Abstract
Background
Moderate alcohol consumption is largely believed to be cardioprotective, while red wine is hypothesized to offer benefit in part due to the pro-angiogenic and antioxidant properties of polyphenols. We investigated the cardiovascular effects of both red wine and vodka in a swine model of endothelial dysfunction.
Methods
Twenty-seven male Yorkshire swine fed a high-fat/cholesterol diet were divided into three groups and received either no alcohol (Control), red wine, or vodka. After seven weeks, myocardial perfusion was measured, and ventricular tissue was analyzed for microvascular reactivity, and immunohistochemical studies.
Results
There were no differences in myocardial perfusion, in arteriolar or capillary density, or in VEGF expression among groups. Total protein oxidation as well as expression of superoxide dismutase-1 and -2 (SOD1, SOD2) and NADPH-oxidase (NOX2) was decreased in both treatment groups compared to controls. Endothelium-dependent microvessel relaxation, however, was significantly improved only in the red wine-supplemented group.
Conclusions
Supplementation with both red wine and vodka decreased oxidative stress by several measures, implicating the effects of ethanol in reducing oxidative stress in the myocardium. However, it was only in the red wine-supplemented group that an improvement in microvessel function was observed. This suggests that a component of red wine, independent of ethanol, possibly a polyphenol such as resveratrol, may confer cardioprotection by normalizing endothelial dysfunction induced by an atherogenic diet.
Keywords: Alcohol, Ethanol, Polyphenols, Antioxidants, Cardiovascular disease, Endothelial function, Oxidative stress
Introduction
Alcohol consumption is largely believed to be cardioprotective when consumed in moderation, with consistent findings of a J- or U-shaped response associating one to three drinks a day with a decrease in cardiovascular mortality when compared to either abstinence from alcohol or heavy alcohol consumption.(1–3) Mechanistically, alcohol has been implicated to be anti-atherogenic, to inhibit lipid oxidation, to increase HDL or “good cholesterol,” and to decrease inflammatory factors; and clinically has been related to a lower prevalence of coronary artery disease (CAD) as well as a reduced risk of death from CAD.(2–5) However, fewer studies have looked at how these cardioprotective effects may differ based on the type of alcoholic beverage consumed, or the effects of other components of alcohol-containing beverages. The consumption of red wine specifically, has been implicated to have several cardioprotective properties. In fact, the term “French paradox” was coined in the early 1990s describing the phenomenon that despite a generally atherogenic diet, the French have a relatively low incidence of cardiovascular disease as compared to the rest of the Western world.(6) These cardioprotective properties have not only been associated with the alcohol in red wine but with other components as well, specifically polyphenols such as resveratrol.(6, 7) In fact, more recent studies have attributed cardioprotective effects of red wine to these polyphenolic compounds, apart from the presence of alcohol.(7–9)
Beneficial cardiovascular effects associated with polyphenols such as resveratrol, include anti-inflammatory, lipid-lowering, pro-angiogenic, and even anti-cancer properties. However, despite enormous marketing and development in the private sector of resveratrol supplements, published medical literature remains controversial or inconclusive.(10, 11) Previous large animal experiments in our laboratory have focused on purified oral resveratrol supplementation in the setting of chronic myocardial ischemia and hypercholesterolemia. These swine experiments did associate high-dose oral resveratrol supplementation of 100mg/kg/day with improved myocardial perfusion to ischemic regions, as well as preserved microvascular coronary vessel function.(12) However, this same group of studies suggested that despite positively modifying cardiovascular risk factors and improving myocardial perfusion, that resveratrol was in fact anti-angiogenic at these high doses.(13, 14)
The current study uses this same large-animal model of diet-induced hypercholesterolemia with the intent to examine the cardiovascular effects of ethanol with and without resveratrol (vodka vs. red wine) with both experimental groups receiving an equivalent amount of ethanol. The high-fat/cholesterol diet used in the current study has previously been shown to induce endothelial dysfunction and increase oxidative stress in swine.(15)
