-oncogenic effector of RhoA/C.
Significance: Disrupting Rho/IQGAP interactions with prenylation inhibitors may be a useful adjunct for breast cancer treatment.
Keywords: Breast Cancer, Cell Migration, Cell Proliferation, Protein-Protein Interactions, Rho GTPases, IQGAP1, Rho Effector, Rho Isoforms
Abstract
We performed a proteomics screen for Rho isoform-specific binding proteins to clarify the tumor-promoting effects of RhoA and C that contrast with the tumor-suppressive effects of RhoB. We found that the IQ-motif-containing GTPase-activating protein IQGAP1 interacts directly with GTP-bound, prenylated RhoA and RhoC, but not with RhoB. Co-immunoprecipitation of IQGAP1 with endogenous RhoA/C was enhanced when RhoA/C were activated by epidermal growth factor (EGF) or transfection of a constitutively active guanine nucleotide exchange factor (GEF). Overexpression of IQGAP1 increased GTP-loading of RhoA/C, while siRNA-mediated depletion of IQGAP1 prevented endogenous RhoA/C activation by growth factors. IQGAP1 knockdown also reduced the amount of GTP bound to GTPase-deficient RhoA/C mutants, suggesting that IQGAP enhances Rho activation by GEF(s) or stabilizes Rho-GTP. IQGAP1 depletion in MDA-MB-231 breast cancer cells blocked EGF- and RhoA-induced stimulation of DNA synthesis. Infecting cells with adenovirus encoding constitutively active RhoAL63 and measuring absolute amounts of RhoA-GTP in infected cells demonstrated that the lack of RhoAL63-induced DNA synthesis in IQGAP1-depleted cells was not due to reduced GTP-bound RhoA. These data suggested that IQGAP1 functions downstream of RhoA. Overexpression of IQGAP1 in MDA-MB-231 cells increased DNA synthesis irrespective of siRNA-mediated RhoA knockdown. Breast cancer cell motility was increased by expressing a constitutively-active RhoCV14 mutant or overexpressing IQGAP1. EGF- or RhoC-induced migration required IQGAP1, but IQGAP1-stimulated migration independently of RhoC, placing IQGAP1 downstream of RhoC. We conclude that IQGAP1 acts both upstream of RhoA/C, regulating their activation state, and downstream of RhoA/C, mediating their effects on breast cancer cell proliferation and migration, respectively.
Introduction
The Rho family of small GTPases includes Rho, Rac, and Cdc42. Rho family proteins cycle between an active, GTP-bound form and an inactive, GDP-bound form, and regulate multiple cellular processes such as growth, survival, and cellular motility, through interaction with various effector molecules (1). Cycling of Rho family proteins between the GTP- and GDP-bound state is regulated by guanine nucleotide exchange factors (GEFs)5 promoting GTP loading, and GTPase-activating proteins (GAPs) causing de-activation (1). RhoA and C are oncogenic and linked to increased cancer cell proliferation and invasion/metastasis, whereas RhoB has tumor suppressor properties, inhibiting colony formation and promoting apoptosis (1, 2). Correspondingly, RhoA and C are frequently overexpressed in human cancers, whereas RhoB is often undetectable or lower in cancer cells compared with surroundding normal tissue (1, 3). RhoA is required for cell transformation by oncogenic Ras, and constitutively active RhoA can stimulate DNA synthesis and cell cycle progression (1, 3, 4). Overexpression of RhoC in human mammary epithelial cells promotes anchorage-independent growth, invasiveness, and tumor formation in mice, whereas siRNA-mediated knock-down of RhoC in breast cancer cells inhibits proliferation, invasiveness, and tumor growth in nude mice (3, 5–8). Studies in RhoC-deficient mice demonstrate an important role of RhoC in tumor cell metastasis (9). In contrast, RhoB inhibits Ras transformation, and RhoB-deficient mice show enhanced growth of Ras-transformed tumor cells and increased carcinogen-induced tumorigenesis (10, 11).
Despite the opposing effects of RhoA and C compared with RhoB, the three Rho isoforms have 85% sequence identity. Moreover, RhoB differs in only one amino acid from RhoA and C within the core effector binding domain, but sequences outside this domain contribute to effector protein binding (2, 12). Most Rho effector proteins bind to all three Rho isoforms, e.g. Rho-kinases, protein kinase N, rhotekin, and mDia (2, 13). Few Rho isoform-specific effector proteins have been identified to date, and none adequately explain the tumor-promoting effects of RhoA/C versus tumor-suppressing effects of RhoB (2, 14–16).
The IQ-motif-containing GTPase-activating protein IQGAP1 is a multi-domain scaffold protein which binds Cdc42 and Rac1, but IQGAP inhibits, rather than activates, GTP hydrolysis by both proteins (17–19). In addition to binding to Cdc42 and Rac1, IQGAP1 interacts with multiple other proteins, and regulates a wide range of cellular processes, such as cell growth and survival, as well as cytoskeletal organization, motility, and cell-cell adhesion (17, 20, 21). Like RhoA and C, IQGAP1 has oncogenic properties and is up-regulated in many cancers, including breast, lung, ovarian, and gastric cancers (17, 20). Forced overexpression of IQGAP1 enhances breast cancer cell proliferation, motility, and invasion, whereas siRNA-mediated knock-down has the opposite effect (22–24). IQGAP1-deficient mice develop normally, but display gastric hyperplasia and polyps (25). Analogous to RhoA and C, IQGAP1 plays an important tumor-promoting role in breast cancer (22), but IQGAP1 has not been observed to bind Rho A or C (18, 19, 26–28).
To better understand the molecular basis for the opposing effects of RhoA/C and RhoB, we searched for RhoA/C- and RhoB-specific interaction proteins using a proteomics screen. We found IQGAP1 is a specific binding partner for GTP-bound RhoA and C, but not RhoB, and is a key modulator of RhoA and C in regulating breast cancer cell proliferation and motility.
EXPERIMENTAL PROCEDURES
Antibodies and DNA Constructs
Murine monoclonal antibodies directed against RhoA (sc-418) and tubulin, and rabbit polyclonal antibodies anti-HA and Myc epitopes were from Santa Cruz Biotechnology (Santa Cruz, CA). The RhoC-specific antibody (#3430) was from Cell Signaling Technology (Danvers, MA), and IQGAP1 antibody was from Millipore (Billerica, MA). Bromo-deoxyuridine (BrdU), Flag-epitope antibodies, Flag M2 affinity gel, and Flag peptide were from Sigma-Aldrich Corp. Epidermal growth factor (EGF) was from Calbiochem. Glutathione-S-transferase (GST)-tagged rhotekin Rho binding domain (RBD) was purified from bacteria as described previously (29, 30). For the proteomics screen, cDNAs encoding RhoAV14, RhoBV14, and RhoCV14 were placed downstream of an EE-epitope tag, followed by a TEV protease cleavage site and a Flag epitope tag [5′-GAA GCC GAC GGA GCC AAG ATG GAA GAA GAA GAA TAT ATG CCT ATG GAA GCG GCG GCC GAG ATT CTT TAT TTT CAG GGC GGA GAC TAC AAA GAC GAT GAC GAC AAG GGG ATC CCC-3′]. Vectors encoding Flag-tagged RhoA (wild type, L63, and N19), RhoA (V14,C190) and the prenylation-deficient mutant RhoA (V14,S190) were generated as described previously (29, 31). Vectors encoding hemagglutinin (HA)-tagged RhoC (wild type and V14) were purchased from the University of Misouri-Rolla cDNA Resource Center (www.cdna.org). A vector encoding full-length human IQGAP1 cDNA was purchased from Open Biosystems (Lafayette, CO; Clone ID# 40073370). Mammalian expression vectors encoding IQGAP1 with an N-terminal Myc- or Flag epitope tag, and bacterial expression vectors encoding IQGAP-N (amino acids 1–862) or IQGAP-C (863–1657) fused to GST were generated by PCR. Adenoviral vectors encoding LacZ (control), RhoCV14, or IQGAP1 were produced as described (32). All PCR-generated constructs were sequenced. Adenovirus encoding HA-tagged RhoAL63 was provided by Dr. J. H. Brown of UCSD (33).
Cell Culture
MDA-MB-231 breast cancer cells and 293T human embryonal kidney cells were from the American Tissue Culture Collection and were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS).
Co-immunoprecipitation and Proteomics Screen
Expression vectors encoding Flag-and EE-tagged RhoAV14, RhoBV14, RhoCV14, or empty vector were transfected into 293T cells using calcium phosphate precipitation and 50 μg of DNA per 150-mm dish. After 48 h, cells were lysed in 0.5% Triton X-100, 50 mm NaHEPES pH 7.4, 100 mm NaCl, 1 mm MgCl2, 0.1 mm EDTA, 0.1 mm EGTA, 0.5 mm Na3VO4, 10 mm β-glycerolphosphate, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. After centrifugation at 16,000 × g for 30 min, supernatants were subjected to immunoprecipitation with FLAG M2 affinity gel, and proteins were eluted with 100 μg/ml FLAG peptide in lysis buffer. In some cases, proteins were subjected to repeat immunoprecipitation with anti-EE epitope antibody, and eluted with TEV protease prior to analysis by denaturing SDS-polyacrylamide gel electrophoresis (PAGE, 7% gel). Proteins were visualized by silver staining using the SilverSNAP Stain Kit II from Pierce/Thermo Scientific. Bands that were uniquely and reproducibly associated with RhoA and C were excised and sent to the Stanford University Mass Spectrometry Facility (mass-spec.stanford.edu) for protein identification by in-gel trypsinization, liquid chromatography, and tandem mass spectrometry.
