Abstract
Recent studies suggest that abstinent cannabis users show deficits on neurocognitive laboratory tasks of impulsive behavior. But results are mixed and less is known on the performance of non-treatment seeking, young adult cannabis users. Importantly, relationships between performance on measures of impulsive behavior and symptoms of cannabis addiction remain relatively unexplored. We compared young adult current cannabis users (CU, n = 65) and non-using controls (NU, n = 65) on several laboratory measures of impulsive behavior, as well as on a measure of episodic memory commonly impacted by cannabis use. The CU group performed more poorly than the NU group on the Hopkins Verbal Learning Test-Revised Total Immediate Recall and Delayed Recall. No significant differences were observed on the measures of impulsive behavior (i.e., Iowa Gambling Task [IGT], Go-Stop Task, Monetary Choice Questionnaire, Balloon Analogue Risk Task). We examined relationships between neurocognitive performance and symptoms of cannabis use disorder symptoms (DSM-IV CUD) among the CU group, which revealed that poorer IGT performance was associated with more symptoms of DSM-IV CUD. Our results show poorer memory performance among young adult cannabis users relative to healthy controls, but no differences on measures of impulsive behavior. However, performance on a specific type of impulsive behavior (i.e., poorer decision-making) was associated with more cannabis use disorder symptoms. These results provide preliminary evidence to suggest that decision-making deficits may be more strongly associated with problems experienced from cannabis use, rather than solely being a consequence of cannabis use, per se.
Keywords: cannabis, addiction, decision-making, neuropsychology, memory, cognitive effects
About 8% of individuals that try cannabis develop a cannabis use disorder (Anthony, Warner, & Kessler, 1994; Lopez-Quintero et al., 2011). Because of its high prevalence of use, more people meet DSM-IV criteria for substance use disorders from cannabis than for any other illicit drug (SAMHSA, 2009) and more individuals sought substance use treatment for cannabis than any other illicit drug in 2009 (SAMHSA, 2009). Cannabis use continues to increase among adolescents and young adults alongside a decreasing perception of harm and less disapproval of its use (Johnston, O’Malley, Bachman, & Schulenberg, 2010). Importantly, as we describe below, neurocognitive deficits have been documented among heavy cannabis users, primarily in the areas of memory and more recently on laboratory measures of impulsive behavior. However, most studies have relied on treatment seeking samples or on those with diagnoses of cannabis use disorders. Less is known about the performance of community-dwelling, young adults who are not seeking treatment for their cannabis use (who constitute a large proportion of all cannabis users). More importantly, to date, studies have compared neurocognitive performance between cannabis-using and non-using samples, but have not explored how neurocognitive performance relates to symptoms of cannabis use disorder. As we describe below, there are theoretical reasons to suspect that neurocognitive problems with impulsive behavior may be related to more symptoms of cannabis use disorder, as they may contribute to compulsive use of cannabis in the face of negative consequences. The primary aims of this study were two-fold: 1) to compare the performance of non-treatment seeking cannabis users and non-users on neurocognitive laboratory measures of impulsive behavior; 2) to examine the relationship of such measures with severity of cannabis use disorder symptoms in this sample.
Cannabis use is known to alter brain functioning through the binding of its primary psychoactive constituent, Δ-9-tetrahydrocannabinol (THC), to cannabinoid receptors (CB1). CB1 receptors are localized throughout cortex, with high concentrations in prefrontal cortex, anterior cingulate, and striatum: brain structures critical to many neurocognitive functions and implicated in addiction neuropathogenesis. Results from functional imaging studies often show differences in brain activity between abstinent cannabis users and non-users in the prefrontal cortex of adolescents (Jager, Block, Luijten, & Ramsey, 2010; Schweinsburg et al., 2005; Tapert et al., 2007) and adults (Block et al., 2002; Eldreth, Matochik, Cadet, & Bolla, 2004; Gruber & Yurgelun-Todd, 2005; Kanayama, Rogowska, Pope, Gruber, & Yurgelun-Todd, 2004; Pillay et al., 2004). Although neurocognitive deficits are evident during both acute intoxication and during abstinence (Fried & Smith, 2001; Gonzalez, 2007; Gonzalez, Carey, & Grant, 2002; Pope, Gruber, & Yurgeluntodd, 1995; Ranganathan & D’Souza, 2006; Schweinsburg, Brown, & Tapert, 2008; Solowij & Battisti, 2008), substantial controversy exists regarding the nature of the deficits, their magnitude, and their duration. Nonetheless, the most consistent deficits among cannabis users are arguably in episodic memory (Grant, Gonzalez, Carey, Natarajan, & Wolfson, 2003; Harrison G. Pope, Gruber, Hudson, Huestis, & Yurgelun-Todd, 2001; Ranganathan & D’Souza, 2006; Solowij & Battisti, 2008), although such deficits are thought to dissipate after approximately a month of abstinence (Hanson et al., 2010; Medina et al., 2007; Pope, Gruber, Hudson, Huestis, & Yurgelun-Todd, 2001)
Recently, several studies also report that cannabis users demonstrate problems in neurocognitive functions associated with impulsivity: defined as a predisposition toward rapid, unplanned reactions withoutregard to the negative consequences (Moeller, Barratt, Dougherty, Schmitz, & Swann, 2001). Deficits among cannabis users have been reported on measures of motor inhibition, risk taking, and decision-making both in laboratory studies of acute use (Lane, Cherek, Tcheremissine, Lieving, & Pietras, 2005; McDonald, Schleifer, Richards, & de Wit, 2003; Ramaekers, Kauert, et al., 2006; Ramaekers, Moeller, et al., 2006) and cross-sectional studies of cannabis users after varying lengths of recent abstinence (Bolla, Brown, Eldreth, Tate, & Cadet, 2002; Clark, Roiser, Robbins, & Sahakian, 2009; Fernandez-Serrano, Perez-Garcia, Schmidt Rio-Valle, & Verdejo-Garcia, 2009; Hermann et al., 2009; Lamers, Bechara, Rizzo, & Ramaekers, 2006; Verdejo-Garcia et al., 2007; Whitlow et al., 2004). However, results are mixed (Crean, Crane, & Mason, 2011). For example, others have found no differences between cannabis users and controls on measures of delay discounting (Johnson et al., 2010) or among other measures of impulsive behavior after acute cannabis administration (McDonald, Schleifer, Richards, & de Wit, 2003; Vadhan et al., 2007). Most studies to date have focused on samples comprised primarily of individuals in treatment or with cannabis use disorders, have employed only a single neurocognitive measure of impulsive behavior, or only attempt to examine the neurocognitive sequelae of cannabis use. It is important to consider that problems of impulse control have been hypothesized to influence the development of drug addiction (de Wit, 2009; Goldstein & Volkow, 2002; Schepis, Adinoff, & Rao, 2008). Most cannabis users do not meet criteria for cannabis use disorders and vary substantially in their amount of use, as well as to the degree they experience problems from their cannabis use. It is possible that deficits in neurocognitive functions associated with impulsivity may influence the extent to which a cannabis user experiences symptoms of a cannabis use disorder (and therefore problems from cannabis), yet this has been largely unexplored.
