Abstract
Aggregation of expanded polyglutamine (polyQ) repeat containing fragments of the huntingtin (htt) protein may play a key role in Huntington’s disease (HD). Consistent with this hypothesis, two Ser to Asp mutations in the 17 amino acid N-terminal httNT segment abrogate both visible brain aggregates and disease symptoms in a full length Q97 htt mouse model while compromising aggregation kinetics and aggregate morphology in a htt fragment in vitro (Gu et al., Neuron 64, 828–840 (2009)). The httNT segment has been shown to play a critical role in facilitating nucleation of amyloid formation in htt N-terminal exon1 fragments. We show here how these Ser to Asp mutations dramatically affect aggregation kinetics and aggregate structural integrity. First, these negatively charged Ser replacements impair the assembly of the α-helical oligomers that play a critical role in htt amyloid nucleation, thus providing an explanation for reduced amyloid formation rates. Second, these sequence modifications alter aggregate morphology, decrease aggregate stability, and enhance the steric accessibility of the httNT segment within the aggregates. Together these changes make the sequence-modified peptides kinetically and thermodynamically less likely to aggregate and more susceptible, if they do, to post-translational modifications and degradation. These effects also show how phosphorylation of a protein might achieve cellular effects via direct impacts on the protein’s aggregation properties. In fact, preliminary studies on exon1-like molecules containing phosphoryl-Ser residues at positions 13 and 16 show they reduce aggregation rates and generate atypical aggregate morphologies similar to the effects of the Ser to Asp mutants.
Keywords: α-helical oligomers, nucleation, critical concentration, post-translational modification, phosphomimetic
Huntington’s disease 1, 2 is one of ten 3, 4 CAG repeat diseases characterized by the expansion of a polyglutamine (polyQ) stretch in a particular protein. In HD, this polyQ stretch begins at amino acid 18 of the ~3200 amino acid long huntingtin (htt) protein. A hallmark feature of this disease is the presence of ubiquitinylated, aggregated forms of htt fragments in neurons of disease victims and in animal and cell models 5, 6. Additional evidence of a disease role for protein aggregation and/or misfolding comes from observations that disease risk 3 and in vitro aggregation rates 7, 8 both increase with polyQ repeat expansion, as well as from the temporal association of aggregate formation and disease symptoms in some model organisms 1. The precise identities and immediate cellular targets of these implicated toxic, aberrantly folded forms of htt have not been identified.
A dramatic example of the association of brain aggregates with HD symptoms was described by Yang and co-workers 9. This group previously constructed a tg mouse containing a Q97 form of full length htt that exhibited accumulation of cortical and striatal aggregates and HD-like neurological deficits 10. In subsequent work, an identical Q97 construct containing phosphomimetic Ser->Asp point mutations at positions 13 and 16 exhibited no disease symptoms and no accumulated visible aggregates 9. Although these effects might be accounted for via possible cell targeting functions of the N-terminal httNT sequence 11–15 (Fig. 1), we speculated 9 that these Ser to Asp mutations could also alter the physical properties of cellular htt fragments, especially in the context of misfolding and aggregation. Consistent with this, we showed in that paper that the same mutations in a polyQ-containing htt fragment model peptide slow in vitro aggregation rates and yield significantly different aggregate morphologies 9. How the conversion of these two Ser residues to Asp might influence the folding and aggregation properties of such htt fragments, and how these changes might translate into the observed loss of aggregate accumulation in the mouse model, is the main subject of this report.
Figure 1.
Amino acid sequences of the peptides studied.
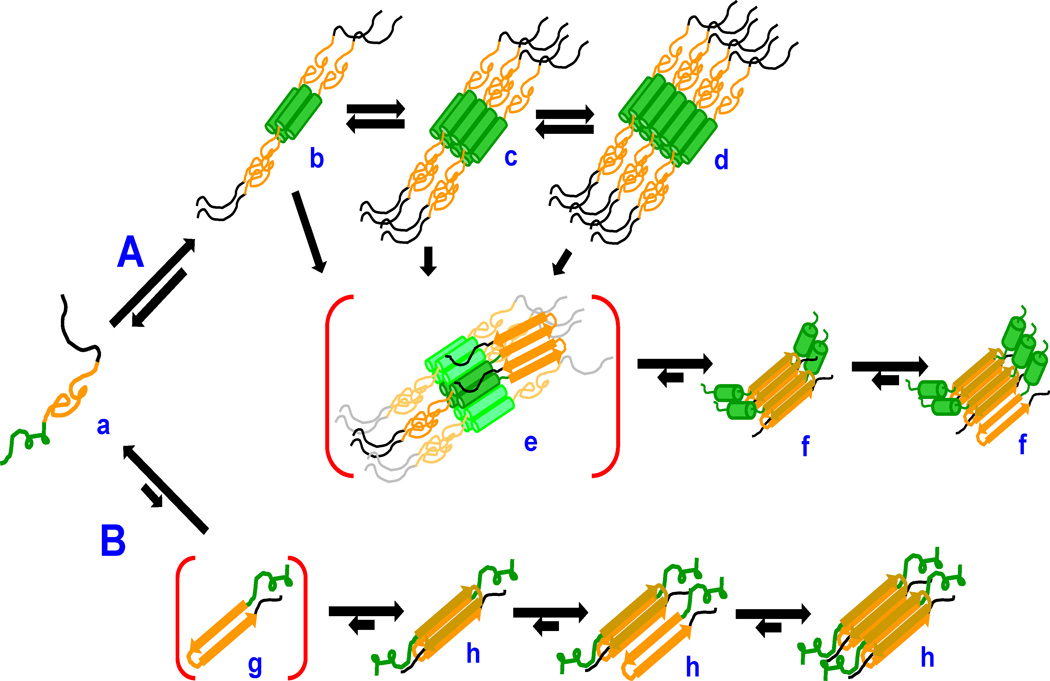
The nucleation mechanisms of polyQ amyloid formation have been analyzed in some detail, both in isolation 16, 17 and in the context of the sequences flanking polyQ in the htt protein 18–22, and both mechanisms are operative in polyQ-containing fragments of htt 23 (Fig. 2). Under most circumstances, the mechanism found in simple expanded polyQ repeat peptides, in which rare amyloid core nuclei are formed directly from within the monomer pool (Fig. 2B), is largely outcompeted in htt fragment aggregation by an alternative mechanism featuring the key involvement of the httNT sequence. In this much more efficient mechanism (Fig. 2A), httNT segments self-associate into α-helix rich tetramers and higher oligomers, which serve to locally concentrate and/or align polyQ segments and facilitate the formation of amyloid nuclei. Interference with the formation of α-helical oligomers suppresses the normally dominant A pathway, allowing enhanced contributions from the B pathway 23.
Figure 2.
Schematic model of htt fragment aggregation mechanisms. PolyQ-containing htt fragments (a; httNT, green; polyQ, orange; Pro-rich, black) can undergo nucleation of polyQ amyloid formation in two ways. In path A, httNT segments undergo reversible formation of α-helical tetramers (b) and higher oligomers (c,d) whose disordered polyQ elements can interact to nucleate amyloid structure (e) and grow by monomer addition (f). In path B, the polyQ segment of monomeric htt fragments (a) can directly undergo reversible assembly into a critical nucleus (g) followed by elongation into amyloid (h). The conformation of httNT within the B pathway aggregates has not been determined.