For co-immunoprecipitation of endogenous Rho with IQGAP1, MDA-MB-231 cells were lysed in the buffer described above, and ∼2 mg of cellular protein was incubated with 6 μg of anti-IQGAP1 antibody.
Western Blots
Western blots were developed with horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence (32). Bar graphs were generated by densitometry scanning using ImageJ software.
DNA and siRNA Transfections and Adenoviral Infections
Cells were transiently transfected using 5 μl of Lipofectamine 2000TM (InVitrogen, San Diego, CA) and 1 μg of DNA or 100 pmol of siRNA per ml of FBS-containing DMEM as described (32). siRNA oligoribonucleotides were from Qiagen (Valencia, CA), and the sequence of the control siRNA targeting green fluorescent protein (GFP) was described previously (34). The other siRNA target sequences were: 5′-AAGTTCTACGGGAAGTAATTG-3′ for IQGAP1; 5′-AAGTCAAGCATTTCTGTCCAA-3′ for RhoA; and 5′-AAGACCTGCCTCCTCATCGTC-3′ for RhoC. The siRNA targeting IQGAP1 was transfected twice 24 h apart; siRNAs targeting RhoA or C were transfected once. Where indicated, cells were infected with adenovirus 6 h after the last siRNA transfection; the multiplicity of infection was titrated to produce amounts of IQGAP1 similar to the amounts of endogenous proteins. For RhoAL63 and RhoCV14, cells were infected with two different viral concentrations sufficient to produce a maximal increase in DNA synthesis or migration, respectively.
Measurement of Rho Activation
Two different methods were used to measure Rho activation. To quantify absolute amounts of GTP bound to transfected, epitope-tagged RhoA, or RhoC constructs, we employed a coupled enzymatic assay using nucleoside diphosphate kinase and luciferase as described previously (29, 35). To determine the activation state of endogenous RhoA or C separately from the other Rho isoforms, we used the Rho binding domain (RBD) of rhotekin to isolate Rho·GTP, and assessed the amount of GTP-bound RhoA or C by Western blotting as described by Ren et al. (30). In some experiments, we assessed the activation state of endogenous RhoA/C using the RBD pull-down assay combined with enzymatic GTP measurements (29).
Migration Assays
For Transwell migration, MDA-MB-231 cells were transfected with the indicated siRNAs as described above, and 6 h later, cells were transferred to 0.1% FBS-containing DMEM. Adenoviral vectors were added as indicated, and 24 h later, 1.5 × 105 cells were plated in DMEM containing 0.2% bovine serum albumin on 8 μm Transwell filters (24 well inserts, BD Biosciences, San Diego, CA) coated with fibronectin (7.5 μg/ml). The wells below the filters were filled with DMEM containing 10% FBS or 20 ng/ml EGF. After incubation for 12–16 h, cells were fixed in 3.7% paraformaldehyde and stained with Crystal violet. Cells remaining on top of the filter were carefully removed with a cotton swab, and cells migrated to the lower surface of the filter were counted.
For a wound-healing assay, cells were transfected as described above, but remained in 10% serum-containing medium and received adenoviral vectors. A scratch was generated 16 h later with a sterile pipette tip, and photographs were taken immediately, and 40 h later. Cells that entered the wound, as defined by the borders on the initial photograph, were counted.
BrdU Incorporation
MDA-MB-231 cells (1 × 104) were plated on glass cover slips in 24-well dishes; cells were transfected with siRNAs, transferred to medium containing 0.1% FBS, and infected with adenovirus as described for Transwell migration. BrdU (200 μm) was added 24 h later with or without EGF (20 ng/ml) for an additional 16 h. Cells were fixed, stained with anti-BrdU antibody, and counterstained with Hoechst 33342 as described (32). At least 300 cells were counted per condition, and results were confirmed by a blinded observer.
Data Analysis
Bar graphs represent the mean ± S.E. of at least three independent experiments. Data were analyzed by one-way analysis of variance with Bonferroni post-test analysis. A p value of <0.05 was considered statistically significant.
RESULTS
IQGAP1 Interacts with RhoA/C but Not RhoB
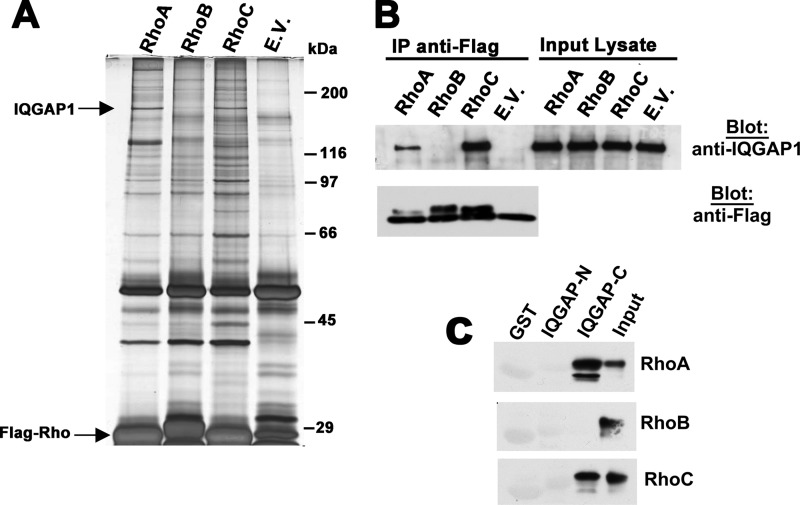
We performed a proteomics screen in 293T cells transfected with equal amounts of epitope-tagged, constitutively active RhoA, B, and C (all with a glycine to valine mutation at position 14) to search for Rho isoform-specific interacting proteins. By mass spectrometric analysis of bands that were reproducibly and differentially associated with anti-epitope immunoprecipitates of the three Rho proteins, we identified IQGAP1 as a 190 kDa protein associated with RhoA and C, but not with RhoB (Fig. 1A). RhoA- and RhoC-specific association with IQGAP was confirmed in experiments in which the double epitope-tagged Rho proteins were re-immunoprecipitated using an antibody specific for the second epitope, but the protein yield was lower (supplemental Fig. S1A).
FIGURE 1.
IQGAP1 interacts with RhoA/C but not RhoB. A, 293T cells were transfected with expression vectors encoding Flag epitope-tagged, constitutively active RhoA, RhoB, and RhoC (each with a glycine to valine substitution at position 14), or empty vector (E.V.), and cell lysates were subjected to immunoprecipitation with anti-Flag antibody. Precipitated proteins were eluted with Flag peptide and analyzed by SDS-PAGE/silver staining. Bands specifically associated with Rho A and C were excised, subjected to trypsin digestion, and analyzed by mass spectrometry. Bands migrating at ∼190 kDa were identified as IQGAP1. B, 293T cells were transfected with the same Rho constructs as in panel A, and anti-Flag immunoprecipitates were analyzed by Western blotting for the presence of endogenous IQGAP1 (top panel). A fraction of the immunoprecipitates was analyzed by blotting with anti-Flag antibody (bottom panel). C, N-terminal or C-terminal halves of IQGAP1 (IQGAP-N (amino acids 1–862) or IQGAP-C (amino acids 863–1657)) were expressed as GST-fusion proteins and purified from bacteria. Beads loaded with GST-IQGAP-N, IQGAP-C, or GST were incubated with Flag-tagged RhoAV14, RhoBV14, or RhoCV14 purified from 293T cells; beads were washed, and analyzed for the presence of RhoA, RhoB, or RhoC, respectively, with 10% input of each Rho protein shown in the last lane.
Co-immunoprecipitation of endogenous IQGAP1 with transfected, constitutively active RhoAV14 and RhoCV14, but not RhoBV14 was also confirmed by Western blotting using an IQGAP1-specific antibody, both in 293T cells (Fig. 1B) and MDA-MB-231 breast cancer cells (supplemental Fig. S1B). To determine whether the interaction between RhoA/C and IQGAP1 was direct, and to establish the IQGAP binding region, we incubated Flag-tagged RhoAV14 or RhoCV14 purified from 293T cells with GST-tagged IQGAP-N (amino acids 1–862) or IQGAP-C (amino acids 863–1657) purified from bacteria, with GST serving as a negative control. RhoA and C bound specifically to the C-terminal half, but not the N-terminal half of IQGAP1, whereas RhoB showed no specific binding to either construct (Fig. 1C). These data suggest direct binding of RhoA and C to the C-terminal half of IQGAP1, which contains the GAP-related domain required for CDC42 and Rac binding (20).
