Background: Satellite cell activation is orchestrated by several signals, which induce their differentiation into skeletal muscle fibers.
Results: Obestatin and the GPR39 receptor exert an autocrine role on the control of myogenesis.
Conclusion: Our data indicate that obestatin/GPR39 is an injury-regulated signal that functions as a myogenic regenerative system.
Significance: Strategies to enhance obestatin-mediated signaling could be useful in treating trauma-induced muscle injuries and skeletal muscle myopathies.
Keywords: Cell Biology, Cell Differentiation, Muscle, Muscle Regeneration, Myogenesis
Abstract
The maintenance and repair of skeletal muscle are attributable to an elaborate interaction between extrinsic and intrinsic regulatory signals that regulate the myogenic process. In the present work, we showed that obestatin, a 23-amino acid peptide encoded by the ghrelin gene, and the GPR39 receptor are expressed in rat skeletal muscle and are up-regulated upon experimental injury. To define their roles in muscle regeneration, L6E9 cells were used to perform in vitro assays. For the in vivo assays, skeletal muscle tissue was obtained from male rats and maintained under continuous subcutaneous infusion of obestatin. In differentiating L6E9 cells, preproghrelin expression and correspondingly obestatin increased during myogenesis being sustained throughout terminal differentiation. Autocrine action was demonstrated by neutralization of the endogenous obestatin secreted by differentiating L6E9 cells using a specific anti-obestatin antibody. Knockdown experiments by preproghrelin siRNA confirmed the contribution of obestatin to the myogenic program. Furthermore, GPR39 siRNA reduced obestatin action and myogenic differentiation. Exogenous obestatin stimulation was also shown to regulate myoblast migration and proliferation. Furthermore, the addition of obestatin to the differentiation medium increased myogenic differentiation of L6E9 cells. The relevance of the actions of obestatin was confirmed in vivo by the up-regulation of Pax-7, MyoD, Myf5, Myf6, myogenin, and myosin heavy chain (MHC) in obestatin-infused rats when compared with saline-infused rats. These data elucidate a novel mechanism whereby the obestatin/GPR39 system is coordinately regulated as part of the myogenic program and operates as an autocrine signal regulating skeletal myogenesis.
Introduction
The regenerative potential of adult skeletal muscle is mainly attributed to the heterogeneous population of muscle stem cells known as satellite cells (1, 2). These pluripotent cells, located in a niche beneath the basal lamina and closely juxtaposed to muscle fiber, are normally mitotically quiescent but can be induced to enter the cell cycle by weight bearing, trauma, and specific disease states. Activated satellite cells give rise to a transient amplifying population of myogenic precursor cells that undergo several rounds of division before migrating to the site of damage to enter terminal differentiation (3, 4). Furthermore, satellite cells also possess the ability to undergo self-renewal to restock the quiescent satellite cell population (4). At the molecular level, a broad spectrum of signals orchestrates the activation of satellite cells and repopulation of the quiescent pool. These signals include the following: basic fibroblast growth factor (5), hepatocyte growth factor (6), epidermal growth factor (EGF (7)), insulin-like growth factor-I (IGF-I 5 (8)), myostatin (9), Wnt glycoproteins (10, 11), brain-derived neurotrophic factor (BDNF (12)), calcitonin (13), α-chemokine stromal-derived factor 1 (SDF-1 (14)), TNF ligand TWEAK (15), Notch ligand Delta (16), integrin VLA-4 (17), M-cadherin (18), VEGF (19), PDGF (20), IL-6 (21), and angiopoietin 1 (22). These signals determine the intracellular pathways that converge on a series of transcription and chromatin-remodeling factors to define the gene and microRNA expression program that delimit myogenic identity (2, 4). Myogenic transcription factors are organized in hierarchical gene expression networks that are spatiotemporally activated or inhibited during lineage progression. In particular, the muscle regulatory factor family (MRF), MyoD, Myf5, myogenin, and MRF4 are essential for myoblast lineage commitment. The MRF in conjunction with other transcriptional regulators induces the expression of muscle-specific genes, such as myosin heavy chain (MHC), that determine terminal myogenic differentiation (2, 4, 23).
Regarding the extrinsic regulation of adult myogenesis, the IGF family and the insulin-like growth factor 1 receptor (IGF1R) appear to play a key role in regulating skeletal muscle growth and myogenesis and in maintaining homeostasis in adult muscle tissues (24). The IGF/IGF1R system stimulates both myoblast proliferation and myoblast differentiation, which are two mutually exclusive biological events during myogenesis. Intriguingly, the IGF/IGF1R system also plays a key role in adipogenesis (25). This dual activity is determined by the cell type-specific context in which differential activation of signaling pathways provides an important switching function that determines the cell fate in the differentiation process (26–28). Recent works from our laboratory have uncovered that insulin, through IGF1R, triggers the expression of obestatin during adipocyte differentiation as a key target for preadipocyte commitment to the adipocyte lineage (29, 30). Obestatin, a 23-amino acid peptide derived from a polypeptide called preproghrelin, precursor of ghrelin, was originally isolated from the stomach. It was found to be a circulating peptide whose secretion is pulsatile with an ultradian rhythmicity similar to the ghrelin and growth hormone secretion (31). It was reported to be the ligand for the orphan receptor GPR39, which belongs to the family of the ghrelin and motilin receptors (32). The autocrine role on adipogenesis was further supported by the effect of exogenous obestatin on the expression of master regulators of the adipocyte fate and, consequently, lipid accumulation (30). This action appears to be related to Akt activation and its downstream targets, mammalian target of rapamycin (mTOR) and S6K1. The relevance of obestatin as a regulator of adipocyte metabolism was supported by AS160 phosphorylation, GLUT4 translocation, and increased glucose uptake. In addition to the adipogenic function, obestatin is a multifunctional peptide with roles in pancreatic homeostasis (33) and cancer (34, 35), and it is associated with the activation of MAPK and Akt signaling pathways (34, 35). The bidirectional activity of IFG/IGF1R on adipogenesis and myogenesis, the common mesenchymal precursor for muscle and fat cells, and the availability of new data on preproghrelin as the source of key autocrine factors for adipogenesis prompted us to perform further studies on the role of obestatin as an autocrine/paracrine factor in the regulation of myogenesis and regeneration following muscle damage. Based on the cardiotoxin (CTX) injury model, we found that the obestatin/GPR39 system is up-regulated in damaged muscle. Using several cellular strategies, we analyzed the mitogenic and myogenic capabilities of this system. Finally, in vivo studies confirmed the role of obestatin in the regulation of myogenesis in adult skeletal muscle.
EXPERIMENTAL PROCEDURES
Materials
Rat/mouse obestatin was obtained from California Peptide Research (Napa, CA). Anti-pAkt hydrophobic motif Ser-473 (HM(Ser-473)), anti-Akt, anti-pERK1/2(Thr-202/Tyr-204), anti-ERK 1/2, anti-pp38(Tyr-182), anti-p38, and anti-tubulin antibodies were from Cell Signaling Technology (Beverly, MA). Anti-GPR39, anti-MHC, anti-p21, and anti-obestatin (for obestatin neutralization assays) antibodies were obtained from Abcam (Cambridge, UK). Anti-preproghrelin antibody was obtained from Phoenix Pharmaceuticals (Burlingame, CA). Anti-myogenin, anti-MyoD, anti-Myf5, anti-Pax-7, anti-Myf6, anti-Six-1, anti-VEGF, anti-VEGF-R2 (flK1), and anti-PEDF antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). For immunohistochemistry, anti-obestatin antibody was from Alpha Diagnostic International Inc. (San Antonio, TX). FITC-conjugated goat anti-mouse antibody was from Invitrogen. Preproghrelin, GPR39, and control siRNAs were obtained from Thermo Fisher Scientific (Dharmacon). Secondary antibodies and enhanced chemiluminescence detection system were from Thermo Fisher Scientific (Pierce). ALZET® osmotic minipumps (model 1003D) were purchased from DURECT Corp. (Cupertino, CA). All other chemical reagents were from Sigma.