In this study, we compared the neurocognitive performance of a carefully selected community sample of young adult current cannabis users that identified cannabis as their drug of choice and non-using controls. Participants were assessed on several commonly-used laboratory measures of impulsive behavior, including measures of decision-making, intertemporal choice (delay discounting), risk-taking, and motor inhibition. They also completed a measure of episodic memory. Episodic memory was assessed to determine if cannabis use among our community, non-treatment seeking sample of cannabis users was sufficient to manifest with memory deficits – a common finding in other studies of cannabis use and neurocognitive functioning. Finally, we examined relationships between the measures of neurocognitive performance and severity of cannabis use disorder symptoms. We hypothesized that cannabis users would demonstrate poorer performance on measures of impulsive behavior and episodic memory when compared to non-users. More importantly, we anticipated that poorer performance on neurocognitive measures of impulsive behavior, would be associated with more severe symptoms of cannabis use disorders.
Methods
Participants
Participants were 65 current cannabis users (CU group) and 65 non-users (NU group) ages 17 to 24 recruited from the Chicago metropolitan area through flyers placed throughout the community and through word-of-mouth. Participants were part of a study on neurocognitive functioning among young adult cannabis users (PI: RG) and a small subset (5%) of the final enrolled sample were recruited from a program project on trajectories to tobacco use (PI: RM). Participant demographic information is presented in Table 1.
Table 1.
Demographics and Mental Health by Group
| NU (n = 65) | CU (n = 65) | p-value | |
|---|---|---|---|
| Age | 20.3 (2.0) | 20.8 (1.8) | .15 |
| Sex (male) | 51% | 65% | .11 |
| Estimated FSIQ | 104 (10.8) | 102.6 (9.9) | .42 |
| Years of Education | 13.6 (1.8) | 13.5 (1.6) | .76 |
| Ethnicity/Race | .55 | ||
| Caucasian | 43% | 43% | |
| African-American | 31% | 37% | |
| Hispanic | 11% | 12% | |
| Other | 15% | 8% | |
| Annual Household Income in Thousands (Md, IQR) | $75 [$45, $150] | $70 [$40, $145] | .71 |
| Mother’s Education | 13.9 (3.7) | 14.3 (2.8) | .55 |
| BDI-2 (Md, IQR) | 4 [1, 9] | 5 [2, 8] | .56 |
| BAI (Md, IQR) | 4 [1, 8] | 5 [2, 9] | .20 |
| WURS, % over ADHD cutpoint | 5% | 6% | 1.0 |
| BIS-11 | 57.9 (9.5) | 59.1 (9.8) | .48 |
Note: all values are means and standard deviations, unless otherwise noted; participants were between 17 to 24 years of age; Md, Median; IQR, interquartile range; FSIQ, Full Scale IQ; BDI-2, Beck Depression Inventory-2nd Edition; BAI, Beck Anxiety Inventory; WURS, Wender-Utah Rating Scale; BIS, Barratt Impulsiveness Scale-11th version
Inclusion and exclusion criteria were selected to minimize confounds to neurocognitive performance. Inclusion criteria for the entire sample were having more than 8 years of education, being fluent in English, and estimated full scale IQ greater than 75. Participants were excluded if they were on current psychotropic medications, had a history of any neurological disorder (including open head injury, closed head injury with loss of consciousness for greater than 10 minutes, epilepsy, brain tumor, cerebrovascular accident, or other systemic medical disorder known to adversely affect brain functioning) or self-reported a history of a diagnosed mental illness (including major depression, bipolar disorder, schizophrenia or other psychotic disorders, ADHD, developmental disorder, or learning disability). Four participants in the CU group and three in the NU group, obtained scores exceeding the clinical cutpoint suggestive of ADHD on the Wender-Utah Rating Scale (WURS), but reported no history of ADHD or a clinical history suggestive of ADHD and were therefore retained for analyses. In order to minimize substance use confounds and to obtain a relatively homogenous group of current CU and NU, participants were also excluded if they reported use of any substance more than 10 times in their lifetime or any use at all during the 30 days prior to their evaluation (other than cannabis use among the CU group and alcohol, nicotine, and hallucinogens across both groups), history of DSM-IV lifetime alcohol dependence or drinking more than 3 drinks per day on average during the 30 days prior to their evaluation, or DSM-IV diagnoses of lifetime abuse or dependence for any other substance (other than alcohol or nicotine). One participant in the CU group and one in the NU group that did not meet criteria suggestive of an alcohol use disorder at screening were later found to meet criteria for lifetime history for alcohol dependence during their study visit; however, the days since they last met alcohol dependence criteria were very remote (730 days ago and 1460 days ago), thus their data was retained for analyses. Inclusion criteria specific for participants in the CU group were identifying cannabis as their drug of choice, use of cannabis more than 200 times, use of cannabis at least 4 times per week during their peak use, and use in the 45 days prior to their evaluation. Cannabis users were asked to abstain for at least 24 hours prior to their evaluation to minimize acute effects or withdrawal symptoms. Inclusion criteria specific to the NU group were use of cannabis less than 10 times, use of cannabis never more than 4 times per week, and no cannabis use during the 90 days prior to their evaluation. Seventy-seven percent of participants in the CU group and no participant in the NU group tested positive for cannabis on a 10-panel rapid urine toxicology drug test (10-panel Drug Check Cup; Express Diagnostics, Blue Earth, Minnesota), which had a 50 ng/ml limit of detection for 11-nor-Δ9-THC-9-COOH. No participant tested positive for any other drug. Alcohol breath levels and behavioral observation during the study visit suggested no significant and recent alcohol use (AlcoMate Prestige Model AL6000; Palisades Park, NJ). Detailed information on participant substance use history is presented in Table 2.
Table 2.