The IQGAP1/RhoA Interaction Requires GTP Binding and Prenylation of RhoA
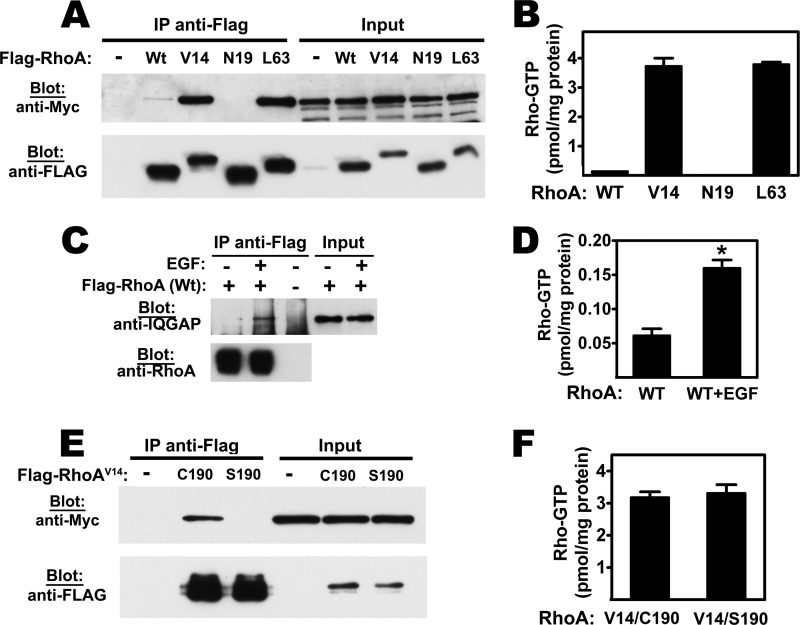
To determine if the RhoA/IQGAP1 interaction was dependent on the activation state of RhoA, we transfected cells with expression vectors encoding Flag-tagged wild type RhoA, constitutively active RhoAV14 and RhoAL63, or dominant-negative RhoAN19. IQGAP1 was strongly associated with RhoAV14 and RhoAL63, but it was barely detectable in immunoprecipitates of wild type RhoA, and not associated with RhoAN19 (Fig. 2A). Fig. 2B shows absolute amounts of GTP bound to transfected RhoA, as measured in anti-Flag immunoprecipitates using an enzymatic assay and normalized to mg of cellular protein. RhoAV14 and RhoAL63 were highly GTP-bound, consistent with their defective GTPase activity (29), but the amount of GTP bound to wild type RhoA and RhoAN19 was about 1/20th and 1/200th as much, respectively (Fig. 2B). The amount of GTP bound to RhoAN19 was at the detection limit, consistent with preferential GDP binding by this mutant protein (4). These data suggest that IQGAP1 binds to RhoA in the active, GTP-bound state. In fact, IQGAP1 association with wild type RhoA was strongly enhanced, when serum-starved cells were treated with EGF to induce RhoA activation (Fig. 2, C and D).
FIGURE 2.
The IQGAP1/RhoA interaction requires GTP binding and prenylation of RhoA. A, 293T cells were co-transfected with Myc epitope-tagged IQGAP1 and Flag-tagged RhoA constructs encoding wild type (Wt), constitutively active (V14 or L63), dominant-negative (N19) RhoA proteins, or empty vector. Anti-Flag immunoprecipitates were analyzed by Western blotting with anti-Myc antibody for the presence of IQGAP1 (top panel), or with anti-Flag antibody for the amounts of RhoA (bottom panel). Input lysates (2.5%) are shown in the right lanes. B, cells were transfected with IQGAP1 and the Rho constructs described in panel A; absolute amounts of GTP bound to anti-Flag immunoprecipitates were measured using a luciferase-based assay, as described under “Experimental Procedures.” C and D, cells were transfected with IQGAP1 and Flag-tagged wild type RhoA as in panel A, but cells were serum-deprived for 8 h and treated with EGF (20 ng/ml) for 5 min. In panel C, anti-Flag immunoprecipitates were analyzed by Western blotting for the presence of IQGAP1, and in panel D, GTP bound to anti-Flag immunoprecipitates was measured enzymatically as in panel B. E and F, 293T cells were co-transfected with Myc-tagged IQGAP1 and vectors encoding Flag-tagged RhoA(V14) in its normal prenylated form (C190 is the cysteine modified by geranyl-geranylation), or with a mutation in the prenylation signal (S190); anti-Flag immunoprecipitates were probed for the association of IQGAP1 (E) or for the amount of GTP bound to RhoA(V14/C190) and RhoA(V14/S190) (F).
To test whether the RhoA/IQGAP1 interaction required C-terminal prenylation of RhoA, we used a double mutant RhoAV14,S190 construct, which is GTPase-deficient and lacks the C-terminal cysteine 190 required for post-translational lipid modification (31). IQGAP did not bind to the prenylation-deficient, GTPase-deficient RhoA protein (Fig. 2E), although the amount of GTP bound to this protein was similar to the amount of GTP bound to its prenylated counterpart (Fig. 2F). Thus, IQGAP1 preferentially binds to prenylated, GTP-bound RhoA in intact cells.
Interaction of Endogenous RhoA and RhoC with IQGAP1
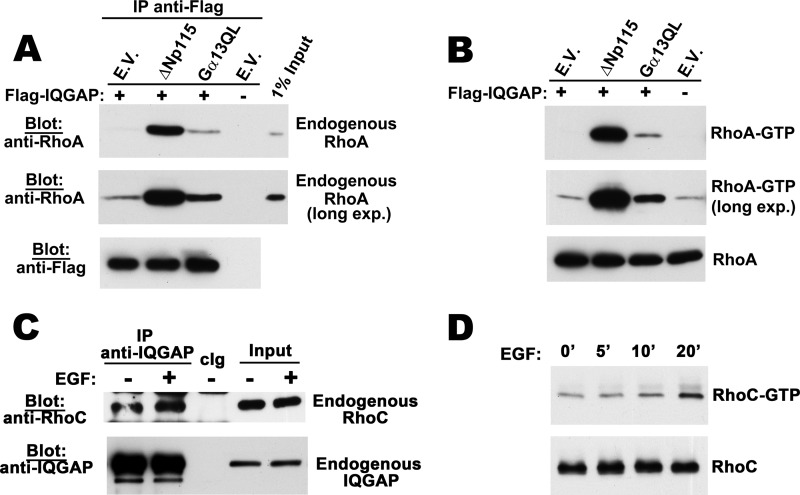
To increase the amount of endogenous RhoA in the GTP-bound form, we transfected 293T cells with the constitutively-active Rho guanine nucleotide exchange factor ΔNp115-RhoGEF or the heterotrimeric G protein Gα13(QL). In several cell types, Gα13(QL) stimulates p115-RhoGEF activity, thereby activating RhoA (29, 35). In the absence of the activators, endogenous RhoA was barely detectable in IQGAP1 immunoprecipitates, but RhoA/IQGAP1 complexes were greatly enhanced in cells co-transfected with the constitutively active p115 or Gα13 constructs, consistent with the increase in endogenous GTP-bound RhoA seen in the transfected cells (Fig. 3, A and B).
FIGURE 3.
Co-immunoprecipitation of endogenous RhoA and RhoC with IQGAP1. A, 293T cells were co-transfected with Flag-tagged IQGAP1 and either empty vector, constitutively active ΔNp115 Rho-GEF, or Gα13QL as indicated. Cell lysates were subjected to immunoprecipitation with anti-Flag antibody; precipitated proteins were eluted with Flag peptide and analyzed by Western blotting with antibody specific for RhoA (top two panels) or anti-Flag antibody (bottom panel). B, cells were transfected as in panel A, and GTP-bound endogenous Rho was isolated from cell lysates using the RBD of Rhotekin as described under “Experimental Procedures”; endogenous RhoA bound to the beads was analyzed by Western blotting with a RhoA-specific antibody (top two panels). Cell lysates were also directly analyzed by Western blotting for total RhoA (lower panel). C, MDA-MB-231 cells were serum-starved for 24 h prior to stimulation with EGF for 20 min. Cell lysates were subjected to immunoprecipitation with IQGAP1-specific antibody or control IgG (cIg), and precipitates were analyzed by Western blotting with antibodies specific for RhoC (top panel) or IQGAP1 (bottom panel). 1% input lysates was analyzed in parallel. D, MDA-MB-231 cells were treated with EGF for the indicated times; GTP-bound endogenous Rho was isolated by RBD-pulldown, and RhoC-GTP bound to the beads or total RhoC were detected by Western blotting as described in panel B for RhoA.
When we treated MDA-MB-231 breast cancer cells with EGF to stimulate GTP loading of endogenous RhoA, we detected small amounts of RhoA in IQGAP1 immunoprecipitates (supplemental Fig. S2, A and B). Treating MDA-MB-231 cells with EGF also increased GTP loading of RhoC, albeit with slower kinetics (Fig. 3D). Endogenous RhoC was associated with IQGAP1 in serum-starved cells, but EGF treatment increased the amount of RhoC in IQGAP1 immunoprecipitates (Fig. 3C).