Cell Culture and Differentiation Induction of L6E9 Myoblasts
Rat L6E9 myoblasts were cultured as described by the supplier (European Collection of Cell Cultures (ECACC), Wiltshire, UK). L6E9 myoblasts were maintained in growth medium (GM) containing DMEM supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 units/ml streptomycin. For routine differentiation, cells were grown to 80% confluence, and GM was replaced with differentiation medium (DM, DMEM supplemented with 2% FBS, 100 units/ml penicillin, and 100 units/ml streptomycin) for 6 days unless otherwise stated.
Quantitative RT-PCR
For quantitative RT-PCR, total RNA was isolated with TRIzol (Invitrogen) and DNA-free kit (Invitrogen, Applied Biosystems/Ambion) to generate first-strand cDNA synthesis using a high-capacity cDNA reverse transcription kit (Applied Biosystems). Quantitative RT-PCR was performed using an ABI PRISM 7300 HT sequence detection system (Applied Biosystems). For the analysis of the preproghrelin gene, β-actin was used as the housekeeping gene (TaqMan: Applied Biosystems). The -fold change in gene expression was calculated using the 2−ΔΔCt relative quantitation method according to the manufacturer's guidelines (Applied Biosystems).
Immunoblot Analysis
Tissue samples or cells were directly lysed in ice-cold radioimmune precipitation buffer (50 mm Tris-HCl (pH 7.2), 150 mm NaCl, 1 mm EDTA, 1% (v/v) Nonidet P-40, 0.25% (w/v) sodium deoxycholate, protease inhibitor mixture (Sigma), and phosphatase inhibitor mixture (Sigma). Lysates were clarified by centrifugation (14,000 × g for 15 min at 4 °C), and the protein concentration was quantified using the QuantiProTM BCA assay kit (Sigma). For immunoblotting, equal amounts of protein were fractionated by SDS-PAGE and transferred onto nitrocellulose membranes. Immunoreactive bands were detected by enhanced chemiluminescence (Pierce ECL Western blotting Substrate; Thermo Fisher Scientific, Pierce).
Immunocytochemistry/Histochemistry
L6E9 cells (myoblasts or myotubes) cultured on coverslips were fixed in 96% ethanol for immunocytochemistry analysis. Muscle samples were mounted in tissue freezing medium (tragacanth paste) and snap-frozen in nitrogen-cooled isopentane. Sections, 10 μm thick, were mounted on HistoBond adhesion microslides (Marienfeld, Lauda-Königshofen, Germany). Cells or tissue samples were consecutively incubated with: 1) primary antibody at a dilution of 1:100 in Dako ChemMate antibody diluent (Dako, Glostrup, Denmark); 2) EnVision peroxidase rabbit (Dako, Carpentaria, CA) used as the detection system; and 3) 3,3′-diaminobenzidine-tetrahydrochloride (Dako liquid DAB + substrate-chromogen system). Samples were counterstained weakly with Harris' hematoxylin. Immunohistochemistry controls were performed by applying the primary antibody plus control antigen peptide (10 nmol/ml) to positive samples. For similar analyses, serial cryostat sections were stained with hematoxylin/eosin, anti-obestatin, anti-GPR39, anti-MyoD, and anti-myogenin.
For immunofluorescence analysis, L6E9 cells were cultured on coverslips and differentiated into myotubes under DM supplemented with obestatin (5 nm) for 6 days. Intact cells were fixed with 4% buffered paraformaldehyde-PBS for 15 min, washed, permeabilized, and blocked with PBT (1% Triton X-100, 1% Tween 20, 5% heat-inactivated normal goat serum, 0.2% BSA in PBS) for 30 min and then incubated with anti-MHC mouse antibody diluted in PBT (1:400) overnight at 4 °C. After three washes with PBS, cells were incubated with the secondary antibody (FITC-conjugate goat anti-mouse antibody) in PBT (1:1000) for 45 min at 37 °C. DAPI was used to counterstain the cell nuclei (Invitrogen). Digital images of cell cultures were acquired with a Leica TCS-SP2 spectral confocal microscope (Leica Microsystems, Heidelberg, Germany). Five fields from three independent experiments were randomly taken for each treatment. The differentiation grade was evaluated based on the number of MHC-positive cells above the total number of nuclei. The number of nuclei within individual myotubes (≥2 nuclei) was counted for 20–50 myotubes. Myotubes were grouped into two categories, those with 2–3 nuclei and those with 4 or more nuclei, and the percentage of myotubes in each category was calculated relative to untreated cells (36). For quantification of myofiber cross-section area, cryostat sections were stained with hematoxylin/eosin, and five randomly chosen microscopic fields from two different sections in each tissue block were examined using ImageJ64 analysis software.
Small Interfering RNA (siRNA) Silencing of Gene Expression
Chemically synthesized double-stranded siRNA duplexes targeting either preproghrelin or GPR39 were selected from ON-TARGETplus SMARTpool siRNA from Thermo Fisher Scientific (Dharmacon; preproghrelin, CCAAGAAGCCACCAGCUAA, CUGCUGACUUACAAAUAAA, CAGAGGAGGAGCUGGAAAU, and UCAAAGAGGCGCCAGCUAA; GPR39, ACAAGGGACUCAACUGUAA, UUACGCAGGUAUUGCAGAA, CCUGCAAGCUCCACACACGUU, and AGUUUGGCUUGUUCAGAUA). An ON-TARGETplus nontargeting siRNA was used as a control for all siRNA experiments. L6E9 cells were transfected with Lipofectamine 2000 (Invitrogen).
Invasion/Migration Assay
L6E9 cells were seeded on 6-well plates, grown to 100% confluence in GM, and then wounded with a sterile pipette tip to remove cells by linear scratches. The cells were washed and maintained in GM or GM + 5 nm obestatin. The progress of migration was photographed immediately after injury and at 8 and 24 h after wounding, near the crossing point. The wound was calculated by tracing along the border of the scratch using the ImageJ64 analysis software and using the following equation: percentage of wound closure = ((wound area (0 h) − wound area (× h))/wound area (0 h)) × 100 (37).
Animals
Adult male Sprague-Dawley rats (250 g; n = 20) were housed in 12-h light/12-h dark cycles with free access to standard rat chow diet and water. ALZET minipumps (model 1003D) were subcutaneously implanted. The animals were assigned to one of two matched experimental groups (n = 10/group): 1) 72-h minipump subcutaneously implanted group (containing obestatin (300 nmol/kg body weight/24 h)); and 2) 72-h minipump subcutaneous control group (containing saline). These minipumps hold 100 μl of volume and deliver 1 μl/h. After 72 h, rats were sacrificed, and the soleus and gastrocnemius muscle tissues were snap-frozen in liquid nitrogen for Western blot analysis. All experimental procedures were approved by the Animal Care Committee at the Universidad de Santiago de Compostela in accordance with European Union normative for the use of experimental animals.