Substance Use Parameters by Group
| NU (n = 65) | CU (n = 65) | |
|---|---|---|
| Current (30 day) DSM-IV SUD | ||
| Alcohol Abuse | 3% | 8% |
| Cannabis Abuse** | 0 | 32% |
| Cannabis Dependence** | 0 | 29% |
| Lifetime DSM-IV SUD | ||
| Alcohol Abuse* | 8% | 23% |
| Alcohol Dependence | 2% | 2% |
| Cannabis Abuse** | 0 | 42% |
| Cannabis Dependence** | 0 | 34% |
| Cannabis Use Parameters | ||
| DSM-IV CUD Symptoms (Md, IQR) | - | 1 [0, 3] |
| Marijuana Severity Index | - | 10.0 (4.3) |
| Age at cannabis use onset | - | 15.6 (3.1) |
| Years of cannabis use | - | 5.0 (2.4) |
| % THC+** | 0 | 77% |
| Days since last use (Md, IQR) | ||
| Alcohol** | 14 [4, 30], n = 60 | 6 [3.5, 14], n = 65 |
| Nicotine** | 14 [4, 450], n = 23 | 2 [1, 8.75], n = 54 |
| Cannabis** | 720 [365, 1278], n = 17 | 3 [2, 4.5], n = 65 |
| Lifetime (Md, IQR) | ||
| Alcoholic drinks** | 108 [13, 498] | 492 [163, 1254] |
| Cigarette Packs** | 0 [0, 0.1] | 68.4 [1.1, 291] |
| Cannabis joints** | 0 [0, 0] | 270 [102, 815] |
| 12 months (Md, IQR) | ||
| Alcoholic drinks** | 24 [4, 120] | 108 [34, 258] |
| Cigarettes** | 0 [0, 0] | 3 [0, 36] |
| Cannabis joints** | 0 [0, 0] | 60 [26, 216] |
| 30 days (Md, IQR) | ||
| Alcohol drinks* | 4 [0, 14] | 10 [2, 20] |
| Cigarettes** | 0 [0, 0] | 0.35 [0, 3] |
| Cannabis joints** | 0 [0, 0] | 6 [3, 18] |
p<.05,
p<.01
Note: All values are means and standard deviations unless otherwise noted; NU, non-users; CU, cannabis users; DSM-IV SUD, Diagnostic and Statistical Manual IV Substance Use Disorder diagnosis; CUD, Cannabis Use Disorder; THC+, positive rapid urine toxicology testing for cannabis; Md, Median; IQR, interquartile range; -, not applicable
Procedures and Measures
The study was approved by the University of Illinois Chicago Institutional Review Board. All participants provided informed consent (or assent and parental consent where appropriate). Participants were administered a counterbalanced battery of tests that included structured interviews, self-report questionnaires, and neurocognitive tests. All participants received a cash payment for participating in the study.
Demographics, medical history, and mental health
Premorbid full scale IQ was estimated with the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001). Current symptoms of depression and anxiety were assessed with the Beck Depression Inventory – 2nd Edition (BDI-II; Beck, Steer, Ball, & Ranieri, 1996) and the Beck Anxiety Inventory (BAI; (Beck & Steer, 1990), respectively. The Wender-Utah Rating Scale (WURS; Ward, Wender, & Reimherr, 1993) was used to evaluate symptoms associated with ADHD. The Barratt Impulsiveness Scale-11 (BIS) assessed impulsive personality traits (Patton, Stanford, & Barratt, 1995). The Mood Disorders portion of the Structured Clinical Interview for DSM-IV disorders (SCID) assessed for lifetime and current (past 30 days) diagnoses of Major Depression and Bipolar Disorder (First, Spitzer, Gibbon, & Williams, 2002).
Substance use history
All participants completed a detailed semi-structured interview that incorporated methods of the time-line followback procedure and assessed patterns of substance use for 13 different substance classes, similar to methods employed in other studies (e.g., Gonzalez, 2004; Rippeth et al., 2004). For each substance queried, participants were asked about frequency and quantity of use across various epochs in their lifetime to arrive at estimates of cumulative lifetime use, as well as amount and frequency of use in the 12 months and in the 30 days prior to their evaluation. The substance use module of the Structured Clinical Interview for DSM-IV (SCID) was administered to diagnose the presence of alcohol and substance use disorders during participants’ lifetime and in the 30 days prior to their evaluation (First et al., 2002). We assessed for the presence and severity of symptoms associated with cannabis addiction with the Marijuana Severity Index (MSI; Alexander, 2003): a 31 yes/no forced-choice questionnaire on problematic patterns of cannabis use that a participant has “ever” experienced from cannabis use. We also quantified severity of cannabis addiction by tabulating the total number of current DSM-IV symptoms of cannabis abuse and dependence endorsed by a participant in the 30 days prior to their evaluation (DSM-IV CUD symptoms).
Laboratory measures of neurocognitive functioning
The Iowa Gambling Task (IGT; Bechara, Damasio, Damasio, & Anderson, 1994) was developed to quantify the poor judgment and impulsive decision-making typically observed among patients with lesions of the orbitofrontal cortex. It is deemed a measure of “decision-making” and is thought to assess a bias toward immediate over longer-term rewards. Participants make 100 choices from a computerized display of four card decks, with each choice followed by a win of some money and sometimes also a loss. Participants are not informed that two of the decks most frequently result in small rewards and few losses (“good” decks). Choices from the other decks more frequently result in larger rewards but also larger losses (“bad” decks). Choices primarily from the “good decks” yield overall positive net scores by the end of the task, whereas choices primarily from “bad decks” will yield a net loss. Substance users typically show poorer performances than healthy controls (Bechara et al., 2001; Grant, Contoreggi, & London, 2000). We used the total net score (choices from good decks minus bad decks) as our outcome measure, with higher scores indicating better decision-making.
The Balloon Analogue Risk Task (BART) is a laboratory measure of risk taking (Lejuez et al., 2002). Participants are shown a graphic of a deflated balloon on a computer screen and are instructed to press a key to “inflate” the balloon. Participants earn $0.05 with each key press. They can collect the total money accumulated at any time and move on to the next trial, which starts with another deflated balloon. A balloon may “pop” after a key press, with a probability unknown to the participant, and all money earned during that trial would be lost. The BART is often performed more poorly by substance users (Crowley, Raymond, Mikulich-Gilbertson, Thompson, & Lejuez, 2006; Fernie, Cole, Goudie, & Field, 2010). The outcome measure is the “adjusted” average number of pumps, which excludes the number of pumps on balloons that explode. Higher scores are suggestive of greater risk taking.
The Monetary Choice Questionnaire (MCQ; Kirby, Petry, & Bickel, 1999) is a self-report measure of intertemporal choice that consists of 27 fixed hypothetical choices between smaller immediate rewards and larger delayed rewards. The MCQ assesses delay discounting by allowing estimation of the degree to which an individual reduces their perceived value of a reward as the time delay to obtaining that reward increases. Delay discounting is characterized by Mazur’s hyperbolic function (Mazur, Commons, Mazur, Nevin, & Rachlin, 1987): Vd = A/(1 + kD), where Vd is the value of a present value of a delayed reward (A) at a given delay (D). The parameter k quantifies individual differences in delay discounting, with a higher value indicating steeper discounting, and log-transformed k values were used as our outcome measure using established methods (Kirby et al., 1999). Higher k values have been shown among substance users compared to healthy controls (Kirby et al., 1999; Madden, Petry, Badger, & Bickel, 1997; Petry, Bickel, & Arnett, 1998).