IQGAP1 Regulates RhoA/C Activation
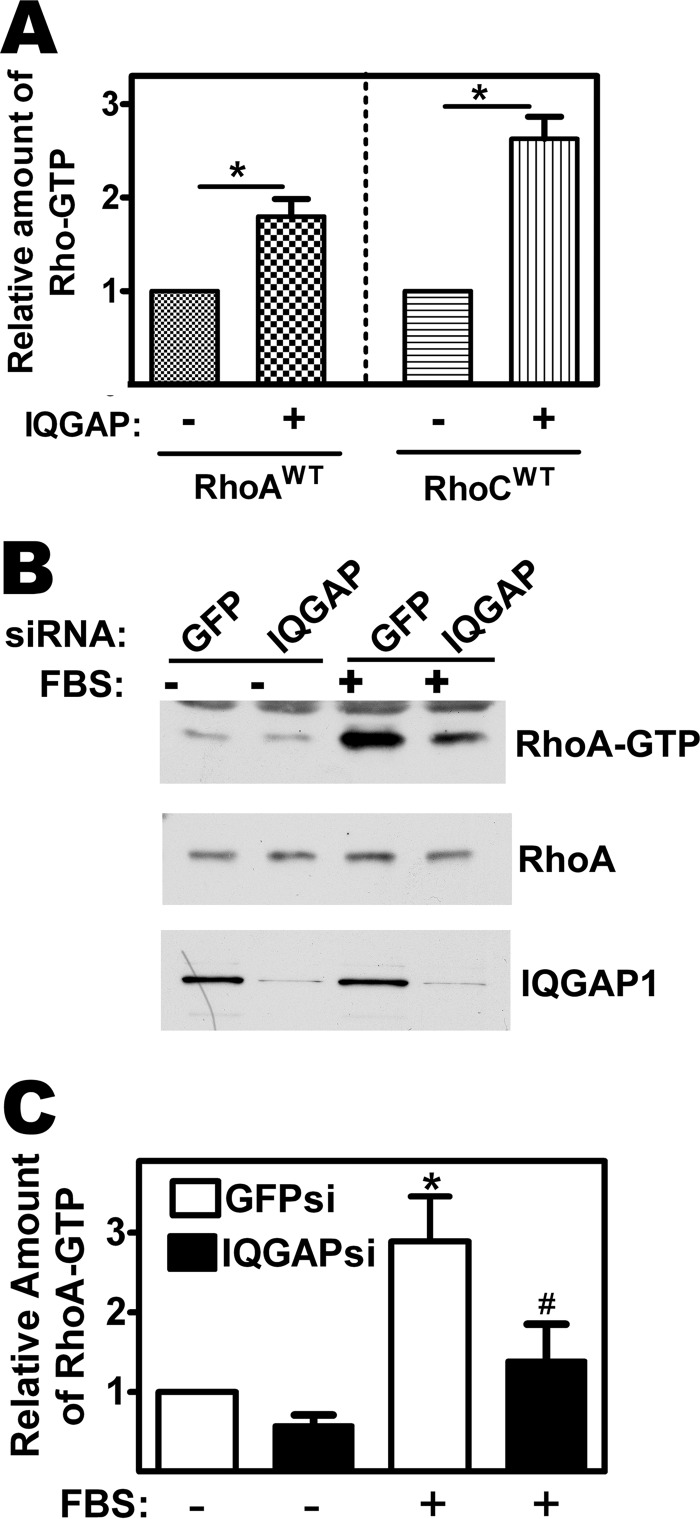
Because binding of Rho-GTP to IQGAP1 could modulate RhoA/C interaction with GEFs or GAPs, we examined whether altering the amount of IQGAP1 in cells would affect Rho-GTP levels. When 293T cells were co-transfected with epitope-tagged wild type RhoA and IQGAP1, the amount of GTP bound to the Rho protein almost doubled compared with control cells transfected with RhoA plus empty vector (Fig. 4A, left panel). Similar results were obtained with RhoC (Fig. 4A, right panel).
FIGURE 4.
IQGAP1 regulates RhoA activation. A, 293T cells were co-transfected with expression vector encoding HA epitope-tagged wild type RhoA or RhoC and either empty vector or vector encoding Flag-tagged IQGAP1. Cell lysates were subjected to immunoprecipitation with anti-epitope antibody, and GTP bound to the immunoprecipitates was quantified as described in Fig. 2B. The amount of Rho-GTP found in cells transfected with empty vector was assigned a value of one; *, p < 0.05 for the comparison between the presence and absence of IQGAP1. B, MDA-MB-231 cells were transfected with siRNA specific for IQGAP1 or GFP (control) as described under “Experimental Procedures”; cells were serum-starved for 24 h, and stimulated with 20% FBS for 5 min to stimulate Rho activation. GTP-bound Rho was isolated from cell lysates as described in Fig. 3B. RhoA-GTP bound to Rhotekin-RBD was assessed by Western blotting with a RhoA-specific antibody (top panel). Cell lysates were also directly analyzed by Western blotting for total RhoA (middle panel, 1% of input) and IQGAP1 (lower panel). C, Western blots from three independent experiments performed as in panel B were analyzed by densitometry, and the amount of endogenous RhoA-GTP (normalized to total RhoA) present in serum-starved, GFP siRNA-transfected cells was assigned a value of 1. *, p < 0.05 for the comparison to serum-starved, GFP siRNA-transfected cells; #, p < 0.05 for the comparison to serum-stimulated, GFP siRNA-transfected cells.
In contrast, when MDA-MB-231 cells were transfected with an siRNA specific for IQGAP1, depletion of IQGAP1 greatly reduced RhoA activation in serum-stimulated cells (Fig. 4, B and C). There was a trend toward lower basal RhoA-GTP levels in IQGAP1-depleted cells compared with cells transfected with control siRNA, but this difference did not reach statistical significance. Similarly, IQGAP1 depletion blocked RhoC activation in EGF-treated cells (supplemental Fig. S2C). Thus, IQGAP1 over-expression enhanced Rho-GTP levels, whereas IQGAP1 knock-down largely prevented growth factor stimulation of RhoA/C activity, suggesting that IQGAP1 either stabilizes RhoA/C in the GTP-bound form or promotes RhoA/C activation by GEFs.
RhoA-induced Proliferation of Breast Cancer Cells Requires IQGAP1
In some breast cancers, RhoA, RhoC, or IQGAP1 are overexpressed compared with normal tissues, and all three proteins promote proliferation of breast cancer cells in vitro and stimulate tumorigenesis in vivo (1, 5, 7, 20, 22, 36, 37). To determine if IQGAP1 is necessary for RhoA to regulate cell proliferation (or vice versa), we used an siRNA approach.
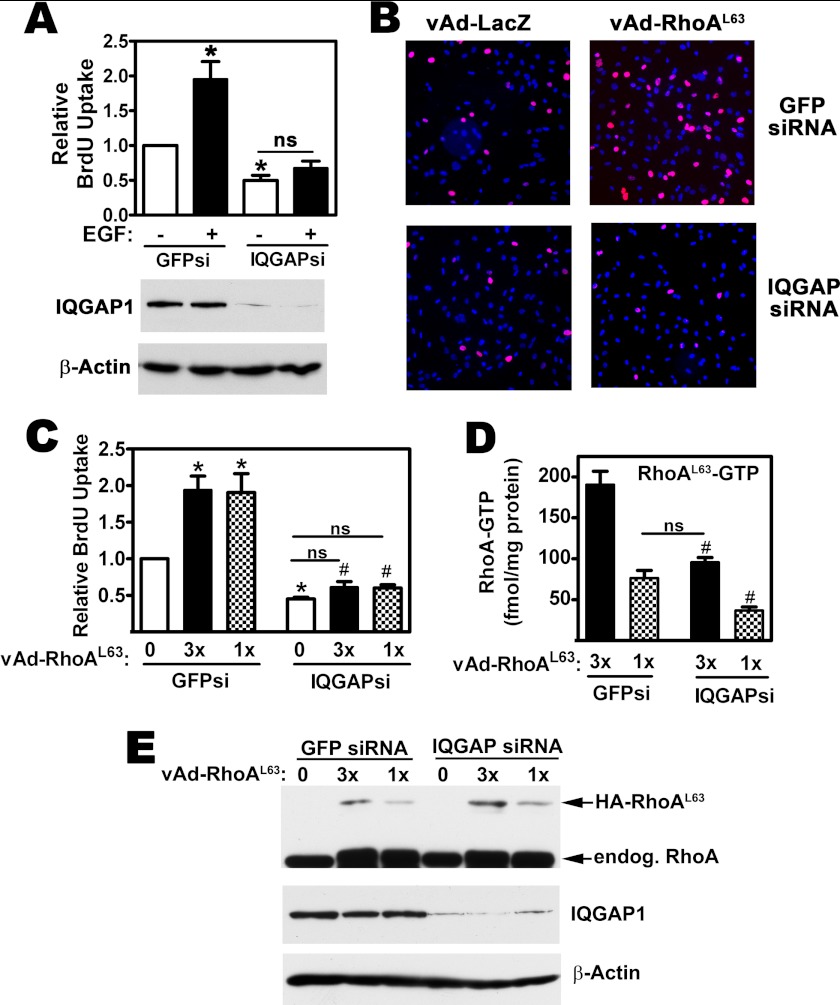
IQGAP1 depletion inhibited DNA synthesis in MDA-MB-231 cells by ∼50%, as assessed by nuclear BrdU uptake (Fig. 5, A and C, open bars, and Fig. 5B, compare top and bottom left panels); similar results have been reported in MCF-7 breast cancer cells (22). In MDA-MB-231 cells transfected with a control siRNA-targeting GFP, treatment with EGF or expression of RhoAL63 doubled the number of cells in S-phase (Fig. 5A, filled bars, for EGF effects; Fig. 5B right panels and Fig. 5C, filled and dotted bars for the effects of RhoAL63). However, in IQGAP1-depleted cells, neither EGF treatment, nor expression of RhoAL63 was able to increase DNA synthesis (Western blots in Fig. 5, A and E show efficient IQGAP1 depletion, and Fig. 5E also shows expression of virally transduced and endogenous RhoA).
FIGURE 5.