Muscle Injury Model
Gastrocnemius muscles from rats were impaired, along their entire length, with four intramuscular injections per muscle with CTX (10 μm CTX; 2 μl/g body weight) or equal volume of PBS (38) to ensure equal distribution throughout the muscle. At different times after injury (6, 24, 48, and 72 h; n = 5 per time point), rats were sacrificed, and gastrocnemius muscles were dissected and divided for histological and Western blot analyses. For histologic analysis, muscle tissue samples were mounted in tissue freezing medium (tragacanth paste) and frozen in isopentane. For Western blot analysis, muscles were snap-frozen in liquid nitrogen. This procedure was approved by the Animal Care Committee at the Universidad de Santiago de Compostela.
Data Analysis
All values are presented as the means ± S.E. Student t tests were performed to assess the statistical significance of two-way analysis. For multiple comparisons, analysis of variance was employed. p < 0.05 was considered as statistically significant (*).
RESULTS
The Obestatin/GPR39 System Is Expressed in Adult Skeletal Muscle
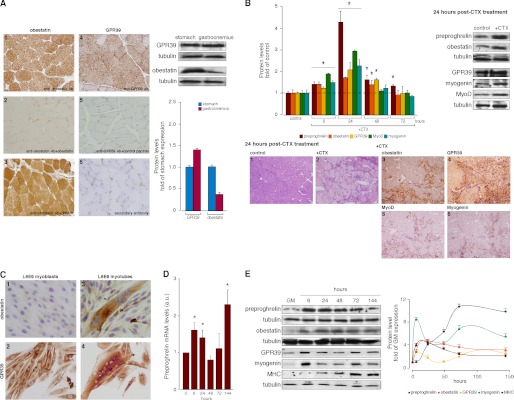
As seen in Fig. 1A, the expression of the obestatin/GPR39 system was detected in adult gastrocnemius skeletal muscle from male ad libitum rats by immunohistochemistry (Fig. 1, A.1 and A.4, respectively). No immunostaining was found when obestatin or GPR39 antibodies were preadsorbed with homologous peptides (Fig. 1, A.2 and A.5, respectively). In contrast, immunostaining was observed when obestatin antibody was preadsorbed with ghrelin peptide (Fig. 1A.3). A comparative study with immunoblot analysis demonstrated moderate obestatin expression in the gastrocnemius when compared with the stomach (∼0.4-fold; Fig. 1A, right panel). In contrast, higher GPR39 expression in the gastrocnemius than in the stomach was detected (∼1.4-fold).
FIGURE 1.
A, immunohistochemical detection of obestatin (A.1) and GPR39 (A.4) in gastrocnemius muscle (objective magnification 20×). Preadsorption controls were performed, applying the primary antibody plus obestatin (A.2), ghrelin (A.3), or GPR39 control peptide (A.5) (10 nmol/ml per control peptide) to positive samples. Immunohistochemistry was also performed in the absence of primary antibody (A.6). Right panel, immunoblot analysis of obestatin and GPR39 of extracts from stomach and gastrocnemius muscle of control rats (n = 5). Results were expressed as a -fold of protein levels in the stomach. B, preproghrelin, obestatin, and GPR39 expression in the CTX model of gastrocnemius muscle injury. Left panel, relative protein expression of preproghrelin, obestatin, GPR39, MyoD, and myogenin in CTX- or PBS-injected gastrocnemius muscle was assessed at the indicated time points by immunoblot (n = 5 per group and time point). Protein expression was expressed as a -fold over PBS control muscle. Right panel, representative immunoblot images of gastrocnemius muscle sections 24 h after PBS or CTX injection. Lower panel, representative images of gastrocnemius muscle sections 24 h after PBS or CTX injection: hematoxylin/eosin (B1, control, and B2, +CTX); immunohistochemical detection of obestatin, GPR39, MyoD, and myogenin after CTX injection (B3, B4, B5, and B6, respectively; objective magnification 20×). C, immunocytochemical detection of obestatin and GPR39 in myoblast and myotube L6E9 cells (objective magnification 40×). D, relative mRNA transcripts of preproghrelin in the course of L6E9 myogenesis. mRNA was quantified by real-time PCR and expressed as arbitrary units (n = 3). E, immunoblot analysis of preproghrelin, obestatin, GPR39, myogenin, and MHC expression in the course of L6E9 myogenesis. Protein level was expressed as a -fold over control cells in GM (n = 3). Immunoblots in A and B are representative of the mean value. Immunoblots in E are representative of three independent experiments. Data were expressed as the mean ± S.E. obtained from intensity scans of independent experiments. *, p < 0.05 versus control values.
Obestatin/GPR39 System Is Up-regulated in Skeletal Muscle upon CTX Injury
To examine the obestatin/GPR39 regulation in a model of skeletal muscle injury and regeneration, CTX solution (10 μm CTX; 2 μl/g body weight) (39) or an equal volume of PBS was injected into the gastrocnemius muscle. Tissues were analyzed at 6, 24, 48, and 72 h after treatment (n = 5 at each time point). As shown in Fig. 1B, immunoblot analysis revealed that the maximal expression of preproghrelin (∼4.5-fold), obestatin (∼1.7-fold), and GPR39 (∼2.1-fold) occurred at 24 h after CTX treatment. Maximal protein expression of the myogenic nuclear factors MyoD (∼2.9-fold) and myogenin (∼2.4-fold) paralleled with the expression of preproghrelin, obestatin, and GPR39 at the 24-h time point (Fig. 1B, left panel). Immunohistochemical analysis of obestatin, GPR39, MyoD, and myogenin was also performed at the 24-h time point (Fig. 1, B.3–B.6). The strongest signal for both obestatin (Fig. 1B.3) and GPR39 (Fig. 1B.4) occurred in regenerated myofibers (Fig. 1, B.5 and B.6).
Obestatin Is Up-regulated during Myogenic Differentiation
To elucidate the role of this system in myogenesis, an in vitro cell culture model that closely recapitulates the formation and maintenance of skeletal muscle was utilized: the rat skeletal muscle L6 myoblasts (subclone E9, L6E9 cells) (40). Upon reaching confluence, serum was withdrawn to induce the differentiation of L6E9 cells from proliferative myoblasts into terminally differentiated myotubes to permit fusion and form multinucleated cells that express markers of differentiated skeletal muscle. As shown in Fig. 1C, L6E9 myotubes, obtained during a 6-day differentiation period, showed a stronger obestatin expression when compared with that observed in L6E9 myoblast cells (Fig. 1C.3 when compared with Fig. 1C.1). In contrast, GPR39 was detected in L6E9 in both myoblast and myotube cells (Fig. 1, C.2 and C.4, respectively). These results led us to focus on preproghrelin expression during myogenesis as a source of obestatin. Preproghrelin expression was examined at the mRNA and protein levels by real-time PCR and immunoblot, respectively, from samples taken at the proliferative state and throughout differentiation. Within 6 h of differentiation, the preproghrelin mRNA levels experienced a rapid increase (∼1.6-fold; Fig. 1D), which subsequently returned to basal level values by 48 h of induction. Transcript expression increased again from 72 h, reaching maximal levels at 144 h after induction of differentiation (∼2.3-fold). As shown in Fig. 1E, immunoblot analysis confirmed the dynamic regulation of preproghrelin expression at the time of differentiation into myotubes, with a rapid increase at the 6-h time point, reaching a maximum at 24 h after induction of differentiation (∼4.4-fold) concomitant with the rise of preproghrelin mRNA expression. Intriguingly, preproghrelin protein expression began to decrease 48 h after induction of differentiation (∼2.1-fold at 144 h), remaining at sustained but reduced levels during terminal differentiation; however, the levels remained higher than in myoblast cells (Fig. 1E). Obestatin peptide expression closely paralleled the expression of preproghrelin (∼4.5- and 3.0-fold at 24 and 144 h, respectively). GPR39 protein expression showed maximal expression levels at two time points: 6 and 144 h (∼2.9- and 2.6-fold, respectively), concomitant with maximum expression levels of the myogenic transcription factor myogenin (Fig. 1E). The expression of MHC reached maximal levels 72 h after induction, which marked the progression of terminal differentiation (Fig. 1E).