The GoStop Task (Dougherty, Mathias, Marsh, & Jagar, 2005) is a computerized stop signal task (Logan, Cowan, & Davis, 1984) that assesses the participant’s ability to stop an already initiated motor response. Participants were asked to quickly press a key on the computer keyboard on Go (or No-Stop) trials: whenever a 5-digit number presented in black font was identical to the previously presented number. On some trials, the font color of the second matching number changes to red and participants are to withhold a response. A total of 80 Stop, 80 No-Stop, and 160 Novel trials (non-matching number in black) are administered in pseudo-random order. The latency from stimulus onset to the appearance of the Stop trial were 150, 250, 350, or 450 ms (20 trials of each), with longer latencies expected to be associated with more inhibition failures. Individuals with substance use disorders are known to exhibit more inhibition failures than healthy controls (Acheson, Richard, Mathias, & Dougherty, 2011; Dougherty, Marsh-Richard, Hatzis, Nouvion, & Mathias, 2008; Fillmore & Rush, 2002; Reynolds et al., 2007). We used the total number of correct inhibitions minus number of “misses” on the Go trials. This was done to adjust for individuals who responded to fewer trials (either on purpose or through inattention) and would otherwise have obtained a misleadingly high number of correct inhibitions. Higher scores were indicative of better inhibitory control.
The Hopkins Verbal Learning Test – Revised (HVLT; Benedict, Schretlen, Groninger, & Brandt, 1998) is a test of episodic verbal memory that consists of three groups of four (nonconsecutive) semantically associated words. Participants are asked to immediately repeat words after each of three trials, and then again after a 25 minute delay. Finally, participants are administered a forced choice recognition trial. Total Immediate Recall was calculated as the total number of words correctly recalled across learning trials, whereas Delayed Recall was the number of correct words spontaneously recalled after the 25-minute delay. Recognition Discriminability was the number of words correctly identified on the forced-choice recognition trial minus any false alarm errors. All scores were generated using published normative data (Benedict et al., 1998).
General Statistical Approach
All analyses were carried out using JMP 9.0 (SAS, Carey, NC). Data were inspected for non-normal distribution and outliers. When appropriate, square-root transformations or nonparametric procedures were used with data that violated assumptions of parametric procedures (these included all measures on amount of substance use). MCQ k values underwent log transformation. In order to control for the influence of 12 month alcohol and nicotine use for between-group comparisons on neurocognitive performance, both variables were first regressed on each of the neurocognitive measures in order to obtain residuals. Comparisons between the CU and NU on neurocognitive performance were conducted on the residualized variables.
Results
Demographics, Mental Health, Substance Use and other Potential Confounds
The CU and NU groups showed no statistically significant differences in age, sex, race/ethnicity, years of education, mother’s years of education, household income, estimated full scale IQ (WTAR), current symptoms of depression (BDI-II) or anxiety (BAI), lifetime DSM-IV diagnosis of major depression (SCID), impulsive personality traits (BIS), and prevalence of possible ADHD (WURS), all p-values > .10 (Table 1). No participant met DSM-IV criteria for Bipolar Disorder and none met criteria for Major Depression in the 30 days prior to their evaluation.
No participant met DSM-IV criteria for current or lifetime abuse or dependence for cocaine, sedatives, stimulants, opiates, or hallucinogens, and none reported injection drug use. Furthermore, none met current diagnosis for alcohol dependence. Detailed substance use parameters are reported in Table 2. Compared to the NU group, the CU group reported a higher prevalence of past alcohol abuse (Chi-square = 5.91, p = .02). When comparing the CU and NU groups on amount of use for various substances, square-root transformations were employed to improve the distribution of the variables. The CU group reported more use of alcohol, nicotine, and cannabis during their lifetime, last 12 months, and last 30 days (all p-values ≤ .01). Similarly, among those reporting use of alcohol, nicotine, or cannabis, those in the CU group reported more recent use than those in the NU group for all three substances (all p-values < .01, See Table 2). Among the CU group, neurocognitive performance was not significantly correlated with days since last use of alcohol (p-values > .10), nicotine (p-values > .32), or cannabis (p-values > .41). The lack of associations may be due to our inclusion/exclusion criteria resulting in a fairly homogenous sample. No participant in the NU group reported other substance use, with the exception of one participant reporting a one-time use of a non-prescription opiate. Participants in the CU group were more likely to have experimented with other substances, but reported use of other substances was less than 10 lifetime occasions (median occasions of use were < 7 times across all substances) and no CU participant reported use of any other illicit substance within the 30 days prior to the evaluation (Md days since last use of each substance = 365 days). These results suggested that only use of nicotine and alcohol use were of sufficient recency and magnitude to warrant controlling for them in our analyses. When considering which of the estimates of amounts of nicotine and alcohol use to employ as covariates, we decided that amount of use in the last 12 months would be optimal, as it would capture the severity of use while being less susceptible to recent and perhaps uncharacteristic changes in use when compared to last 30 day use. Similarly, use in the last 12 months would be less confounded by a participant’s age than cumulative lifetime use, as an older participant could obtain higher cumulative amounts of use despite less frequent and more distal use than a younger participant. Thus the influence of 12 month alcohol and nicotine use were controlled for in our between-group analyses by regressing both variables on each neurocognitive measure and using the residuals as the outcome measure. Multiple linear regressions with 12 month alcohol and nicotine use as independent variables were conducted separately using each neurocognitive measure as the dependent variable. Twelve-month nicotine use was significantly associated with MCQ-K (t = 2.12, β = .18, p = .04) and HVLT Total Immediate Recall (t = 2.26, β = −.20, p = .03), whereas alcohol use was significantly associated with IGT performance (t = 2.04, β = .18, p = .04).
