Abstract
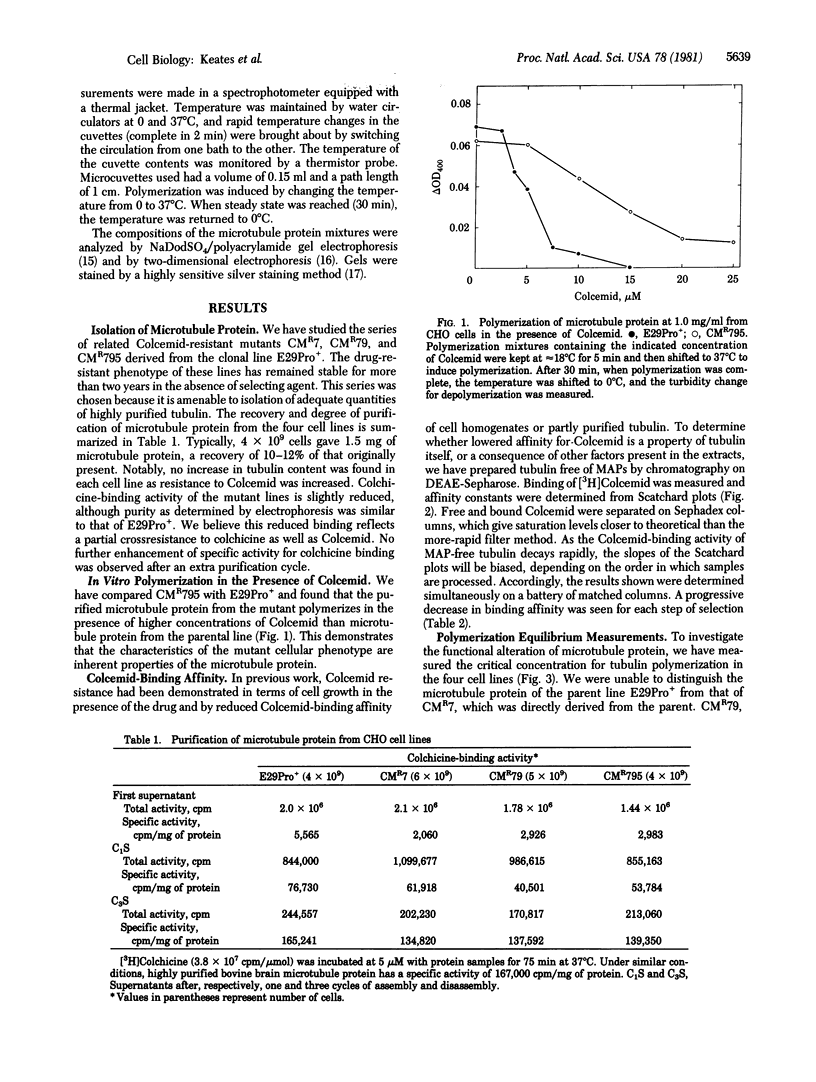
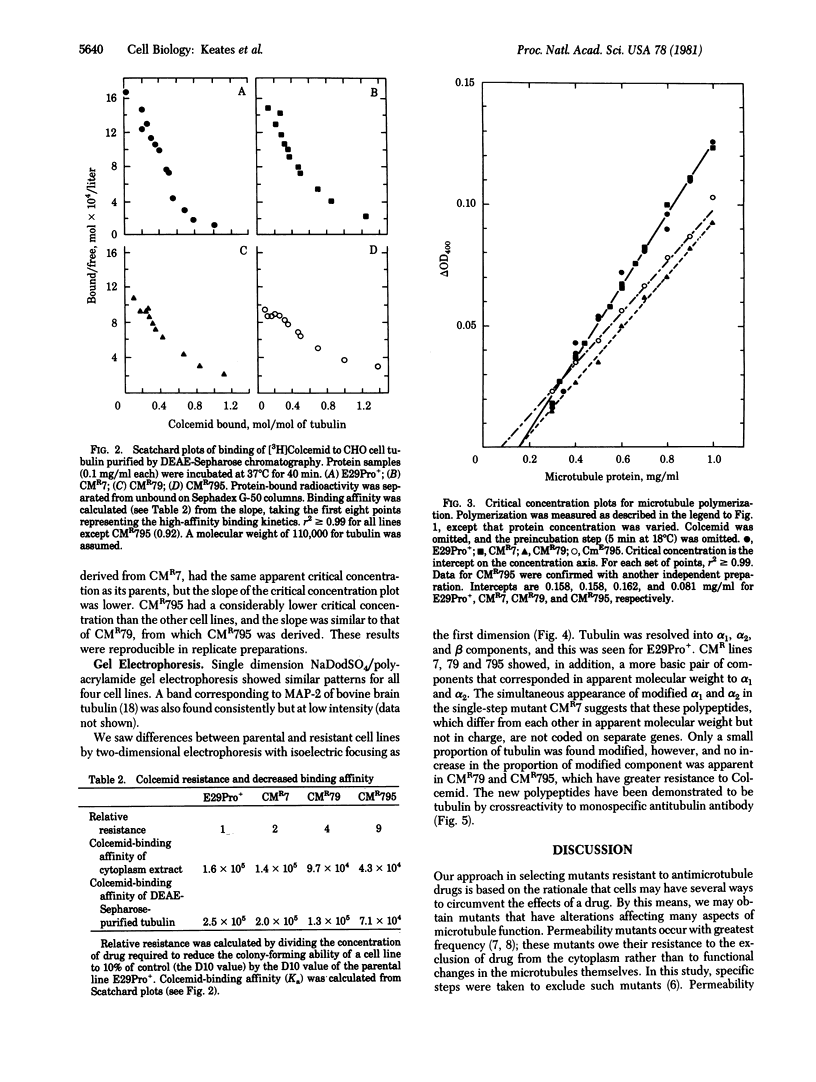
We have examined mutant lines of Chinese hamster ovary cells that have increased resistance to the antimicrotubule drug Colcemid. Analysis of the functional properties of purified microtubule protein indicates that increased tolerance to the drug in vivo is reflected in altered properties of microtubules and tubulin in vitro. In this study, we have examined one series of related mutants and have found different microtubule alterations associated with each selection step. These changes include decreased Colcemid-binding affinity, an altered electrophoretic pattern of tubulin subcomponents, increased resistance to Colcemid inhibition of polymerization in vitro and, in one case, a decreased critical concentration for microtubule assembly. Characterized mutants of the class described here will be useful for probing the regulation of microtubule assembly in vivo.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borisy G. G. A rapid method for quantitative determination of microtubule protein using DEAE-cellulose filters. Anal Biochem. 1972 Dec;50(2):373–385. doi: 10.1016/0003-2697(72)90046-2. [DOI] [PubMed] [Google Scholar]
- Bryan J. A quantitative analysis of microtubule elongation. J Cell Biol. 1976 Dec;71(3):749–767. doi: 10.1083/jcb.71.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral F., Sobel M. E., Gottesman M. M. CHO mutants resistant to colchicine, colcemid or griseofulvin have an altered beta-tubulin. Cell. 1980 May;20(1):29–36. doi: 10.1016/0092-8674(80)90231-7. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Connolly J. A., Kalnins V. I., Ling V. Microtubules in colcemid-resistant mutants of CHO cells. Exp Cell Res. 1981 Mar;132(1):147–155. doi: 10.1016/0014-4827(81)90091-4. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Borisy G. G. Kinetic analysis of microtubule self-assembly in vitro. J Mol Biol. 1977 Nov 25;117(1):1–31. doi: 10.1016/0022-2836(77)90020-1. [DOI] [PubMed] [Google Scholar]