Autocrine/Paracrine Role of Obestatin on Myogenesis
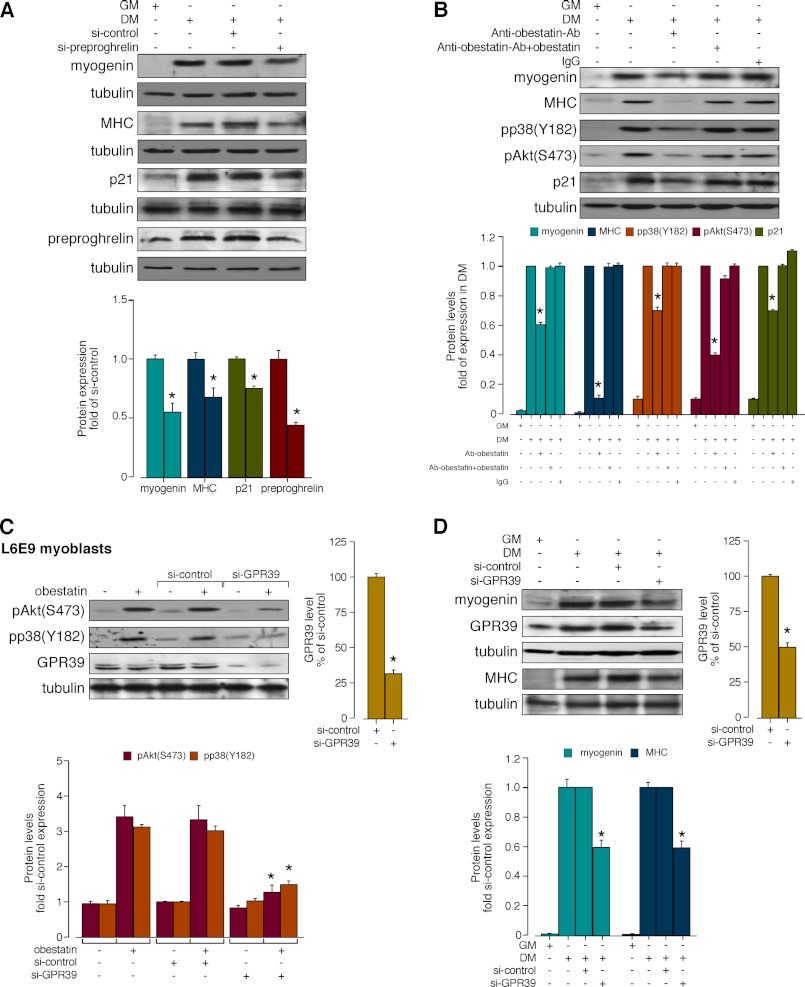
The effect of acute obestatin deficiency was first determined by preproghrelin knockdown by means of siRNA prior to induction of myogenic differentiation. Under these conditions, the constructs decreased preproghrelin level by 57 ± 2% relative to siRNA control (si-control; Fig. 2A) during a 96 h-differentiation period. Silencing of preproghrelin (si-preproghrelin) decreased myogenin and MHC expression levels with respect to si-control (46 ± 6 and 33 ± 7%, respectively; Fig. 2A). Correspondingly, the cell cycle arrest protein p21 expression was decreased in the presence of si-preproghrelin (26 ± 2%; Fig. 2A). The individual autocrine role of obestatin was tested using a neutralizing obestatin antibody (Ab) (30). L6E9 cells were maintained in GM, DM, anti-obestatin Ab (5 μg/μl) in DM, obestatin + anti-obestatin Ab (1:1, w/w) in DM, or preimmune IgG (5 μg/μl) in DM during a 6-day differentiation period in which DM plus reactive were replaced every 24 h. As shown in Fig. 2B, anti-obestatin Ab reduced myogenin and MHC expression levels with respect to standard differentiation conditions (40 ± 3 and 89 ± 2%, respectively), whereas obestatin + anti-obestatin Ab or preimmune IgG treatments, used as Ab controls, did not affect the differentiation marker levels. Simultaneously, Akt phosphorylation at the C-terminal hydrophobic motif (Ser-473) (HM(Ser-473); pAkt(Ser-473)) and p38 phosphorylation at Tyr-182 (pp38(Tyr-183)) showed significant decrease in the presence of the obestatin neutralizing Ab (60 ± 2 and 30 ± 3%, respectively; Fig. 2B). Furthermore, the presence of the anti-obestatin Ab reduced p21 expression (32 ± 1%; Fig. 2B).
FIGURE 2.
A, effect of siRNA depletion of preproghrelin on myogenesis. L6E9 cells were transfected with preproghrelin siRNA prior to induction of myogenesis for 96 h. Expression of myogenin, MHC, p21, and preproghrelin was denoted as a -fold of the respective levels in control siRNA-transfected cells (n = 3). B, autocrine role of obestatin on myogenesis. Differentiating L6E9 cells were maintained in DM, DM supplemented with anti-obestatin antibody (5 μg/μl; anti-obestatin Ab), obestatin + anti-obestatin Ab (1:1, w/w), or preimmune IgG (5 μg/μl) for 6 days. Expression of pp38(Tyr-182), pAkt(Ser-473), or p21 is expressed as a -fold of respective expressions in DM control (n = 6). C, effect of GPR39 knockdown by siRNA on obestatin-activated pAkt(Ser-473) and pp38(Tyr-182) in L6E9 myoblast cells (5 nm, 5 min). GPR39 was expressed as a -fold of its level to control siRNA-transfected cells (n = 3). Activation of Akt and p38 was expressed relative to control siRNA-transfected cells. D, effect of siRNA depletion of GPR39 on myogenesis. L6E9 cells were transfected with GPR39 siRNA prior to induction of myogenesis for 96 h. Expression of myogenin and MHC was expressed as a -fold of the respective levels to control siRNA-transfected cells (n = 3). Immunoblots in A, B, C, and D are representative of three independent experiments. Data were expressed as the mean ± S.E. obtained from intensity scans of independent experiments. *, p < 0.05 versus control values.
GPR39 Regulates Myogenesis
First, the effect of acute GPR39 deficiency on obestatin signaling was determined by treatment of L6E9 myoblast cells with siRNA (si-GPR39). Under these conditions, the constructs decreased GPR39 expression by 69 ± 2% (Fig. 2C). In the presence of si-control, obestatin-activated Akt(Ser-473) and p38(Tyr-182) phosphorylation was identical to levels observed with untransfected cells. Silencing of GPR39 decreased subsequent pAkt(Ser-473) and pp38(Tyr-182) with respect to si-control by 61 ± 2 and 52 ± 1% with treatment of obestatin (5 nm, 10 min; Fig. 2C), respectively. Second, the effect of acute GPR39 deficiency on myogenesis was further evaluated on myogenic differentiation. Under these conditions, the constructs decreased GPR39 expression by 50 ± 3% (Fig. 2D) during a 96-h differentiation period. The GPR39 knockdown decreased the expression levels of myogenin and MHC by 40 ± 5 and 41 ± 4% with respect to si-control, respectively (Fig. 2D).