In this paper we show that these Ser to Asp mutations within httNT influence aggregation rates, not by abrogating the α-helical oligomer pathway, but by decreasing its efficiency. We show that, in spite of ssNMR data showing aggregate secondary structures similar to fragments with WT httNT, Ser-modified htt fragment aggregates exhibit substantial differences from WT in EM morphologies, in decreased stabilities toward dissociation, and in increased susceptibility of their httNT segments to trypsin cleavage. These differences suggest several possible explanations for the dramatic phenotypes of the S13D/S16D tg mice. We also show that authentic phosphoryl-serine residues at positions 13 and 16 of polyQ-containing htt fragments exhibit aggregation properties similar to those of the corresponding Ser->Asp mutants, suggesting that this commonly used phosphomimetic mutation faithfully captures the aggregation-modifying effects of httNT phosphorylation.
RESULTS
Phosphorylation of httNT serine residues impairs aggregation
To assess the importance of the negative charges introduced in the Ser to Asp mutations, as well as the ability of these mutations to mimic the effects of Ser phosphorylation, we obtained httNTQ37P10K2 (F17W) peptides with one or both of the Ser residues at positions 13 and 16 phosphorylated (Fig. 1) and assessed their effects on overall aggregation kinetics (Fig. 3a). We found that, like the S13D/S16D mutant (●), the doubly phosphorylated S13pS/S16pS peptide (●) exhibits substantially reduced aggregation compared to the WT peptide (●), with a greater rate suppression effect than that of the S13D/S16D mutant (Fig. 3a). Furthermore, overall aggregation rates of the single phosphoryl-Ser peptides S13pS (○) and S16pS (○) are also slower than the WT rate (●) and similar to S13D/S16D rate (●). Phosphorylation of these Ser residues also had effects similar to that of the S13D/S16D mutations 9 on aggregate morphologies in the EM. Like the S13D/S16D mutant peptides (Fig. 3 d–g), the intermediate and final aggregates of the double (Fig. 3, h–j) and single (Fig. 3, k–n) phosphoryl-Ser peptides resemble the protofibrillar and oligomeric intermediates observed on incubation of polyQ-containing htt fragments with WT httNT (Fig. 3c), and do not resemble mature fibrils (Fig. 3b). The results show that the Ser->Asp mutations are, in fact, good mimics of the disrupting effects of Ser phosphorylation on the ability of the httNT sequence to stimulate polyQ aggregation, producing reduced aggregation kinetics and final morphologies that resemble those normally seen for reaction intermediates. This emphasizes the role of the introduced negative charges on these modified properties.
Figure 3.
Aggregation of phosphoryl-Ser derivatives of httNTQ37P10K2 peptides. (a) Sedimentation analysis of httNTQ37P10K2 peptides in PBSA at 37°C by sedimentation analysis. WT (F17W) (●, 19 µM); S13D/S16D (●, 34 µM); S13p( ○, 21 µM); S16p (○, 20 µM); S13pS/S16pS (●, 19.5 µM). (b–r) Electron micrographs of the aggregates of different httNTQ37P10K2 peptides isolated at different time-points. Aggregates of: WT (F17W) at 47 hrs (b) and after 1 hr (c); S13D/S16D after 100 hrs (d–g); S13pS/S16pS after 163 hrs (hd–j); S13p (kd–l) and S16p (md–n), both at 33 hrs; S13D/S16D aggregates at 1.5 hrs (od–p) and S13pS/S16pS aggregates at 2 hrs (qd–r). Scale bar = 50 nm.
Serine modifications affect oligomerization rates but not monomer conformations
One hypothesis for the molecular basis of HD posits that polyQ expansion leads to an alternative folded state within monomers that is responsible for pathology 22. To probe for effects of httNT serine modifications on the conformations of polyQ-containing htt fragments, we conducted solution spectroscopy experiments, working at low concentrations that disfavor helical bundle association and oligomerization 20 (Fig. 4a). We found no significant differences in the CD spectra of freshly disaggregated (Methods) solutions of the WT (––––), S13D/S16D (––––) and S13pS/S16pS (––––) versions of httNTQ37P10K2 (Fig. 4a). Furthermore, using FRET probes at positions -1 and 17 (Fig. 1) in the httNTQ37P10K2 background, at 30 µM in PBS at pH 7.4, we found no difference in the estimates of average -1 to 17 distance for the WT (31.9 ± 0.1 Å) and S13D/S16D (31.8 ± 0.3 Å) peptides. Thus, there is no indication from these low resolution methods that these Ser modifications within httNT influence monomer conformation.
Figure 4.
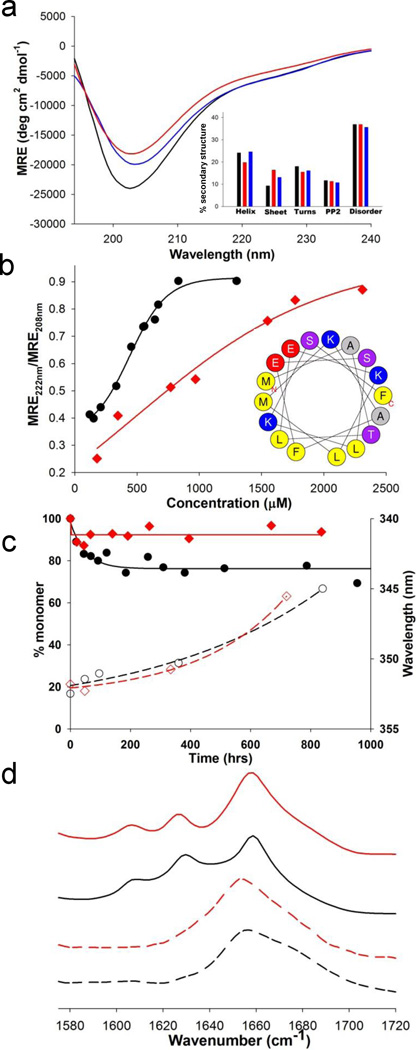
Structural consequences of Ser modification during aggregation. (a) CD spectra of httNTQ37P10K2 peptides (WT (black), S13D/S16D (red) and S13pS/S16pS (blue)) at ~ 35 µM in 10 mM Tris.HCl, pH 7.4; inset, secondary structure composition estimates from the CD curves (Methods). (b) Concentration dependence of α-helix structure in isolated httNT peptides of WT (●) and S13D/S16D (♦) sequence; inset, helical wheel analysis of httNT, with alanine residues in gray, hydrophobic amino acids in yellow, acidic residues in red, basic residues in blue, and neutral hydrophilic residues in violet. (c) Aggregation kinetics of httNTQ8K2 peptides by sedimentation (WT (●), S13D/S16D (♦)) and by Trp fluorescence of isolated aggregates (WT (○), S13D/S16D (◊)). (d) FTIR spectra of the aggregates of the httNTQ8 peptides isolated at different times: WT at 48hrs (-----) and 840 hrs (––––); S13D/S16D at 48hrs (----) and 720 hrs (––––).
In contrast, we found that negatively charged residues at positions 13 and 16 impart a thermodynamic barrier onto the first step in the amyloid formation mechanism (Fig. 2A), oligomer formation. While the WT httNT sequence (●) undergoes concentration dependent, reversible assembly into α-helix rich oligomers with a mid-point of about 0.5 mM, the S13D/S16D mutant (♦) exhibits a much less cooperative assembly curve with a midpoint of about 0.9 mM (Fig. 4b). Furthermore, in peptides of the sequence httNTQ8K2 (Fig. 1), a background that supports very slow httNT-mediated nucleation of polyQ amyloid formation 20, the S13D/S16D mutation (♦) greatly diminishes the time-dependent formation of sedimentable oligomeric intermediates compared to WT httNT (●) (Fig. 4c).
