Abstract
Cigarette smoking continues to be the most preventable cause of illness and death and has been linked to the development and prognosis of cancer. Current smokers have higher levels of inflammation than nonsmokers, and inflammation can remain elevated in former smokers even years following cessation. Inflammation can also be enhanced by stress. This study examined cortisol and inflammatory responses to a laboratory stressor in breast cancer survivors who formerly smoked compared to their counterparts who had never smoked. Participants included 89 women (age = 51.6 ± 8.9 years) who had completed treatment for stage 0–IIIA breast cancer within the past three years and were at least two months post surgery, radiation or chemotherapy, whichever occurred last. Cortisol and interleukin-6 (IL-6) were evaluated in response to a standardized laboratory speech and mental arithmetic stressor. Former (n=25) and never (n=64) smokers did not differ by cancer stage, cancer treatment, comorbidities, time since cancer treatment, depression, or stress. Despite having similar cortisol responses to the stressor, former smokers had exaggerated IL-6 responses two hours post-stressor compared to never smokers. This effect persisted after controlling for age, BMI, time since treatment, education, and antidepressant use. An exaggerated and prolonged inflammatory response to stress could be one mechanism underlying the persistent inflammation observed in former smokers.
Keywords: persistent inflammation, smoking, stress, cancer survivors, interleukin-6 (IL-6), cortisol, glucocorticoid resistance
1. Introduction
Cigarette smoking continues to be the most preventable cause of illness and death and has been linked to the development and prognosis of cancer. Between 1997 and 2001, more than 450,000 deaths resulted from cigarette smoking each year (Center for Disease Control and Prevention, 2005). On average, adults who smoke cigarettes die 14 years earlier than nonsmokers (Doll et al., 2004). Dysregulated immune function, including chronic inflammation, may underlie the increased risk of developing smoking-related chronic diseases such as cancer and premature death (Cross et al., 1999).
Cigarette smoking boosts systemic inflammation (Das, 1985). For example, current smokers have higher basal levels of C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor-alpha (TNF-α) compared to individuals who have never smoked (Bermudez et al., 2002; Bo et al., 2005; Haddy et al., 2005; Wannamethee et al., 2005; Nazmi et al., 2008). Furthermore, CRP and IL-6 levels increase with greater smoking exposure as indexed by number of cigarettes smoked per day or pack years (Mendall et al., 2000; Bazzano et al., 2003; Helmersson et al., 2005; Wannamethee et al., 2007).
Some data suggest that inflammation may remain elevated even years after smoking cessation. For example, compared to never smokers, CRP levels were higher in former smokers 10–20 years following smoking cessation (Frohlich et al., 2003; Wannamethee et al., 2005). Similarly, past smokers had elevated IL-6 levels compared to nonsmokers (Wannamethee et al., 2007). Reasons behind persistent inflammation remain unclear; it has been suggested that systemic hypoxia and tissue damage may continue driving elevated inflammation as the body recovers from chronic exposure to over 7000 inhaled chemicals (Agusti et al., 2003; United States Department of Health and Human Services (USDHHS), 2010).
This study addressed an additional possibility, the hypothesis that inflammatory responses to acute stress may be enhanced among former smokers. Cortisol, a primary stress hormone, inhibits immune cell activity by binding to glucocorticoid receptors and reducing cytokine production (Brattsand and Linden, 1996; Barnes, 1998). However, chronically elevated cortisol can lead to glucocorticoid resistance, such that immune cells down-regulate the expression of glucocorticoid receptors (Webster and Cidlowski, 1994; Webster et al., 2002). In turn, this down-regulation leads to increased inflammation because cortisol cannot effectively dampen excessive cytokine production (Miller et al., 2002).
Cigarette smoking has been linked to alterations in hypothalamic-pituitary-adrenal (HPA) activity. Nicotine, the addictive component in tobacco, can stimulate the HPA axis and result in greater cortisol release (Balfour, 1989; Mendelson et al., 2005). Among smokers, both basal cortisol levels and the cortisol awakening response were greater than nonsmokers’ cortisol levels (Rohleder and Kirschbaum, 2006; Steptoe and Ussher, 2006). Smokers exhibited a blunted cortisol response to a laboratory stressor compared to nonsmokers (Kirschbaum et al., 1993b; Kirschbaum et al., 1994; al'Absi et al., 2003; Childs and de Wit, 2009). Furthermore, smokers can develop glucocorticoid resistance (Pedersen et al., 1996; Barnes and Adcock, 2009).
However, despite the evidence of HPA dysregulation in current smokers, it is unclear whether these alterations continue after smoking cessation. The current study investigates this possibility by comparing responses of former and never smokers to a laboratory stressor. To our knowledge, this investigation is the first study to compare HPA responses to an acute stressor in former and never smokers.
A breast cancer diagnosis and its treatment are typically stressful, and many breast cancer survivors continue to report significant distress following treatment (Härtl et al., 2003; McGregor and Antoni, 2009). Prior stress exposure and/or depression may enhance stress-induced inflammatory responses (Glaser et al., 2003; Pace et al., 2006). Thus, using a sample of breast cancer survivors to examine acute stress responses may offer an opportunity to investigate how past smoking may affect physiology. Accordingly, we investigated cortisol and IL-6 responses to an acute laboratory stressor in former and never smokers. We hypothesized that former smokers would have a reduced or blunted cortisol response to the acute stressor compared to never smokers. In addition, we expected that the IL-6 response would be larger in former smokers than never smokers.
2. Methods
2.1 Participants
The study data were drawn from the baseline sample (prior to randomization) of a clinical trial assessing the impact of yoga on fatigue and inflammation in breast cancer survivors. We used all available participants who had provided baseline data, except for 9 current smokers because the group was too small to make meaningful conclusions, 2 participants who did not have baseline IL-6 levels, and 5 participants were removed due to lack of both IL-6 assessments post-stressor. In addition, one never smoker had IL-6 levels 3 standard deviations above the mean at all three time points; therefore, this participant’s data were dropped from all analyses.
