Abstract
Myocardial infarction (MI) produces a collagen scar, altering the local microenvironment and impeding cardiac function. Cell therapy is a promising therapeutic option to replace the billions of myocytes lost following MI. Despite early successes, chronic function remains impaired and is likely a result of poor cellular retention, proliferation, and differentiation/maturation. While some efforts to deliver cells with scaffolds attempt to address these shortcomings, they lack the natural cues required for optimal cell function. The goal of this study was to determine whether a naturally-derived cardiac extracellular matrix (cECM) could enhance cardiac progenitor cell (CPC) function in vitro. CPCs were isolated via magnetic sorting of c-kit+ cells and were grown on plates coated with either cECM or collagen I (COL). Our results show an increase in early cardiomyocyte markers on cECM compared to COL, as well as corresponding protein expression later. CPCs show stronger serum-induced proliferation on cECM as compared to COL, as well as increased resistance to apoptosis following serum-starvation. Finally, a microfluidic adhesion assay demonstrated stronger adhesion of CPCs to cECM compared with COL. These data suggest that cECM may be optimal for CPC therapeutic delivery, as well as provide potential mechanisms for the shortcomings in naked cell therapy.
Keywords: ECM (extracellular matrix), Progenitor Cell, Cell adhesion, Cell proliferation, Gene expression
1. Introduction
Cardiovascular disease is the leading cause of death in the United States. There were an estimated 1.5 million cases of myocardial infarction (MI) in 2011[1]. Following MI in animal models, there is a 40-60% reduction in myocyte number in the myocardium with billions of myocytes being lost within the first several days [2, 3]. These myocytes are not replaced and this results in extensive inflammation and fibrosis, leading to loss of contractility. Fibroblasts within the damaged tissue proliferate and secrete high levels of collagen to prevent the heart from rupture, ultimately leading to heart failure. The only comprehensive cure for heart failure is cardiac transplant, which is greatly limited by the number of available donor hearts. This has forced clinicians to find new ways to improve chronic cardiac function such as the use of beta-blockers, angiotensin receptor blockers, and other pharmacological interventions [4]. While these therapies may sustain cardiac function, they do little to regenerate functional tissue.
Cellular therapy has shown early success as a potential treatment for improving acute cardiac function post-MI [5-8]. Mesenchymal stem cell injection into the infarcted myocardium shows decreased fibrosis and improvement in certain heart function parameters [5, 9]. While exciting, this finding was not due to reconstitution of the myocardium, but attributed to increased angiogenesis [8]. In 2003, the heart was found to have a population of stem/progenitor cells capable of cardiac differentiation, termed cardiac progenitor cells (CPCs) [10]. These cells are clonogenic, self-renewing, and capable of differentiation into the 4 major cardiac cell types (cardiomyocyte, endothelial, smooth muscle, fibroblast), [11, 12]. For these reasons, and because CPCs do not form teratomas in cell therapies, they are a good candidate for repairing the myocardium. Intramyocardial injections of CPCs have shown improvements in cardiac function after injury, potentially through myocardial regeneration [10-14]. Phase 1 clinical trials are underway with injection of autologous CPCs and are promising [15]. However, while many cell therapy trials show acute success, improvements in chronic function remain a challenge. This is most likely due to the fact that local delivery of cells faces several shortcomings such as poor retention of the cells in the myocardium, reduced survival, and poor differentiation and maturation of implanted cells [16]. Due to these issues, the mechanisms by which positive effects have been seen are controversial (i.e. paracrine factors vs. regeneration) [9, 16].
Cellular phenotypes are influenced by their microenvironment. Matrix stiffness [15, 17, 18], organization [19], and biochemistry [19-21] have been shown to influence cell fate. These signals are transduced intracellularly through receptor-ligand interactions, mainly integrins [22]. It is important to consider that these trends are likely to be matrix and cell type specific. By providing cells with an ideal microenvironment, it is plausible that the cells will have a more favorable outcome (i.e. improved survival, proliferation, differentiation). This is achieved either in vitro by culturing cells on a matrix or in vivo by administering cells within a matrix that can assemble into a 3-dimensional scaffold. Injectable biomaterials have the potential attractive cell delivery vehicles as they can provide a suitable microenvironment and can potentially be delivered via minimally invasive catheters [23]. Cellular therapies have been combined with various matrices to treat MI [24-30]. The major disadvantage of the currently used biomaterials for myocardial regeneration is that they lack the complexity and specificity of the native myocardial extracellular matrix [31].
In this study, a naturally-derived, porcine cardiac extracellular matrix (cECM) was examined for the ability to improve CPC function. Our hypothesis was centered on the fact that this would mimic the biochemical cues of a healthy myocardium, while collagen would represent both the diseased area and a commonly used cell delivery vehicle [30]. Our results demonstrate that CPCs prefer the naturally-derived cECM over collagen as measured by cardiomyogenic gene expression, cell survival, proliferation, and adhesion.
2. Materials and Methods
2.1 CPC isolation
CPCs were isolated from adult male Sprague-Dawley rats (about 250g) by removing the heart and homogenizing the tissue, as approved by Emory University’s Institute Animal Care and Use Committee. The tissue homogenate was further digested with type-2 collagenase (1 mg/mL in Hank’s Balanced Salt Solution (HBSS); Worthington Biochemical) and passed through a 70 μm filter. Cells were then incubated with Dynabeads (Dynal) conjugated to a c-kit antibody (Santa Cruz H-300) prior to magnetic sorting. Sorted cells were plated on a T-75 tissue culture flask and expanded to confluence. Following isolation, CPCs were characterized by flow cytometric analysis of c-kit (Santa Cruz H-300), multi-drug resistance protein (MDR; Santa Cruz H-241), Gata-4 (Santa Cruz H-112) and Nkx2.5 (Santa Cruz H-114). Only clones with >90% c-kit expression were used for subsequent studies. Supplemental figure 1 shows representative flow cytometry histograms for c-kit, MDR, Gata-4, and Nxk2.5.