Methods
Animal Model
Starting at 4 weeks of age, 27 male Yorkshire miniswine (Parson’s Research, Amherst, MA) were fed a 500 g/day high-fat/cholesterol diet throughout the 11-week experiment to simulate conditions of coronary artery disease (CAD) and induce endothelial dysfunction. The hypercholesterolemic diet consisted of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate, and 75% regular chow (Sinclair Research, Columbia, MO). All animals had unlimited access to drinking water. After 4 weeks of diet modification, all animals underwent ameroid constrictor placement as previously described.(14)
Post-operatively, animals were divided into three groups according to diet supplementation. The high-cholesterol control group (HCC, n=9) continued the high-fat/cholesterol diet for the remaining 7 weeks of the experiment. High-cholesterol wine pigs were supplemented with 375 mL of red wine (2009 Pinot Noir, Black Mountain Vineyard, Napa and Sonoma, CA) daily (12.5% EtOH/V, HCW, n=9). High-cholesterol vodka pigs were supplemented with 112 mL of vodka (Rubinoff Vodka, Somerville, MA) daily (40% EtOH/V, HCV, n=9).
Seven weeks after initial operation, at 15 weeks of age, all animals underwent a final non-survival operation via a median sternotomy. Cardiac harvest included the collection of tissue from the remote, normal left ventricle (NV). A small transmural section of myocardium (approximately 200 μg) was placed into Krebs solution on ice for microvessel studies to be conducted on the same day. A second transmural section of similar size was collected for shadow labeling and perfusion analysis (as described below). The remaining myocardium was immediately snap-frozen in liquid nitrogen for immunohistochemical analysis and Western blot studies.
All experiments were approved by the Rhode Island Hospital Institutional Animal Care and Use Committees. Animals were cared for in accordance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals.”(16)
Myocardial Blood Flow
Prior to cardiac harvest, isotope-labeled microspheres (BioPhysics Assay Laboratory Inc.) were injected into the left atrium for perfusion analysis as described previously.(12) Briefly 1.5 × 107 gold microspheres (5 ml) were injected into the left atrium at the time of ameroid placement during temporary LCx occlusion to identify the NV and area at risk (AAR) through shadow labeling. For perfusion analysis, Lutetium and Europium microspheres were injected into the left atrium during the final operation during conditions of rest and demand pacing at 150 beats/min (bpm) with an external pacing pulse generator (Medtronic, Minneapolis, MN) while simultaneously withdrawing arterial blood from the femoral artery catheter with the Harvard PHD 2000 automated syringe pump (Harvard Apparatus, Holliston MA). NV myocardium samples and blood samples were dried at 60° C for > 48 h. Microsphere density in each sample was calculated with a gamma counter after exposure to neutron beam radiation (Biophysics Assay Laboratory). Myocardial blood flow to each sample was calculated using: Blood flow = (withdrawal rate/tissue weight) × (tissue microsphere count/blood microsphere count).
Vessel Density
12-μm-thick sections of frozen myocardial tissue from the NV were formalin-fixed and incubated with goat antibody against endothelium-specific CD31 (R&D Systems, Minneapolis, MN) followed by DyLight-conjugated anti-goat secondary antibody (Jackson ImmunoResearch, West Grove, PA) for capillary density, or with mouse antibody against smooth muscle actin (SMA, Abcam, Cambridge, MA) followed by DyLight-conjugated anti-mouse secondary antibody (Jackson ImmunoResearch) for arteriolar density. Microscopic images of sections were digitally captured and analyzed for capillary density (CD31-positive structures between 5 and 25 μm2 in cross-sectional area) and arteriolar density (SMA-positive structures that co-stained with CD31) as previously described,(17) and analyzed using Image J software (National Institutes of Health, Bethesda, MD). Arteriolar density was calculated as arteriolar vessel count per high-powered field (HPF) while capillary density was reported as the percent of a representative HPF occupied by CD31 staining capillaries. Researchers were blinded to the study group during data analysis.