Women were recruited through breast cancer clinics, community fliers, and media announcements. Eligible women completed treatment for stage 0–IIIA breast cancer within the past three years (except for selective estrogen receptor modulators or aromatase inhibitors) and were at least two months post surgery, radiation, or chemotherapy, whichever occurred last. Screening exclusions included a prior history of breast or any other cancer except basal or squamous cell, more than five hours a week of vigorous physical exercise, current yoga practice, diabetes, uncontrolled hypertension, evidence of liver or kidney failure, and symptomatic ischemic heart disease. The Ohio State Biomedical Cancer Research Review Committee approved the project; all subjects gave written informed consent prior to participation.
2.2 Procedure
Women arrived at a hospital research unit at 0830h. A nurse inserted a catheter into each participant’s arm to collect a fasting blood sample after measuring blood pressure, temperature, weight, and height. Following consumption of a standardized breakfast, the women were allowed to relax for 20 minutes and provided a baseline saliva sample immediately following the relaxation period.
Next, the women completed the Trier Social Stress Test (TSST; Kirschbaum et al., 1993a), a well-validated laboratory stressor that provokes reliable physiological responses (Kudielka et al., 2004). For the speech, participants were told to imagine that they had an interview for a new job; they were given 10 minutes to prepare a speech about why they were the best candidate. Following speech preparation, participants were escorted to another room where they saw a microphone, video camera, and an audience panel. They were told that the panel would not be responsive except to track time and was trained in behavioral observation to rate their speech’s content and style. Participants delivered their 5 minute speech and were asked to perform several serial subtraction tasks for an additional 5 minutes. The serial subtraction task requires participants to subtract one or two digit numbers repeatedly from a four digit number under pressure to perform well.
Saliva samples were collected immediately after the stressor and 30, 45, 60, 75, and 120 minutes post-stressor. Nurses drew blood 45 and 120 minutes after the stressor had ended. Participants continued to fill out questionnaires following the stressor until the final blood draw and saliva collection was complete.
The blood draw collection times were selected based on the meta-analysis by Steptoe and colleagues (2007). Although the authors note that the inflammatory response to stress appears to be delayed, the exact time course has yet to be established (Steptoe et al., 2007). After reviewing the study designs included in the meta-analysis, we found that 6 of 7 studies collected blood between 40–45 minutes post-stressor and found significant increases in IL-6 (Altemus et al., 2001; Steptoe et al., 2001; Steptoe et al., 2002; Kunz-Ebrecht et al., 2003; Owen et al., 2003; von Kanel et al., 2006) and only 2 of 4 studies found significant IL-6 changes at 30 minutes (Brydon et al., 2005; Edwards et al., 2006). Therefore, we used the 45 and 120 minute post-stressor collection times.
2.3 Biological Assays
Saliva samples were collected using a salivette (Sarstedt, Newton, North Carolina), an untreated sterile cotton roll that was placed in the subject’s mouth for approximately 2 minutes or upon saturation. Samples were placed in a −24°C freezer after collection and analyzed using the Cortisol Coat-A-Count radioimmunoassay (Siemens Medical Solutions Diagnostics, Los Angeles). The lowest limit of detection was .025 µg/dL. The intra-assay and inter-assay coefficients of variation were 4.3% and 5.2%, respectively.
IL-6 levels were determined using Quantikine High Sensitivity Immunoassay kits (R&D Systems, Minneapolis, MN) according to the kit instructions. Serum samples were stored in a −80°C freezer until assay and were run undiluted in duplicate. The sensitivity of the kit to detect IL-6 was 0.039 pg/mL. Intra-assay coefficient of variation ranged from 6.9–7.8% and inter-assay coefficient variation ranged from 6.5–9.6%.
2.4 Measures
Participants answered questions about their age, race, highest level of education, and current medication use. Smoking history was assessed using questions adapted from the National Social Life, Health, and Aging Project (Drum et al., 2009). Pack-years was calculated as the average number of packs per day times the number of years smoked. Time since smoking cessation was calculated by subtracting the date participants stopped smoking from the date of their visit. Following participants’ authorization, medical records were used to confirm cancer stage, type of treatment(s), and treatment end date(s). Body mass index (BMI; kg/m2) was calculated from height and weight data collected during the visit. Time since treatment was calculated by subtracting the date of last treatment from the date of their visit. Participants provided data on current medication use.
The Charlson index, the most widely used comorbidity index for predicting mortality among cancer patients, was used to assess comorbidities (Charlson et al., 1994). The measure assigns weights to 19 comorbid conditions based on their potential influence on one-year mortality in breast cancer patients. For example, moderate to severe renal disease would receive a higher number on the index than diabetes.
The 14-item Perceived Stress Scale (PSS) was used to assess global stress levels (Cohen et al., 1983). It measures the degree to which individuals rate the current state of their lives as stressful. The scale assesses how overloaded, unpredictable, and uncontrolled respondents feel about their lives. In the analyses, PSS scores were used as a continuous variable.
The Center for Epidemiological Studies-Depression scale (CES-D) is a widely used 20-item scale assessing depressive symptomatology (Radloff, 1977). Population norms provide cutoffs for varying levels of depression, with higher scores signifying more depressive symptoms (Roberts and Vernon, 1983; Basco et al., 1997). It has been widely used with cancer populations (Demark-Wahnefried et al., 2003). In the analyses, CES-D scores were used as a continuous variable. In table 1, we also provide the number of participants with CES-D score greater than 16, the cut-off suggesting clinical depression.
Table 1.