Obestatin Enhances Myogenic Differentiation
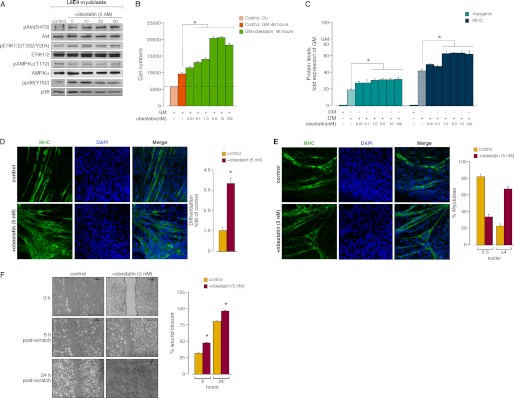
To ascertain the influence of obestatin on myogenesis, the effect of obestatin (0.001–100 nm) on intracellular targets leading to either proliferation (ERK1/2) or differentiation (p38 and Akt) was first assayed in L6E9 myoblasts. pAkt(Ser-473), pp38(Tyr-182), and pERK1/2(Thr-202/Tyr-204) were observed using different concentrations of obestatin (0.001–100 nm), and the level of phosphorylation peaked at 5 nm in L6E9 myoblasts (data not shown). Fig. 3A shows that myoblast treatment with obestatin (5 nm) elicited a time-dependent increase in pAkt(Ser-473), pERK1/2(Thr-202/Tyr-204), and pp38(Tyr-182) protein levels, which reached a maximum at 10 min. Mitogenic action was then explored by dose-effect experiments performed on myoblasts treated with a range of obestatin concentrations (0.01–100 nm) in GM (proliferating conditions; Fig. 3B). Maximal effect in the cell number was observed at 5 nm obestatin (∼2.2-fold). This mitogenic effect was compared with that induced by ghrelin, under the same range of concentrations (0.01–100 nm) in GM. As shown in supplemental Fig. 1S, A, ghrelin showed proliferative capability in L6E9 myoblast cells, although its effect was lower than that of obestatin for all doses tested.
FIGURE 3.
A, time course of the effect of obestatin (5 nm) in L6E9 myoblast cells on pAkt(Ser-473), pERK1/2(Thr-202/Tyr-204), pAMPK(Thr-172), and pp38(Tyr-182) (n = 3). AMPK, AMP-activated protein kinase. B, dose-response effect of obestatin (0.01–100 nm) on L6E9 myoblast cell proliferation (48 h, n = 5). Co: cells initially seeded. Control: cells maintained 48 h in GM. C, dose-response effect of obestatin (0.01–100 nm) on differentiating L6E9 cells for 6 days (n = 6). Levels of myogenin and MHC were represented as a -fold of respective expression in GM. D, left panel, immunofluorescence detection of MHC and DAPI (objective magnification 20×) in L6E9 myotube cells under DM (control) or DM + obestatin (5 nm) at the 6-day point after stimulation. Right panel, the differentiation grade was evaluated based on the number of MHC-positive cells above total nuclei and expressed as a -fold of control. E, representative images used to determined the number of nuclei within individual myotubes (at least two nuclei) in L6E9 myotube cells under DM (control) or DM + obestatin (5 nm) at the 6-day point after stimulation (left panel, objective magnification 20×). Myotubes were grouped into two categories, and the percentage of myotubes in each category was determined (right panel). F, left panel, L6E9 myoblast cells at 100% confluence were wounded with a sterile pipette tip to remove cells. Photographs were taken (objective magnification 10×) at 0, 8, and 24 h after injury. Right panel, wound closure was evaluated using the equation described under “Experimental Procedures.” Immunoblots in A and C are representative of three and six independent experiments, respectively. For these panels, data were expressed as the mean ± S.E. obtained from intensity scans of independent experiments. For panels D and E, data were expressed as the mean ± S.E. as described under “Experimental Procedures.” *, p < 0.05 versus control values.
To assess the activity of obestatin as a promoter of the differentiation process, L6E9 cells were switched to DM supplemented with obestatin at a range of concentrations (0.001–100 nm) for 6 days. As shown in Fig. 3C, the protein levels of myogenin and MHC, as detected by immunoblot, were dose-dependent and up-regulated with the maximal effect at 5 nm for both differentiation markers (∼1.7- and 1.6-fold, respectively). The possibility that obestatin accelerates differentiation was also investigated (DM + 5 nm obestatin for 6 days). Immunoblot analyses revealed normal kinetics with enhanced protein level of both myogenin and MHC when compared with control cells (DM for 6 days; supplemental Fig. 1S, B). These results were confirmed by quantifying the differentiation grade by immunofluorescence. After 6 days, obestatin-treated cells (DM + 5 nm obestatin) increased by ∼250% in myotube number when compared with control cells (DM; Fig. 3D). Moreover, myotubes were grouped into two categories, those with 2–3 nuclei and those with 4 or more nuclei (36). As shown in Fig. 3E, ∼22% of control myotubes contained ≥4 nuclei, versus ∼67% of obestatin-treated cells (DM + 5 nm obestatin). This effect represents an increase of ∼3.4-fold respect to control cells (DM). Next, we performed invasion/migration assays on L6E9 myoblast cells supplemented with obestatin (5 nm). Confluent monolayers of cells were physically wounded with a scratch and allowed to migrate to heal for 8 and 24 h, eliminating the possibility of proliferation accounting for the wound closure. As shown in Fig. 3D, at 8 h after initial scratch, obestatin-treated cells exhibited an ∼53% increase in the invasion/migration capability when compared with the control. At 24 h after scratch, the increase was ∼21% for obestatin-treated cells when compared with control.
To confirm the observation that obestatin activates the differentiation program, the ability of this peptide to activate the differentiation program under GM (GM + 5 nm obestatin) was evaluated in L6E9 myoblast cells for 6 days. The protein levels of myogenin and MHC, as detected by immunoblot, were significantly increased when compared with control cells in GM (∼4.2- and 14-fold, respectively; supplemental Fig. 1S, C). The expression for both differentiation markers was induced to similar levels upon 6 days of treatment with ghrelin (5 nm; supplemental Fig. 1S, C).
Obestatin Regulates Myogenic Markers in Vivo
Based on the short half-life of this peptide (∼22 min), osmotic minipumps were selected for in vivo obestatin administration (41). Male rats received a 72-h continuous subcutaneous infusion of obestatin (300 nmol/kg of body weight/24 h) (30), and dissected gastrocnemius and soleus tissues were processed for immunoblot analysis. Levels of Pax-7, Myf5, MyoD, myogenin, Myf6, and MHC were up-regulated in gastrocnemius and soleus muscle tissues from obestatin-treated rats when compared with control tissues (Fig. 4A). After 72 h of infusion, a significant increase in myofiber diameters was observed in gastrocnemius muscle (Fig. 4A, right panel). A minor effect was observed in the soleus myofiber area (Fig. 4A, right panel). Moreover, obestatin treatment resulted in a significant up-regulation of the VEGF and VEGF-R2 expression in both gastrocnemius and soleus muscle when compared with controls (Fig. 4B). Conversely, the PEDF expression level was significantly down-regulated in gastrocnemius and soleus muscle under obestatin treatment (Fig. 4B).
FIGURE 4.
Effect of 72-h continuous subcutaneous infusion of obestatin (300 nmol/kg of body weight/24 h; n = 10) on myogenic markers (MyoD, Myf5, Pax-7, myogenin, Myf6, and MHC) and myofiber cross-section area (A) and VEGF, VEGFR2, and PEDF expression (B). Protein levels were expressed as a -fold of control obtained from a 72-h minipump subcutaneously implanted in saline group (n = 10 per group). Immunoblots in A and B are representative of mean values from each rat group. In A, hematoxylin/eosin-stained gastrocnemius (right, upper panel) and soleus (right, lower panel) sections are representative of the mean value of total cross-section area. Data were expressed as the mean ± S.E. *, p < 0.05 versus control values.