2.2 Decellularized cardiac extracellular matrix (cECM) generation
Decellularized porcine ventricular extracellular matrix was obtained and processed into a cell culture coating as previously described [32, 33]. Briefly, porcine ventricular tissue was isolated and cut into small rectangular pieces, rinsed in phosphate buffered saline (PBS, Fisher), and decellularized using 1% sodium dodecyl sulfate (SDS, Fisher) for 4-5 days. The decellularized cECM was then rinsed with Triton X-100 (Integra Chemical Company) for 30 minutes, DI water overnight, frozen at −80°C overnight, lyophilized (Labconco) overnight, and milled into a fine powder. The powder was digested using pepsin at 1 mg/ml in 0.1M HCl (Fisher) for at least 54 hours prior to use, as modified from a previously published protocol, at a ratio of 10:1 of ECM matrix to pepsin [34]. The material was then raised to a basic pH by adding 1 M NaOH (Fisher), and brought to a salt concentration of 1× PBS through the addition of 10X PBS. Then, the material was brought to physiological pH of 7.4 using HCl and NaOH, and diluted to 6 mg/ml using 1× PBS. The cECM was then frozen at −80°C overnight, lyophilized for 24 hours (Labconco) and stored at −80°C prior to use.
2.3 Cell culture
Matrix solutions were made by reconstituting cECM in sterile water and then diluting to 1 mg/mL in 100 mM acetic acid. Collagen I (COL; rat tail, Invitrogen) was diluted to 1 mg/ml in 100 mM acetic acid. Tissue culture plastic plates were coated with cECM or COL and incubated for 1 hour at 37°C to allow adsorption. Coated plates were then washed twice with 1× PBS to remove acetic acid. CPCs were seeded on top of the coated plates and incubated in the appropriate medium for the desired timepoints (see subsequent methods sections for details specific to each experiment).
2.4 RNA and protein isolation
Cell culture was performed as described above in 6-well tissue culture plastic plates coated with 500 μl of the appropriate matrix. Two wells were prepared for each condition with 5 ×105 cells per well. Cells were cultured in treatment media (Ham’s F−12 (Mediatech) + 0.1 μg/mL bFGF (Sigma) + 1× ITS (Cellgro) + 1× Penicillin-Streptomycin-Glutamine (P/S/G, Cellgro)) and media was exchanged every 48 hours. Cells were harvested 2 and 7 days following plating with Trizol (Invitrogen) for isolation of RNA and protein. The Trizol solution was frozen at −80°C until RNA isolation was performed. RNA and protein extraction were performed according to the manufacturer’s protocol. Samples were stored at −20°C.
2.5 Reverse transcription and quantitative real-time PCR
RNA quantification and purity was determined by absorbance readings at 260 and 280 nm by a BioTek Synergy2 Spectrophotometer. Reverse transcription was performed with M-MLV (Invitrogen) as follows. Samples were prepared with 2 μg RNA and 0.1 μg hexamers (Thermo Scientific), 0.1 μg oligo dTs (Fermentas), 25 nmol dNTPS (Fermentas) and RNase free water for a final volume of 12 μL. No-template controls were performed by replacing RNA content with RNase free water. Samples were heated at 65°C for 5 minutes to denature the RNA, followed by 25°C for 10 minutes to allow hexamers and oligos to anneal. First strand buffer (1× final concentration, Invitrogen), 0.2 μmol DTT (Invitrogen), 40 units RNaseOUT Inhibitor (Invitrogen) and 200 units M-MLV (Invitrogen) were added to each sample. Samples were heated at 37°C for 60 minutes for reverse transcription, followed by 70°C for 15 minutes to inactivate the enzyme. cDNA products were stored at −20°C.
Gene expression was measured by quantitative real-time PCR on an Applied Biosystems StepOne Plus Real-Time PCR System. Reaction mixtures contained 7.5 μL Power SYBR Green (Invitrogen), 5.1 μL RNase free water (Hyclone), 1.4 μL of the appropriate primer at 1μM (IDT) and 1 μL 1:5 cDNA (total volume = 15 μL). The running protocol was 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds, for all SYBR Green Primers. A melt-curve was calculated at 2°C intervals with the same cycling conditions. Each sample was run in duplicate. A Taqman gene expression assay was performed to quantify GAPDH expression (Applied Biosystems; 50°C for 2 minutes, 95°C for 10 minutes and then 40 cycles of 95°C for 15 seconds followed by 60°C for 1 minute). Results are normalized to GAPDH and expressed as fold change for cECM relative to COL (ΔΔCt). Primer sequences are listed in Table 1.
Table 1.
Real-time PCR Primers
| Name | Forward 5′-3′ | Reverse 5′-3′ |
|---|---|---|
| α-Myosin Heavy Chain (α-mhc) |
AACGCCCAAGCCCACTTGAA | CATTGGCACGGACTGCGTCA |
| troponinT (tropT) |
AAGGCCAAAGTCACCGGGCG | TCGGGTGCCTGGCAAGACCT |
| troponinC (tropC) |
GATCTCTTCCGCATGTTTGACA | TGGCCTGCAGCATCATCTT |
| gata-4 | ACCTGCTACAGCAGGGTTGGT | TTCTAGCACAACTGCAAGCATGGC |
| nkx2.5 | CAAGTGCTCTCCTGCTTTCC | GGCTTTGTCCAGCTCCACT |
| Smooth muscle (sm) α-actin |
CCCAGATTCAGGAACAGCAT | GTTAGCAAGGTCGGATGCTC |
| Smooth muscle (sm) 22α |
AGCCAGTGAAGGTGCCTGAGAAC | TGCCCAAAGCCATTAGAGTCCTC |
| Fibroblast specific protein 1 (fsp) |
GAGGAGGCCCTGGATGTAAT | CTTCATTGTCCCTGTTGCTG |
| von Willebrand Factor (vwf) |
CCCACCGGATGGCTAGGTATT | GAGGCGGATCTGTTTGAGGTT |
| tie2 | TGCCACCATCACTCAATACCA | AGGCTGGGTTGCTTGATCCTp |
For array studies, cDNA from 3 separate studies was pooled to a total of 1 μg per plate. Extracellular matrix and adhesion molecule gene array plates were purchased from Qiagen (SABiosciences) and gene levels were normalized to beta-actin housekeeping gene. Data were compared using the logΔΔCt to plot COL vs. ECM and changes ± 2.5-fold were considered significantly up- or downregulated.