RhoA-induced MDA-MB-231 cell proliferation requires IQGAP1. A, MDA-MB-231 cells were transfected with siRNAs specific for GFP (GFPsi) or IQGAP1 (IQGAPsi) as described in Fig. 4B; some cells were treated with EGF (20 ng/ml). Cell proliferation was assessed by BrdU uptake, identified by immunofluorescence staining as described under “Experimental Procedures,” and DNA was counter-stained with Hoechst 33342. The percentage of BrdU-positive nuclei found in GFP siRNA-transfected, control cells was assigned a value of one (*, p < 0.05 for the comparison to control cells; ns, non-significant). Below: IQGAP1 expression was analyzed by Western blotting in cells that were transfected in parallel, with β-actin serving as a loading control. B, MDA-MB-231 cell were transfected as in panel A, but cells were additionally infected with adenoviral vectors encoding LacZ (vAd-LacZ, control) or constitutively active RhoAL63 (vAd-RhoAL63), as indicated. Immunofluorescence staining for BrdU uptake is shown in red. C, siRNA-transfected cells were infected with two different concentrations of virus encoding HA-tagged RhoAL63 (1 or 3×) or LacZ virus (“0”). The percentage of BrdU-positive nuclei found in control cells (GFP siRNA-transfected cells infected with LacZ virus) was assigned a value of one (*, p < 0.05 for the comparison to GFP siRNA-transfected cells infected with LacZ virus; #, p < 0.05 for the comparison to GFP siRNA-transfected cells infected with the same amount of RhoAL63 virus). D, cells were siRNA-transfected and Rho virus infected as in panel C, and the amount of GTP bound to RhoAL63 was measured in anti-HA immunoprecipitates as described under “Experimental Procedures” ( #, p < 0.05 for the comparison to GFP siRNA-transfected cells infected with the same amount of RhoAL63 virus). E, cell lysates from cells treated as in panel C were analyzed by Western blotting using antibodies specific for RhoA (top), IQGAP1 (middle), or β-actin (bottom panel). Note that the HA epitope-tagged RhoA L63 migrates with a higher apparent molecular weight than endogenous RhoA.
We infected cells with two different concentrations of adenoviral vector encoding the constitutively active RhoAL63 to produce low and high amounts of GTP-bound RhoA, but even the lower dose of virus with less activated RhoA was sufficient to induce maximal stimulation of DNA synthesis in the presence of control siRNA (compare Fig. 5, C and D, filled and dotted bars on the left). In contrast, both doses of RhoAL63 were completely ineffective in IQGAP1-depleted cells (Fig. 5C, filled and dotted bars on the right). IQGAP1 depletion significantly decreased the amount of GTP bound to RhoAL63, suggesting that IQGAP1 is necessary for Rho-GEF-mediated GTP loading of the GTPase-deficient RhoL63 protein (Fig. 5D). However, in IQGAP-depleted cells infected with the higher (3×) virus concentration, the amount of GTP-bound RhoAL63 per mg cellular protein was similar to the amount of GTP-bound RhoAL63 found in control siRNA-transfected cells infected with the lower (1×) virus concentration (Fig. 5D). Since control siRNA-transfected cells showed a robust proliferative response to the 1× dose of RhoAL63 virus, we conclude that reduction in GTP-bound RhoAL63 does not explain the lack of response to RhoAL63 in IQGAP1-depleted cells. Thus, IQGAP1 appears to act as a RhoA effector inducing proliferation.
IQGAP1 Can Promote Proliferation Independently of RhoA
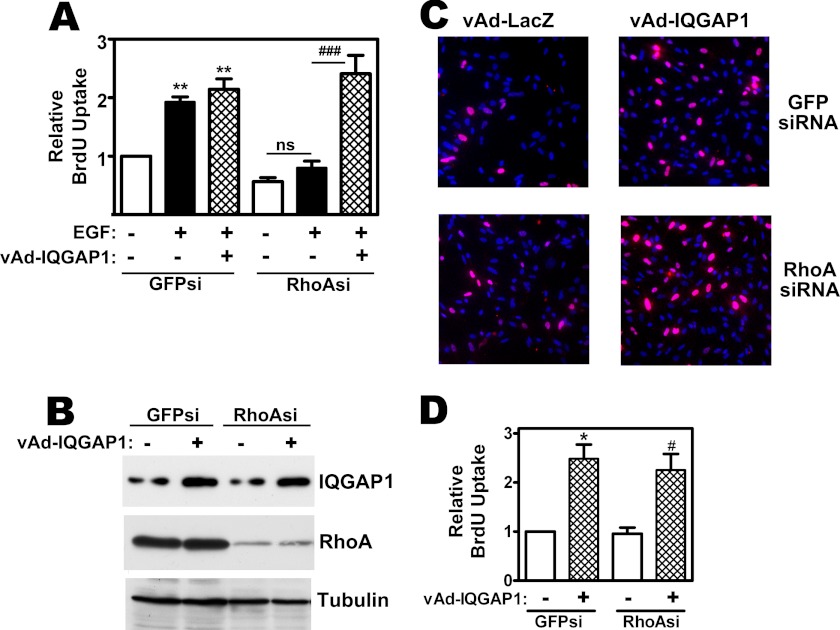
To test if increased levels of IQGAP1 can enhance cell proliferation independently of RhoA, we examined the effect of IQGAP1 overexpression in RhoA-depleted cells. RhoA depletion prevented EGF-induced DNA synthesis in MDA-MB-231 cells, but viral overexpression of IQGAP1 restored BrdU incorporation in EGF-treated cells (Fig. 6A; RhoA depletion and viral IQGAP1 expression is shown in Fig. 6B). Even in the absence of EGF, over-expression of IQGAP1 increased BrdU incorporation about 2-fold, and the effect was the same in MDA-MB-231 cells transfected with control or RhoA-specific siRNAs (Fig. 6, C and D). Thus, proliferation induced by IQGAP1 overexpression is independent of RhoA, but RhoA requires IQGAP1 to stimulate proliferation, placing IQGAP1 downstream of RhoA. Similar results were obtained with RhoCV14 (not shown).
FIGURE 6.
IQGAP1-induced DNA synthesis in MDA-MB-231 cells is independent of RhoA. A, MDA-MB-231 cells were transfected with siRNA specific for GFP (GFPsi) or RhoA (RhoAsi); cells were additionally infected with adenoviral vectors encoding LacZ (control, −) or IQGAP1 (vAd-IQGAP1). Cells were serum-deprived for 24 h and received EGF as indicated; cell proliferation was assessed as in Fig. 5 (**, p < 0.01 for the comparison to GFP siRNA-transfected control cells infected with LacZ virus; ###, p < 0.001 for the comparison between cells infected with LacZ virus versus IQGAP1 virus; ns, non-significant). B, cells were treated as in A, and cell lysates were analyzed by Western blotting for IQGAP1 (top), RhoA (middle), or α-tubulin (bottom panel). C and D, MDA-MB-231 cells were siRNA transfected and infected with control or IQGAP1 virus as in panel A, but were not treated with EGF. BrdU immunofluorescence staining is shown in red (C), and three independent experiments are summarized in D (*,# p < 0.05 for the comparison between cells infected with LacZ virus versus IQGAP1 virus).
RhoC-induced Migration of Breast Cancer Cells Requires IQGAP1
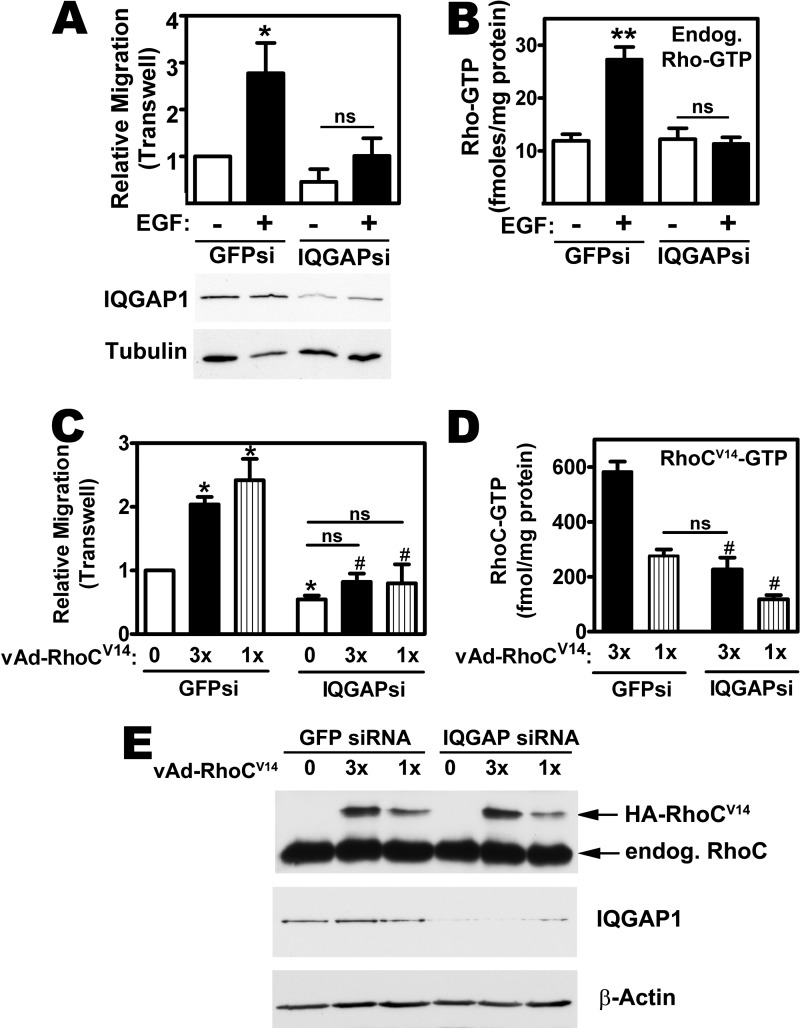
Overexpression of wild type RhoC in human mammary epithelial cells is sufficient to induce a motile and invasive phenotype, and expression of a constitutively active RhoCV14 has more dramatic effects (5, 8). IQGAP1 also promotes breast cancer motility and invasion, in vitro and in vivo (22–24). To determine if the two proteins operate in a linear pathway, we performed migration assays in siRNA-transfected MDA-MB-231cells. EGF treatment or viral expression of constitutively-active RhoCV14 stimulated transwell migration in control siRNA-transfected cells, but had minimal effects in cells transfected with IQGAP1-specific siRNA (Fig. 7A, filled bars, for EGF effects; Fig. 7C, filled and striped bars for the effects of RhoCV14; Fig. 7E shows IQGAP depletion and expression of virally transduced and endogenous RhoC).