Differences in Neurocognitive Performance between Cannabis Users and Nonusers
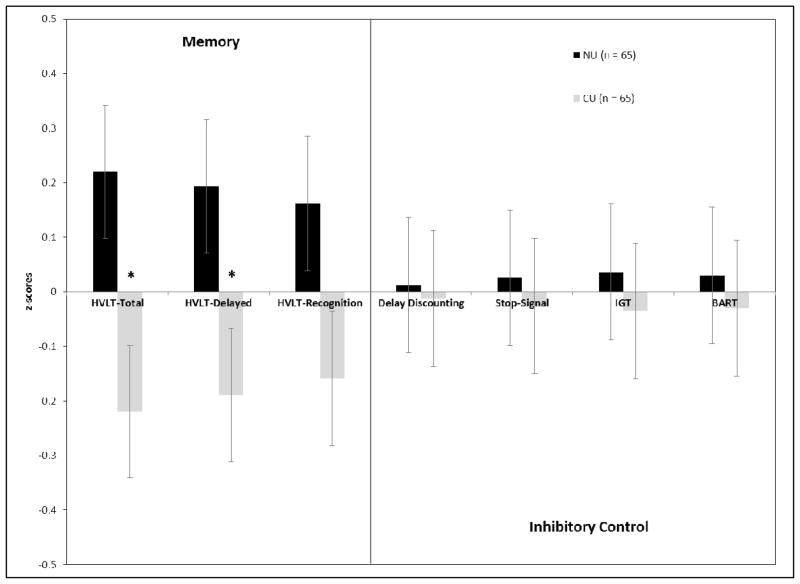
One-way ANOVAs were conducted employing group (CU or NU) as the independent variable and one index of neurocognitive performance as the dependent variable (using the residuals obtained after regressing 12 month alcohol and nicotine use). Statistically significant differences in mean performance between the CU and NU groups were observed for HVLT Total Immediate Recall (F1, 128 = 5.61, Cohen’s d = −.42, p = .019) and HVLT Delayed Recall (F1, 128 = 4.38, Cohen’s d = −.37, p = .038), with the CU group performing more poorly on both tasks. No statistically significant differences were observed for HVLT Recognition Discriminability (F1, 128 = 3.89, p = .051), IGT net score (F1, 128 = .92, p = .34), MCQ k (F1, 128 = .41, p = .52), Go-Stop (F1, 128 = .019, p = .89), or BART performance (F1, 128 = .045, p = .83); see Table 3. Figure 1 presents the results of these analyses using z-scores calculated from the means and standard deviations of the entire sample on each of the neurocognitive tasks. Significant differences in HVLT-Delayed recall did not emerge when controlling for performance on HVLT Total Immediate Recall (p = .94), suggesting that differences in HVLT performance was due to problems with the acquisition of new information, rather than recall. HVLT Total Immediate and Delayed Recall were strongly correlated within the NU (r = .73, p < .001) and the CU group (r = .72, p < .001).
Table 3.
Neurocognitive Differences by Group
| NU (n = 65) | CU (n = 65) | p-value | |
|---|---|---|---|
| Episodic Memory | |||
| HVLT-R Total Immediate Recall | −.26 (1.16) | −.82 (1.33) | .019 |
| HVLT-R Delayed Recall | −.41 (1.13) | −.89 (1.30) | .038 |
| HVLT-R Recognition Discriminability | −.10 (2.63) | −.13 (1.08) | .069 |
| Inhibitory Control | |||
| IGT Net Score | 8.13 (29.49) | 6.06 (28.87) | .34 |
| Go-Stop (correct inhibitions – misses) | 23.09 (17.47) | 22.14 (19.57) | .89 |
| MCQ k | .05 (.05) | .05 (.05) | .52 |
| BART (adjusted pumps) | 32.0 (12.2) | 32.7 (11.8) | .83 |
Note: All values are means and standard deviations; NU, non-users; CU, cannabis users; HVLT, Hopkins Verbal Learning Test – Revised; IGT, Iowa Gambling Task; MCQ, Monetary Choice Questionnaire; BART, Balloon Analogue Risk Task.
Figure 1. Neurocognitive Performance by Group.
Note: The values presented in the graph are mean z-scores (and standard errors) calculated for each group on each task using the average performance of the entire sample. This allows us to present performance across all tasks on the same graph. The NU and CU groups differed significantly only on HVLT-R Total Immediate Recall (HVLT Total) and HVLT-R Delayed Recall (HVLT-Delayed).
*p-value < .05.
Relationships between Neurocognitive Performance and Symptoms of Cannabis Use Disorders
We conducted two linear regressions among the CU group with performance on each of the neurocognitive measures as independent variables, amount of alcohol and nicotine use in the last 12 months as covariates, and one of the two measures of cannabis addiction symptoms (MSI and DSM-IV CUD symptoms) as dependent variables. To prevent multiIcollinearity, only one of the three possible HVLT indices, HVLT Total Immediate Recall, was included in the model, as it evidenced the largest effect in prior between-group analyses. No statistically significant associations were observed between any of the neurocognitive measures and MSI (all p-values > .05). However, poorer IGT performance was associated with more DSM-IV CUD symptoms (β = −.30, p = .03). No other neurocognitive task was found to be associated with DSM-IV CUD symptoms (all p-values > .42). Because DSM-IV CUD symptoms are count data and were not normally distributed, we conducted these analyses again using Poisson regression with control for overdispersion (Gardner, Mulvey, & Shaw, 1995; Karazsia & van Dulmen, 2008) and obtained the same results – only IGT net score was significantly associated with DSM-IV CUD symptoms (p = .02).
Additional Exploratory Analyses
Pairwise Pearson correlations among the neurocognitive measures of impulsive behavior revealed no significant correlations (r-values ranged from .07 to .16; p-values ranged from .07 to .69).
In order to further explore the relationship between cannabis use and DSM-IV symptoms of cannabis use disorders, we examined bivariate correlations between three parameters of cannabis use (total lifetime use, use in last 12 months, and use in the last 30 days) and the total number of DSM-IV cannabis use disorder symptoms (DSM-IV CUD) in the CU group. All three parameters exhibited statistically significant, moderate correlations with DSM-IV CUD (Lifetime: Rho = .41, p = .0007; 12 months: Rho = .53, p < .0001; 30 days: Rho = .52, p < .0001).
Discussion
In this study we examined if a community sample of non-treatment seeking young adult, current cannabis users (CU) demonstrated deficits on neurocognitive laboratory tasks of impulsive behavior compared to non-using controls (NU). We also assessed their episodic memory to replicate common findings of poorer memory among cannabis users. More importantly, we examined if neurocognitive performance was associated with the severity of cannabis use disorder symptoms endorsed by the cannabis users. Consistent with the current literature, we found significantly poorer episodic memory performance among the CU group, which appeared to be due to problems with the acquisition of new information. Contrary to our hypotheses, we found no evidence of deficits on laboratory measures of impulsive behavior among the CU. Nonetheless, significant associations between IGT performance and DSM-IV symptoms of cannabis use disorders (DSM-IV CUD) were observed. Our results suggest that deficits in neurocognitive measures of impulsive behavior may not be as prominent as memory deficits among non-treatment seeking cannabis users. However, problems specifically with decision-making appear to be associated with more symptoms of cannabis use disorders, indicating that individual differences in decision-making may place cannabis users at greater risk for cannabis use disorders and to potentially experience more problems from their cannabis use.