2.6 Western blot
Protein quantification was performed by microBCA (Thermo Scientific) according to the manufacturer’s protocol. Samples were prepared by adding 30 μg protein to appropriate amounts of 3× Laemmli buffer and water to yield a final volume of 25 μL and then boiled for 8 minutes at 95°C. Each sample was then loaded on 12% SDS-PAGE gel. NovexSharp (Invitrogen) protein ladder was loaded at 15 μL. Electrophoresis was performed and gels were transferred to a nitrocellulose membrane. Membranes were immediately blocked with 5% milk in Tris-buffered saline with 1% Tween-20 (TBS-T) overnight at 4°C. Membranes were washed 3 times in 1× TBS-T, then immersed in a 1:1000 primary antibody (Nk×2.5: Santa Cruz H−114, rabbit; Gata−4: Santa Cruz H−112, rabbit; GAPDH: Santa Cruz FL−335, rabbit). All antibody solutions were made in 5% milk in 1× TBS-T and incubated with membranes overnight at 4°C prior to 3 washes with 1× TBS-T. Membranes were incubated at room temperature for 1 hour in 1:5000 secondary antibody. For all cases, the secondary antibody was HRP-conjugated goat anti-rabbit (Bio-rad). Membranes were exposed on film or Kodak imager and results were quantified with ImageJ and are expressed as fold change for cECM/COL.
2.7 Determination of proliferation
Cell culture plates were prepared as described above with 125 μL of the appropriate matrix per well of a 24-well plate. CPCs were seeded at a density of 2000 cells per well to allow growth, while removing the possibility of contact inhibition [17]. The cells were incubated in serum-rich treatment medium (Ham’s F-12 (Mediatech) + 10% FBS (Hyclone) + 0.1 μg/mL bFGF (Sigma) + 1× ITS (Cellgro) + 1× P/S/G (Cellgro)). Following 48 hour incubation, the medium was discarded and the cells were lifted from culture plates with TrypLE Express (Invitrogen). The cell solution was diluted 1:100 in Isoton II (Beckman Coulter) and cells were immediately counted in triplicate in a Coulter Counter. Results are expressed as fold change in cell number as final count/initial seed number.
2.8 Microfluidic adhesion assays
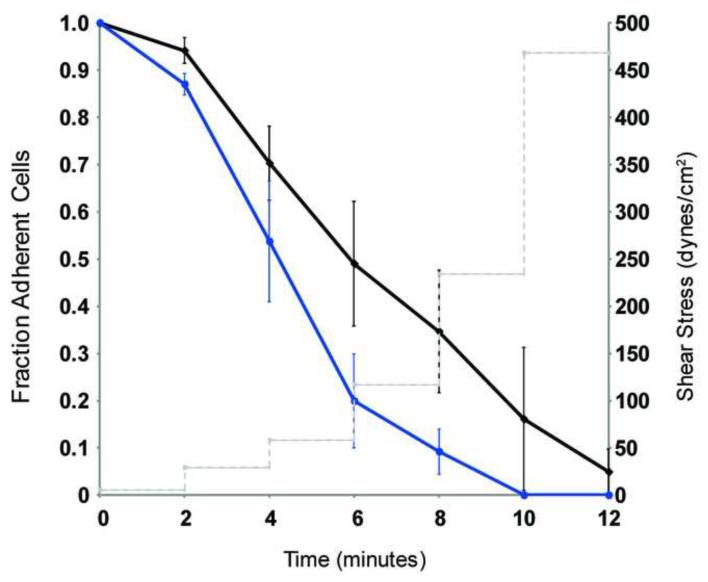
Individual channels of the microfluidic devices [35] were filled simultaneously with protein solutions of interest, including collagen I (Invitrogen, rat tail), cECM, fibronectin (BD Biosciences, human) and laminin (BD Biosciences, mouse) at 10 μg/mL. Devices were then incubated for 3 hours at 37°C; the channels were rinsed with PBS and blocked with 2% BSA for 0.5 hours at 37°C. CPCs were then seeded at 1×106 cells/mL in growth media (Ham’s F-12 (Mediatech) + 0.1 μg/mL bFGF (Sigma) + 10% FBS (Hyclone) + 1× P/S/G (Cellgro)) and allowed to adhere for 3 hours at 37°C, and then subjected to step-wise increments of shear stresses from 0 to ~470 dynes/cm2 for 12 minutes. Results are reported as the fraction of adherent cells over time normalized to starting cell number.
2.9 Annexin V staining
Cell culture plates were prepared as described above with 125 μL of the appropriate matrix per well of a 24-well plate. CPCs were seeded at a density of 50,000 cells per well, in triplicate. Cells were cultured in serum-free media (Ham’s F-12 (Mediatech) + 1× P/S/G (Cellgro)). Cells that were not serum-deprived were stained with AnnexinV Alexa Fluor® 647 (Invitrogen) as a negative control. After 12 hour incubation, media from each well was collected and TrypLE Express (Invitrogen) was added to lift the cells from the well. The lifted cells were added to the respective collected media. Cells were centrifuged at >1500 rpm for 5 minutes. The supernatant was removed and the cells were washed with cold 1× PBS. After centrifugation at >1500 rpm for 5 minutes, the supernatant was again removed and the cells were resuspended in 50 μL of Annexin binding buffer. The cell solution was then incubated with 5 μL of AnnexinV Alexa Fluor® 647 (Invitrogen) at room temperature for 15 minutes. After incubation, 400 μL of Annexin binding buffer was added and samples were mixed gently and kept on ice. Cells were analyzed immediately by flow cytometry.
2.10 Statistical Analysis
Graphpad Prism 3 Software was used as indicated in figure legends. Student’s t-test with Welch’s Correction or paired t-test was performed. P-values of less than 0.05 were considered significant.