Microvessel Studies
Coronary arterioles (80 – 180 μm diameter) were isolated from the NV samples (kept in Krebs solution on nice from harvest earlier the same day) and placed in a microvessel chamber. Vessels were preconstricted by 30–60% of the baseline diameter with the thromboxane-A2 analog U46619 (0.1 – 1.0 μM) and then treated with the endothelium-dependent vasodilator adenosine diphosphate (ADP, 10−9 to 10−4 mol/L), or the endothelium-independent vasodilator sodium nitroprusside (SNP, 10−9 to 10−4 mol/L). Responses were defined as % relaxation of the preconstricted diameter as previously described.(17) All reagents were obtained from Sigma Aldrich (St. Louis, MO). Researchers were blinded to study group during microvessel studies and data analysis.
Myocardial Lysate Preparation
Myocardial lysis buffer was prepared by adding 1 protease inhibitor tablet (Roche Diagnostics, Indianapolis, IN), 250 μL each of phosphatase inhibitor 2 and 3 cocktails (Sigma-Aldrich), and 320 μL of 2M sodium fluoride to 25 mL of radio-immunoprecipitation assay buffer (RIPA Buffer, Boston BioProducts, Ashland, MA) at 4° C. The RIPA soluble myocardial lysate fraction was then prepared by homogenizing 150 mg of previously frozen (−80° C) myocardial tissue from the NV in 1.0 mL of the above prepared RIPA buffer at 4° C using 6 × 2.0 mm diameter zirconium-oxide beads (Next Advance, Inc. Averill Park, NY) in the Bullet Blender Blue tissue homogenizer (Next Advance) for two, 5-minute cycles. Lysates were then incubated at 4° C for 30 minutes and centrifuged at 14,000 x g for 10 minutes at 4° C. The supernatant was designated as the RIPA soluble fraction. Prepared lysates were stored in liquid nitrogen. Total protein concentrations for all lysates were determined by Micro BCA Protein Assay Kit (Thermo Scientific, Rockford, IL) and Synergy Mx multi-mode microplate reader 7191000 (BioTek Instruments, Inc. Winooski, VT).
Protein Expression and Protein Oxidative Stress
Sixty micrograms of total protein from the RIPA soluble myocardial lysate fraction made from the NV was fractionated by SDS-PAGE using the NuPage Novex Bis-Tris Mini Gel system (Invitrogen, Carlsbad, CA) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). Membranes were incubated at 4° C overnight with antibodies against Cu/Zn super oxide dismutase 1 (SOD1, Enzo Life Sciences, Inc. Farmingdale, NY), Mn superoxide dismutase 2 (SOD2, Enzo Life Sciences), NADPH Oxidase (NOX2, Sigma-Aldrich, St. Louis, MO), vascular endothelial growth factor (VEGF, Abcam, Cambridge, MA), endothelium nitric oxide synthase (eNOS, Cell Signaling, Danvers, MA), and phospho-eNOS (p-eNOS, Cell Signaling) at dilutions recommended by the manufacturer followed by the appropriate HRP-linked secondary antibodies (1:4000 – 1:8000, Jackson Immunoresearch, West Grove, PA). Immune complexes were visualized via electrochemiluminescense (ECL) and digitally photographed using GeneSnap software (Syngene, Cambridge, England), in conjunction with the GBOX Chemi XT16 digital image capture system (Syngene). Raw data from uncompressed tiff digital images were analyzed with Image J software to quantify light densiometry for each sample at the appropriate molecular weight. Densiometry results for each group was averaged and expressed in fold change (FC) as compared to the HCC mean using Microsoft Excel Software (Microsoft, Redmond, WA). To ensure and correct for equal protein loading, membranes were stained with Ponceau acid red, a validated method for protein loading control,(18) or by expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Cell Signaling), a constitutively expressed housekeeping protein, for eNOS and p-eNOS, with adjustments made during analysis as necessary.