Although we found no between-group differences on neurocognitive measures of impulsive behavior in our sample, other studies have reported such deficits. However, a recent synthesis of studies on cannabis use and executive functions (which include laboratory measures of impulsive behavior) suggest that findings vary substantially depending on the specific measures employed and whether cannabis users are tested while acutely intoxicated or when abstinent (Crean, Crane, & Mason, 2011). A factor that may have contributed to the lack of differences between CU and NU on laboratory measures of impulsive behavior in our study may have been our stringent inclusion criteria, which required that all cannabis users identify cannabis as their drug of choice, used cannabis recently, and had very minimal history of other drug use, with the exception of alcohol and nicotine use (both of which were controlled for in our analyses). Moreover, our study did not require for our CU participants to be treatment-seeking or to meet criteria for a cannabis use disorder. We were also careful to exclude individuals with numerous other mental health, substance use, and neurological confounds. It is important to note that despite this careful control of confounds, the CU group continued to show significantly poorer memory performance compared to the NU group, of a magnitude consistent with (or larger than) those observed among samples of older, long term, heavy cannabis users (e.g., Grant et al., 2003). Thus, even though cannabis users in our sample may not have used as heavily as those in other studies, cannabis use in our non-treatment seeking sample was clearly sufficient to still manifest with neurocognitive deficits in episodic memory relative to controls.
Although the CU and NU groups did not differ in decision-making performance, poorer IGT performance was significantly associated with more DSM-IV CUD symptoms. This is an interesting finding, especially when considering the lack of differences between the NU and CU groups on IGT performance. Although speculative in the absence of a prospective longitudinal design, it is possible that poorer decision-making may be more relevant to whether an individual experiences symptoms of a cannabis use disorder rather than being a direct consequence of cannabis use, per se. In this study we find that cannabis consumption is associated with poorer episodic memory performance, but not with decision-making. In contrast, decision-making is related to symptoms of cannabis use disorder, but memory performance is not. It is conceivable that an individual with intact decision-making may be more likely than an individual with impaired decision-making to inhibit using cannabis in situations more likely to lead to significant adverse consequences (e.g., driving, working, or in school), thus making the latter more likely to experience symptoms of a cannabis use disorder. Theoretically, two individuals with similar amounts of cannabis use may experience differing degrees of problems from their use. Our findings suggest that problems with decision-making may have an influence on the amount of significant problems experienced by cannabis users (as reflected by DSM-IV symptoms of cannabis use disorders). We acknowledge that both cannabis consumption and symptoms of cannabis use disorder are related; however, their correlation is not invariably strong. This is likely due to the fact that abuse/dependence is defined by several symptoms, which do not require escalating use. In our own sample, symptoms of cannabis addiction and amount of cannabis use were only moderately correlated (Rho = .41 to .53). It is important to note that relationships between laboratory measures of impulsive behavior were not invariably related to symptoms of cannabis use disorder. Our results suggest that of the constructs examined, problems with decision-making may be most pertinent in influencing the magnitude of current cannabis use disorder symptoms. A future study will focus on decision-making and the problems that individuals specifically report from their cannabis use.
Several characteristics of our study need to be considered when interpreting our findings. First, it is important to underscore that the cross-sectional design of the study prevents us from establishing causation or determining any clear temporal relationships among our variables. Secondly, the CU group showed higher amounts of alcohol and nicotine use, and was more likely to have a history of other experimental drug use, compared to the NU group. In addition to adequately matching groups and excluding individuals with a history of drug abuse/dependence and current alcohol dependence, recent heavy alcohol use, and frequent or recent use of other substances, we also controlled statistically for amount of alcohol and nicotine use to address these common group differences and to better isolate the effects of cannabis. We also note that we found no relationships between neurocognitive measures and the MSI. This may be due to the MSI querying about behaviors that have happened “ever” in a participant’s life, in contrast to the DSM-IV CUD variable that specifically asked about symptoms in the last 30 days. Furthermore, it is worth noting that not all participants in the cannabis-using group tested positive for THC or its metabolites on urine toxicology testing. This is not surprising given the levels of detection of the urinalysis and the fact that THC metabolites accumulate in adipose tissue and have erratic elimination that may be affected by many factors (e.g., chronicity and amount of use, recent exercise, water intake, BMI, resting metabolic rate, and diet). Thus, it is possible for a positive test to be obtained after several weeks of use or a recent user may yield a negative test result under some circumstances. Because we included both cannabis users and non-users in our study, we do not think that non-cannabis using participants were motivated to falsely endorse recent cannabis use.
It is also important to consider that our sample consisted of 17 to 24 year olds. We focused on recruiting emerging adults because this is the population where our findings may have the most impact, given the high prevalence of cannabis use in this age range. Furthermore, in this age range it is likely to find participants with minimal use of other illicit drugs. However, our results may not generalize to other age groups. We must also consider that in the age range sampled in the current study, the brain is continuing to undergo important neuromaturational changes. For example, cortical development peaks around 12 to 14 years of age and continues to decrease in volume and thickness into emerging adulthood with synaptic pruning of gray-matter density occurring first in more primary sensorimotor areas and last in higher-order association areas like the prefrontal cortex (Giedd et al., 1999; Gogtay et al., 2004). Although the CU and NU group were well-matched on age, participants in the CU group showed some variability in their age at first cannabis use which may be a more relevance to neurocognitive functioning than the participants age at testing (see reviews by Crane, Schuster, Fusar-Poli, & Gonzalez, in press; Schweinsburg, Brown, & Tapert, 2008). This important issue will require more careful consideration and investigation in future studies along with the emerging evidence of important sex-differences in the neurocognitive performance, brain activity, and morphometry of male and female cannabis users (McQueeny et al., 2011; Medina et al., 2009).
Our study benefited from including multiple neurocognitive measures of impulsive behavior; however, only the IGT revealed significant relationships with cannabis use disorder symptoms. Although the laboratory measures of impulsive behavior we employed are conceptually related, they assess multiple underlying processes that oftentimes are weakly correlated (Castellanos & Tannock, 2002; Dougherty et al., 2009; Monterosso, Ehrman, Napier, O’Brien, & Childress, 2001; Nigg, 2000). Indeed, we found that these measures were not significantly correlated in our sample. In future studies, it will be important to include specifically multiple measures of decision-making to determine if our findings are specific to the IGT, affect only some aspects of decision-making, or are associated with decision-making in general. Furthermore, like many other studies that employ similar measures we provided participants with hypothetical earnings on the tasks that involved rewards, rather than real money. Although we cannot rule out the possibility that our results may have been different if we provided tangible incentives based on performance, studies comparing real to hypothetical money reinforcement on IGT generally suggest no significant overall differences (Bowman & Turnbull, 2003; Fernie & Tunney, 2006), but may have some consequences depending on the sample and task parameters (Fernie & Tunney, 2006; Vadhan, Hart, Haney, van Gorp, & Foltin, 2009). However, the standardized and normed version of the IGT (Bechara, 2007), as well as the methods used in seminal studies (Bechara, Damasio, Damasio, & Anderson, 1994; Grant, Contoreggi, & London, 2000) also employ hypothetical money. In contrast, many studies with the BART or with measures of delay discounting employ some type of incentive, although several studies suggest that these tasks remains valid with hypothetical rewards (Benjamin & Robbins, 2007; Lagorio & Madden, 2005; Madden, Begotka, Raiff, & Kastern, 2003; Madden et al., 2004).