3. Results
3.1 Gene expression analysis of CPC differentiation
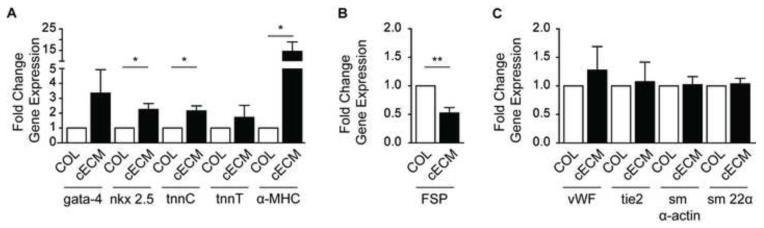
In order to measure the effects of cECM on CPCs cardiogenic differentiation, real-time quantitative PCR transcription was performed. CPCs were analyzed for each potential lineage: gata-4, nkx-2.5, α-myosin heavy chain (α-mhc), troponinC, troponinT (cardiomyocyte); smooth muscle (sm) α-actin, sm22α (smooth muscle); von Willbrand Factor (vwf), tie2 (endothelial); and fibroblast-specific protein 1 (FSP; fibroblast). As shown in Figure 1A, culture of CPCs on cECM significantly (p<0.05) increased the expression of early cardiomyocyte markers, nkx-2.5 (2.3 ± 0.4-fold), α-mhc (14.6 ± 4.4-fold), and troponinC (2.4 ± 0.2-fold) as compared with cells cultured on collagen I (COL). While there was a trend for increased gata-4 and troponinT, they did not reach significance. In addition, there was a significant decrease in the expression of the fibroblastic marker FSP (0.5 ± 0.1-fold) in cells cultured on cECM compared with COL (p<0.01, Figure 1B). No significant differences were seen for the selected endothelial and smooth muscle markers as shown in Figure 1C. When extended to day 7, there was no further increase in smooth muscle or fibroblastic markers (data not shown). Further, to determine whether this response was tissue-specific, we examined gene expression changes in cells cultured on adipose-derived ECM as described in [36]. In contrast to cECM, there was no significant increase in any cardiac marker in cells cultured on adipose ECM compared with collagen (Supplemental Figure 2). Additionally, the decrease seen in FSP in cells cultured on cECM was not seen in cells cultured on adipose ECM. These data suggest that cells seeded on cECM demonstrate enhanced differentiation or maturation toward the cardiac lineage and decreased maturation toward the fibroblastic lineage as compared to COL.
Figure 1. Cardiogenic gene expression of CPCs cultured on cECM and COL.
Cardiac progenitor cells (CPCs) were cultured on cECM (black bars) or COL (white bars) for 2 days and cardiomyocyte (A), fibroblast (B) and endothelial and smooth muscle (C) lineage markers evaluated by qPCR. Results were normalized to GAPDH and expressed as a fold change for cECM over COL (ΔΔCt) and reported as a mean ± SEM. Unpaired student’s t-test; *p<0.05, **p<0.01, n=4-6. COL = collagen, cECM = cardiac decellularized extracellular matrix, tnn = troponin, mhc = myosin heavy chain, fsp = fibroblast specific protein, vwf = von Willebrand factor, sm = smooth muscle, GAPDH = Glyceraldehyde-3-phosphate dehydrogenase.
3.2 Western Analysis of CPC cardiomyogenesis
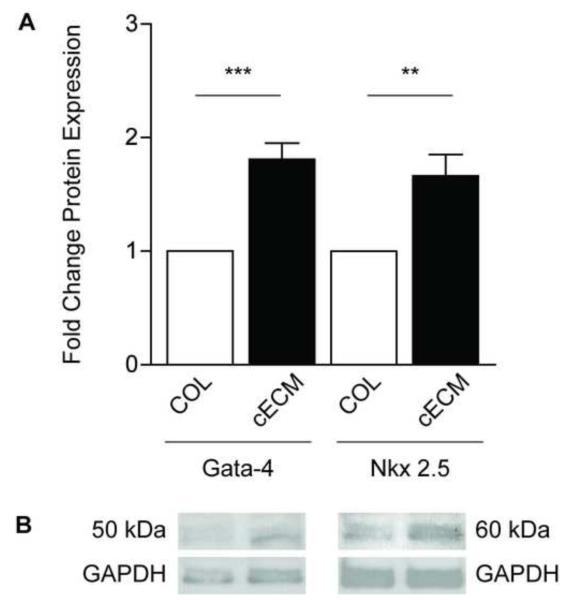
To determine if gene expression changes were followed by protein changes, Western blot analysis was performed on protein samples collected from CPCs cultured on either cECM or COL for 7 days. Figure 2 shows representative blots (b) probed for the cardiomyocyte markers Gata-4 and Nkx2.5 along with grouped data (a). CPCs cultured on cECM had significantly higher (p<0.001) levels of Gata-4 as compared to COL (1.8 ± 0.1-fold) after normalization to GAPDH. A similar increase was seen for Nkx2.5 (1.6 ± 0.2-fold; p<0.05) in cells cultured on cECM compared to COL. These data demonstrate that significant changes in cardiomyogenic gene expression lead to subsequent increases in protein levels.
Figure 2. Western analysis of cardiac protein expression.
Protein was isolated from cardiac progenitor cells cultured on cECM (black bars) or COL (white bars) for 7 days. Grouped data (A) and representative blots (B) are shown as mean ± SEM. Images were quantified with ImageJ and protein expression was normalized to GAPDH. Unpaired student’s t-test; **p<0.01, ***p<0.001; n=4-6. COL = collagen, cECM = cardiac decellularized extracellular matrix, GAPDH = Glyceraldehyde-3-phosphate dehydrogenase.
3.3 Proliferation of CPCs
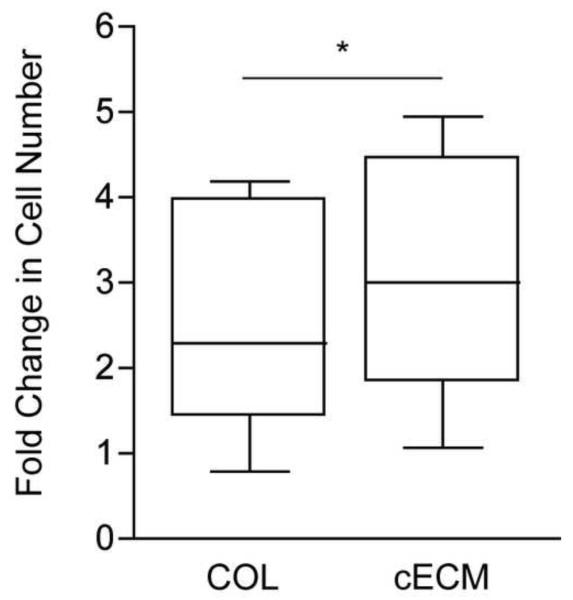
In order to determine the effect of cECM on CPC proliferation, cells were cultured on cECM or COL in the presence of serum and cell count was measured 48 hours later. As the grouped data in Figure 3 demonstrate, there was a significant (p<0.05) 35% increase in proliferation of CPCs on cECM when compared to those seeded on COL (cECM = 2.9-fold over initial seeding, COL = 2.3-fold). In addition, we examined this response using adipose ECM to determine the role of tissue-specificity. As the data in supplemental figure 2 demonstrate, there was no significant increase in proliferation in cells cultured on adipose ECM compared with collagen. These data show that cECM is a better substrate for CPC proliferation as compared to COL.