At first glance, the abilities of negatively charged groups at positions 13 and 16 to reduce the formation of httNT α-helix-rich oligomers is somewhat puzzling. The helical wheel diagram of httNT 12, 13 (Fig. 4b inset) shows a strong amphipathic nature, and recent results suggest that the hydrophobic face of this helix plays a major role in oligomer stabilization (R. Mishra and R. Wetzel, Ms. in preparation). However, Ser13 and Ser16 are on the hydrophilic face of the α-helix, which is predicted to be solvent exposed and therefore not expected to play a major role in oligomer formation. One possible explanation of the surprising ability of these mutations to suppress α-helical oligomer formation is that this hydrophilic face may be involved in packing of α-helical tetramers into higher order oligomers that might be required for stabilizing oligomers and ultimately for amyloid nucleation. Alternatively, charge repulsion between positions 13 and 16 in the neighboring turns of the helix might destabilize α-helix formation and hence affect overall oligomer stability.
For both WT and S13D/S16D httNTQ8K2 sequences, the oligomers formed are α-helix-rich by FTIR (Fig. 4d). In spite of the much less favorable and slower formation of α-helix rich aggregates, the S13D/S16D httNTQ8K2 peptide is able to undergo nucleation of β-rich, amyloid-like structure with similar kinetics to WT httNT. This is shown by the previously described 19, 20 transition, within the aggregate pool, of the Trp at position 17 from a solvent exposed (λemm ~351–353nm) to a solvent-excluded (λemm ~345nm) environment (Fig. 4c, compare WT (○) to S13D/S16D (◊)), and in development of FTIR bands, including β-sheet (1625–1630 cm−1), characteristic of polyQ amyloid structure (Fig. 4d, compare WT (––––) to S13D/S16D (––––)).
Serine modifications and polyQ amyloid nucleation and elongation
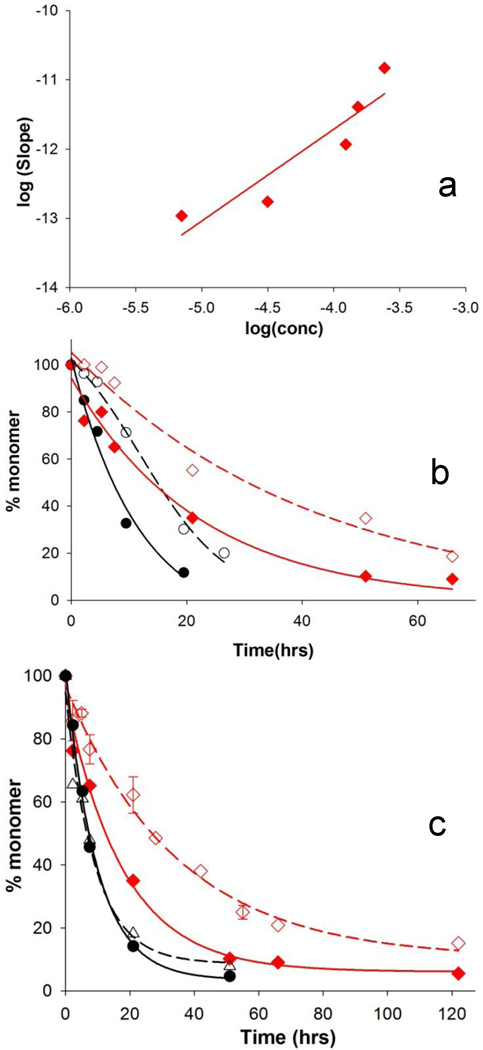
Collection of time dependent spectroscopic changes, as described above, is a useful approach for gauging aggregate structure in short polyQ-containing htt fragments, but is problematic for rapidly aggregating, long polyQ versions due to the difficulty in collecting useful amounts of early pre-nucleation aggregates. We therefore used other probes to assess the mechanistic impact of serine modifications in fragments with disease-relevant repeat lengths. The concentration dependence of initial aggregation rate has proved a good gauge of nucleation mechanism in this system. Thus, peptides undergoing nucleation without help from the httNT flanking sequence (i.e., Fig. 2B) typically exhibit log-log slopes in the range of 3 or higher 16–18, 21, 24, 25, while peptides undergoing nucleation facilitated by a flanking httNT sequence (i.e., Fig. 2A) exhibit slopes in the range of ~1 19, 21. We found that the slope of an analogous log-log plot for the S13D/S16D mutant of httNTQ37P10K2 is ~ 1.3 (Fig. 5a), consistent with a nucleation mechanism that is predominantly httNT-mediated (Fig. 2A).
Figure 5.
Aggregation kinetics consequences of S13D/S16D mutation in httNTQ37P10K2. (a) Concentration dependence of initial phase of the aggregation reaction. (b) Inhibition of aggregation by 1:1 ratio of httNTQ3 to peptide. WT alone (●, 50 µM) and with inhibitor (○, 48 µM) ; S13D/S16D alone (♦, 43 µM) and with inhibitor (◊, 68 µM). (c) Inhibition of aggregation by ~ 5-fold excess of elongation inhibitor PGQ9P 1,2,3 K8 compared to peptide. WT alone (●, 22 µM) and with inhibitor (○, 26 µM); S13D/S16D alone (♦, 43 µM) and with inhibitor (◊, 34 µM).
Another useful probe is the response of the aggregation reaction to mechanism-specific aggregation inhibitors. Recently we reported that peptides consisting of httNT with few if any attached Gln residues are able to transiently inhibit httNT mediated amyloid nucleation by co-assembling with polyQ-containing htt fragments into a mixed α-helix-rich oligomer, thereby reducing the local polyQ concentration in the oligomers and hence the amyloid nucleation efficiency 21. We find that, in fact, the S13D/S16D mutant of httNTQ37P10K2 responds to inhibition by an httNTQ3 peptide with a similar retardation of aggregation onset as the WT peptide (Fig. 5b). Thus, consistent with the slope of the log-log plot (Fig. 5a), sensitivity to httNT inhibition indicates a major role for the httNT domain in facilitating the aggregation of the S13D/S16D mutant. We also previously described inhibitors such as PGQ9P1,2,3K8 (Fig. 1). Such peptides inhibit the elongation of amyloid-like aggregates of simple polyQ and, because of the importance of elongation in the nucleation process, also suppress spontaneous amyloid formation by simple polyQ peptides 22, 26. Presumably because the nucleation of httNT containing polyQ peptides is dominated by httNT-mediated oligomer formation, we find that the polyQ amyloid elongation inhibitor PGQ9P1,2,3K8 gives no detectible inhibition of spontaneous aggregation of WT httNTQ37P10K2 (Fig. 5c). Interestingly, however, this inhibitor does inhibit spontaneous aggregation of the S13D/S16D mutant (Fig. 5c). Further studies will be required to elucidate the mechanistic basis of this effect. However, the different responses of the WT and S13D/S16D peptides to the PGQ9P1,2,3K8 inhibitor provides further evidence for the mechanistic impact of the negatively charged residues in httNT on aggregation.
Overall these mechanistic probes support the view that Ser modifications in httNT do not alter the fundamental, httNT-mediated nucleation mechanism of htt N-terminal fragments (Fig. 2A), but rather influence the efficiency of this mechanism. The reductions in overall aggregation rate previously reported for the S13D/S16D mutant 9, and reported here for the phosphoryl-Ser peptides (Fig. 3a), appear to be primarily due to less favorable formation of the α-helix-rich oligomeric intermediates that are the springboard for amyloid nucleation (Fig. 2A).