In summary, this study extends findings of neurocognitive performance among cannabis users and, to our knowledge, is the first to specifically examine how laboratory measures of impulsive behavior relate to symptoms of cannabis use disorder. Our results support the notion that problems with decision-making may influence the degree to which a cannabis user experiences symptoms of a cannabis use disorder. Future studies will examine if measures of decision-making are valuable for identifying cannabis users who are likely to experience significant negative consequences from its use. Investigations of cognitive strategies for remediation of decision-making deficits may also prove fruitful in ameliorating cannabis use disorders. However, the important question of whether problems of decision-making are an antecedent or consequence of drug use, which has also been raised by others (e.g., (de Wit, 2009; Goldstein, Alia-Klein, Cottone, & Volkow, 2006), remains unanswered. It is possible that problems with inhibition, risk taking, and decision-making may serve as a risk factor for the development of substance use disorders (de Wit, 2009; Goldstein & Volkow, 2002; Schepis et al., 2008), as they may make it more difficult to resist the urge to continue using a drug even when its use is harmful (Bechara, 2005). This question will need to be addressed more directly through longitudinal studies, similar to those that have been employed by others to demonstrate relationships between externalizing behaviors and future development of substance use disorders (Giancola, Moss, Martin, Kirisci, & Tarter, 1996; Kirisci, Tarter, Reynolds, & Vanyukov, 2006; Tarter, Kirisci, Habeych, Reynolds, & Vanyukov, 2004; Tarter et al., 2003) and alcohol use (Deckel & Hesselbrock, 1996; Norman et al.).
Acknowledgments
This publication was supported by Grant Number K23DA023560 and R01DA031176 (PI: Gonzalez) and F31DA032244 (PI: Schuster) from the National Institute on Drug Abuse (NIDA), as well as P01 CA098262 (PI: Mermelstein) from the National Cancer Institute (NCI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- Acheson A, Richard DM, Mathias CW, Dougherty DM. Adults with a family history of alcohol related problems are more impulsive on measures of response initiation and response inhibition. Drug and Alcohol Dependence. 2011;117(2–3):198–203. doi: 10.1016/j.drugalcdep.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D. A Marijuana Screening Inventory (Experimental Version): Description and Preliminary Psychometric Properties. The American Journal of Drug and Alcohol Abuse. 2003;29(3):619–646. doi: 10.1081/ada-120023462. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Experimental and Clinical Psychopharmacology. 1994;2(3):244–268. [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A. The Iowa Gambling Task Professional Manual. Boca Raton, FL: Psychological Assessment Resources; 2007. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. San Antonio: X: The Psychological Corporation; 1990. [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test--Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12(1):43–55. [Google Scholar]
- Benjamin AM, Robbins SJ. The role of framing effects in performance on the Balloon Analogue Risk Task (BART) Personality and Individual Differences. 2007;43(2):221–230. [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, Andreasen NC. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacology Biochemistry and Behavior. 2002;72(1–2):237–250. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bowman CH, Turnbull OH. Real versus facsimile reinforcers on the Iowa Gambling Task. Brain and Cognition. 2003;53(2):207–210. doi: 10.1016/s0278-2626(03)00111-8. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Reviews Neuroscience. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Clark L, Roiser JP, Robbins TW, Sahakian BJ. Disrupted ‘reflection’ impulsivity in cannabis users but not current or former ecstasy users. Journal of Psychopharmacology. 2009;23(1):14–22. doi: 10.1177/0269881108089587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane N, Schuster RM, Fusar-Poli P, Gonzalez R. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Current Drug Abuse Reviews. doi: 10.1007/s11065-012-9222-1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. An Evidence Based Review of Acute and Long-Term Effects of Cannabis Use on Executive Cognitive Functions. Journal of Addiction Medicine. 2011;5(1):1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley TJ, Raymond KM, Mikulich-Gilbertson SK, Thompson LL, Lejuez CW. A risk-taking “set” in a novel task among adolescents with serious conduct and substance problems. Journal of the American Academy of Child and Adolescenct Psychiatry. 2006;45(2):175–183. doi: 10.1097/01.chi.0000188893.60551.31. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction Biology. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckel AW, Hesselbrock V. Behavioral and Cognitive Measurements Predict Scores on the MAST: A 3-Year Prospective Study. Alcoholism: Clinical and Experimental Research. 1996;20(7):1173–1178. doi: 10.1111/j.1530-0277.1996.tb01107.x. [DOI] [PubMed] [Google Scholar]
- Dougherty D, Mathias C, Marsh D, Jagar A. Laboratory behavioral measures of impulsivity. Behavior Research Methods. 2005;37(1):82–90. doi: 10.3758/bf03206401. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh-Richard DM, Hatzis ES, Nouvion SO, Mathias CW. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug and Alcohol Dependence. 2008;96(1–2):111–120. doi: 10.1016/j.drugalcdep.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh-Richard DM, Furr RM, Nouvion SO, Dawes MA. Distinctions in behavioral impulsivity: implications for substance abuse research. Addictive Disorders & Their Treatment. 2009;8(2):61–73. doi: 10.1097/ADT.0b013e318172e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23(3):914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Perez-Garcia M, Schmidt Rio-Valle J, Verdejo-Garcia A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. Journal of Psychopharmacology. 2009;24(9):1317–32. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- Fernie G, Cole JC, Goudie AJ, Field M. Risk-taking but not response inhibition or delay discounting predict alcohol consumption in social drinkers. Drug and Alcohol Dependence. 2010;112(1–2):54–61. doi: 10.1016/j.drugalcdep.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Fernie G, Tunney RJ. Some decks are better than others: the effect of reinforcer type and task instructions on learning in the Iowa Gambling Task. Brain and Cognition. 2006;60(1):94–102. doi: 10.1016/j.bandc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug and Alcohol Dependence. 2002;66(3):265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). Version 2.0. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fried PA, Smith AM. A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicology and Teratology. 2001;23(1):1–11. doi: 10.1016/s0892-0362(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Gardner W, Mulvey EP, Shaw EC. Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychological Bulletin. 1995;118(3):392–404. doi: 10.1037/0033-2909.118.3.392. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Moss HB, Martin CS, Kirisci L, Tarter RE. Executive cognitive functioning predicts reactive aggression in boys at high risk for substance abuse: a prospective study. Alcohol Clinical and Experimental Research. 1996;20(4):740–744. doi: 10.1111/j.1530-0277.1996.tb01680.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R, Alia-Klein N, Cottone L, Volkow N. The orbitofrontal cortex in drug addiction. In: Zald DH, Rauch Scott L, editors. The Orbitofrontal Cortex. Oxford University Press; 2006. pp. 481–522. [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychology Review. 2007;17(3):347–361. doi: 10.1007/s11065-007-9036-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Carey C, Grant I. Nonacute (Residual) neuropsychological effects of cannabis use: a qualitative analysis and systematic review. Journal of Clinical Pharmacology. 2002;42(11):48S–57S. doi: 10.1002/j.1552-4604.2002.tb06003.x. [DOI] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. Journal of the International Neuropsychological Society. 2003;9(5):679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroirnaging of marijuana smokers during inhibitory processing: A pilot investigation. Cognitive Brain Research. 