Figure 3. Serum-induced proliferation of CPCs.
Cardiac progenitor cells (CPCs) seeded on cECM (right) or COL (left) were cultured for 48 hours. Fold change in cell number was calculated as the final cell count divided by the number of cells seeded as determine by Coulter counting. Box and whisker plots show the mean, quartiles ± SEM. Paired student’s t-test, *p<0.05; n=7. COL = collagen, cECM = cardiac decellularized extracellular matrix.
3.4 Survival of CPCs
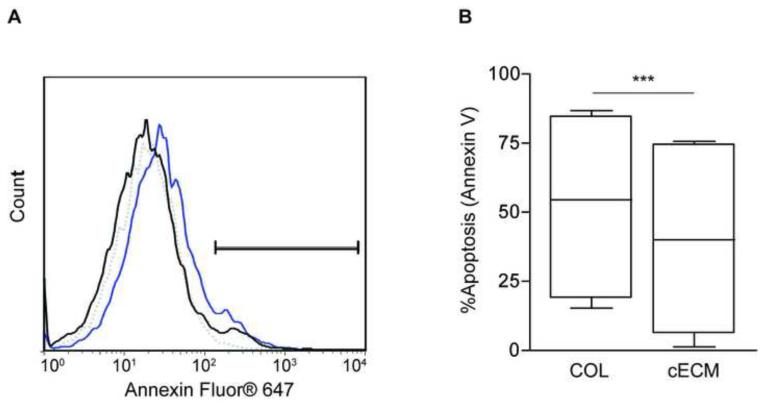
To evaluate the effects of cECM on CPC survival, Annexin V staining was performed after CPCs seeded on either cECM or COL were serum-starved for 12 hours. Figure 4a shows a representative histogram of Annexin V staining, illustrating decreased apoptosis for CPCs cultured on cECM as compared to COL. Grouped data demonstrate a significant reduction in percent apoptosis for cells cultured on cECM (40% ± 14%), as compared to COL (53% ± 14%; p<0.001; Figure 4b). These data show a significant improvement in survival for cells cultured on cECM as compared to COL.
Figure 4. Survival of serum-deprived CPCs cultured on cECM and COL.
Cardiac progenitor cells (CPCs) cultured on cECM or COL were serum-deprived for 12 hours, then harvested for Annexin V staining. Representative histograms of Annexin V staining for CPCs cultured on cECM (black) and COL (blue) are shown (A). Gating was based on CPCs that were not serum-deprived (dotted line). Box and whisker plots (B) show mean, quartile ± SEM for grouped data. Paired student’s t-test; ***p<0.001; n=6. COL = collagen, cECM = cardiac decellularized extracellular matrix.
3.5 Adhesion of CPCs
To determine if CPCs adhered more strongly to cECM or COL, microfluidic adhesion assays were performed under increasing levels of shear stress. The grouped data in Figure 5 show that CPCs cultured on cECM adhere more strongly as compared to CPCs cultured on COL as represented by the higher fraction of adherent cells over increasing shear stresses. The force at which 50% of the cells were removed was 120 dynes/cm2 for cells cultured on cECM compared with 60 dynes/cm2 for COL. CPCs cultured on fibronectin and laminin adhere to their substrate with a similar strength to CPCs cultured on COL (data not shown). These data provide evidence that CPCs adhere more tightly to cECM as compared to COL.
Figure 5. CPC adhesion to cECM and COL.
Cardiac progenitor cell (CPC) adherence to cECM (black) and COL (blue) was determined by microfluidic adhesion assay where cells were subjected to increasing shear stresses. Grouped data shows mean ± SEM fraction of adherent cells (left-axis) over time with increasing shear stresses (dotted line, right-axis). CPC adherence to fibronectin and laminin were similar to COL (data not shown).
3.6 PCR-array data
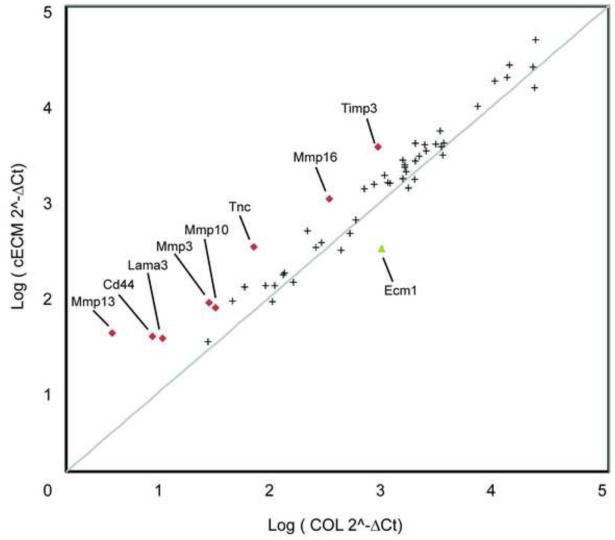
In order to investigate the global regulation of extracellular-matrix related proteins in CPCs, cells were cultured on cECM or COL for 48 hours and samples were pooled from 3 experiments for gene array analysis and presented in Figure 6. Extracted data demonstrated >2.5-fold increases in lama3 (laminin 5; 3.55-fold), mmp3 (3.20-fold), mmp10 (2.46-fold), mmp13 (11.79-fold), mmp16 (3.25-fold), timp3 (4.17-fold), and tnc (TenascinC; 4.92-fold). Interestingly, col1a1 was detected in COL cultured CPCs but not in cECM-cultured cells. CD44, a receptor for hyaluronic acid was also increased in cECM compared to COL (4.63-fold). These data demonstrate that cells cultured on cECM display enhanced expression of collagenases, as well as increased expression of laminin, suggesting extensive remodeling of the extracellular environment.
Figure 6. Extracellular matrix and adhesion molecule PCR array.