Serine modifications and aggregate structure and stability
Previously we reported magic angle spinning (MAS) ssNMR data on mature amyloid fibrils of httNTQ30P10K2 peptides that confirmed the expected β-structure in many of the polyQ Gln residues while surprisingly showing that residues 4–11 of the httNT reside in stable α-helix 27. We acquired the corresponding S13D/S16D peptide containing U-13C,15N-labeling of particular residues within the α-helix, the polyQ segment and the intervening linker region, then prepared mature fibrils and collected MAS ssNMR data. We used chemical shift indexing (CSI) relative to data on proteins of known structure to infer secondary structure at the labeled positions 28. The results (Fig. 6) show that the labeled sites in S13D/S16D fibrils have chemical shifts, and thus structures, that are remarkably similar to that of the WT httNT peptide. Thus, the Lys6 and Ala10 residues of S13D/S16D exhibit the same kind of strong α-helical signal as found in the WT peptide aggregates. For Leu14, despite a slightly increased indication of α-helicity for the Cα shift alone, the overall chemical shift pattern for this residue in the S13D/S16D protein, as in the WT protein, is not indicative of a well-defined secondary structure. Note that electrostatic or structural effects due to the mutation of the neighboring S13 may affect the observed chemical shifts and in part explain these chemical shift changes. Finally, as in the previously reported aggregates with WT httNT, a specifically-labeled Gln18 is found in two chemically distinct environments, both of which are β-conformations. The S13D/S16D aggregates exhibit an essentially identical split β profile. Thus, in spite of significant differences in EM morphology, the S13D/S16D peptide aggregates are indistinguishable at the level of secondary structure from the WT httNT aggregates, at least at the labeled residues. This is consistent with the above kinetics data suggesting an unchanged aggregation mechanism compared with the WT sequence.
Figure 6.
Structural consequences of Ser modification in mature httNTQ30P10K2 aggregates. Graphical representation of the 13C secondary chemical shifts (Δδ) obtained for indicated labeled residues, based on MAS ssNMR on mature aggregates from S13D/S16D (top) and WT (bottom) peptides. Δδ values were determined as differences from random coil chemical shifts for CO, Cα, and Cβ sites. The local secondary structure inferred from those values is shown. Red bars represent α-helicity, whereas blue bars β-sheet structure. Black bars indicate undetermined secondary structure. Split bars for Gln18 indicate two sets of observed NMR signals associated with two conformations, both β.
To complement these ssNMR results, we performed other tests probing for changes in aggregate structure and stability introduced by serine modifications. We exposed final aggregates of various httNTQ37P10K2 peptides, after fractionation from any non-aggregated monomers by centrifugation, to limited trypsin proteolysis - a sensitive, if low resolution, probe of amyloid structure 29, and analyzed the results by LC-MS. We found that mature WT aggregates are relatively stable to cleavage within the httNT segment, with only about 10% of the peptides being cleaved, all at Lys6 (Fig. 7a, black bars). In contrast, about 30% of the peptides in S13D/S16D aggregates (red bars), and nearly 50% of the peptides in S13pS/S16pS aggregates (blue bars), are cleaved by trypsin exposure under the same conditions. Interestingly, the single phosphoryl-Ser mutants, S13pS and S16pS, are both about 30% cleaved, the same level as the S13D/S16D aggregates; thus the sensitivity of these modified Ser aggregates to trypsin cleavage rank very similarly to their relative formation rates in the kinetics analysis (Fig. 3a). We also examined the trypsin cleavage sensitivities of the aggregates of some of these peptides isolated relatively early (30–35% completion) in the aggregation reactions. We observed trypsin susceptibilities in these early/intermediate aggregates very similar to those in the mature aggregates (Fig. 7a).
Figure 7.
Structural consequences of Ser modification in mature httNTQ37P10K2 aggregates. (a) Fraction of molecules cleaved within httNT after exposure to trypsin in various mature aggregates (WT, black bars; S13pS/S16pS, blue bars; S13D/S16D, red bars; S13pS, red hatched bar; S16pS, blue hatched bar). (b) Sedimentation assays of aggregation kinetics and plateau levels of monomer for WT (●), S13D/S16D (♦) and S13pS/S16pS (▲) peptides in PBS at 37 °C. Inset: Dissociation of S13D/S16D aggregates in PBS at 37 °C by sedimentation assay.
In addition to a greater amount of cleaved material, we also observed “deeper” cleavages in the digests of fibrils from the httNT mutated peptides. Thus, while the small amount of cleavage of WT fibrils was all at Lys6, cleavage of fibrils from mutated httNT peptides occurred at both Lys6 and Lys9. About equal amounts of fragments from cleavage at positions 6 and 9 were obtained on digestion of the S13D/S16D mutant fibrils, while the position 9 fragment predominated in the digest of the S13pS/S16pS fibrils.
The changes in aggregate stabilities suggested by the limited proteolysis experiments are consistent with a robust measure of global stability 30, 31, the concentration of monomeric peptide remaining when the aggregation reaction reaches equilibrium. This residual monomer concentration, which typically can be reached both in the aggregate association and dissociation directions 17, 30, is essentially the critical concentration, or Cr, for the polymerization reaction, and hence is linked to the thermodynamic favorability of aggregate elongation 30, 31. We found a surprisingly dramatic effect of the Ser modifications within httNT on this measure of aggregate stability. We found that the aggregation reaction of WT httNTQ37P10K2 reaches equilibrium, with a Cr in the 0.3 µM range after about 50 hrs, with no further change up to 200 hrs (Fig. 7b, ●). In comparison, the corresponding S13D/S16D peptide aggregation reaction reaches a plateau in monomer concentration in the 2.5 – 3.0 µM range after about 100 hrs, with no further change out to 1400 hrs (Fig. 7b, ♦). A similar monomer concentration of 2 µM is reached when S13D/S16D aggregates are diluted and given the opportunity to dissociate toward equilibrium (Fig. 7b inset), showing the robustness of the measurement as a quantity associated with a dynamic equilibrium. The S13pS/S16pS peptide requires about 200 hrs to reach equilibrium where it indicates a Cr value in the 4.5 – 6.0 µM range (Fig. 7b, ▲). Based on these values, we calculated the aggregation destabilization (ΔΔGag) 31 attributable to the negative charges introduced at the Ser residues to be ~1.3–1.4 kcal/mol for the S13D/S16D mutation and ~1.7–1.8 kcal/mol for the S13pS/S16pS peptide. Since the Cr value is the concentration below which aggregation cannot occur, these Cr values may have important practical consequences for cellular aggregate accumulation.
DISCUSSION
Protein phosphorylation and dephosphorylation play an enormous role in cell regulation and cell pathway dysfunction linked to disease 32. Phosphorylation of the protein huntingtin 33, 34, especially at Ser421 35 and within the N-terminal httNT sequence 14, 15, 36, 37, has been implicated in controlling a variety of this protein’s cellular activities. Phosphorylation at serines 13 and 16 has been implicated in enhanced degradation of htt by the proteasome in cell models 14, in suppressed accumulation of htt aggregates in a mouse model 9, and in enhanced htt transport into the nucleus 14, 15, 37. The dramatic effects on aggregate accumulation and HD phenotype of the phosphomimetic S13D and S16D mutations in the BACHD mouse model suggest that persistent phosphorylation of these residues in the cell could play an equally strong role in abrogating the effects of an expanded polyQ sequence in huntingtin. The possible impact of partial and/or transient phosphorylation is more difficult to assess from the phosphomimetic results.
Regardless of the implications for a strong role for phosphorylation, the dramatic effects of the S13D/S16D mutations in mice offer an opportunity to explore elements of the HD disease mechanism. That is, it is clear that mutation of these Ser residues to Asp, at only two positions out of over 3,000 residues in full length htt, dramatically neutralizes the aggregation effects of a Q97 repeat in this system 9. We therefore focused our efforts on understanding how this double mutation influences various aspects of htt aggregation. Our previous results showed that replacement of Ser13 and Ser16 with Asp significantly reduced the rate of amyloid formation in a htt exon1-like peptide and dramatically changed the morphology of the aggregates 9. In that paper we speculated that diminution of the aggregation rate could affect the accumulation of aggregates by allowing the cellular mechanisms of aggregate eradication 38, 39 to better keep up with aggregation. Here we describe the underlying molecular basis of this diminution in aggregation rate, as well as some potentially equally important differences conferred on the product fibrils. We also show that the Ser to Asp mutation is in fact a reasonable approximation for the effect of stable, 100% phosphorylation of these two Ser residues on amyloid formation rates and amyloid structure.