DISCUSSION
In the present study, we have shown that healthy skeletal muscle expresses obestatin and GPR39, and this expression strikingly increased upon muscle injury. In vitro, obestatin was preferentially expressed by L6E9 myotubes, whereas GPR39 was equally expressed in both myoblast and myotube cells. Expression of both obestatin and GPR39 was coordinately up-regulated during the early stages of myogenesis, and their levels remained sustained throughout terminal differentiation of L6E9 cells. Functionally, blocking obestatin during myogenesis induced a decrease in myogenic-associated markers (myogenin, MHC). Silencing of preproghrelin or GPR39 expression produced similar results. Obestatin stimulation of undifferentiated L6E9 cells promoted mitogenic activity and migration function of myoblasts. Furthermore, this peptide promoted myogenic differentiation, increasing the expression of myogenic markers, without changes to the kinetics of differentiation. Additionally, these results were supported by in vivo testing of the gastrocnemius and soleus muscle obtained from male rats under continuous subcutaneous infusion of obestatin. The testing of this peptide showed an up-regulation of a set of transcription factors regulating myogenesis: (i) markers associated with satellite cell activation: Pax-7 and Myf5; (ii) markers associated with early differentiation: MyoD, myogenin, and Myf6; and (iii) a marker associated with late differentiation: MHC. Altogether, these observations demonstrated that the obestatin/GPR39 system is involved in myogenesis and that obestatin is expressed by differentiating myogenic precursors to function in an autocrine manner.
After injury, satellite cells are activated and enter the cell cycle to proliferate, and eventually, they fuse to replace degenerated muscle fibers, preserving muscle structure and function. Several signals, derived both from damaged fibers and from infiltrating cells, are involved in satellite cell activation (1, 42). From the data presented thus far, obestatin should be incorporated into the list of myogenic regulatory factors derived from damaged fibers. Based on its overexpression in muscle injury, its up-regulation during myogenesis, and its promoter role on myogenic differentiation and fusion of L6E9 cells, it is possible to speculate that obestatin exerts an autocrine effect on the skeletal myogenic process. The fact that preproghrelin expression increased in the course of differentiation and remained sustained throughout terminal differentiation of L6E9 cells supports this hypothesis. Furthermore, preproghrelin knockdown experiments uncovered its contribution to myogenesis, although it would implicate both ghrelin and obestatin. The myogenic role of obestatin is supported by neutralization of this peptide with a specific antibody in L6E9 cells, which reduced its myogenic potential through myogenin and MHC down-regulation. Further support for a myogenic role for obestatin is demonstrated by the effect on the activation and proliferation of myoblasts, with the resulting growth arrest by contact and initiation of myogenesis, e.g. myogenesis activation of proliferating myoblasts in GM. The increased expression of myogenic regulator factors, Pax-7, Myf5, MyoD, myogenin, Myf6, and MHC (23), associated with subcutaneous infusion of obestatin in rats reinforces this suggestion. Considering the myogenic characteristics of ghrelin and des-acyl ghrelin previously described (44), we hypothesized that preproghrelin-derived peptides mutually contribute to myogenesis as pro-myogenic factors, as corroborated by the mitogenic and myogenic roles for ghrelin and obestatin described in the present work. Although the myogenic characteristics of ghrelin found in this study are in agreement with previous works (44), the mitogenic action of ghrelin appears to be in contradiction with a previous study showing inhibition of C2C12 myoblast cells (44). Even if the role of ghrelin and des-acyl ghrelin in mitogenesis remains unclear and varies depending on the nature of the study, the myogenic properties of preproghrelin-derived peptides reinforce our hypothesis.
Obestatin has multiple functional properties, and its capacity to stimulate myoblast proliferation, migration, and differentiation in vitro is particularly interesting. In L6E9 myoblasts, obestatin was shown to enhance ERK, Akt, and p38 activities. These pathways are activated by regeneration signals and demonstrate the ability of muscle progenitors to execute different stages of the regeneration program: (a) the ERK/MAP pathway activates cell proliferation and migration (45, 46); (b) the Akt pathway promotes cell proliferation, survival, and differentiation (47); and (c) the p38 pathway stimulates cell cycle arrest and terminal differentiation (48). The convergence of these pathways at the chromatin level defines the mechanism for integration of mitogenic and myogenic signals to direct the transition from quiescence to terminal differentiation (49). Thus, the range of obestatin functions, together with its expression in normal and regenerating skeletal muscle, is consistent with a role in regulating myofiber formation and regeneration. In this regard, obestatin shows functional similarity with the insulin-like growth factors (IGF-I and IGF-II) because both factors have the capacity to promote both proliferation and differentiation of muscle cells (45, 50).
Silencing of GPR39 expression in L6E9 myoblast cells demonstrated that this G protein-coupled receptor is the extracellular target for obestatin action. Although there is ongoing controversy regarding GPR39 (51, 52), our findings are consistent with the role of this receptor on obestatin signaling in preadipocyte 3T3-L1 cells (29, 30) and in gastrointestinal and adipose tissues (32). The expression of GPR39 in L6E9 cells and its changes during myogenesis further suggest a role for GPR39 in this process. Definitive evidence for the requirement of GPR39 in myogenesis came from the observation that its knockdown by siRNA significantly diminished the myogenic capacity of L6E9 cells. Furthermore, the preproghrelin expression up-regulation and, as a consequence, the increase of obestatin biosynthesis and secretion by myotubes, together with its faint expression in myoblasts, lead us to speculate that obestatin might exert a paracrine role on myoblasts via a GPR39-dependent mechanism. Similarly, the obestatin/GPR39 system exerts an autocrine/paracrine control on adipogenesis (30). Combined, these all point to the role of obestatin/GPR39 signaling as a key component for proper skeletal and adipose tissue development. In fact, our recent results underscored a key role for preproghrelin-derived peptides on adipogenesis through an autocrine/paracrine mechanism (29).
Male rats under continuous subcutaneous infusion of obestatin showed a modified expression of transcription factors that regulate the progression through the myogenic lineage in soleus and gastrocnemius muscle tissue. Up-regulation of Pax-7 expression might be indicative of a control on satellite cell activation to enhance myogenic specification and/or to induce self-renewal (4, 53, 54). It is known that this transcription factor is maintained in satellite cells and proliferating myoblasts but is down-regulated before differentiation (53–56). Furthermore, the expression of myogenic regulatory factors is coordinated by this factor. Indeed, up-regulation of MyoD and Myf5 was observed in obestatin-treated rats, denoting the conversion of satellite cells into myoblast (4), although it is not possible to rule out a role for this peptide in the replenishment of the satellite cell pool. MyoD and Myf5 are required for the expression of myogenic precursors and act upstream of Myf6 and myogenin. Accordingly, Myf6 and myogenin were up-regulated, denoting myoblast differentiation (2). Moreover, MHC was observed to be up-regulated, as an indication of myogenic differentiation. Additionally, obestatin was shown to increase the expression of VEGF and its receptor isoform VEGFR2 in this tissue. Conversely, PEDF expression was down-regulated. The expression and role of VEGF as an autocrine myogenic factor in muscle was previously described (57). Furthermore, VEGFR2 was shown to be up-regulated during myogenic differentiation (57). In addition to its myogenic action, it is important to note its known angiogenic role (58). This latter effect is important in attracting distant vessels to the myoblast differentiation sites. This implies that VEGF, produced by differentiating myocytes, provides the needed vascularization of developing skeletal muscle. This is further supported by down-regulation of the anti-angiogenic factor PEDF (43). All together, our data support the view that obestatin regulates the “hierarchy” of transcription factors involved in the control of the different stages of the myogenic program, including activation, proliferation, and differentiation, exerting a role in the expression of VEGF/VEGFR2 for the potential regulation of the angiogenic process in skeletal muscle tissue.