Cardiac progenitor cells (CPCs) were cultured on either cECM (y-axis) or COL (x-axis) for 2 days and 3 samples from each condition were pooled for a total of 1 μg cDNA. PCR array plates were purchased from Qiagen (SABiosciences). Results are presented as logΔΔCt and considered significantly up- (red diamonds) or down- (green triangle) regulated for ± 2.5-fold changes for cECM compared to COL. Gray line represents no change in gene expression between conditions
4. Discussion
Adult stem cell delivery is a promising therapy that has shown improvements in early clinical trials. Despite these exciting preliminary trials, problems still exist in the retention, survival, and maturation of implanted cells [16]. While natural and synthetic materials may have the potential to improve some of these parameters, great care must be taken to ensure that the implanted cells receive the proper signals to support their function. In this report, we identify a naturally-derived, decellularized cardiac extracellular matrix (cECM) that is capable of enhancing cardiac progenitor cell (CPC) adhesion, growth, survival, and maturation as compared with the commonly used matrix collagen I (COL). While the original 3D structure is lost when preparing the liquid form of cECM, the liquid matrix still retains ECM proteins and peptide fragments, and thus many of the original biochemical cues, providing a mimic of the adult heart ECM [32, 33].
In order to repair the infarcted myocardium, it is expected that new cardiomyocytes must be generated and that the continued deposition of a collagen scar must be slowed. CPCs are capable of differentiating into all cardiac cell types [11, 12], though their function can be enhanced by soluble factors and genetic manipulation. Here, we show that culture of CPCs on cECM increases the expression of early cardiac markers after 2 days of treatment [37]. Of these markers, two (Gata-4 and Nkx2.5) were chosen for examination at the protein level after 7 days of treatment. A significant increase in Gata-4 and Nkx2.5 protein levels was demonstrated through Western blot. While we do not present evidence of functional cardiomyogenesis, we show cECM increases the propensity of CPCs to become cardiomyocyte precursors. It is unlikely that this short time period would show significant differences in cardiomyogenesis. Longer periods of genetic analysis were not performed as the initial matrix is likely replaced after 3 days [38, 39]. In fact, our PCR array data suggest that CPCs cultured on cECM may actively replace collagen in the matrix via upregulation of collagenases such as MMP3,10,13, and 16. Additionally, there was a >3.5-fold increase in laminin, as well as smaller increases in collagen IV and fibronectin (~1.5-2-fold) suggesting the deposition of a more complex extracellular matrix. Increases in these cardiac markers, specifically the more mature α-MHC and troponins, are well associated with increased maturation of progenitor cells [40, 41]. MHC and troponin expression is known to follow Nkx2.5 and Gata-4 in development, and cells with increased expression of these markers demonstrate improved regenerative capacity. In agreement with these results, we previously demonstrated enhanced maturation of human embryonic stem cell derived cardiomyocytes when plated on cECM compared to gelatin, as indicated by increased multi-cellular organization and desmosome formation [32]. While this is an exciting result, human embryonic stem cells have the disadvantage of potentially forming teratomas in vivo.
No statistically significant differences in the expression of smooth muscle and early endothelial genes were observed, suggesting that cECM treatment is not more or less likely to push CPCs toward these lineages than COL. Lack of maturation to the endothelial and smooth muscle lineage may affect implanted cardiomyocyte function as endothelial cells play a critical role in cardiomyocyte survival. While this may be a concern, recent studies from our laboratory and others demonstrate robust endothelial and smooth muscle cell population of implanted matrices, and thus implanted cells may still receive the signals they require [20, 33, 42]. Additionally, a lack of increase in endothelial and smooth muscle markers does not necessarily mean CPCs do not differentiate/mature to these lineages, as our comparisons are to COL. Of significant note in our study, is the reduction in fibroblast specific protein 1 (FSP), a fibroblastic marker, after two days of culture on cECM compared to COL. Fibroblasts are largely responsible for depositing collagens in the myocardium following MI, and a reduction in FSP may correlate to a reduction in collagen production; however this was outside the scope of this study. Once again, our PCR data confirms this potential phenotype through upregulation of collagenases and deposition of more contractile collagen isoforms (collagen III and IV).
Our studies also demonstrate cECM to be a better substrate for proliferation of CPCs than COL. This may be important for cell transplantation, when many cells are lost or diffuse away from the site of injection [16]. While most matrices would inhibit this loss, enhanced proliferation is an added benefit as it may increase the likelihood for tissue repair by brute numbers. Unlike embryonic stem cells, CPCs have not been shown to induce tumor formation upon injection making their enhanced proliferation less of a concern [43]. Aside from cECM’s conceivable use as a delivery vehicle, another potential use for cECM is for the expansion of these cells in vitro following tissue harvest. Current clinical protocols for autologous CPC therapy call for the removal of patient tissue biopsies, followed by isolation and expansion of the c-kit+ fraction. This now takes up to 3 months for patients to receive their own cells back, and tissue damage/loss is still occurring in this time [44, 45]. Culture of cells on cECM during expansion could lead to faster implantation times for patients and improve functional recovery. In conjunction with enhanced proliferation, cECM provides protection to CPCs under stress from serum-starvation. A 12% reduction in apoptosis as seen in our studies on cECM compared to COL is quite significant. In a clinical setting, this could translate to more than 100,000 additional viable cells as a patient receives a dose of 1 million cells [44]. These results show promise for future work, as CPCs injected within a cECM hydrogel into the infarcted myocardium may be better primed to survive the harsh conditions than cells injected with COL.
This study compares cECM to COL and does not include other matrix components, or the use of tissue culture plastic as a control. COL was chosen due to its abundance in both the myocardium following infarction, as well as its use as a cell delivery vehicle. In future work, it would be relevant to examine the effects of other single protein matrix components on CPC differentiation proliferation and survival. Collagen IV and laminin are present in CPC niches, while collagen III and fibronectin are also present in the myocardium post-MI [46]. Additionally, how cells respond on tissue culture plastic was not examined in this study as the response would be largely irrelevant for the reason discussed above. We did not examine how these cells respond in 3-dimensional culture, and it is possible that behaviors do not mimic results seen in 2-dimensional coating experiments. While this may mimic the conditions under which CPCs would be cultured if cECM is used for pre-conditioning, it does not adequately address the proposed in vivo model in which CPCs are injected with cECM to form a 3-dimensional hydrogel in vivo. Moreover, there is also a possibility that CPCs may be cultured in 3D using this material and may behave quite differently than seen in our study.