In principle, there are a number of mechanistic steps that might be targeted by httNT sequence changes to explain observed aggregation rate reductions. These include control of monomer conformation, oligomer formation, amyloid nucleation and amyloid elongation. Our studies show that only one of these mechanisms is consistent with the data. We found that the S13D/S16D mutation does not appreciably affect the ensemble monomeric conformation of the httNTQ37P10K2 peptides based on CD (Fig. 4a) and FRET (Results). (In contrast, Truant and colleagues described CD experiments showing significant reductions of α-helix in httNT peptides modified on the Ser residues, which they interpreted as evidence for altered monomer conformations 15. The different results may be due to sample disaggregation procedures, peptide sequence background used, or peptide concentrations used.) The httNT Ser modifications also do not appear to change the fundamental amyloid nucleation mechanism, which is equally dependent, for both WT and S13D/S16D peptides, on the initial formation of httNT-mediated α-helix rich oligomers (Fig. 5 a,b). Likewise, by using short polyQ versions of exon1-like peptides we show that the S13D/S16D mutations have no great effect on the amyloid nucleation and elongation reactions once α-helix-rich oligomers have formed (Fig. 4 c,d). In contrast to these negative findings, we observed that httNT segments containing the S13D/S16D mutations are thermodynamically impaired in their assembly into α-helix-rich oligomers (Fig. 4 b,c). Thus, the most likely source of the observed diminished spontaneous aggregation rates is a reduced ability to form the α-helix-rich tetramers and higher aggregates that serve as the species within which polyQ amyloid structure is nucleated 19, 20, 22 (Fig. 2).
These results for Ser to Asp mutations within httNT on the solution structure and reactions of exon1-like monomers are replicated in peptides in which one or both of the Ser residues are phosphorylated. Thus, the approximate secondary structural distribution within the monomer ensemble of the S13pS/S16pS peptide is indistinguishable from both the WT httNT sequence and the S13D/S16D double mutant (Fig. 4a). Likewise, aggregation of httNTQ37P10K2 peptides in which one or both of the httNT Ser residues are phosphorylated is slowed dramatically (Fig. 3a); in fact the S13pS/S16pS peptide aggregates substantially less rapidly than the S13D/S16D peptide, presumably due to the more highly charged nature of the phosphoryl groups compared with carboxylate groups.
Thus, we obtained consistent data suggesting that the homogeneous, stable phosphorylation of Ser13 and Ser16, or their replacement with Asp residues, is expected to lead to slower kinetics of formation of amyloid fibrils due to the reduced stability of the transient oligomers that are required for amyloid nucleation. By slowing amyloid formation rates, these sequence alterations might allow cellular processes to better manage aggregate formation and therefore suppress aggregate accumulation. It also seemed possible, however, that alterations in the structures and properties of the product fibrils once formed might contribute to the tg mouse phenotype. We therefore compared aggregate structure and properties for WT peptides and peptides with modifications at residues 13 and 16.
We examined the final amyloid-like aggregates grown from the S13D/S16D httNTQ37P10K2 peptide by ssNMR and found that, just as in WT fibrils 27, residues 4–11 exist in α-helix, residue 14 is in irregular structure, and Gln18 is in β-sheet (Fig. 6). This result confirms the importance of α-helix formation by httNT in the aggregation mechanism as well as showing no major difference in residue specific secondary structural preferences between WT and mutant aggregates. In spite of these similar secondary structural profiles, however, further examination showed significant differences in structure and properties between WT and httNT-mutated exon1 fibrils.
For example, electron micrographs clearly show a difference in aggregate morphology 9 (Fig. 3). Thus, while WT httNT exon1 fibrils preparations are essentially homogeneous, exhibiting long, single fibrils with rough edges (Fig. 3b), final aggregates from peptides containing either Ser to Asp mutations or phosphoryl-Ser in the httNT segment are a mixture of morphologies including large bundles of thin filaments (Fig. 3 d,h), amorphous aggregates (Fig. 3 e,j), and short, protofibril-like structures (Fig. 3 f,g,i) reminiscent of an early reaction intermediate in the WT reaction (Fig. 3c).
Additionally, the final aggregates from httNTQ37P10K2 peptides containing either S13D/S16D or S13pS/S16pS replacements are dramatically more susceptible to trypsin (Fig. 7a). Thus, WT fibrils subjected to limited trypsin digestion suffer less than 10% cleavage, all of which occurs at residue 6, the most distal Lys residue from the polyQ amyloid core. In contrast, S13D/S16D final aggregates under the same conditions are cleaved in about 30% of the constituent molecules, with a distribution of about equal portions of cleavage at Lys6 and Lys 9. Finally, final aggregates of the S13pS/S16pS peptide are cleaved within httNT in almost 50% of the constituent peptides, and most of the cleavages occur at Lys9 nearer the polyQ amyloid core. This suggests significantly greater access by trypsin, and therefore perhaps other enzymes, to the httNT segments within the aggregates containing negative charges at the normal Ser positions. This suggests that these aggregates may be more susceptible to in vivo proteolysis than WT aggregates. In addition, this indication of different degrees of steric access might be relevant to the known ability of htt fragments to be ubiquitinylated at the N-terminus 11, 14 and to suspicions that ubiquitin-targeted proteasomal degradation plays a large role in the cell’s ability to remove htt aggregates 1. If ubiquitinylating enzymes have greater access to the Lys residues that are the targets of ubiquitin modification in the aggregates of phosphomimetic and phosphorylated peptides, compared to WT, this might suggest a greater ability of the ubiquitin system to target mutant aggregates for proteasome degradation, consistent with the lack of inclusion accumulation in the S13D/S16D BACHD mice.
Finally, the measured Cr values for various exon1 fragments suggest that mutations introducing negative charges at positions 13 and 16 of httNT abrogate the positive contribution of httNT to fibril stability. Thus, we previously reported Cr values for K2Q36P10K2 and K2Q40P10K2 peptide fibrils in the 2–4 µM range under the same growth conditions used in the experiments described here 18. The covalent attachment of httNT to such molecules, in the WT peptide httNTQ37P10K2, reduces the Cr to the 0.3 µM range (Results). However, the Cr of a similar molecule containing negative charges at positions 13 and 16 reduces stability to Cr values of ~ 2.5 µM for the S13D/S16D sequence and ~5 µM for the S13pS/S16pS sequence (Results). Thus, introduction of these negative charges destabilizes htt exon1-like fibrils to a range essentially identical to fibrils from similar sequences lacking httNT entirely. The implications for exon1 fibril structure are not entirely clear. Part of the stabilization contributed by httNT could derive from the involvement of the aromatic residue at position 17 in β-structure 27, and part could derive from helix-helix interactions. In any case, it is clear that the negative residues at positions 13 and 16 somehow compromise these httNT contributions and that this may have consequences for aggregate stability within the cell. These results are consistent with several recent model peptide studies demonstrating a general ability of phosphorylation to modify the kinetics and product structure of amyloid formation 40–42.