- Keates R. A., Mason G. B. Inhibition of microtubule polymerization by the tubulin-colchicine complex: inhibition of spontaneous assembly. Can J Biochem. 1981 May;59(5):361–370. doi: 10.1139/o81-050. [DOI] [PubMed] [Google Scholar]
- Kirschner M. W. Microtubule assembly and nucleation. Int Rev Cytol. 1978;54:1–71. doi: 10.1016/s0074-7696(08)60164-3. [DOI] [PubMed] [Google Scholar]
- Kowit J. D., Fulton C. Purification and properties of flagellar outer doublet tubulin from Naegleria gruberi and a radioimmune assay for tubulin. J Biol Chem. 1974 Jun 10;249(11):3638–3646. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ling V., Aubin J. E., Chase A., Sarangi F. Mutants of Chinese hamster ovary (CHO) cells with altered colcemid-binding affinity. Cell. 1979 Oct;18(2):423–430. doi: 10.1016/0092-8674(79)90061-8. [DOI] [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Rauch C. T., Wilson L. Mechanism of colchicine-dimer addition to microtubule ends: implications for the microtubule polymerization mechanism. Biochemistry. 1980 Nov 25;19(24):5550–5557. doi: 10.1021/bi00565a014. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Addition of colchicine--tubulin complex to microtubule ends: the mechanism of substoichiometric colchicine poisoning. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3466–3470. doi: 10.1073/pnas.74.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle B. W., Doenges K. H., Bryan J. Assembly of tubulin from cultured cells and comparison with the neurotubulin model. Cell. 1977 Nov;12(3):573–586. doi: 10.1016/0092-8674(77)90258-6. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Schimke R. T., Urlaub G., Chasin L. A. Amplified dihydrofolate reductase genes are localized to a homogeneously staining region of a single chromosome in a methotrexate-resistant Chinese hamster ovary cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5553–5556. doi: 10.1073/pnas.75.11.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez J., Fellous A., Francon J., Lennon A. M. Competitive inhibition of colchicine binding to tubulin by microtubule-associated proteins. Proc Natl Acad Sci U S A. 1979 Jan;76(1):86–90. doi: 10.1073/pnas.76.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Morris N. R. A beta-tubulin mutation in Aspergillus nidulans that blocks microtubule function without blocking assembly. Cell. 1981 Jun;24(3):837–845. doi: 10.1016/0092-8674(81)90109-4. [DOI] [PubMed] [Google Scholar]