Blotting for total protein oxidation was carried out via Oxyblot Protein Oxidation Detection kit (Millipore) using 10 μg of myocardial lysate from the NV for each sample following the protocol provided by the manufacturer. Membranes were developed in ECL, and analyzed and quantified as described above for Western blots. To ensure and correct for equal protein loading, membranes were probed with GAPDH, with adjustments were made to analysis as necessary. Researchers were blinded to the study group during data analysis for both Western blot and total protein oxidation assay.
Statistical Analysis
All results were reported as mean ± standard error of the mean (SEM). Myocardial perfusion data, immunohistochemistry, Western blot and protein oxidative stress results were analyzed by one-way analysis of variance among groups (ANOVA) followed by a post-hoc Bonferroni test to measure variance between groups while microvessel relaxation results were analyzed by two-way ANOVA followed by post-hoc Bonferroni tests using GraphPad Prism 5.0 Software (GraphPad Software Inc. San Diego, CA).
Results
Experimental Model
One animal from the HCW group died 9 days after ameroid placement. Necropsy did not reveal any obvious reason for death (which was likely due to ventricular arrhythmia) and the animal was excluded from analysis. Two animals from the HCC group died. One death occurred after jugular venous blood draw, presumably from a tension pneumothorax, and the other died during ameroid placement secondary to technical intraoperative complications. Both were excluded from analysis, and replaced with new control animals. All animals consumed the total content of each meal and any meal supplement (red wine or vodka mixed into chow). There were no obvious behavioral changes in any specific animal or group.
Myocardial Perfusion
There were no differences in myocardial perfusion either at rest, or during demand pacing (Fig. 1).
Figure 1.
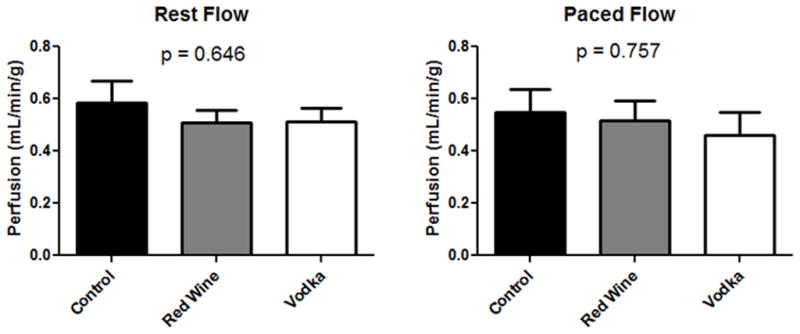
Myocardial Blood Flow to the left ventricle both at rest and during demand pacing of 150 bpm.
Vessel Density
Immunohistochemical analysis did not reveal any significant differences in arteriolar density or in capillary density among groups (Fig. 2).
Figure 2.
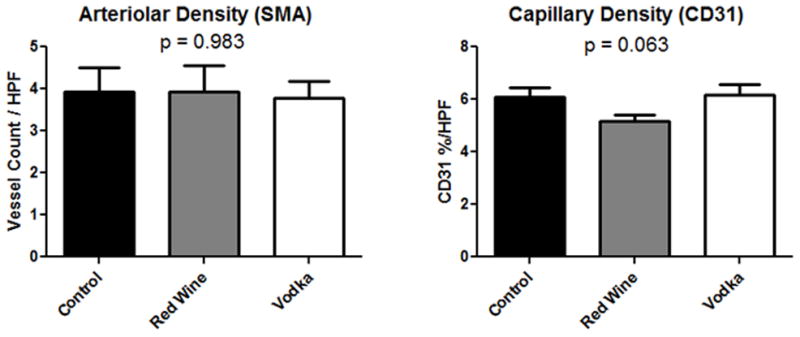
Vessel Density in the left ventricle. Arteriolar density identified by structures of the appropriate size co-staining for smooth muscle actin (SMA) and CD31, whereas capillary structures stain for CD31 alone. HPF = high powered field.