Sample characteristics [mean (SD) or frequency (%)] by smoking history group.
| Never Smokers (N=64) |
Former Smokers (N=25) |
P-value | |
|---|---|---|---|
| Age (years) | 51.2 (10.2) | 52.0 (7.5) | 0.71 |
| BMI (kg/m2) | 26.8 (5.3) | 28.1 (5.7) | 0.32 |
| Race (Caucasian) | 56 (89%) | 22 (88%) | 1.00 |
| Education | 0.03 | ||
| High school or less | 4 (6%) | 2 (8%) | |
| Some college | 10 (16%) | 10 (40%) | |
| College graduate | 25 (39%) | 3 (12%) | |
| Graduate or professional training | 25 (39%) | 10 (40%) | |
| Psychological measures | |||
| Perceived stress | 22.6 (7.5) | 22.2 (6.7) | 0.81 |
| Fatigue | 12.2 (20.6) | 16.3 (21.8) | 0.41 |
| Depressive symptoms | 10.5 (7.5) | 9.3 (8.0) | 0.50 |
| CES-D score > 16 | 14 (22%) | 5 (20%) | 0.85 |
| Sleep score (PSQI) | 7.5 (3.8) | 6.7 (2.8) | 0.37 |
| Number of comorbidities | 1.00 | ||
| 0 | 59 (92%) | 24 (96%) | |
| 1 | 3 (5%) | 1 (4%) | |
| 2 | 2 (3%) | 0 (0%) | |
| Inflammation-related medication use | |||
| Antidepressants | 17 (27%) | 14 (56%) | 0.01 |
| Aspirin | 11 (17%) | 5 (20%) | 0.76 |
| NSAIDs | 21 (33%) | 9 (36%) | 0.78 |
| Oral steroids | 5 (8%) | 1 (4%) | 1.00 |
| Statins | 9 (14%) | 2 (8%) | 0.72 |
| Menopausal Statusa | 0.83 | ||
| Pre-menopause | 5 (9%) | 2 (9%) | |
| Peri-menopause | 9 (16%) | 2 (9%) | |
| Post-menopause | 41 (75%) | 19 (82%) | |
| Past smoking behaviors | |||
| Pack-years | -- | 8.8 (9.9) | |
| Time since smoking cessation (years) | -- | 18.7 (11.1) |
SD = standard deviation; BMI = body mass index; kg/m2 = kilograms per meter squared; CES-D = Center for Epidemiological Studies-Depression; PSQI = Pittsburgh Sleep Quality Index; NSAIDs = non-steroidal anti-inflammatory drugs
Eleven participants did not provide their menopausal status; therefore, sample sizes by smoking status are 55 never smokers and 23 former smokers.
The Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) is a 30-item scale that assesses five dimensions of fatigue (Stein et al., 1998; Stein et al., 2004). The total score represents the sum of four subscales (general fatigue, physical fatigue, emotional fatigue, and mental fatigue) minus the vigor subscale. In the analyses, total fatigue scores were used as a continuous variable.
The Pittsburgh Sleep Quality Index (PSQI) assessed sleep quality and sleep disturbances (Buysse et al., 1989). The scale has good internal consistency (Cronbach α of 0.85) and high test-retest reliability (r = .86) over a 45 day period (Backhaus et al., 2002). In the analyses, total sleep scores were used as a continuous variable.
2.5 Statistical Methods
The IL-6 and cortisol responses post-stress were compared between former and never smokers, as well as the change from 45 minutes post stress to 120 minutes post stress. Since several observations were measured within each subject, linear mixed models were used to test the effects of former smoking status while taking into the account the correlation within subject (Diggle et al., 2002). An unstructured variance-covariance structure was fitted to estimate error variance. These models were fit using PROC MIXED in SAS with a REPEATED statement (SAS Institute V. 9.2, Cary, NC, USA). Models were adjusted on baseline values and included age, BMI, time since treatment, education, antidepressant use, smoking history, and time post-stressor (categorical) as well as the interaction between smoking history and time. Antidepressant use (1=user vs. 0=nonuser) was an aggregate variable that included use of tricyclics, selective serotonin reuptake inhibitors (SSRIs), and monoamine oxidase inhibitors (MAOIs). IL-6 values were natural log-transformed and cortisol values were log-10 transformed so that residuals were approximately normally distributed. Comparisons between former and never smokers on demographic variables were made using two sample t-tests for means, and Fisher’s exact test for categorical variables. A two-sided significance level of α=0.05 was used for all tests.
3. Results
3.1 Characteristics of sample
The final analysis sample consisted of 89 subjects who had information on current and past smoking status as well as baseline (pre-stressor) IL-6 and cortisol data. IL-6 data were complete for 85 subjects (96%) and cortisol data were complete for 84 subjects (94%); the remaining subjects had intermittent missing values but were included in the mixed-model analyses.
Sample characteristics for 64 never smokers and 25 former smokers are summarized in Table 1. Cancer-specific characteristics for the sample are presented by smoking history group in Table 2. Former smokers and never smokers were similar on almost all characteristics. The participants were very healthy; only 6 participants reported having comorbidities including squamous cell carcinoma, asthma, and myocardial infarction. The sample was primarily Caucasian, and cancer stage was equally distributed between smoking history groups (p=0.95). Former smokers were more likely to have less education (p=0.03) and more likely to use antidepressants (p=0.01) compared to never smokers, and thus education and antidepressant use were included as potential confounders in all regression models.
Table 2.