- Pittz E. P., Lee J. C., Bablouzian B., Townend R., Timasheff S. N. Light scattering and differential refractometry. Methods Enzymol. 1973;27:209–256. doi: 10.1016/s0076-6879(73)27012-x. [DOI] [PubMed] [Google Scholar]
- Raff E. C. The control of microtubule assembly in vivo. Int Rev Cytol. 1979;59:1–96. doi: 10.1016/s0074-7696(08)61660-5. [DOI] [PubMed] [Google Scholar]
- Schmitt H., Atlas D. Specific affinity labelling of tubulin with bromocolchicine. J Mol Biol. 1976 Apr 25;102(4):743–758. doi: 10.1016/0022-2836(76)90289-8. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Rudolph S. A., Rosenbaum J. L., Greengard P. Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci U S A. 1975 Jan;72(1):177–181. doi: 10.1073/pnas.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht H., Ringel I., Szasz J. The co-polymerization of tubulin and tubulin chochicine complex in the absence and presence of associated proteins. J Biol Chem. 1980 Oct 10;255(19):9138–9148. [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Sánchez F., Natzle J. E., Cleveland D. W., Kirschner M. W., McCarthy B. J. A dispersed multigene family encoding tubulin in Drosophila melanogaster. Cell. 1980 Dec;22(3):845–854. doi: 10.1016/0092-8674(80)90561-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]
- Wilson L., Friedkin M. The biochemical events of mitosis. II. The in vivo and in vitro binding of colchicine in grasshopper embryos and its possible relation to inhibition of mitosis. Biochemistry. 1967 Oct;6(10):3126–3135. doi: 10.1021/bi00862a021. [DOI] [PubMed] [Google Scholar]
- Wilson L. Properties of colchicine binding protein from chick embryo brain. Interactions with vinca alkaloids and podophyllotoxin. Biochemistry. 1970 Dec 8;9(25):4999–5007. doi: 10.1021/bi00827a026. [DOI] [PubMed] [Google Scholar]