In conclusion, we propose that the obestatin/GPR39 system signaling is involved in muscle regeneration, in which obestatin exerts an autocrine function to control the myogenic differentiation program, thereby implicating its potential for use as a therapeutic agent for the treatment of trauma-induced muscle injuries or skeletal muscle myopathies.
Supplementary Material
This work was supported in part by grants from Instituto de Salud Carlos III (ISCIII; Ministerio de Economía y Competitividad, Spain; Grant PS09/02202), Ministerio de Economía y Competitividad (Grant SAF2010-20451), Xunta de Galicia (Grant INCITE09918374PR), and Fundación Mutua Madrileña.

This article contains supplemental Fig. 1S.
- IGF
- insulin-like growth factor
- IGF1R
- IGF 1 receptor
- MRF
- muscle regulatory factor family
- CTX
- cardiotoxin
- PEDF
- pigment epithelium-derived factor
- GM
- growth medium
- DM
- differentiation medium
- Ab
- antibody
- p
- phosphorylated.
REFERENCES
- 1. Chargé S. B., Rudnicki M. A. (2004) Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84, 209–238 [DOI] [PubMed] [Google Scholar]
- 2. Tedesco F. S., Dellavalle A., Diaz-Manera J., Messina G., Cossu G. (2010) Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J. Clin. Invest. 120, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuang S., Gillespie M. A., Rudnicki M. A. (2008) Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell 2, 22–31 [DOI] [PubMed] [Google Scholar]
- 4. Bentzinger C. F., Wang Y. X., Rudnicki M. A. (2012) Building muscle: molecular regulation of myogenesis Cold Spring Harb. Perspect. Biol. 4, pii: a008342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DiMario J., Buffinger N., Yamada S., Strohman R. C. (1989) Fibroblast growth factor in the extracellular matrix of dystrophic (mdx) mouse muscle. Science 244, 688–690 [DOI] [PubMed] [Google Scholar]
- 6. Tatsumi R., Anderson J. E., Nevoret C. J., Halevy O., Allen R. E. (1998) HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev. Biol. 194, 114–128 [DOI] [PubMed] [Google Scholar]
- 7. Golding J. P., Calderbank E., Partridge T. A., Beauchamp J. R. (2007) Skeletal muscle stem cells express anti-apoptotic ErbB receptors during activation from quiescence. Exp. Cell Res. 313, 341–356 [DOI] [PubMed] [Google Scholar]
- 8. Machida S., Booth F. W. (2004) Insulin-like growth factor 1 and muscle growth: implication for satellite cell proliferation. Proc. Nutr. Soc. 63, 337–340 [DOI] [PubMed] [Google Scholar]
- 9. McCroskery S., Thomas M., Maxwell L., Sharma M., Kambadur R. (2003) Myostatin negatively regulates satellite cell activation and self-renewal. J. Cell Biol. 162, 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brack A. S., Conboy M. J., Roy S., Lee M., Kuo C. J., Keller C., Rando T. A. (2007) Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 [DOI] [PubMed] [Google Scholar]
- 11. Polesskaya A., Seale P., Rudnicki M. A. (2003) Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell 113, 841–852 [DOI] [PubMed] [Google Scholar]
- 12. Mousavi K., Jasmin B. J. (2006) BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J. Neurosci. 26, 5739–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fukada S., Uezumi A., Ikemoto M., Masuda S., Segawa M., Tanimura N., Yamamoto H., Miyagoe-Suzuki Y., Takeda S. (2007) Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 25, 2448–2459 [DOI] [PubMed] [Google Scholar]
- 14. Ratajczak M. Z., Majka M., Kucia M., Drukala J., Pietrzkowski Z., Peiper S., Janowska-Wieczorek A. (2003) Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells 21, 363–371 [DOI] [PubMed] [Google Scholar]
- 15. Girgenrath M., Weng S., Kostek C. A., Browning B., Wang M., Brown S. A., Winkles J. A., Michaelson J. S., Allaire N., Schneider P., Scott M. L., Hsu Y. M., Yagita H., Flavell R. A., Miller J. B., Burkly L. C., Zheng T. S. (2006) TWEAK, via its receptor Fn14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration. EMBO J. 25, 5826–5839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conboy I. M., Conboy M. J., Smythe G. M., Rando T. A. (2003) Notch-mediated restoration of regenerative potential to aged muscl. Science 302, 1575–1577 [DOI] [PubMed] [Google Scholar]
- 17. Rosen G. D., Sanes J. R., LaChance R., Cunningham J. M., Roman J., Dean D. C. (1992) Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell 69, 1107–1119 [DOI] [PubMed] [Google Scholar]
- 18. Irintchev A., Zeschnigk M., Starzinski-Powitz A, Wernig A. (1994) Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev. Dyn. 199, 326–337 [DOI] [PubMed] [Google Scholar]
- 19. Germani A., Di Carlo A., Mangoni A., Straino S., Giacinti C., Turrini P., Biglioli P., Capogrossi M. C. (2003) Vascular endothelial growth factor modulates skeletal myoblast function. Am. J. Pathol. 163, 1417–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sejersen T., Betsholtz C., Sjölund M., Heldin C. H., Westermark B., Thyberg J. (1986) Rat skeletal myoblasts and arterial smooth muscle cells express the gene for the A chain but not the gene for the B chain (c-sis) of platelet-derived growth factor (PDGF) and produce a PDGF-like protein. Proc. Natl. Acad. Sci. U.S.A. 83, 6844–6848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cantini M., Massimino M. L., Rapizzi E., Rossini K., Catani C., Dalla Libera L., Carraro U. (1995) Human satellite cell proliferation in vitro is regulated by autocrine secretion of IL-6 stimulated by a soluble factor(s) released by activated monocytes. Biochem. Biophys. Res. Commun. 216, 49–53 [DOI] [PubMed] [Google Scholar]
- 22. Abou-Khalil R., Le Grand F., Pallafacchina G., Valable S., Authier F. J., Rudnicki M. A., Gherardi R. K., Germain S., Chretien F., Sotiropoulos A., Lafuste P., Montarras D., Chazaud B. (2009) Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell 5, 298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Braun T., Gautel M. (2011) Transcriptional mechanisms regulating skeletal muscle differentiation, growth, and homeostasis. Nat. Rev. Mol. Cell Biol. 12, 349–361 [DOI] [PubMed] [Google Scholar]
- 24. Clemmons D. R. (2009) Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol. Metab. 20, 349–356 [DOI] [PubMed] [Google Scholar]
- 25. Rosen E. D., MacDougald O. A. (2006) Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7, 885–896 [DOI] [PubMed] [Google Scholar]
- 26. Sordella R., Jiang W., Chen G. C., Curto M., Settleman J. (2003) Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell 113, 147–158 [DOI] [PubMed] [Google Scholar]
- 27. Shefer G., Wleklinski-Lee M., Yablonka-Reuveni Z. (2004) Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J. Cell Sci. 117, 5393–5404 [DOI] [PubMed] [Google Scholar]
- 28. Park K. W., Halperin D. S., Tontonoz P. (2008) Before they were fat: adipocyte progenitors. Cell Metab. 8, 454–457 [DOI] [PubMed] [Google Scholar]
- 29. Gurriarán-Rodríguez U., Al-Massadi O., Crujeiras A. B., Mosteiro C. S., Amil-Diz M., Beiroa D., Nogueiras R., Seoane L. M., Gallego R., Pazos Y., Casanueva F. F., Camiña J. P. (2011) Preproghrelin expression is a key target for insulin action on adipogenesis. J. Endocrinol. 210, R1–7 [DOI] [PubMed] [Google Scholar]