Microvessel Reactivity
Endothelium-dependent relaxation to ADP did vary significantly between groups (ANOVA p = 0.0026) with a more robust relaxation response in the HCW group as compared to either the HCV group or control group. See Figure 3 for Bonferroni post-test analysis between groups at specific dosing. The microvessel response to the endothelium-independent relaxation to SNP did not significantly differ between groups (Fig. 3).
Figure 3.
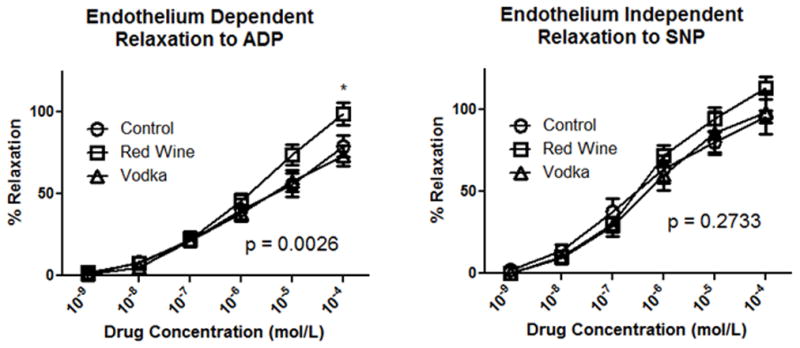
Microvessel Reactivity in the left ventricle in response to adenosine diphosphate (ADP) and sodium nitroprusside (SNP). P value reported for two-way ANOVA while * denotes p < 0.05 for post-test at given concentration.
Protein Oxidative Stress and Protein Expression
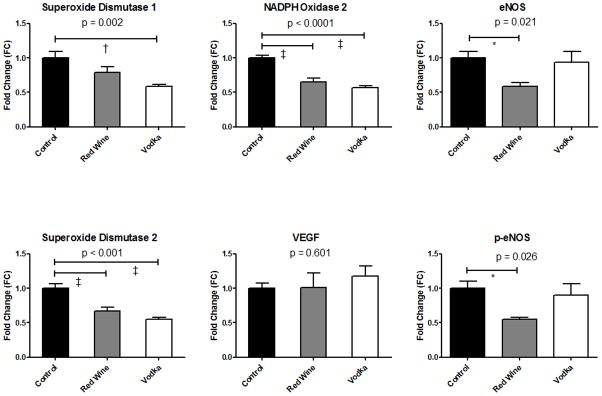
Total protein oxidation was significantly lower in the HCW and HCV groups as compared to the controls (Fig. 4). Expression of superoxide dismutase, an antioxidant which catalyzes the dismutation of superoxide into oxygen and hydrogen peroxide, varied significantly among groups with SOD1 expression lowest in the HCV group, and SOD2 expression significantly lower in both the HCW and HCV groups as compared to controls. Expression of NADPH oxidase (NOX2), a generator of the reactive free-radical, superoxide, was also significantly lower in both treatment groups (HCW, HCV) as compared to controls. VEGF expression did not vary significantly among groups, while expression of both total and phosphorylated eNOS was significantly lower in the HCW group as compared to controls (Fig. 5).
Figure 4.