FIGURE 7.
RhoC-induced MDA-MB231 cell migration requires IQGAP1. A, MDA-MB-231 cells were transfected with siRNAs specific for GFP or IQGAP1; cell migration was assessed in a transwell assay with some cells receiving EGF in the lower chamber, as described under “Experimental Procedures.” The number of cells migrating under control conditions (GFP siRNA-transfected, no EGF) was assigned a value of one (*, p < 0.05 for the comparison to control; ns, non-significant). IQGAP knock-down was assessed by Western blotting (below). B, cells were transfected as in panel A, and some cells were stimulated with EGF for 20 min. Endogenous, GPT-bound Rho proteins were isolated by RBD-pulldown assay and absolute amounts of GTP bound to the beads were measured using the luciferase-based assay described under “Experimental Procedures” (**, p < 0.01 for the comparison to control). C, MDA-MB-231 cells were siRNA transfected as in panel A, but were infected with LacZ virus (0) or with two different concentrations of adenovirus encoding HA-tagged RhoCV14 (1 and 3×). Cell migration was assessed by transwell assay with all cells receiving FBS in the lower chamber. The number of cells migrating under control conditions (GFP siRNA-transfected and LacZ virus-infected) was assigned a value of one (*, p < 0.05 for the comparison to control; #, p < 0.05 for the comparison to GFP siRNA-transfected cells infected with the same amount of RhoCV14 virus). D, cells were treated as in panel C, and the amount of GTP bound to RhoCV14 was measured in anti-HA immunoprecipitates as described under “Experimental Procedures” (#, p < 0.05 for the comparison to GFP siRNA-transfected cells infected with the same amount of RhoAL63 virus). E, cell lysates from cells treated as in panel C were analyzed by Western blotting with antibodies specific for RhoC (top panel, note that the HA epitope-tagged RhoCV14 migrates with a higher apparent molecular weight than endogenous RhoC), IQGAP1 (middle panel), and β-actin (bottom panel).
IQGAP1 knockdown prevented the EGF-induced increase in endogenous Rho-GTP levels (Fig. 7B), and reduced the level of GTP bound to RhoCV14 by ∼50% (Fig. 7D). However, the amount of GTP-bound RhoCV14 in IQGAP1-depleted cells infected with a high (3×) virus concentration was similar to the amount of GTP-bound RhoCV14 in control cells infected with 1× virus; thus RhoC-GTP remained high enough to expect an increase in migration (compare Fig. 7, C and D). We conclude that IQGAP1 is required for RhoC-induced migration, and that reduction in RhoCV14-GTP levels does not explain the lack of migratory response to RhoCV14 in IQGAP1-depleted cells.
IQGAP1 Can Promote Migration Independently of RhoC
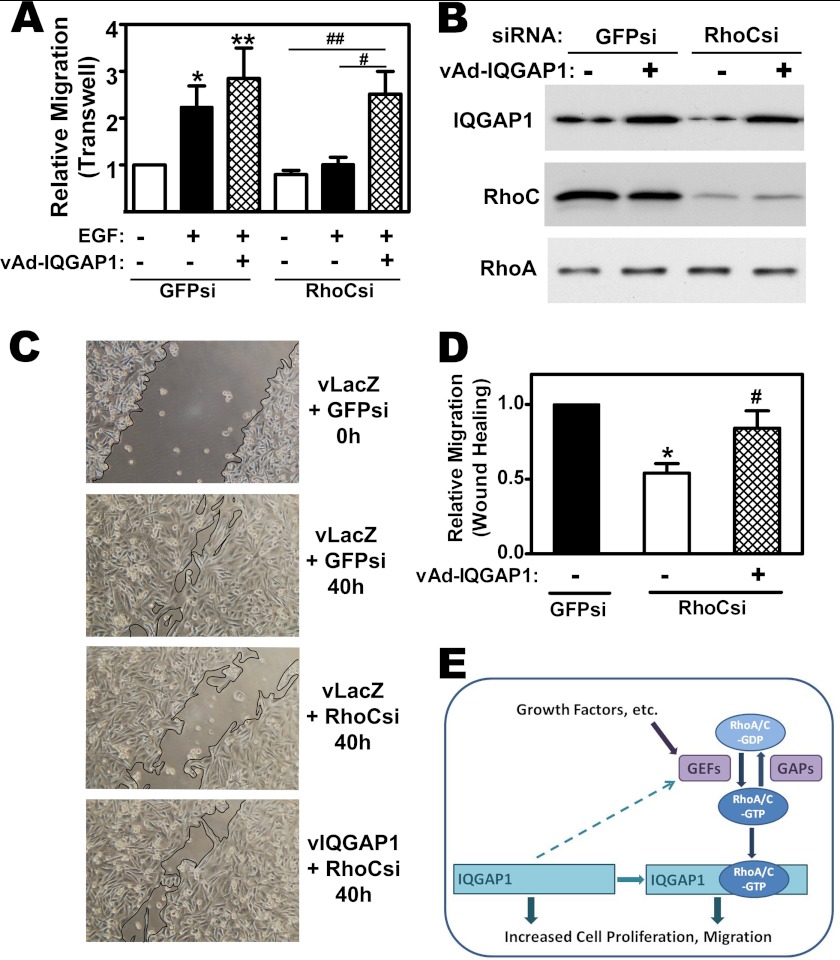
RhoC knockdown blocked EGF-induced transwell migration in MDA-MB-231 cells (Fig. 8A, open and filled bars), consistent with results reported in other breast cancer cell lines (5–7, 38). However, IQGAP1 overexpression restored EGF-induced migration in RhoC-deficient cells (Fig. 8A, cross-hatched bars; RhoC depletion and IQGAP1 expression is shown in Fig. 8B).
FIGURE 8.
IQGAP1 can restore migration in RhoC-depleted cells; IQGAP1 functions both upstream and downstream of RhoA and C. A, MDA-MB-231 cells were transfected with siRNAs specific for GFP or RhoC, and infected with adenovirus encoding LacZ or IQGAP1; cell migration was measured as in Fig. 7A, with some cells receiving EGF in the lower chamber (*, p < 0.05 or **, p < 0.01 for the comparison to control; #, p < 0.05 or ##, p < 0.01 for the comparison between LacZ and IQGAP1 virus in RhoC siRNA-transfected cells). B, cells were treated as in A, and cell lysates were analyzed by Western blotting with antibodies specific for IQGAP1 (top), RhoC (middle), or RhoA (bottom panel). C, cells were siRNA transfected and infected with virus encoding LacZ (vLacZ) or IQGAP1 (vIQGAP1) as in panel A, but migration was measured in a wound-closure assay, with photographs taken immediately, and 40 h after cells were scratched off the plate. D, summary of three experiments performed as in C; the number of cells migrating into the wound in the control culture (GFP siRNA-transfected and LacZ virus-infected) was assigned a value of one (*, p < 0.05 for the comparison to control; #, p < 0.05 for the comparison between LacZ and IQGAP1 virus in RhoC siRNA-transfected cells). E, model depicting IQGAP1 binding GTP-bound RhoA/C and mediating RhoA and RhoC-induced DNA synthesis and migration, respectively. The activation state of RhoA/C is regulated by GEFs and GAPs; growth factors stimulate GEFs, and IQGAP1 appears to enhance Rho activation by GEFs. Interaction of RhoA/C-GDP with Rho guanine nucleotide dissociation inhibitors is not shown in this figure. IQGAP1 has no intrinsic GAP activity, and IQGAP1 overexpression leads to increased cell proliferation and migration through RhoA/C-independent mechanisms.
We also assessed migration in a wound closure assay: RhoC depletion inhibited wound closure, and IQGAP1 overexpression enhanced wound closure in RhoC-deficient cells (Fig. 8, C and D). Thus, overexpression of IQGAP is sufficient to induce migration of MDA-MB-231 cells independently of RhoC, but RhoC-induced migration requires IQGAP1, suggesting that IQGAP1 is an effector of RhoC (Fig. 8E).
DISCUSSION
We identified IQGAP1 as a Rho isoform-specific binding protein that may represent the first Rho isoform-specific effector to explain, at least in part, the differential effects of RhoA and C versus RhoB on tumor cell growth and motility (1, 2). IQGAP1 is a scaffolding protein that links multiple signaling pathways implicated in cancer: it regulates cell growth through interactions with growth factor receptors, B-Raf/mitogen-activated protein kinases, and Akt/mTOR; it modulates cell adhesions via β-catenin/E-cadherin interactions; and it stimulates cell motility via cytoskeletal rearrangements that involve binding of F-actin, N-WASp, mDia, CDC42, and Rac1 (20, 26–28, 39–41). Our findings now add RhoA and C to the small GTPases that directly bind IQGAP1. Sakurai-Yageta et al. (28) did not co-immunoprecipitate RhoAL63 with IQGAP1 from transfected 293 cells, but this was in the absence of magnesium, and we found magnesium to be essential for the Rho/IQGAP interaction.