Cancer-specific sample characteristics [mean (SD) or frequency (%)] by smoking history group.
| Never Smokers (N=64) |
Former Smokers (N=25) |
P-value | |
|---|---|---|---|
| Cancer stage | 0.95 | ||
| Stage 0 | 6 (9%) | 2 (8%) | |
| Stage I | 23 (36%) | 11 (44%) | |
| Stage II | 27 (42%) | 10 (40%) | |
| Stage IIIA | 8 (13%) | 2 (8%) | |
| Cancer treatment | 0.11 | ||
| Surgery only | 11 (17%) | 1 (4%) | |
| Surgery + chemo | 16 (25%) | 12 (48%) | |
| Surgery + rad | 11 (17%) | 5 (20%) | |
| Surgery + chemo + rad | 26 (41%) | 7 (28%) | |
| Time since treatment ended (months) | 11.3 (7.8) | 10.5 (6.8) | 0.68 |
| Cancer-specific medications | |||
| SERMs | 20 (31%) | 8 (32%) | 0.95 |
| Aromatase Inhibitors | 24 (38%) | 10 (40%) | 0.83 |
chemo = chemotherapy; rad = radiation; SERMs = selective estrogen receptor modulators
3.2 Salivary cortisol response to TSST
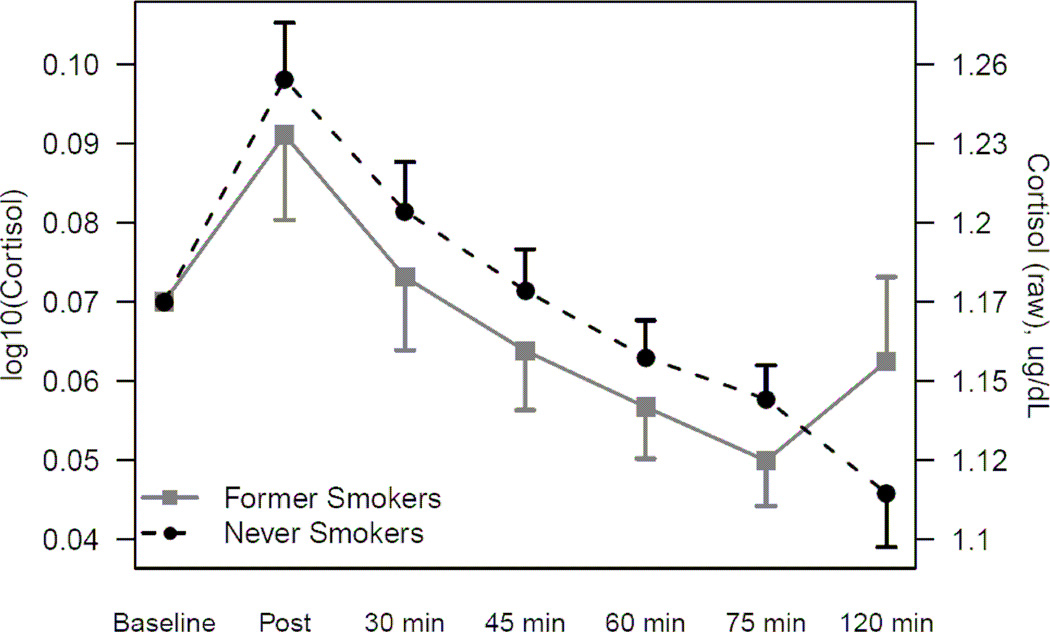
Overall there was a significant increase in salivary cortisol immediately after the stressor and significant subsequent decrease over the two hours post-stressor (p<0.001 for both). Former smokers’ salivary cortisol response did not differ from never smokers (p=0.54). The raw cortisol data are presented in Table 3 for never and former smokers. Figure 1 shows the cortisol (log10 transformed and raw) response to the laboratory stressor for never and former smokers, adjusted for baseline cortisol, age, BMI, time since treatment, education, and use of antidepressants. Education and antidepressant use were not significant predictors and their exclusion did not modify the outcome of model.
Table 3.
Mean (SD) of cortisol and IL-6 levels for never and former smokers across the laboratory session.
| Never Smokers (N=64) |
Former Smokers (N=25) |
P-value | |
|---|---|---|---|
| Salivary Cortisol (µg/dL)* | |||
| Baseline | 1.18 (0.07) | 1.20 (0.21) | 0.64 |
| Post stress | 1.27 (0.19) | 1.25 (0.23) | 0.61 |
| 30 minutes post stress | 1.21 (0.14) | 1.19 (0.17) | 0.41 |
| 45 minutes post stress | 1.18 (0.11) | 1.16 (0.14) | 0.40 |
| 60 minutes post stress | 1.16 (0.10) | 1.14 (0.10) | 0.48 |
| 75 minutes post stress | 1.15 (0.08) | 1.13 (0.08) | 0.27 |
| 120 minutes post stress | 1.11 (0.06) | 1.23 (0.61) | 0.20 |
| IL-6 (pg/mL)* | |||
| Baseline | 2.01 (2.06) | 1.74 (1.78) | 0.45 |
| 45 minutes post stress | 2.09 (1.87) | 1.87 (1.85) | 0.29 |
| 120 minutes post stress | 2.57 (2.05) | 2.82 (2.25) | 0.51 |
SD = standard deviation; IL-6 = Interleukin-6
The raw values are displayed for clarity and all tests were conducted on log transformed data.
Figure 1.
Log-transformed (log10) and raw mean cortisol response to laboratory stressor (and standard errors) for never and former smokers. Results from a mixed model adjusted for baseline cortisol level, age, BMI, time from treatment to visit, education, and antidepressant use.