2005;23(1):107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addictive Behaviors. 2010;35(11):970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Lemenager T, Gelbke J, Welzel H, Skopp G, Mann K. Decision making of heavy cannabis users on the Iowa Gambling Task: stronger association with THC of hair analysis than with personality traits of the Tridimensional Personality Questionnaire. European Addiction Research. 2009;15(2):94–98. doi: 10.1159/000189788. [DOI] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Cannabis Use and Memory Brain Function in Adolescent Boys: A Cross-Sectional Multicenter Functional Magnetic Resonance Imaging Study. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(6):561–572. e563. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F, Moore BA, Badger GJ, Budney AJ. Delay discounting in current and former marijuana-dependent individuals. Experimental and Clinical Psychopharmacology. 2010;18(1):99–107. doi: 10.1037/a0018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future Study. Rockville, MD: 2010. [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology. 2004;176(3–4):239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Karazsia BT, van Dulmen MH. Regression models for count data: illustrations using longitudinal predictors of childhood injury. Journal of pediatric psychology. 2008;33(10):1076–1084. doi: 10.1093/jpepsy/jsn055. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology-General. 1999;128(1):78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Tarter RE, Reynolds M, Vanyukov M. Individual differences in childhood neurobehavior disinhibition predict decision to desist substance use during adolescence and substance use disorder in young adulthood: a prospective study. Addictive Behaviors. 2006;31(4):686–696. doi: 10.1016/j.addbeh.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Lamers CTJ, Bechara A, Rizzo M, Ramaekers JG. Cognitive function and mood in MDMA/THC users, THC users and non-drug using controls. Journal of Psychopharmacology. 2006;20(2):302–311. doi: 10.1177/0269881106059495. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Tcheremissine OV, Lieving LM, Pietras CJ. Acute marijuana effects on human risk taking. Neuropsychopharmacology. 2005;30(4):800–809. doi: 10.1038/sj.npp.1300620. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) Journal of Experimiental Psychology Applied. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. Journal of Experimental Psychology Human Perception and Performance. 1984;10(2):276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Lagorio CH, Madden GJ. Delay discounting of real and hypothetical rewards III: steady-state assessments, forced-choice trials, and all real rewards. Behavioural processes. 2005;69(2):173–187. doi: 10.1016/j.beproc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, Perez de los Cobos J, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug and Alcohol Dependence. 2011;115(1–2):120–130. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Begotka AM, Raiff BR, Kastern LL. Delay discounting of real and hypothetical rewards. Experimental and Clinical Psychopharmacology. 2003;11(2):139–145. doi: 10.1037/1064-1297.11.2.139. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: Drug and monetary rewards. Experimental and Clinical Psychopharmacology. 1997;5(3):256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Raiff BR, Lagorio CH, Begotka AM, Mueller AM, Hehli DJ, Wegener AA. Delay discounting of potentially real and hypothetical rewards: II. Between- and within-subject comparisons. Experimental and Clinical Psychopharmacology. 2004;12(4):251–261. doi: 10.1037/1064-1297.12.4.251. [DOI] [PubMed] [Google Scholar]
- Mazur JE, Commons ML, Mazur JE, Nevin JA, Rachlin H. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analysis of Behavior: Vol. 5. The effect of delay and of intervening events on reinforcement value. Hillsdale: Erlbaum; 1987. pp. 55–73. [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28(7):1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Padula CB, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behavioral Brain Research. 2011;224(1):128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007;13(5):807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K, McQueeny T, Nagel B, Hanson K, Yang T, Tapert S. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addiction biology. 2009;14(4):457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric Aspects of Impulsivity. American Journal of Psychiatry. 2001;158(11):1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ehrman R, Napier KL, O’Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: do they measure the same construct? Addiction. 2001;96(12):1825–1837. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On Inhibition/Disinhibition in Developmental Psychopathology: Views From Cognitive and Personality Psychology and a Working Inhibition Taxonomy. Psychological Bulletin. 2000;126(2):220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence. 2011;119(3):216–23. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93(5):729–738. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- Pillay SS, Rogowska J, Kanayama G, Jon DI, Gruber S, Simpson N, Yurgelun-Todd DA. Neurophysiology of motor function following cannabis discontinuation in chronic cannabis smokers: an fMRI study. Drug and Alcohol Dependence. 2004;76(3):261–271. doi: 10.1016/j.drugalcdep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry. 2001;58(10):909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Yurgeluntodd D. The residual neuropsychological effects of cannabis - the current status of research. Drug and Alcohol Dependence. 1995;38(1):25–34. doi: 10.1016/0376-8716(95)01097-i. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, van RP, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31(10):2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Moeller MR, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of Delta(9)-THC concentration in serum and oral fluid: Limits of impairment. Drug and Alcohol Dependence. 2006;85(2):114–122. doi: 10.1016/j.drugalcdep.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D’Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology. 2006;188(4):425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Patak M, Shroff P, Penfold RB, Melanko S, Duhig AM. Laboratory and self-report assessments of impulsive behavior in adolescent daily smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2007;15(3):264–271. doi: 10.1037/1064-1297.15.3.264. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Schepis TS, Adinoff B, Rao U. Neurobiological processes in adolescent addictive disorders. American Journal of Addiction. 2008;17(1):6–23. doi: 10.1080/10550490701756146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Current Drug Abuse Reviews. 2008;1(1):99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug and Alcohol Dependence. 2005;79(2):201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Current Drug Abuse Reviews. 2008;1(1):81–98. doi: 10.2174/1874473710801010081. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health, 2009. 2009. [Google Scholar]
- Tapert S, Schweinsburg A, Drummond S, Paulus M, Brown S, Yang T, Frank L. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194(2):173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug and Alcohol Dependence. 2004;73(2):121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. American Journal of Psychiatry. 2003;160(6):1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, van Gorp WG, Gunderson EW, Haney M, Foltin RW. Acute effects of smoked marijuana on decision making, as assessed by a modified gambling task, in experienced marijuana users. Journal of Clinical and Experimental Neuropsychology. 2007;29(4):357–364. doi: 10.1080/13803390600693615. [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, Haney M, van Gorp WG, Foltin RW. Decision-making in long-term cocaine users: Effects of a cash monetary contingency on Gambling task performance. Drug and Alcohol Dependence. 2009;102(1–3):95–101. doi: 10.1016/j.drugalcdep.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug and Alcohol Dependence. 2007;90:2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Amercian Journal of Psychiatry. 1993;150(6):885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug and Alcohol Dependence. 2004;76(1):107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]