Other workers who examined the interaction of IQGAP1 with CDC42 and Rac1 used bacterially expressed, unprenylated Rho family proteins as affinity ligands, which may explain why they did not detect IQGAP1 binding to RhoA (18, 19, 26, 27). To our knowledge, the effect of prenylation on CDC42 and Rac1 binding to IQGAP1 has not been tested; CDC42 and Rac1 appear to bind IQGAP1 in the absence of lipid modifications, but prenylation could affect binding affinity. Prenylation of Rho proteins is also required for proper subcellular localization, and differences in RhoA/C versus RhoB subcellular localization may partly explain why RhoB does not interact with IQGAP1 in intact cells (2). However, more work is needed to explain why purified RhoB does not bind IQGAP1.
CDC42 and Rac1 bind to the GAP-related domain located in the C-terminal half of IQGAP1, and binding involves primarily the switch I and II regions of CDC42 and Rac1 (42). Since the switch regions of Rho family proteins are highly conserved (43), and we found that RhoA and C bind to the C-terminal half of IQGAP1, it is likely that they also bind to the GAP-related domain.
We found that IQGAP1 binds to RhoA only in the GTP-bound, active form, supporting the notion that it acts as an effector of the small GTPase. Similarly, IQGAP1 binds preferentially to GTP-bound CDC42 and Rac1, although variable degrees of IQGAP1 binding to GDP-bound CDC42 and Rac1 have been reported (18, 26, 27). IQGAP1 inhibits the intrinsic GTPase activity of CDC42 in vitro, stabilizing the protein in the GTP-bound form, and overexpression of IQGAP1 increases the pool of GTP-bound CDC42, but effects on Rac1-GTP are controversial (44, 45). The effect of IQGAP1 on CDC42 (and Rac1) GTP levels may explain why overexpression of IQGAP1 can increase cell proliferation and migration independently of RhoA/C (Fig. 8E).
We observed that overexpression of IQGAP1 increased GTP binding to RhoA and C, while IQGAP1 depletion prevented growth factor-induced activation of endogenous RhoA and RhoC. Similarly, a dominant-negative IQGAP1, missing the GAP-related domain required for Rac1 and CDC42 binding, decreases CDC42- and Rac-GTP levels and blocks CDC42 activation by bradykinin (44). We found a trend toward lower basal RhoA-GTP levels in IQGAP1 siRNA-transfected cells, which did not reach statistical significance; this may be due to the fact that basal RhoA-GTP levels in serum-starved cells were extremely low and difficult to measure. That IQGAP1 knock-down impaired growth factor-induced RhoA/C activation by Rho-GEFs suggests IQGAP1 may facilitate RhoA/C access to GEFs (Fig. 8E). Alternatively, IQGAP1 may stabilize RhoA-GTP by shielding the protein from GAPs, as suggested for Rac1 and CDC42 (44, 45). We favor the first explanation, because IQGAP1 depletion decreased GTP binding to the GTPase-deficient RhoAL63 and RhoCV14 mutants.
Expression of RhoAL63 or RhoCV14 increased DNA synthesis in MDA-MB-231 cells, similar to effects of RhoCV14 seen in MCF10A cells (8). RhoA and C stimulate cell cycle progression by increasing cyclin D expression; this occurs through multiple pathways that may involve IQGAP1 (1, 8, 46). In addition, RhoA down-regulates the cell cycle-dependent kinase inhibitor p21WAF1, which is crucial for promotion of G1-S transition (1, 46). Our data show that effects of RhoA and RhoC on breast cancer proliferation required IQGAP1, and suggest that IQGAP1 acts as a Rho effector in this pathway (Fig. 8E). While IQGAP1 depletion affected GTP-binding to RhoAL63, residual amounts of GTP-bound RhoA remained high enough to allow us to conclude that lack of RhoA-induced proliferation in IQGAP-depleted cells could not be explained by a reduction in RhoA-GTP.
IQGAP1 overexpression stimulated DNA synthesis even in RhoA-depleted MDA-MB-231 cells, indicating that IQGAP1 affects cell proliferation independently of RhoA, most likely through its interactions with growth factor receptors, B-Raf/mitogen-activated protein kinases, and Akt/mTOR (20, 40, 41). When overexpressed in MCF7 cells, IQGAP1 also increases soft agar colony formation and tumor growth in mice (22).
Expression of RhoCV14 increased migration of MDA-MB-231 cells, consistent with previous reports in another breast cancer cell line (8). We found that the RhoC effect was blocked in IQGAP1-depleted cells, but again, this finding was not explained by the change in RhoC GTP-loading, suggesting that IQGAP acts as an effector for RhoC-induced migration (Fig. 8E). RhoC and IQGAP1 have both been linked to increased cancer cell motility and invasion; high RhoC or IQGAP1 expression strongly correlates with invasion and metastasis in several human cancers, including breast cancer (1, 17, 20, 36). RhoC overexpression is sufficient to enhance metastasis in poorly metastatic melanoma cells (47), and RhoC-null mice have a reduced number and size of lung metastasis in a murine breast cancer model (9). RhoC is overexpressed in 90% of inflammatory breast cancers, which exhibit a highly invasive and metastatic phenotype, and RhoC knock-down inhibits motility and invasiveness of inflammatory breast cancer-derived cell lines (5, 6, 38). The effects of RhoA knockdown on breast cancer migration are more varied (6, 38). We did not find a significant effect of RhoAL63 on the motility of MDA-MB-231 cells, and a dominant-negative RhoA does not inhibit IQGAP1-induced migration in MCF7 cells (23). However, RhoA co-localizes with IQGAP1 to membrane protrusions termed invadopodia at the leading edge of invading breast cancer cells, and Rho A regulates the interaction of IQGAP1 with proteins involved in matrix metalloproteinase secretion (28). In glioma cells, IQGAP1 co-localizes with the small GTPase ADP-ribosylation factor-6 (ARF6) at the leading edge of invasive cells, and association of IQGAP1 with ARF6 and Rac1 is required for hepatocyte growth factor-induced cell migration (48). Thus, IQGAP1 appears to accumulate at the leading edge of migrating cells, but depending on the cell type, different IQGAP-interacting proteins may mediate different aspects of cell polarization, migration, and invasion.
In conclusion, we found that IQGAP1 acts both upstream of RhoA/C to regulate their activation state, and down-stream, as an effector mediating increased breast cancer cell proliferation and migration. Since RhoA and C contribute both to the loss of growth control and the invasive potential of multiple cancer types, they represent an attractive target for cancer therapy (1). The findings presented here enhance our understanding of the role of RhoA/C and IQGAP1 in cancer biology, and provide a rationale for the use of prenylation inhibitors in the treatment of breast cancer (1).
Supplementary Material
Acknowledgments
We thank Dr. Joan H. Brown for generous gift of RhoAL63 adenovirus and Dr. Y. Chen for helping prepare the IQGAP1 and RhoCV14 adenovirus.
This work was supported, in whole or in part, by National Institutes of Health Grants K22-CA124517 (to D. C.) and R01-AR051300 (to R. B. P.).

This article contains supplemental Figs. S1 and S2.
- GEF
- guanine nucleotide exchange factor
- BrdU
- bromo-deoxyuridine
- EGF
- epidermal growth factor
- GAP
- GTPase-activating protein
- IQGAP
- IQ-motif-containing GTPase-activating protein
- RBD
- rho binding domain.