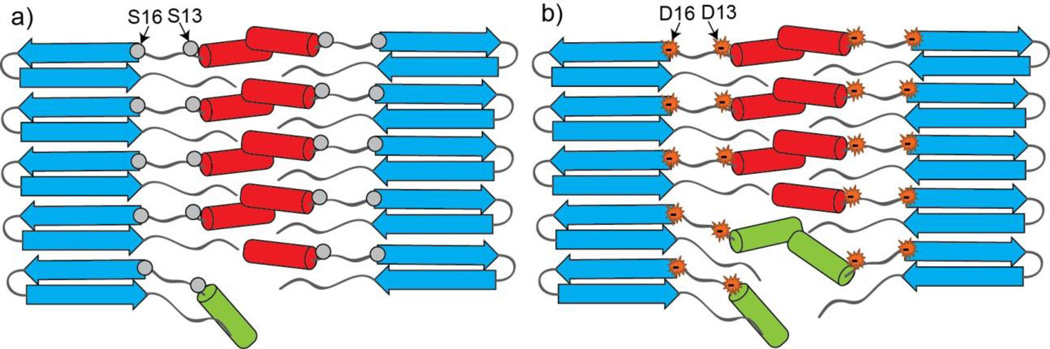
Together, the results of analysis of aggregate structures and properties suggests that placing negative charges at positions 13 and 16 decreases aggregate thermodynamic stability (both in the early httNT-mediated oligomers and the mature fibrils) as well as increases the accessibility of the httNT terminus to enzyme modification. Although these results may appear to be inconsistent with the ssNMR results showing approximately equivalent secondary structures for the WT and S13D/S16D aggregates, we suggest that the results merely reflect the different methods’ complementary insights into fibril structure, dynamics and stability. Thus, the ssNMR experiments are exquisitely sensitive to the local structure of the immobilized material (i.e., the atomic structure of the protofilaments), but lack direct information on non-local interactions such as changes in morphology as seen by EM (i.e., supramolecular assembly of smaller units into fibrils). The ssNMR experiments also detect the overall signals of the bulk sample, in particular the most rigid parts of the molecule (see also ref. 27). These points are brought out by the hypothetical model shown in Figure 8.
Figure 8.
A model for the htt exon1-like fibril structure, integrating observations from ssNMR, EM and trypsin cleavage. In the figures, polyQ β-sheets are in blue, more rigid httNT α-helices are in red, and more mobile httNT α-helices are in green. Positions 13 and 16 are in a segment lacking secondary structure 27. The figure shows how increased mobility (green helices) caused by charge repulsion (b) leads to greater exposure of httNT sequences to trypsin access compared to more stably packed WT httNT (a). In both WT and mutated httNT, the bulk of the httNT helices (red) remain stably involved in structure and detectible by MAS ssNMR.
The Figure 8 model (as well as the Fig. 2f model) proposes that the httNT segments of peptides within a filament interact with and stabilize each other. It also proposes that the httNT segments may be largely responsible for the filament-filament interactions required to build up the thicker fibrils seen for WT exon1 like aggregates (Fig. 3b). This model is similar to a recently proposed model for the role of httNT in fibril structure based on EPR data 43. Since the addition of negative charges in httNT occurs in the ‘linker’ part of the peptide between the β-sheet and α-helix in the mature fibrils 27, it will not necessarily disturb the α-helix in residues 4–11 observed by ssNMR in the final aggregates. At the same time, charge repulsion could introduce destabilization that manifests in the EM, Cr and trypsin data presented. Thus, repulsion could interfere with fibril assembly resulting in the accumulation of filaments (compare panel b to panels d and h in Figure 2). Second, charge repulsion could reduce the httNT contribution to fibril stability, as observed in the Cr values (Fig. 7b). Third, charge repulsion could lead to more dynamic unfolding-refolding of the α-helices, at least at certain locations such as fibril ends, thus increasing the sensitivity of httNT to trypsin (Fig. 7a). The model in Figure 8 may account for why only a subset of the httNT segments (the green helices in the figure) are trypsin-sensitive, being in an environment (fibril termini in the schematic) that is particularly sensitive to charge repulsion effects. The model also suggests that the bulk of the httNT segments in both WT and S13D/S16D fibrils are expected to be found in α-helix in the MAS ssNMR experiments.
Taken together, our studies on the S13D/S16D peptide indicate a number of possible biophysical explanations for the absence of inclusions in the tg mice carrying these mutations. First, the mutations decrease the tendency of the disordered httNT sequence to engage in α-helical tetramer formation, reducing the steady state concentration of helical oligomers and consequently the rate of amyloid nucleation and growth. Second, the mature aggregates produced are less thermodynamically stable, to the extent that there is less of a driving force for their formation in the cell. Third, the httNT segment in these aggregates is more accessible to trypsin, and perhaps to other enzymes such as ubiquitinylating enzymes, potentially providing a biophysical mechanism leading to increased proteasomal degradation.
Mutations of Ser or Thr residues to Asp or Glu residues have long been successfully used as ribosomally programmable mimics of phosphorylated residues, in spite of the dramatically different sizes and pKas of carboxylic acid and phosphoric acid moieties. Our results show that Asp mutations can also be reasonable mimics of the effects of phosphorylation of Ser residues on protein aggregate formation rates and properties. Many substrates for phosphorylation are intrinsically disordered proteins, and the mechanisms by which phosphorylation imparts cellular effects have a strong protein folding component 44. Our results suggest one feasible pathway by which protein phosphorylation might generally impact protein misfolding and aggregation, by directly modulating the long term viability of disordered proteins in the cell. More specifically, the data provide a consistent mechanism to explain the lack of aggregate accumulation, and potentially the lack of toxicity, of S13D/S16D htt in a mouse model of HD. Since these Ser modifications suppress amyloid formation by first suppressing oligomer formation, the results suggest that α-helix rich oligomers, while not visible in the mouse brain, also remain among the list of suspects as possible toxic agents in HD.
MATERIALS AND METHODS
Materials
Water (HPLC grade), acetonitrile (99.8% HPLC grade), HFIP (99.5%, spectrophotometric grade), and formic acid were from Acros Organics, TFA (99.5% purity) from Pierce, and thioflavin T was from Sigma. Chemically synthesized peptides (Fig. S1) were obtained from either the Keck Biotechnology Center at Yale University (http://keck.med.yale.edu/ssps/) or from GenScript, Inc. and purified on an Agilent Zorbax C3 reverse phase HPLC column before use. Most peptides contained a conservative Phe->Trp replacement at position 17 previously shown to have little effect on aggregation rates 19.
General Methods
All peptides were rigorously disaggregated as described 45, 46. Peptide concentrations were determined by analytical HPLC based on standard curves 45 from stock solutions of purified peptides calibrated as described 20. For Trp fluorescence, FTIR analysis and trypsin digestion studies, aggregates were isolated from reaction mixtures at specified time points by centrifugation at 21,000 × g for 45 mins and the pellets were washed 3 times by resuspension and resedimentation with PBS to remove traces of TFA and the other solutes. Aggregate concentrations were determined directly 45 or estimated from the measured monomer concentrations of aggregation reactions. A sedimentation/analytical HPLC assay 45 was used to determine aggregation and aggregate dissociation 17, 30 kinetics. Trypsin limited proteolysis was performed as described 46 on aggregates isolated as described above using 12 hrs incubation at a 1:25 weight ratio of trypsin to aggregate in 50 mM Tris.HCl, pH 7.0 at 37 °C. Transmission electron microscopy was on uranyl acetate negative stained samples as described 17, 46.