3.3 Serum IL-6 response to TSST
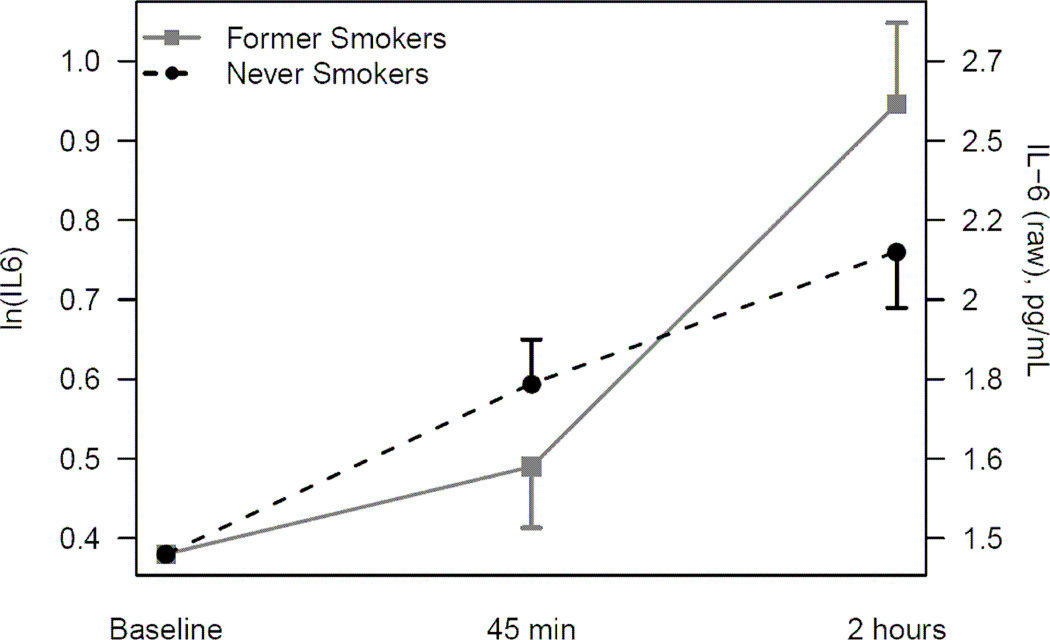
Overall there was a significant IL-6 increase in response to the acute stressor (p<.001). Figure 2 displays the average IL-6 (natural log transformed and raw) response to the laboratory stressor for never smokers and former smokers, adjusted for baseline IL-6, age, BMI, time since cancer treatment, education, and antidepressant use. The raw IL-6 levels are presented in Table 3 for never and former smokers. There were significant differences between never smokers and former smokers in their IL-6 responses to stress (Table 4). Former smokers’ IL-6 levels rose less steeply 45 minutes following the stressor compared to never smokers, though the difference was not significant (p=0.25). However, from 45 minutes to two hours post stressor, former smokers had a significantly larger increase in IL-6 compared to never smokers (p=0.01). Education and antidepressant use were not significant predictors and their exclusion did not modify the outcome of model.
Figure 2.
Natural log-transformed (ln) and raw mean IL-6 response to laboratory stressor (and standard errors) for never and former smokers. Results from a mixed model adjusted for baseline IL-6 level, age, BMI, time from treatment to visit, education, and antidepressant use.
TABLE 4.
Group effects on IL-6 outcomes after a laboratory stressor (natural log transformed).
| Covariate-adjusted least squares mean (SE) | |||||
|---|---|---|---|---|---|
| Time post- stressor |
Never Smokers (N=64) |
Former Smokers (N=25) |
Group difference |
95% CI | P-value |
| 45 minutes | 0.59 (0.06) | 0.49 (0.08) | −0.10 (0.09) | −0.28 to 0.08 | 0.25 |
| 120 minutes | 0.76 (0.07) | 0.95 (0.10) | 0.19 (0.12) | −0.05 to 0.43 | 0.13 |
| Changea | 0.17 (0.06) | 0.46 (0.09) | 0.29 (0.10) | 0.07 to 0.51 | 0.01 |
IL-6 = Interleukin-6; SE = standard error; CI = confidence interval
Note: All outcomes are natural log-transformed. Least square means are adjusted on baseline value, age, BMI, time from treatment to visit, education and usage of antidepressants.
Change is from 45 minutes post-stress to 120 minutes post-stress.
As several other factors may affect IL-6 levels, we also investigated the effects of other possible confounders, including race, cancer stage, comorbidities, sleep, perceived stress, depression, and fatigue. Since sleep, stress, depressive symptoms, and fatigue were highly correlated, these variables were entered individually into the adjusted regression models. None of these variables were significantly associated with the IL-6 response post-stressor, and adding these covariates had negligible effects on estimates presented in Table 4 and Figure 2.
3.4 Former smoking behavior characteristics
Secondary analyses examined the effects of lifetime tobacco exposure (e.g., pack years) and time since smoking cessation on baseline IL-6 levels and the IL-6 response to the stressor for former smokers. When controlling for age and BMI, neither time since quitting (ps>.61) nor total pack years (ps>.22) was significantly associated with baseline IL-6 levels or the IL-6 response to the stressor (results not shown).
4. Discussion
Among our sample of breast cancer survivors, exposure to the TSST led to significant cortisol and IL-6 increases in both groups. However, smoking history modified the IL-6 response, despite similar cortisol responses between former and never smokers. Specifically, compared to individuals who had never smoked, past smokers’ IL-6 response was exaggerated and more prolonged at 2 hours post-stress. This result remained significant even after controlling for use of antidepressants and psychological factors such as perceived stress, depression, and fatigue. Among former smokers, neither lifetime tobacco exposure nor time since smoking cessation was related to their baseline IL-6 levels or heightened and persistent IL-6 stress response.
Cigarette smoking exposes individuals to more than 7000 chemicals, including heavy metals such as cadmium (USDHHS, 2010). In vitro cadmium exposure can induce cytokine production from lung epithelial and immune cells. For example, cadmium exposure increased production of IL-8, IL-6, and interferon-gamma (IFN-γ) (Krocova et al., 2000; Låg et al., 2010; Cormet-Boyaka et al., 2011). Cadmium has a half life of 15–30 years (Jin et al., 1998; Rennolds et al., 2010). Therefore, long term heavy metal exposure could be a factor contributing to persistent inflammation observed in former smokers. Due to the bidirectional communication between the immune and neuroendocrine systems, it is possible the continued presence of heavy metals could perpetually modulate acute stress responses.
Current smokers have elevated basal cortisol and an attenuated cortisol response to an acute stressor compared to nonsmokers (Kirschbaum et al., 1993b; al'Absi et al., 2003; Rohleder and Kirschbaum, 2006; Steptoe and Ussher, 2006); suggesting dysregulated diurnal rhythmicity and stress reactivity of the HPA axis. Baseline cortisol levels did not differ between former and never smokers. Additionally, the baseline cortisol levels for both groups fall within the published reference range (0.094–1.515 ug/dL) for females 31–70 year old (Aardal and Holm, 1995).