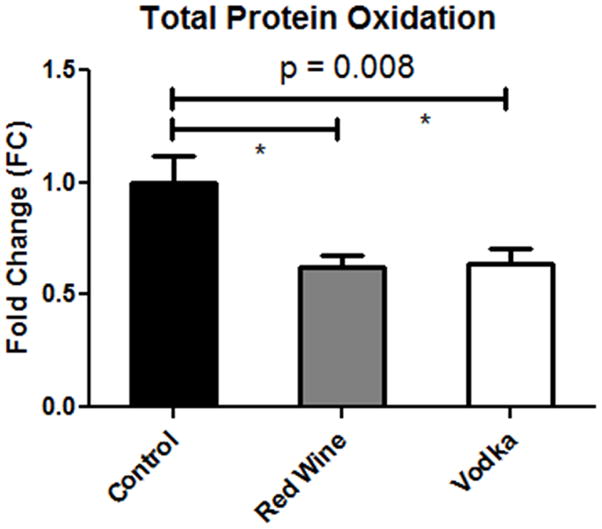
Total Protein Oxidation in the left ventricular myocardium. P value reported for one-way ANOVA while * denotes p < 0.05 between groups.
Figure 5.
Protein Expression as measured by Western blot of left ventricular myocardial lysates. P value reported for one-way ANOVA. Post-test analysis between groups: * = p < 0.05, † = p < 0.01, ‡ = p < 0.001.
Discussion
In this study of diet-induced endothelial dysfunction, the two experimental groups had their high-fat/cholesterol diet supplemented with either vodka or red wine receiving the same amount of ethanol daily. There were no differences in myocardial perfusion among groups, nor were there any differences in vascular ingrowth as measured by immunohistochemical staining for arterioles and capillaries, or in VEGF expression. Upon further examination of coronary microvessel reactivity and pathways of oxidative stress, clear differences were observed not only between the controls and the groups receiving alcohol, but also between the vodka-supplemented and red wine-supplemented groups. A significant reduction in total protein oxidation was observed in both treatment groups (red wine and vodka) as compared to controls. Protein expression of NADPH oxidase (NOX2), a producer of superoxide and a major contributor to the development of atherosclerosis,(19) was also significantly decreased in both the treatment groups. Thus, these observed anti-oxidant effects appear the result of ethanol exposure, as both treatment groups were receiving equivalent amounts of ethanol, and no difference existed between the two treatment groups in terms of total protein oxidation or NOX2 expression. The expression of cytoplasmic (SOD1) and mitochondrial (SOD2) superoxide dismutase was also decreased in the treatment groups. Though a reduction in expression of these vital antioxidants in general may represent either a deficiency in the catalyzed dismutation of superoxide, or simply decreased expression secondary to decreased oxidation in the cell, the current study would appear to be a manifestation of the later, as we observed concomitant reductions of total protein oxidation and of producers of superoxide. These findings correlate with the known effect of moderate alcohol consumption to raise plasma levels of HDL,(5) and the natural antioxidant effects of HDL such as the inhibition of the oxidation of low density lipoprotein (LDL).(20)
Analysis of microvascular function did yield differences between the two treatment groups. As described previously, our laboratory has demonstrated this high-fat/cholesterol, atherogenic diet to induce measurable endothelial dysfunction in swine.(15) Thus, the finding of improved endothelium-dependent vasorelaxation in the current study observed in the red wine-supplemented animals at higher doses of ADP as compared to either the controls, or vodka-supplemented animals does offer insight into the potential benefits of red wine independent of the presence of alcohol. Previous studies in Yorkshire swine fed this same diet demonstrated high-dose, purified resveratrol (100 mg/kg/day) supplementation to also preserve endothelium-dependent coronary microvascular function,(12) suggesting that resveratrol, even at the comparatively lower doses expected in red wine, may prevent endothelial dysfunction caused by an atherogenic diet. However, these results must be interpreted with caution, as resveratrol is by no means the only compound in red wine other than ethanol, and is in fact, not even the only polyphenol present in red wine. That being said, there is rapidly growing evidence in both animal studies (2, 11, 12) and clinical trials (21) suggesting that resveratrol improves endothelial function. In contrast to previous studies demonstrating improved endothelial function with resveratrol administration (12), we observed a decrease in both total and phosphorylated eNOS in the red wine treatment group, although improved endothelium-dependent vasorelaxation was still observed. Granted, the study in reference used a much higher dose of resveratrol; however, one would still anticipate higher expression of activated (phosphorylated) eNOS in conjunction with improved microvascular function. There are several possible explanations for this paradoxical finding. One possibility is that the “physiologic” dose of resveratrol found in red wine, or some other acting agent in the compound, increases the sensitivity of the endothelium to nitric oxide (NO), thus necessitating lower (in this case ½ as much) available NO in order to increase endothelium-dependent vasorelaxation. Another possibility is that the improvements observed in microvascular reactivity in the red wine group is the sequelae of upregulation of another vasoactive pathway such as cyclooxygenase, causing a decrease in the eNOS pathway through negative feedback. A third possibility could be through an increase in bioavailable NO in the red wine treated group secondary to a decrease in oxidative stress and free radicals, thus requiring less expression of eNOS and p-eNOS. However, if the third scenario were the case, then we would also have expected these same changes in the vodka-treated animals, and this was not observed.