Spectroscopic methods
Far-UV CD measurements were performed on a JASCO J-810 spectropolarimeter using a 0.1 mm or 1 mm path length cuvette. Samples for CD measurement were prepared as described 46. CD spectra were analyzed using the CONTINLL program 47 from the CDPro package (lamar.colostate.edu/~sreeram/CDPro) in which the SP37A reference set (ibasis 5) was used to estimate the amount of secondary structure. For FTIR analysis, aggregates isolated from 1–2 mM reactions were analyzed on an ABB Bomem FTIR instrument. Spectra were obtained by averaging a total of 400 scans collected at RT with 4 cm-1 resolution. Residual buffer absorption was subtracted and spectral components were identified from second-derivative minima using PROTA software (Biotools, Inc.). Tryptophan fluorescence measurements on aggregates were as described 46. Methods for acquiring ss NMR data have been described 27.
Limited trypsin digestion
For quantification of trypsin digestion, aggregates were isolated by centrifugation, resuspended in buffer, and the aggregate concentration determined by dissolving an aliquot in formic acid and quantifying by the HPLC assay. These aggregates were then incubated at concentrations of 25–50 µg/ml in 50 mM Tris-HCl, pH 7.0 for 12–16 hrs at 37 °C in the presence of trypsin at a 1:25 weight ratio of trypsin (SEQUENZ-Trypsin, Worthington Biochemical Corp.) to protein. At the end of the incubation, the aggregates were isolated by sedimentation, washed twice with 50mM Tris-HCl pH 7 buffer, then dissolved in formic acid and injected onto LC-MS (Agilent 1100 electrospray). Unique elution positions were obtained for full-length peptide and for peptides cleaved at positions 6 and 9. Structures of these peptides were confirmed by MS (see Supplemental Table 1). To quantify digestion, the A215 peaks of the peaks were integrated to determine the relative amounts of intact and nicked httNTQ37P10K2. The ratio of the area of the fragment(s) to the total (i.e., intact plus fragment(s)) peptide area was then used to determine the fraction of the total aggregate that was cleaved.
FRET measurements
FRET efficiencies were calculated based on a ratiometric approach on the basis of measured donor (ID) and acceptor (IA) fluorescence intensities from the protein tagged with a pair of FRET fluorophores (see Supplemental Methods).
Supplementary Material
HIGHLIGHTS.
S13D/S16D mutations in ~3200 amino acid huntingtin rescues mice from polyQ toxicity
In peptide models S->D and S->pS at 13 and 16 reduce aggregation kinetics
These mutations suppress α-helical tetramerization required for amyloid nucleation
Within the aggregates, they weaken stability and enhance access to the N-terminus
Serine modification may thus reduce aggregate burden by several biophysical means
ACKNOWLEDGMENTS
We thank Drs. James Conway and Alexander Makhov for access to the Structural Biology Dept. cryo-EM facility, and acknowledge funding support from NIH grant R01 AG019322 (to R. W. and P. v.d.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol Rev. 2010;90:905–981. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]
- 2.Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 3.Bates GP, Benn C. The polyglutamine diseases. In: Bates GP, Harper PS, Jones L, editors. Huntington's Disease. Oxford, UK: Oxford University Press; 2002. pp. 429–472. [Google Scholar]
- 4.Wilburn B, Rudnicki DD, Zhao J, Weitz TM, Cheng Y, Gu XF, Greiner E, Park CS, Wang N, Sopher BL, La Spada AR, Osmand A, Margolis RL, Sun YE, Yang XW. An antisense CAG repeat transcript at JPH3 locus mediates expanded polyglutamine protein toxicity in Huntington's disease-like 2 mice. Neuron. 2011;70:427–440. doi: 10.1016/j.neuron.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 6.DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 7.Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, Bates GP, Davies SW, Lehrach H, Wanker EE. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell. 1997;90:549–558. doi: 10.1016/s0092-8674(00)80514-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Berthelier V, Yang W, Wetzel R. Polyglutamine aggregation behavior in vitro supports a recruitment mechanism of cytotoxicity. J Mol Biol. 2001;311:173–182. doi: 10.1006/jmbi.2001.4850. [DOI] [PubMed] [Google Scholar]
- 9.Gu X, Greiner ER, Mishra R, Kodali R, Osmand A, Finkbeiner S, Steffan JS, Thompson LM, Wetzel R, Yang XW. Serines 13 and 16 are critical determinants of full-length human mutant huntingtin induced disease pathogenesis in HD mice. Neuron. 2009;64:828–840. doi: 10.1016/j.neuron.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray M, Shirasaki DI, Cepeda C, Andre VM, Wilburn B, Lu XH, Tao J, Yamazaki I, Li SH, Sun YE, Li XJ, Levine MS, Yang XW. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci. 2008;28:6182–6195. doi: 10.1523/JNEUROSCI.0857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steffan JS, Agrawal N, Pallos J, Rockabrand E, Trotman LC, Slepko N, Illes K, Lukacsovich T, Zhu YZ, Cattaneo E, Pandolfi PP, Thompson LM, Marsh JL. SUMO modification of Huntingtin and Huntington's disease pathology. Science. 2004;304:100–104. doi: 10.1126/science.1092194. [DOI] [PubMed] [Google Scholar]
- 12.Atwal RS, Xia J, Pinchev D, Taylor J, Epand RM, Truant R. Huntingtin has a membrane association signal that can modulate huntingtin aggregation, nuclear entry and toxicity. Hum Mol Genet. 2007;16:2600–2615. doi: 10.1093/hmg/ddm217. [DOI] [PubMed] [Google Scholar]
- 13.Rockabrand E, Slepko N, Pantalone A, Nukala VN, Kazantsev A, Marsh JL, Sullivan PG, Steffan JS, Sensi SL, Thompson LM. The first 17 amino acids of Huntingtin modulate its sub-cellular localization, aggregation and effects on calcium homeostasis. Hum Mol Genet. 2007;16:61–77. doi: 10.1093/hmg/ddl440. [DOI] [PubMed] [Google Scholar]
- 14.Thompson LM, Aiken CT, Kaltenbach LS, Agrawal N, Illes K, Khoshnan A, Martinez-Vincente M, Arrasate M, JG OS-R, Khashwji H, Lukacsovich T, Zhu YZ, Lau AL, Massey A, Hayden MR, Zeitlin SO, Finkbeiner S, Green KN, Laferla FM, Bates G, Huang L, Patterson PH, Lo DC, Cuervo AM, Marsh JL, Steffan JS. IKK phosphorylates Huntingtin and targets it for degradation by the proteasome and lysosome. J Cell Biol. 2009;187:1083–1099. doi: 10.1083/jcb.200909067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atwal RS, Desmond CR, Caron N, Maiuri T, Xia JR, Sipione S, Truant R. Kinase inhibitors modulate huntingtin cell localization and toxicity. Nature Chemical Biology. 2011;7:453–460. doi: 10.1038/nchembio.582. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Ferrone F, Wetzel R. Huntington's Disease age-of-onset linked to polyglutamine aggregation nucleation. Proc. Natl. Acad. Sci. USA. 2002;99:11884–11889. doi: 10.1073/pnas.182276099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kar K, Jayaraman M, Sahoo B, Kodali R, Wetzel R. Critical nucleus size for disease-related polyglutamine aggregation is repeat-length dependent. Nat Struct Mol Biol. 2011;18:328–336. doi: 10.1038/nsmb.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharyya A, Thakur AK, Chellgren VM, Thiagarajan G, Williams AD, Chellgren BW, Creamer TP, Wetzel R. Oligoproline effects on polyglutamine conformation and aggregation. J Mol Biol. 2006;355:524–535. doi: 10.1016/j.jmb.