Our study is the first to investigate the cortisol response to an acute stressor in former smokers compared to never smokers. Although it did not reach statistical significance, former smokers’ cortisol response was lower than never smokers, mirroring the pattern observed when comparing current and nonsmokers. Based on our data, the estimated effect size of Cohen’s d=0.3 would require 176 participants per group to have 80% power to detect significant differences in the cortisol stress response between never and former smokers. Therefore, we cautiously interpret our nonsignificant cortisol finding and suggest that future studies address this question with a larger sample.
Cortisol is immunoregulatory and can limit inflammatory stress responses. However, our data show that despite similar cortisol responses, former smokers had an exaggerated IL-6 response compared to never smokers. Glucocorticoid resistance, characterized by a reduction in lymphocytes’ sensitivity to cortisol, may offer a possible explanation for the significant IL-6 group differences without significant cortisol differences.
Smokers can develop glucococorticoid resistance (Pedersen et al., 1996; Barnes and Adcock, 2009). For example, when compared to nonsmoking asthmatics, asthmatic smokers were unable to control their symptoms using traditional glucocorticoid therapy (Chaudhuri et al., 2003). Future studies should investigate the presence of glucocorticoid resistance in healthy smokers compared to healthy never smokers. If glucocorticoid resistance is highly prevalent in current smokers, former smokers may offer a unique opportunity to investigate the time required for glucocorticoid receptor sensitivity to return. If the development of glucocorticoid resistance is not pervasive among smokers, then glucocorticoid resistance might be a clinically useful tool in identifying individuals at a heightened risk of developing inflammatory diseases such as asthma, autoimmune diseases, and cancer.
In our data, baseline IL-6 did not differ between former and never smokers. In addition, former smoking characteristics such as pack years and time since quitting did not affect baseline inflammation levels unlike prior reports (Frohlich et al., 2003; Wannamethee et al., 2005; Wannamethee et al., 2007). For example, Wannamethee and colleagues (2007) found that among nearly 3500 men (60–79 years old), 1999 former smokers had elevated IL-6 levels 20 years following cessation. In contrast, our sample was much smaller and consisted of 25 female former smokers, resulting in limited power to detect a relationship between past smoking behaviors and IL-6 baseline levels.
Smoking status was collected via self-report; assessing salivary cotinine levels would have allowed us to corroborate the self-report data and confirm former smoking status. If any of the former smokers were actually current smokers, the lack of access to tobacco during the laboratory session could have influenced the stress IL-6 responses. However, given the average time lapse since smoking, we believe the participants’ self-reports on smoking status. Our breast cancer survivor population included a wide range of cancer stages; therefore, differences among cancer stage and subsequent treatment may have enhanced variability in our data. However, in our sample, we did not observe an effect of cancer stage or cancer treatment on self-reported distress or baseline cortisol or IL-6 levels.
Broadly, chronic low grade inflammation is harmful. Individuals who have higher levels of inflammation are at greater risk for Type 2 diabetes, cardiovascular disease, and cancer (Ershler and Keller, 2000). Elevated inflammation is associated with greater all-cause mortality risk (Harris et al., 1999). Importantly, chronic inflammation influences multiple stages of cancer progression including tumor development, invasion, and metastasis (Aggarwal et al., 2006). In breast cancer survivors, elevated inflammation may be especially critical because it has been linked to poorer quality of life and cancer recurrence (Bower, 2007; Pierce et al., 2009).
Repeated excessive inflammatory responses to acute stress may enhance systemic inflammation in former smokers; this heightened reactivity may offer one explanation as to why former smokers still have more illnesses and die sooner compared to never smokers (Härtl et al., 2003; Van den Beuken-van Everdingen et al., 2008). As our data demonstrate, former smokers have an exaggerated and prolonged IL-6 response to acute stress compared to never smokers even when controlling for known psychological confounders, suggesting that an altered stress response may be another mechanism fueling persistent inflammation in former smokers.
Acknowledgement
We appreciate the helpful assistance of the Stress & Health Study lab staff and the Clinical Research Center nursing staff.
Role of the Funding Sources
Work on this paper was supported in part by the Gilbert and Kathryn Mitchell endowment (R. Glaser) and NIH grants CA126857, DE014320, CA131029, NCRR Grant UL1RR025755, which funds the Clinical Research Center, and the Ohio State Comprehensive Cancer Center Core Grant CA016058. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health or any division providing financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Disclaimers: The authors have no financial interests or relationships that pose potential conflicts of interest with this article.
Contributors
Jeanette M. Bennett performed the literature search, developed the hypotheses, lead writer of the manuscript, incorporated all co-authors edits and revisions, and submitted the manuscript.
Ronald Glaser oversaw the completion of cytokine assays and edited the manuscript.
Rebecca R. Andridge supervised data analysis, wrote portions of the manuscript, and edited the manuscript.
Juan Peng conducted statistical analyses and aided in manuscript preparation.
William B. Malarkey is the medical supervisor of the study and edited the manuscript.
Janice K. Kiecolt-Glaser wrote the grant and designed the study, supervised data collection for the study, and helped write the manuscript.
All authors have approved the final manuscript.