As noted previously, one of the limitations of stem cell injection in the infarcted myocardium is the lack of retention of the cells [16]. Microfluidic adhesion assay shows that CPCs adhere more strongly to cECM than COL. Additionally, other single protein ECM components were tested (laminin and fibronectin) and similar adhesion as COL was seen (data not shown). These results suggest that CPCs may interact more tightly with the more complex cECM than single matrix proteins like COL, which may play a role in the other findings in this study. It is unclear if the forces used in this study represent the post-infarct tissue environment as the assay is merely intended to demonstrate cell-material interaction strength. Tighter adhesion to ECM is shown to improve survival, proliferation, and growth of cells as this may lead to enhanced integrin activation [47]. While this study does not determine mechanistic pathways, integrins such as the β1 integrin are critical for cardiac development. Modulation of the β1 integrin negatively affects cardiomyocyte function, post-injury healing, and stem cell differentiation [48, 49]. Additionally, in mesenchymal stem cells, while β1 regulates adhesion to the ECM, αvβ3 may regulate differentiation [50, 51]. Cardiac progenitor cells exist in niches that are rich in laminin, and thus a more complex mix of integrins may regulate different functions [52]. Full compositional characterization of the cECM has not yet been achieved, though initial mass spectrometry studies determined the presence of collagens I-VI, elastin, fibrinogen, fibronectin, laminin, fibrillin-1, lumican, and fibulin-3 and -5 [32]. These components are not surprising given that the myocardium is known to contain collagens I and III, laminin, fibronectin, and elastin [53, 54]. Finally, our array data demonstrates a substantial (>4-fold) increase in tenascinC gene expression. While the role of tenascin in CPCs is unstudied, it plays an important role in the adhesion and mitogen responses of hematopoetic progenitors and this study identifies a potential role for its involvement in the cECM response [55].
Previously, the successful use of cECM as an injectable biomaterial has been established [33, 56]. When injected into the rat myocardium, cECM has shown an immune response comparable to implanted decellularized small intestine submucosa and syngeneic muscle implants [56]. Numerous xenogeneic decellularized ECMs have been cleared by the FDA, are considered biocompatible, and are in clinical use [57]. Appropriate processing however needs to be performed to avoid significant residual DNA and detergents, and xenogeneic antigens are always of concern. Our decellularized cardiac matrix, however, appears to have excellent biocompatibility though further antigenicity and biocompatibility tests are underway prior to clinical translation. Because it is digested rather than in a patch form, it can be used as an injectable hydrogel that self-assembles into a porous and fibrous scaffold in vivo, opening up the possibility of minimally invasive delivery [58]. In fact, recent studies demonstrate the cECM hydrogel can be delivered as a liquid to the myocardium of pigs through a minimally invasive, transendocardial catheter injection, and subsequently form a scaffold in vivo [56]. This liquid form requires the use of pepsin, which does remain in the material, although it is inactivated in the process of the pH adjustment and has previously been used in other FDA approved products. Interestingly, when delivered intramyocardially in rats, cECM demonstrated improved function compared with untreated animals, with an observed increase in cardiomyocytes [56]. The source of the myocytes was not determined, though taken together with our studies it may suggest the mechanism of enhanced endogenous CPC proliferation and differentiation. While not done in this study, stem cells have been delivered to patients via intramyocardial catheters and thus this approach may have great clinical significance. For the myocardium, small intestine submucosa (SIS) and urinary bladder matrix have been examined for treating cardiac wall defects and MI. Although SIS and bladder matrix patches, and a SIS emulsion have resulted in cell infiltration, [33, 59, 60] they have also caused undesirable tissue formation such as adipose and even cartilaginous tissue, [59] potentially due to inappropriate cell-matrix interactions. While the ECM contains similar components across tissues, each tissue does have its own distinct combination of ECM components. Our data with cECM and preliminary results with adipose ECM demonstrate the importance of tissue-specific ECM cues regulating progenitor cell growth, survival, differentiation, and adhesion.
5. Conclusion
We show in this study that when used to culture cardiac progenitor cells in a 2-dimensional culture, a naturally-derived, decellularized cardiac extracellular matrix enhanced their adhesion, maturation, proliferation, and survival compared with collagen alone. Cells respond to their environments and single matrix component coatings and delivery vehicles likely lack the complexity needed to promote proper cell function. Moreover, it is likely the cells need local cues and thus non-tissue-specific materials may not be optimal for cell delivery. These data have implications in both the ex vivo culture of these cells for implantation into patients, as well as the delivery of these cells to the infarcted myocardium to protect them from the overabundance of collagen in the post-MI heart. This is the first study examining the interaction between CPCs and a naturally-derived extracellular matrix and may hold great promise for enhancing the function of this clinically-relevant cell type.