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 19.Thakur AK, Jayaraman M, Mishra R, Thakur M, Chellgren VM, Byeon IJ, Anjum DH, Kodali R, Creamer TP, Conway JF, Gronenborn AM, Wetzel R. Polyglutamine disruption of the huntingtin exon 1 N terminus triggers a complex aggregation mechanism. Nat Struct Mol Biol. 2009;16:380–389. doi: 10.1038/nsmb.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayaraman M, Kodali R, Sahoo B, Thakur AK, Mayasundari A, Mishra R, Peterson CB, Wetzel R. Slow Amyloid Nucleation via alpha-Helix-Rich Oligomeric Intermediates in Short Polyglutamine-Containing Huntingtin Fragments. Journal of Molecular Biology. 2012;415:881–899. doi: 10.1016/j.jmb.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra R, Jayaraman M, Roland BP, Landrum E, Fullam T, Kodali R, Thakur AK, Arduini I, Wetzel R. Inhibiting the Nucleation of Amyloid Structure in a Huntingtin Fragment by Targeting alpha-Helix-Rich Oligomeric Intermediates. Journal of Molecular Biology. 2012;415:900–917. doi: 10.1016/j.jmb.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wetzel R. Physical chemistry of polyglutamine: Intriguing tales of a monotonous sequence. J. Mol. Biol. 2012;421:466–490. doi: 10.1016/j.jmb.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayaraman M, Mishra R, Kodali R, Thakur AK, Koharudin LMI, Gronenborn AM, Wetzel R. Kinetically Competing Huntingtin Aggregation Pathways Control Amyloid Polymorphism and Properties. Biochemistry. 2012;51:2706–2716. doi: 10.1021/bi3000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thakur A, Wetzel R. Mutational analysis of the structural organization of polyglutamine aggregates. Proc Natl Acad Sci U S A. 2002;99:17014–17019. doi: 10.1073/pnas.252523899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayaraman M, Kodali R, Wetzel R. The impact of ataxin-1-like histidine insertions on polyglutamine aggregation. Protein Eng Des Sel. 2009;22:469–478. doi: 10.1093/protein/gzp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thakur AK, Yang W, Wetzel R. Inhibition of polyglutamine aggregate cytotoxicity by a structure-based elongation inhibitor. FASEB J. 2004;18:923–925. doi: 10.1096/fj.03-1238fje. [DOI] [PubMed] [Google Scholar]
- 27.Sivanandam VN, Jayaraman M, Hoop CL, Kodali R, Wetzel R, van der Wel PC. The aggregation-enhancing huntingtin N-terminus is helical in amyloid fibrils. J Am Chem Soc. 2011;133:4558–4566. doi: 10.1021/ja110715f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wishart DS, Sykes BD. The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J Biomol NMR. 1994;4:171–180. doi: 10.1007/BF00175245. [DOI] [PubMed] [Google Scholar]
- 29.Kheterpal I, Williams A, Murphy C, Bledsoe B, Wetzel R. Structural features of the Ab amyloid fibril elucidated by limited proteolysis. Biochem. 2001;40:11757–11767. doi: 10.1021/bi010805z. [DOI] [PubMed] [Google Scholar]
- 30.O'Nuallain B, Shivaprasad S, Kheterpal I, Wetzel R. Thermodynamics of abeta(1–40) amyloid fibril elongation. Biochemistry. 2005;44:12709–12718. doi: 10.1021/bi050927h. [DOI] [PubMed] [Google Scholar]
- 31.Williams AD, Shivaprasad S, Wetzel R. Alanine scanning mutagenesis of Abeta(1–40) amyloid fibril stability. J Mol Biol. 2006;357:1283–1294. doi: 10.1016/j.jmb.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 32.Julien SG, Dube N, Hardy S, Tremblay ML. Inside the human cancer tyrosine phosphatome. Nat Rev Cancer. 2011;11:35–49. doi: 10.1038/nrc2980. [DOI] [PubMed] [Google Scholar]
- 33.Luo S, Vacher C, Davies JE, Rubinsztein DC. Cdk5 phosphorylation of huntingtin reduces its cleavage by caspases: implications for mutant huntingtin toxicity. J Cell Biol. 2005;169:647–656. doi: 10.1083/jcb.200412071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schilling B, Gafni J, Torcassi C, Cong X, Row RH, LaFevre-Bernt MA, Cusack MP, Ratovitski T, Hirschhorn R, Ross CA, Gibson BW, Ellerby LM. Huntingtin phosphorylation sites mapped by mass spectrometry. Modulation of cleavage and toxicity. J Biol Chem. 2006;281:23686–23697. doi: 10.1074/jbc.M513507200. [DOI] [PubMed] [Google Scholar]
- 35.Pardo R, Colin E, Regulier E, Aebischer P, Deglon N, Humbert S, Saudou F. Inhibition of calcineurin by FK506 protects against polyglutamine-huntingtin toxicity through an increase of huntingtin phosphorylation at S421. J Neurosci. 2006;26:1635–1645. doi: 10.1523/JNEUROSCI.3706-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aiken CT, Steffan JS, Guerrero CM, Khashwji H, Lukacsovich T, Simmons D, Purcell JM, Menhaji K, Zhu YZ, Green K, Laferla F, Huang L, Thompson LM, Marsh JL. Phosphorylation of threonine 3: implications for Huntingtin aggregation and neurotoxicity. J Biol Chem. 2009;284:29427–29436. doi: 10.1074/jbc.M109.013193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Havel LS, Wang CE, Wade B, Huang BD, Li SH, Li XJ. Preferential accumulation of N-terminal mutant huntingtin in the nuclei of striatal neurons is regulated by phosphorylation. Human Molecular Genetics. 2011;20:1424–1437. doi: 10.1093/hmg/ddr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waelter S, Boeddrich A, Lurz R, Scherzinger E, Lueder G, Lehrach H, Wanker EE. Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol Biol Cell. 2001;12:1393–1407. doi: 10.1091/mbc.12.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broncel M, Falenski JA, Wagner SC, Hackenberger CP, Koksch B. How post-translational modifications influence amyloid formation: a systematic study of phosphorylation and glycosylation in model peptides. Chemistry. 2010;16:7881–7888. doi: 10.1002/chem.200902452. [DOI] [PubMed] [Google Scholar]
- 41.Valette NM, Radford SE, Harris SA, Warriner SL. Phosphorylation as a Tool To Modulate Aggregation Propensity and To Predict Fibril Architecture. Chembiochem. 2012;13:271–281. doi: 10.1002/cbic.201100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoue M, Konno T, Tainaka K, Nakata E, Yoshida H, Morii T. Positional Effects of Phosphorylation on the Stability and Morphology of Tau-Related Amyloid Fibrils. Biochemistry. 2012;51:1396–1406. doi: 10.1021/bi201451z. [DOI] [PubMed] [Google Scholar]
- 43.Bugg CW, Isas JM, Fischer T, Patterson PH, Langen R. Structural Features and Domain Organization of Huntingtin Fibrils. J Biol Chem. 2012 doi: 10.1074/jbc.M112.353839. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dyson HJ. Expanding the proteome: disordered and alternatively folded proteins. Q Rev Biophys. 2011;44:467–518. doi: 10.1017/S0033583511000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Nuallain B, Thakur AK, Williams AD, Bhattacharyya AM, Chen S, Thiagarajan G, Wetzel R. Kinetics and thermodynamics of amyloid assembly using a high-performance liquid chromatography-based sedimentation assay. Methods Enzymol. 2006;413:34–74. doi: 10.1016/S0076-6879(06)13003-7. [DOI] [PubMed] [Google Scholar]
- 46.Jayaraman M, Thakur AK, Kar K, Kodali R, Wetzel R. Assays for studying nucleated aggregation of polyglutamine proteins. Methods. 2011;53:246–254. doi: 10.1016/j.ymeth.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sreerama N, Woody RW. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal Biochem. 2000;287:252–260. doi: 10.1006/abio.2000.4880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.