References
- Aardal E, Holm AC. Cortisol in saliva-reference ranges and relation to cortisol in serum. Clinical Chemistry and Laboratory Medicine. 1995;33:927–932. doi: 10.1515/cclm.1995.33.12.927. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochemical Pharmacology. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Agusti A, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. European Respiratory Journal. 2003;21:347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74:401–410. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- Altemus M, Rao B, Dhabhar FS, Ding W, Granstein RD. Stress-induced changes skin barrier function in healthy women. Journal of Investigative Dermatology. 2001;117:309–317. doi: 10.1046/j.1523-1747.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of Psychosomatic Research. 2002;53:737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Balfour DJ. Influence of nicotine on the release of monoamines in the brain. Prog Brain Res. 1989;79:165–172. doi: 10.1016/s0079-6123(08)62476-0. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clinical Science. 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. The Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- Basco MR, Krebaum SR, Rush AJ. Outcome measures of depression. In: Strupp HH, Horowitz LM, Lambert MJ, editors. Measuring patient changes in mood, anxiety, and personality disorders. Washington, DC: American Psychological Association; 1997. pp. 207–245. [Google Scholar]
- Bazzano L, He J, Muntner P, Vupputuri S, Whelton PK. Relationship between smoking and novel risk factors for cardiovascular disease in the United States. Annals of Internal Medicine. 2003;138:891–898. doi: 10.7326/0003-4819-138-11-200306030-00010. [DOI] [PubMed] [Google Scholar]
- Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. American Journal of Cardiology. 2002;89:1117–1119. doi: 10.1016/s0002-9149(02)02284-1. [DOI] [PubMed] [Google Scholar]
- Bo S, Gentile L, Ciccone G, Baldi C, Benini L, Dusio F, Lucia C, Forastiere G, Nuti C, Cassader M, Pagano GF. The metabolic syndrome and high C-reactive protein: prevalence and differences by sex in a southern-European population-based cohort. Diabetes-Metabolism Research and Reviews. 2005;21:515–524. doi: 10.1002/dmrr.561. [DOI] [PubMed] [Google Scholar]
- Bower JE. Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain, Behavior, and Immunity. 2007;21:863–871. doi: 10.1016/j.bbi.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brattsand R, Linden M. Cytokine modulation by glucocorticoids: Mechanisms and actions in cellular studies. Alimentary Pharmacology & Therapeutics. 1996;10:81–90. doi: 10.1046/j.1365-2036.1996.22164025.x. [DOI] [PubMed] [Google Scholar]
- Brydon L, Edwards S, Jia HY, Mohamed-Ali V, Zachary I, Martin JF, Steptoe A. Psychological stress activates interleukin-1 beta gene expression in human mononuclear cells. Brain, Behavior, and Immunity. 2005;19:540–546. doi: 10.1016/j.bbi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (CDC) Tobacco use access, and exposure to tobacco in media among middle and high school students – United States, 2004. Morbidity and Mortality Weekly Report. 2005;54:297–301. [PubMed] [Google Scholar]
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of Clinical Epidemiology. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Chaudhuri R, Livingston E, McMahon AD, Thomson L, Borland W, Thomson NC. Cigarette Smoking Impairs the Therapeutic Response to Oral Corticosteroids in Chronic Asthma. Am. J. Respir. Crit. Care Med. 2003;168:1308–1311. doi: 10.1164/rccm.200304-503OC. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacology (Berl) 2009;203:1–12. doi: 10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Cormet-Boyaka E, Jolivette K, Bonnegarde A, Rennolds J, Hassan F, Mehta P, Tridandapani S, Webster-Marketon J, Boyaka PN. An NF-κB-independent and Erk1/2-dependent mechanism controls CXCL8/IL-8 responses of airway epithelial cells to cadmium. Toxicological Sciences. 2011 doi: 10.1093/toxsci/kfr310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross CE, Traber M, Eiserich J, van der Vliet A. Micronutrient antioxidants and smoking. British Medical Bulletin. 1999;55:691–704. doi: 10.1258/0007142991902565. [DOI] [PubMed] [Google Scholar]
- Das I. Raised C-reactive protein levels in serum from smokers. Clinica Chimica Acta. 1985;153:9–13. doi: 10.1016/0009-8981(85)90133-0. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Morey MC, Clipp EC, Pieper CF, Snyder DC, Sloane R, Cohen HJ. Leading the way in exercise and diet (Project LEAD): Intervening to improve function among older breast and prostate cancer survivors. Controlled Clinical Trials. 2003;24:206–223. doi: 10.1016/s0197-2456(02)00266-0. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of Longitudinal Data. 2nd ed. Oxford: Clarendon Press; 2002. [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking 50 years' observations on male British doctors. British Medical Journal. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drum ML, Shiovitz-Ezra S, Gaumer E, Lindau ST. Assessment of Smoking Behaviors and Alcohol Use in the National Social Life, Health, and Aging Project. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 2009;64:I119–I130. doi: 10.1093/geronb/gbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KM, Burns VE, Ring C, Carroll D. Sex differences in the interleukin-6 response to acute psychological stress. Biological Psychology. 2006;71:236–239. doi: 10.1016/j.biopsycho.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Frohlich M, Sund M, Lowel H, Imhof A, Hoffmeister A, Koenig W. Independent association of various smoking characteristics with markers of systemic inflammation in men - Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95) European Heart Journal. 2003;24:1365–1372. doi: 10.1016/s0195-668x(03)00260-4. [DOI] [PubMed] [Google Scholar]
- Glaser R, Robles T, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses following influenza vaccination in older adults. Archives of General Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- Haddy N, Sass C, Maumus S, Marie B, Droesch S, Siest G, Lambert D, Visvikis S. Biological variations, genetic polymorphisms and familial resemblance of TNF-alpha and IL-6 concentrations: STANISLAS cohort. Eur. J. Hum. Genet. 2005;13:109–117. doi: 10.1038/sj.ejhg.5201294. [DOI] [PubMed] [Google Scholar]
- Harris T, Ferrucci L, Tracy R, Corti M, Wacholder S, Ettinger WJ, Heimovitz H, Cohen H, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. American Journal of Medicine. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Härtl K, Janni W, Kästner R, Sommer H, Strobl B, Rack B, Stauber M. Impact of medical and demographic factors on long-term quality of life and body image of breast cancer patients. Annals of oncology. 2003;14:1064–1071. doi: 10.1093/annonc/mdg289. [DOI] [PubMed] [Google Scholar]