In this study of induced endothelial dysfunction with an atherogenic diet, supplementation with both red wine and vodka decreased oxidative stress by several measures, implicating the effects of ethanol in reducing oxidative stress in the myocardium. However, it was only in the red wine-supplemented group that any improvement in microvessel function was observed. This suggests that a component of red wine, independent of ethanol, possibly a polyphenol such as resveratrol, may confer cardioprotection by normalizing endothelial dysfunction induced by an atherogenic diet. These findings are in line with recent and consistent findings in both animal studies and clinical trials and warrant further investigation in regards to the role of alcohol and polyphenol consumption in decreasing the morbidity and mortality of cardiovascular disease.
Acknowledgments
We would like to thank the animal research facility staff at the Rhode Island Hospital for their excellent care of the animals and assistance in the operating room
Footnotes
The authors have no potential conflicts of interest to disclose.
Financial Support and Disclosures: Funding provided by grants from the National Heart, Lung, and Blood Institute (R01HL46716, R01HL69024, and R01HL85647, Dr. Sellke), NIH training grant T32-HL076130 (Dr. Sellke), NIH training grant 5T32-HL076134 (Dr. Lassaletta), NIH training grant 5T32-HL094300 (Dr. Chu, Dr. Elmadhun), NIH training grant T32-HL007734 (Dr. Robich), and through the Thoracic Surgery Foundation for Research and Education Fellowship (Dr. Lassaletta).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eapen DJ, Manocha P, Valiani K, Mantini N, Sperling L, et al. Alcohol and the heart: an ounce of prevention. Curr Treat Options Cardiovasc Med. 2011;13:313–325. doi: 10.1007/s11936-011-0131-z. [DOI] [PubMed] [Google Scholar]
- 2.Lakshman R, Garige M, Gong M, Leckey L, Varatharajalu R, et al. Is alcohol beneficial or harmful for cardioprotection? Genes Nutr. 2010;5:111–120. doi: 10.1007/s12263-009-0161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansel B, Thomas F, Pannier B, Bean K, Kontush A, et al. Relationship between alcohol intake, health and social status and cardiovascular risk factors in the Urban Paris-Ile-de-France Cohort: is the cardioprotective action of alcohol a myth? Eur J Clin Nutr. 2010;64:561–568. doi: 10.1038/ejcn.2010.61. [DOI] [PubMed] [Google Scholar]
- 4.Magnus P, Bakke E, Hoff DA, Hoiseth G, Graff-Iversen S, et al. Controlling for high-density lipoprotein cholesterol does not affect the magnitude of the relationship between alcohol and coronary heart disease. Circulation. 2011;124:2296–2302. doi: 10.1161/CIRCULATIONAHA.111.036491. [DOI] [PubMed] [Google Scholar]
- 5.Brinton EA. Effects of ethanol intake on lipoproteins and atherosclerosis. Curr Opin Lipidol. 2010;21:346–351. doi: 10.1097/MOL.0b013e32833c1f41. [DOI] [PubMed] [Google Scholar]
- 6.Lippi G, Franchini M, Favaloro EJ, Targher G. Moderate red wine consumption and cardiovascular disease risk: beyond the “French paradox”. Semin Thromb Hemost. 2010;36:59–70. doi: 10.1055/s-0030-1248725. [DOI] [PubMed] [Google Scholar]