Supplementary Material
Acknowledgements
Funding sources: This work has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201000043C to MED, a DuPont Young Faculty Award to HL, NHLBI R21HL104493 to KLC, an American Heart Association predoctoral fellowship 11PRE7840078 to AVB. This material is also based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1148903 to KMF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Christman has an equity interest in Ventrix, Inc., a company that may potentially benefit from the research results, and also serves on the company’s Scientific Advisory Board. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olivetti G, Capasso JM, Meggs LG, Sonnenblick EH, Anversa P. Cellular basis of chronic ventricular remodeling after myocardial infarction in rats. Circ Res. 1991;68:856–69. doi: 10.1161/01.res.68.3.856. [DOI] [PubMed] [Google Scholar]
- 3.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–62. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 4.Wang QD, Pernow J, Sjoquist PO, Ryden L. Pharmacological possibilities for protection against myocardial reperfusion injury. Cardiovasc Res. 2002;55:25–37. doi: 10.1016/s0008-6363(02)00261-4. [DOI] [PubMed] [Google Scholar]
- 5.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 6.Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW, et al. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–16. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 7.Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, et al. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–16. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 9.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 11.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 12.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–71. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005;102:8692–7. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Vliet P, Roccio M, Smits AM, van Oorschot AA, Metz CH, van Veen TA, Sluijter JP, et al. Progenitor cells isolated from the human heart: a potential cell source for regenerative therapy. Neth Heart J. 2008;16:163–9. doi: 10.1007/BF03086138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Terrovitis JV, Smith RR, Marban E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–94. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadjipanayi E, Mudera V, Brown RA. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J Tissue Eng Regen Med. 2009;3:77–84. doi: 10.1002/term.136. [DOI] [PubMed] [Google Scholar]
- 18.Wang HB, Dembo M, Wang YL. Substrate fl exibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–50. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 19.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battista S, Guarnieri D, Borselli C, Zeppetelli S, Borzacchiello A, Mayol L, Gerbasio D, et al. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials. 2005;26:6194–207. doi: 10.1016/j.biomaterials.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Miskon A, Mahara A, Uyama H, Yamaoka T. A suspension induction for myocardial differentiation of rat mesenchymal stem cells on various extracellular matrix proteins. Tissue Eng Part C Methods. 2010;16:979–87. doi: 10.1089/ten.TEC.2009.0218. [DOI] [PubMed] [Google Scholar]
- 22.Bratt-Leal AM, Carpenedo RL, McDevitt TC. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol Prog. 2009;25:43–51. doi: 10.1002/btpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mearns BM. Intramyocardial injections are safe. Nat Rev Cardiol. 2009;6:441–2. [Google Scholar]
- 24.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48:907–13. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–50. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kofidis T, de Bruin JL, Hoyt G, Lebl DR, Tanaka M, Yamane T, Chang CP, et al. Injectable bioartificial myocardial tissue for large-scale intramural cell transfer and functional recovery of injured heart muscle. J Thorac Cardiovasc Surg. 2004;128:571–8. doi: 10.1016/j.jtcvs.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation. 2005;112:I173–7. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- 28.Kutschka I, Chen IY, Kofidis T, Arai T, von Degenfeld G, Sheikh AY, Hendry SL, et al. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation. 2006;114:I167–73. doi: 10.1161/CIRCULATIONAHA.105.001297. [DOI] [PubMed] [Google Scholar]
- 29.Thompson CA, Nasseri BA, Makower J, Houser S, McGarry M, Lamson T, Pomerantseva I, et al. Percutaneous transvenous cellular cardiomyoplasty. A novel nonsurgical approach for myocardial cell transplantation. J Am Coll Cardiol. 2003;41:1964–71. doi: 10.1016/s0735-1097(03)00397-8. [DOI] [PubMed] [Google Scholar]
- 30.Zhang P, Zhang H, Wang H, Wei Y, Hu S. Artificial matrix helps neonatal cardiomyocytes restore injured myocardium in rats. Artif Organs. 2006;30:86–93. doi: 10.1111/j.1525-1594.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 31.Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A. 2009;15:205–19. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, Christman KL. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One. 2010;5:e13039. doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–16. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29:1630–7. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Lu H, Koo LY, Wang WM, Lauffenburger DA, Griffith LG, Jensen KF. Microfluidic shear devices for quantitative analysis of cell adhesion. Anal Chem. 2004;76:5257–64. doi: 10.1021/ac049837t. [DOI] [PubMed] [Google Scholar]
- 36.Young DA, Ibrahim DO, Hu D, Christman KL. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta biomaterialia. 2011;7:1040–9. doi: 10.1016/j.actbio.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lien CL, Wu C, Mercer B, Webb R, Richardson JA, Olson EN. Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development. 1999;126:75–84. doi: 10.1242/dev.126.1.75. [DOI] [PubMed] [Google Scholar]
- 38.Cleutjens JP, Kandala JC, Guarda E, Guntaka RV, Weber KT. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol. 1995;27:1281–92. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 39.Li YY, McTiernan CF, Feldman AM. Proinflammatory cytokines regulate tissue inhibitors of metalloproteinases and disintegrin metalloproteinase in cardiac cells. Cardiovasc Res. 1999;42:162–72. doi: 10.1016/s0008-6363(98)00297-1. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–4. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phelps EA, Landazuri N, Thule PM, Taylor WR, Garcia AJ. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci U S A. 2010;107:3323–8. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–57. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 44.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barallobre-Barreiro J, Didangelos A, Schoendube FA, Drozdov I, Yin X, Fernandez-Caggiano M, Willeit P, et al. Proteomics analysis of cardiac extracellular matrix remodeling in a porcine model of ischemia/reperfusion injury. Circulation. 2012;125:789–802. doi: 10.1161/CIRCULATIONAHA.111.056952. [DOI] [PubMed] [Google Scholar]
- 47.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fassler R, Rohwedel J, Maltsev V, Bloch W, Lentini S, Guan K, Gullberg D, et al. Differentiation and integrity of cardiac muscle cells are impaired in the absence of beta 1 integrin. J Cell Sci. 1996;109(Pt 13):2989–99. doi: 10.1242/jcs.109.13.2989. [DOI] [PubMed] [Google Scholar]
- 49.Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, et al. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90:458–64. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 50.Popov C, Radic T, Haasters F, Prall WC, Aszodi A, Gullberg D, Schieker M, et al. Integrins alpha2beta1 and alpha11beta1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death Dis. 2011;2:e186. doi: 10.1038/cddis.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salasznyk RM, Williams WA, Boskey A, Batorsky A, Plopper GE. Adhesion to Vitronectin and Collagen I Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells. J Biomed Biotechnol. 2004;2004:24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–31. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108:1395–403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 54.Rutschow S, Li J, Schultheiss HP, Pauschinger M. Myocardial proteases and matrix remodeling in inflammatory heart disease. Cardiovasc Res. 2006;69:646–56. doi: 10.1016/j.cardiores.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Seiffert M, Beck SC, Schermutzki F, Muller CA, Erickson HP, Klein G. Mitogenic and adhesive effects of tenascin-C on human hematopoietic cells are mediated by various functional domains. Matrix biology: journal of the International Society for Matrix Biology. 1998;17:47–63. doi: 10.1016/s0945-053x(98)90124-x. [DOI] [PubMed] [Google Scholar]
- 56.Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, Wang J, et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012;59:751–63. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–93. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 58.Singelyn JM, Christman KL. Injectable materials for the treatment of myocardial infarction and heart failure: the promise of decellularized matrices. J Cardiovasc Transl Res. 2010;3:478–86. doi: 10.1007/s12265-010-9202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Badylak S, Obermiller J, Geddes L, Matheny R. Extracellular matrix for myocardial repair. Heart Surg Forum. 2003;6:E20–6. doi: 10.1532/hsf.917. [DOI] [PubMed] [Google Scholar]
- 60.Rane AA, Christman KL. Biomaterials for the treatment of myocardial infarction a 5-year update. J Am Coll Cardiol. 2011;58:2615–29. doi: 10.1016/j.jacc.2011.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.