- 30. Gurriarán-Rodríguez U., Al-Massadi O., Roca-Rivada A., Crujeiras A. B., Gallego R., Pardo M., Seoane L. M., Pazos Y., Casanueva F. F., Camiña J. P. (2011) Obestatin as a regulator of adipocyte metabolism and adipogenesis. J. Cell Mol. Med. 15, 1927–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang J. V., Ren P. G., Avsian-Kretchmer O., Luo C. W., Rauch R., Klein C., Hsueh A. J. (2005) Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science 310, 996–999 [DOI] [PubMed] [Google Scholar]
- 32. Zhang J. V., Jahr H., Luo C. W., Klein C., Van Kolen K., Ver Donck L., De A., Baart E., Li J., Moechars D., Hsueh A. J. (2008) Obestatin induction of early-response gene expression in gastrointestinal and adipose tissues and the mediatory role of G protein-coupled receptor, GPR39. Mol. Endocrinol. 22, 1464–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Granata R., Settanni F., Gallo D., Trovato L., Biancone L., Cantaluppi V., Nano R., Annunziata M., Campiglia P., Arnoletti E., Ghè C., Volante M., Papotti M., Muccioli G., Ghigo E. (2008) Obestatin promotes survival of pancreatic β-cells and human islets and induces expression of genes involved in the regulation of β-cell mass and function. Diabetes 57, 967–979 [DOI] [PubMed] [Google Scholar]
- 34. Pazos Y., Alvarez C. J., Camiña J. P., Casanueva F. F. (2007) Stimulation of extracellular signal-regulated kinases and proliferation in the human gastric cancer cells KATO-III by obestatin. Growth Factors 25, 373–381 [DOI] [PubMed] [Google Scholar]
- 35. Alvarez C. J., Lodeiro M., Theodoropoulou M., Camiña J. P., Casanueva F. F., Pazos Y. (2009) Obestatin stimulates Akt signalling in gastric cancer cells through β-arrestin-mediated epidermal growth factor receptor transactivation. Endocr. Relat. Cancer 16, 599–611 [DOI] [PubMed] [Google Scholar]
- 36. Horsley V., Friday B. B., Matteson S., Kegley K. M., Gephart J., Pavlath G. K. (2001) Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J. Cell Biol. 153, 329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goetsch K. P., Niesler C. U. (2011) Optimization of the scratch assay for in vitro skeletal muscle wound healing analysis. Anal. Biochem. 411, 158–160 [DOI] [PubMed] [Google Scholar]
- 38. Toschi A., Severi A., Coletti D., Catizone A., Musarò A., Molinaro M., Nervi C., Adamo S., Scicchitano B. M. (2011) Skeletal muscle regeneration in mice is stimulated by local overexpression of V1a-vasopressin receptor. Mol. Endocrinol. 25, 1661–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zeng L., Akasaki Y., Sato K., Ouchi N., Izumiya Y., Walsh K. (2010) Insulin-like 6 is induced by muscle injury and functions as a regenerative factor. J. Biol. Chem. 285, 36060–36069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nadal-Ginard B. (1978) Commitment, fusion, and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell 15, 855–864 [DOI] [PubMed] [Google Scholar]
- 41. Zizzari P., Longchamps R., Epelbaum J., Bluet-Pajot M. T. (2007) Obestatin partially affects ghrelin stimulation of food intake and growth hormone secretion in rodents. Endocrinology 148, 1648–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ten Broek R. W., Grefte S., Von den Hoff J. W. (2010) Regulatory factors and cell populations involved in skeletal muscle regeneration. J. Cell. Physiol. 224, 7–16 [DOI] [PubMed] [Google Scholar]
- 43. Ek E. T., Dass C. R., Choong P. F. (2006) PEDF: a potential molecular therapeutic target with multiple anti-cancer activities. Trends Mol. Med. 12, 497–502 [DOI] [PubMed] [Google Scholar]
- 44. Filigheddu N., Gnocchi V. F., Coscia M., Cappelli M., Porporato P. E., Taulli R., Traini S., Baldanzi G., Chianale F., Cutrupi S., Arnoletti E., Ghè C., Fubini A., Surico N., Sinigaglia F., Ponzetto C., Muccioli G., Crepaldi T., Graziani A. (2007) Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Mol. Biol. Cell 18, 986–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mourkioti F., Rosenthal N. (2005) IGF-1, inflammation, and stem cells: interactions during muscle regeneration. Trends Immunol. 26, 535–542 [DOI] [PubMed] [Google Scholar]
- 46. Leloup L., Daury L., Mazères G., Cottin P., Brustis J. J. (2007) Involvement of the ERK/MAP kinase signalling pathway in milli-calpain activation and myogenic cell migration. Int. J. Biochem. Cell Biol. 39, 1177–1189 [DOI] [PubMed] [Google Scholar]
- 47. Wu M., Falasca M., Blough E. R. (2011) Akt/protein kinase B in skeletal muscle physiology and pathology. J. Cell Physiol. 226, 29–36 [DOI] [PubMed] [Google Scholar]
- 48. Lluís F., Perdiguero E., Nebreda A. R., Muñoz-Cánoves P. (2006) Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol. 16, 36–44 [DOI] [PubMed] [Google Scholar]
- 49. Serra C., Palacios D., Mozzetta C., Forcales S. V., Morantte I., Ripani M., Jones D. R., Du K., Jhala U. S., Simone C., Puri P. L. (2007) Functional interdependence at the chromatin level between the MKK6/p38 and IGF1/PI3K/AKT pathways during muscle differentiation. Mol. Cell 28, 200–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matheny R. W., Jr., Nindl B. C., Adamo M. L. (2010) Minireview: Mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration. Endocrinology 151, 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chartrel N., Alvear-Perez R., Leprince J., Iturrioz X., Reaux-Le Goazigo A., Audinot V., Chomarat P., Coge F., Nosjean O., Rodriguez M., Galizzi J. P., Boutin J. A., Vaudry H., Llorens-Cortes C. (2007) Comment on “Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake”. Science 315, 766. [DOI] [PubMed] [Google Scholar]
- 52. Holst B., Egerod K. L., Schild E., Vickers S. P., Cheetham S., Gerlach L. O., Storjohann L., Stidsen C. E., Jones R., Beck-Sickinger A. G., Schwartz T. W. (2007) GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology 148, 13–20 [DOI] [PubMed] [Google Scholar]
- 53. Wang Y. X., Rudnicki M. A. (2012) Satellite cells, the engines of muscle repair. Nat. Rev. Mol. Cell Biol. 13, 127–133 [DOI] [PubMed] [Google Scholar]
- 54. Olguin H. C., Olwin B. B. (2004) Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev. Biol. 275, 375–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hu P., Geles K. G., Paik J. H., DePinho R. A., Tjian R. (2008) Codependent activators direct myoblast-specific MyoD transcription. Dev. Cell 15, 534–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McKinnell I. W., Ishibashi J., Le Grand F., Punch V. G., Addicks G. C., Greenblatt J. F., Dilworth F. J., Rudnicki M. A. (2008) Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat. Cell Biol. 10, 77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bryan B. A., Walshe T. E., Mitchell D. C., Havumaki J. S., Saint-Geniez M., Maharaj A. S., Maldonado A. E., D'Amore P. A. (2008) Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. Mol. Biol. Cell 19, 994–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patel-Hett S., D'Amore P. A. (2011) Signal transduction in vasculogenesis and developmental angiogenesis. Int. J. Dev. Biol. 55, 353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.