- 7.Rastija V. An overview of innovations in analysis and beneficial health effects of wine polyphenols. Mini Rev Med Chem. 2011;11:1256–1267. doi: 10.2174/13895575111091256. [DOI] [PubMed] [Google Scholar]
- 8.Vasanthi HR, Parameswari RP, Deleiris J, Das DK. Health benefits of wine and alcohol from neuroprotection to heart health. Front Biosci (Elite Ed) 2012;4:1505–1512. doi: 10.2741/e476. [DOI] [PubMed] [Google Scholar]
- 9.Wu JM, Hsieh TC. Resveratrol: a cardioprotective substance. Ann N Y Acad Sci. 2011;1215:16–21. doi: 10.1111/j.1749-6632.2010.05854.x. [DOI] [PubMed] [Google Scholar]
- 10.Chu LM, Lassaletta AD, Robich MP, Sellke FW. Resveratrol in the Prevention and Treatment of Coronary Artery Disease. Curr Atheroscler Rep. 2011;13:439–446. doi: 10.1007/s11883-011-0202-3. [DOI] [PubMed] [Google Scholar]
- 11.Lassaletta AD, Chu LM, Sellke FW. Therapeutic neovascularization for coronary disease: current state and future prospects. Basic Res Cardiol. 2011;106:897–909. doi: 10.1007/s00395-011-0200-1. [DOI] [PubMed] [Google Scholar]
- 12.Robich MP, Osipov RM, Nezafat R, Feng J, Clements RT, et al. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation. 2010;122:S142–149. doi: 10.1161/CIRCULATIONAHA.109.920132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robich MP, Chu LM, Chaudray M, Nezafat R, Han Y, et al. Anti-angiogenic effect of high-dose resveratrol in a swine model of metabolic syndrome. Surgery. 2010;148:453–462. doi: 10.1016/j.surg.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu LM, Robich MP, Lassaletta AD, Feng J, Laham RJ, et al. Resveratrol supplementation abrogates pro-arteriogenic effects of intramyocardial vascular endothelial growth factor in a hypercholesterolemic swine model of chronic ischemia. Surgery. 2011;150:390–399. doi: 10.1016/j.surg.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, et al. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg. 2006;81:634–641. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 16.Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 17.Clements RT, Sodha NR, Feng J, Boodhwani M, Liu Y, et al. Impaired coronary microvascular dilation correlates with enhanced vascular smooth muscle MLC phosphorylation in diabetes. Microcirculation. 2009;16:193–206. doi: 10.1080/10739680802461950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, et al. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem. 2010;401:318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 19.Ago T, Kuroda J, Kamouchi M, Sadoshima J, Kitazono T. Pathophysiological roles of NADPH oxidase/nox family proteins in the vascular system. -Review and perspective. Circ J. 2011;75:1791–1800. doi: 10.1253/circj.cj-11-0388. [DOI] [PubMed] [Google Scholar]
- 20.Farmer JA, Liao J. Evolving concepts of the role of high-density lipoprotein in protection from atherosclerosis. Curr Atheroscler Rep. 2011;13:107–114. doi: 10.1007/s11883-011-0166-3. [DOI] [PubMed] [Google Scholar]
- 21.Magyar K, Halmosi R, Palfi A, Feher G, Czopf L, et al. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin Hemorheol Microcirc. 2012;50:179–187. doi: 10.3233/CH-2011-1424. [DOI] [PubMed] [Google Scholar]