REFERENCES
- 1. Sahai E., Marshall C. J. (2002) Rho-GTPases and cancer. Nature Rev. Cancer 2, 133–142 [DOI] [PubMed] [Google Scholar]
- 2. Wheeler A. P., Ridley A. J. (2004) Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp. Cell Res. 301, 43–49 [DOI] [PubMed] [Google Scholar]
- 3. Tang Y., Olufemi L., Wang M. T., Nie D. (2008) Role of Rho GTPases in breast cancer. Front. Biosci. 13, 759–776 [DOI] [PubMed] [Google Scholar]
- 4. Qiu R. G., Chen J., McCormick F., Symons M. (1995) A role for Rho in Ras transformation. Proc. Natl. Acad. Sci. U.S.A. 92, 11781–11785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Golen K. L., Wu Z. F., Qiao X. T., Bao L. W., Merajver S. D. (2000) RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 60, 5832–5838 [PubMed] [Google Scholar]
- 6. Simpson K. J., Dugan A. S., Mercurio A. M. (2004) Functional analysis of the contribution of RhoA and RhoC GTPases to invasive breast carcinoma. Cancer Res. 64, 8694–8701 [DOI] [PubMed] [Google Scholar]
- 7. Pillé J. Y., Denoyelle C., Varet J., Bertrand J. R., Soria J., Opolon P., Lu H., Pritchard L. L., Vannier J. P., Malvy C., Soria C., Li H. (2005) Anti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Molecular Therapy 11, 267–274 [DOI] [PubMed] [Google Scholar]
- 8. Wu M., Wu Z. F., Kumar-Sinha C., Chinnaiyan A., Merajver S. D. (2004) RhoC induces differential expression of genes involved in invasion and metastasis in MCF10A breast cells. Breast Cancer Res. Treat. 84, 3–12 [DOI] [PubMed] [Google Scholar]
- 9. Hakem A., Sanchez-Sweatman O., You-Ten A., Duncan G., Wakeham A., Khokha R., Mak T. W. (2005) RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 19, 1974–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu A. X., Rane N., Liu J. P., Prendergast G. C. (2001) RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol. Cell. Biol. 21, 6906–6912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Z., Sun J., Pradines A., Favre G., Adnane J., Sebti S. M. (2000) Both farnesylated and geranylgeranylated RhoB inhibit malignant transformation and suppress human tumor growth in nude mice. J. Biol. Chem. 275, 17974–17978 [DOI] [PubMed] [Google Scholar]
- 12. Karnoub A. E., Symons M., Campbell S. L., Der C. J. (2004) Molecular basis for Rho GTPase signaling specificity. Breast Cancer Res. Treat. 84, 61–71 [DOI] [PubMed] [Google Scholar]
- 13. Lammers M., Meyer S., Kühlmann D., Wittinghofer A. (2008) Specificity of interactions between mDia isoforms and Rho proteins. J. Biol. Chem. 283, 35236–35246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vega F. M., Fruhwirth G., Ng T., Ridley A. J. (2011) RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J. Cell Biol. 193, 655–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mircescu H., Steuve S., Savonet V., Degraef C., Mellor H., Dumont J. E., Maenhaut C., Pirson I. (2002) Identification and characterization of a novel activated RhoB binding protein containing a PDZ domain whose expression is specifically modulated in thyroid cells by cAMP. Eur. J. Biochem. 269, 6241–6249 [DOI] [PubMed] [Google Scholar]
- 16. Zeng P. Y., Rane N., Du W., Chintapalli J., Prendergast G. C. (2003) Role for RhoB and PRK in the suppression of epithelial cell transformation by farnesyltransferase inhibitors. Oncogene 22, 1124–1134 [DOI] [PubMed] [Google Scholar]
- 17. White C. D., Brown M. D., Sacks D. B. (2009) IQGAPs in cancer: a family of scaffold proteins underlying tumorigenesis. FEBS Lett. 583, 1817–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCallum S. J., Wu W. J., Cerione R. A. (1996) Identification of a putative effector for Cdc42Hs with high sequence similarity to the RasGAP-related protein IQGAP1 and a Cdc42Hs binding partner with similarity to IQGAP2. J. Biol. Chem. 271, 21732–21737 [DOI] [PubMed] [Google Scholar]
- 19. Hart M. J., Callow M. G., Souza B., Polakis P. (1996) IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 15, 2997–3005 [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson M., Sharma M., Henderson B. R. (2009) IQGAP1 regulation and roles in cancer. Cell Signal. 21, 1471–1478 [DOI] [PubMed] [Google Scholar]
- 21. Brandt D. T., Grosse R. (2007) Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 8, 1019–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jadeski L., Mataraza J. M., Jeong H. W., Li Z., Sacks D. B. (2008) IQGAP1 stimulates proliferation and enhances tumorigenesis of human breast epithelial cells. J. Biol. Chem. 283, 1008–1017 [DOI] [PubMed] [Google Scholar]
- 23. Mataraza J. M., Briggs M. W., Li Z., Entwistle A., Ridley A. J., Sacks D. B. (2003) IQGAP1 promotes cell motility and invasion. J. Biol. Chem. 278, 41237–41245 [DOI] [PubMed] [Google Scholar]
- 24. Mataraza J. M., Li Z., Jeong H. W., Brown M. D., Sacks D. B. (2007) Multiple proteins mediate IQGAP1-stimulated cell migration. Cell Signal. 19, 1857–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li S., Wang Q., Chakladar A., Bronson R. T., Bernards A. (2000) Gastric hyperplasia in mice lacking the putative Cdc42 effector IQGAP1. Mol. Cell. Biol. 20, 697–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuroda S., Fukata M., Kobayashi K., Nakafuku M., Nomura N., Iwamatsu A., Kaibuchi K. (1996) Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J. Biol. Chem. 271, 23363–23367 [DOI] [PubMed] [Google Scholar]
- 27. McCallum S. J., Erickson J. W., Cerione R. A. (1998) Characterization of the association of the actin-binding protein, IQGAP, and activated Cdc42 with Golgi membranes. J. Biol. Chem. 273, 22537–22544 [DOI] [PubMed] [Google Scholar]
- 28. Sakurai-Yageta M., Recchi C., Le Dez G., Sibarita J. B., Daviet L., Camonis J., D'Souza-Schorey C., Chavrier P. (2008) The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J. Cell Biol. 181, 985–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen J. C., Zhuang S., Nguyen T. H., Boss G. R., Pilz R. B. (2003) Oncogenic Ras leads to Rho activation by activating the mitogen-activated protein kinase pathway and decreasing Rho-GTPase-activating protein activity. J. Biol. Chem. 278, 2807–2818 [DOI] [PubMed] [Google Scholar]
- 30. Ren X. D., Kiosses W. B., Schwartz M. A. (1999) Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Turner S. J., Zhuang S., Zhang T., Boss G. R., Pilz R. B. (2008) Effects of lovastatin on Rho isoform expression, activity, and association with guanine nucleotide dissociation inhibitors. Biochem. Pharmacol. 75, 405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rangaswami H., Schwappacher R., Marathe N., Zhuang S., Casteel D. E., Haas B., Chen Y., Pfeifer A., Kato H., Shattil S., Boss G. R., Pilz R. B. (2010) Cyclic GMP and protein kinase G control a Src-containing mechanosome in osteoblasts. Sci. Signal. 3, ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Del Re D. P., Miyamoto S., Brown J. H. (2008) Focal adhesion kinase as a RhoA-activable signaling scaffold mediating Akt activation and cardiomyocyte protection. J. Biol. Chem. 283, 35622–35629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng Y., Zhuang S., Gloddek J., Tseng C. C., Boss G. R., Pilz R. B. (2006) Regulation of cGMP-dependent protein kinase expression by Rho and Kruppel-like transcription factor-4. J. Biol. Chem. 281, 16951–16961 [DOI] [PubMed] [Google Scholar]
- 35. Gudi T., Chen J. C., Casteel D. E., Seasholtz T. M., Boss G. R., Pilz R. B. (2002) cGMP-dependent protein kinase inhibits serum response element-dependent transcription by inhibiting Rho activation and functions. J. Biol. Chem. 277, 37382–37393 [DOI] [PubMed] [Google Scholar]
- 36. Karlsson R., Pedersen E. D., Wang Z., Brakebusch C. (2009) Rho GTPase function in tumorigenesis. Biochim. Biophys. Acta 1796, 91–98 [DOI] [PubMed] [Google Scholar]
- 37. Bouzahzah B., Albanese C., Ahmed F., Pixley F., Lisanti M. P., Segall J. D., Condeelis J., Joyce D., Minden A., Der C. J., Chan A., Symons M., Pestell R. G. (2001) Rho family GTPases regulate mammary epithelium cell growth and metastasis through distinguishable pathways. Mol. Med. 7, 816–830 [PMC free article] [PubMed] [Google Scholar]
- 38. Wu M., Wu Z. F., Rosenthal D. T., Rhee E. M., Merajver S. D. (2010) Characterization of the roles of RHOC and RHOA GTPases in invasion, motility, and matrix adhesion in inflammatory and aggressive breast cancers. Cancer 116, 2768–2782 [DOI] [PubMed] [Google Scholar]
- 39. Brandt D. T., Marion S., Griffiths G., Watanabe T., Kaibuchi K., Grosse R. (2007) Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J. Cell Biol. 178, 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang J. B., Sonn R., Tekletsadik Y. K., Samorodnitsky D., Osman M. A. (2009) IQGAP1 regulates cell proliferation through a novel CDC42-mTOR pathway. J. Cell Sci. 122, 2024–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. White C. D., Li Z., Dillon D. A., Sacks D. B. (2011) IQGAP1 protein binds human epidermal growth factor receptor 2 (HER2) and modulates trastuzumab resistance. J. Biol. Chem. 286, 29734–29747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Owen D., Campbell L. J., Littlefield K., Evetts K. A., Li Z., Sacks D. B., Lowe P. N., Mott H. R. (2008) The IQGAP1-Rac1 and IQGAP1-Cdc42 interactions: interfaces differ between the complexes. J. Biol. Chem. 283, 1692–1704 [DOI] [PubMed] [Google Scholar]
- 43. Wennerberg K., Der C. J. (2004) Rho-family GTPases: it's not only Rac and Rho (and I like it). J. Cell Sci. 117, 1301–1312 [DOI] [PubMed] [Google Scholar]
- 44. Swart-Mataraza J. M., Li Z., Sacks D. B. (2002) IQGAP1 is a component of Cdc42 signaling to the cytoskeleton. J. Biol. Chem. 277, 24753–24763 [DOI] [PubMed] [Google Scholar]
- 45. Noritake J., Fukata M., Sato K., Nakagawa M., Watanabe T., Izumi N., Wang S., Fukata Y., Kaibuchi K. (2004) Positive role of IQGAP1, an effector of Rac1, in actin-meshwork formation at sites of cell-cell contact. Mol. Biol. Cell 15, 1065–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liberto M., Cobrinik D., Minden A. (2002) Rho regulates p21CIP1, cyclin D1, and checkpoint control in mammary epithelial cells. Oncogene 21, 1590–1599 [DOI] [PubMed] [Google Scholar]
- 47. Clark E. A., Golub T. R., Lander E. S., Hynes R. O. (2000) Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406, 532–535 [DOI] [PubMed] [Google Scholar]
- 48. Hu S., Shi B., Jarzynka M. J., Yiin J. J., D'Souza-Schorey C., Cheng S. Y. (2009) ADP-ribosylation factor 6 regulates glioma cell invasion through the IQ-domain GTPase-activating protein 1-Rac1-mediated pathway. Cancer Res. 69, 794–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.