- Helmersson J, Larsson A, Vessby B, Basu S. Active smoking and a history of smoking are associated with enhanced prostaglandin F-2 alpha, interleukin-6 and F-2-isoprostane formation in elderly men. Atherosclerosis. 2005;181:201–207. doi: 10.1016/j.atherosclerosis.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Jin T, Lu J, Nordberg M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology. 1998;19:529–535. [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The "Trier Social Stress Test": A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993a;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Scherer G, Strasburger CJ. Pituitary and adrenal hormone responses to pharmacological, physical, and psychological stimulation in habitual smokers and nonsmokers. Clin Investig. 1994;72:804–810. doi: 10.1007/BF00180552. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Strasburger CJ, Langkrar J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacol Biochem Behav. 1993b;44:527–531. doi: 10.1016/0091-3057(93)90162-m. [DOI] [PubMed] [Google Scholar]
- Krocova Z, Macela A, Kroca M, Hernychova L. The immunomodulatory effects of lead and cadmium on the cells of immune system in vitro. Toxicology in Vitro. 2000;14:33–40. doi: 10.1016/s0887-2333(99)00089-2. [DOI] [PubMed] [Google Scholar]
- Kudielka B, Schommer N, Hellhammer D, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29:983–992. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain, Behavior, and Immunity. 2003;17:373–383. doi: 10.1016/s0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Låg M, Rodionov D, Øvrevik J, Bakke O, Schwarze PE, Refsnes M. Cadmium-induced inflammatory responses in cells relevant for lung toxicity: Expression and release of cytokines in fibroblasts, epithelial cells and macrophages. Toxicology Letters. 2010;193:252–260. doi: 10.1016/j.toxlet.2010.01.015. [DOI] [PubMed] [Google Scholar]
- McGregor BA, Antoni MH. Psychological intervention and health outcomes among women treated for breast cancer: a review of stress pathways and biological mediators. Brain, Behavior, and Immunity. 2009;23:159–166. doi: 10.1016/j.bbi.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendall MA, Strachan DP, Butland BK, Ballam L, Morris J, Sweetnam PM, Elwood PC. C-reactive protein: relation to total mortality, cardiovascular mortality and cardiovascular risk factors in men. European Heart Journal. 2000;21:1584–1590. doi: 10.1053/euhj.1999.1982. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low-and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30:1751–1763. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Cohen S, Ritchey A. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychology. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Nazmi A, Oliveira IO, Victora CG. Correlates of C-reactive protein levels in young adults: a population-based cohort study of 3827 subjects in Brazil. Brazilian Journal of Medical and Biological Research. 2008;41:357–367. doi: 10.1590/s0100-879x2008000500003. [DOI] [PubMed] [Google Scholar]
- Owen N, Poulton T, Hay FC, Mohamed-Ali V, Steptoe A. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain Behavior and Immunity. 2003;17:286–295. doi: 10.1016/s0889-1591(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry. 2006;163:1630–1632. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pedersen B, Dahl R, Karlstrom R, Peterson C, Venge P. Eosinophil and neutrophil activity in asthma in a one-year trial with inhaled budesonide. The impact of smoking. Am. J. Respir. Crit. Care Med. 1996;153:1519–1529. doi: 10.1164/ajrccm.153.5.8630596. [DOI] [PubMed] [Google Scholar]
- Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, Ulrich CM. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rennolds J, Butler S, Maloney K, Boyaka PN, Davis IC, Knoell DL, Parinandi NL, Cormet-Boyaka E. Cadmium Regulates the Expression of the CFTR Chloride Channel in Human Airway Epithelial Cells. Toxicological Sciences. 2010;116:349–358. doi: 10.1093/toxsci/kfq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R, Vernon S. The Center for Epidemiologic Studies Depression Scale: its use in a community sample. American Journal of Psychiatry. 1983;140:41–46. doi: 10.1176/ajp.140.1.41. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Kirschbaum C. The hypothalamic-pituitary-adrenal (HPA) axis in habitual smokers. Int J Psychophysiol. 2006;59:236–243. doi: 10.1016/j.ijpsycho.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. Journal of Pain and Symptom Management. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Practice. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behavior and Immunity. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht SR, Mohamed-Ali V. Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain, Behavior, and Immunity. 2002;16:774–784. doi: 10.1016/s0889-1591(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Ussher M. Smoking, cortisol and nicotine. Int J Psychophysiol. 2006;59:228–235. doi: 10.1016/j.ijpsycho.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Willemsen G, Owen N, Flower L, Mohamed-Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clinical Science. 2001;101:185–192. [PubMed] [Google Scholar]
- United States Department of Health and Human Services (USDHHS) Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. [PubMed] [Google Scholar]
- Van den Beuken-van Everdingen M, Peters ML, de Rijke JM, Schouten HC, van Kleef M, Patijn J. Concerns of former breast cancer patients about disease recurrence: a validation and prevalence study. Psycho-Oncology. 2008;17:1137–1145. doi: 10.1002/pon.1340. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Kudielka BM, Preckel D, Hanebuth D, Fischer JE. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behavior and Immunity. 2006;20:40–48. doi: 10.1016/j.bbi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Lowe GDO, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. European Heart Journal. 2005;26:1765–1773. doi: 10.1093/eurheartj/ehi183. [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Whincup PH, Rumley A, Lowe GDO. Inter-relationships of interleukin-6, cardiovascular risk factors and the metabolic syndrome among older men. Journal of Thrombosis and Haemostasis. 2007;5:1637–1643. doi: 10.1111/j.1538-7836.2007.02643.x. [DOI] [PubMed] [Google Scholar]
- Webster JC, Cidlowski JA. Downregulation of the glucocorticoid receptor. A mechanism for physiological adaptation to hormones. Annals of the New York Academy of Sciences. 1994;746:216–220. doi: 10.1111/j.1749-6632.1994.tb39238.x. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annual Review of Immunology. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]