Abstract
Background
A recent study of peanut allergic subjects treated with omalizumab generated some results that were concordant with a study of cat allergics being treated with omalizumab. However, there were differences that provided additional insights into the nature of the cellular responses in allergic patients.
Objective
Determine the cause for failure to suppress the allergen-induced basophil response during treatment with omalizumab.
Methods
Peanut allergic patients were treated with omalizumab. Clinical, serological and cellular indices relevant to the response of the patient and their peripheral blood basophils (specific-to-total IgE ratio, cell surface FceRI expression, histamine release responses to anti-IgE Ab or peanut allergen) were obtained at 3 times.
Results
Following treatment, approximately 60% of the subjects’ basophil responses to peanut allergen did not significantly decrease. In 40% of cases, the in vitro basophil response to peanut allergen increased 2–7 fold. The increases were associated with two primary factors, a high (>10%) specific-to-total IgE ratio and an increase in the intrinsic response of the basophil to IgE-mediated stimulation. The extent to which the basophil response to peanut allergen increased was inversely correlated with the improvement in the individual’s ability to tolerate ingestion of peanut.
Conclusion
The basophil response during treatment with omalizumab is a consequence of two competing factors, suppression of allergen-specific IgE on the cell surface vs. increased intrinsic sensitivity to IgE-mediated stimulation. In peanut allergy, the basophil response appears to mitigate against the ability of omalizumab to improve the patients’ tolerance of oral allergen.
Keywords: basophil, anti-IgE, peanut, syk, IgE receptor
Introduction
A longstanding issue in the field of allergic disease is concerned with the relative roles of mast cells and basophils in the expression of this family of diseases. This question was recently explored by using omalizumab to manipulate IgE levels in cat allergic subjects 1. The study design used observations that the basophil response to cat allergen would decrease much faster than the tissue mast cell response when subjects were placed on omalizumab. It was found that when the basophil response was markedly decreased the nasal mast cell response was not but that clinical signs and symptoms during an experimental allergen challenge in the nose were decreased by 50%. One interpretation of this observation was that basophils contributed to the acute response to allergen in the nose.
This experimental design was extended to subjects with peanut allergy 2. As in the previous design, the subjects’ basophil histamine release response to peanut allergen was monitored after the start of omalizumab. When the response decreased to 20% of the pre-omalizumab baseline, subjects were brought back to the clinic for the first post-treatment double-blinded oral food challenge. Unexpectedly, approximately 60% of the subjects’ basophils did not show a decreased response to peanut allergen when considering the entirety of the peanut dose response curve, although there were decreases at the lowest suboptimal levels of stimulation. There appear to be reasons for these results that this report will explore because they speak to a newly discovered characteristic of the system. In the aforementioned study of cat allergic patients it was observed that while the basophil response to cat allergen generally decreased after starting treatment, the distribution of the decrease in the treated patients was broad. In that study, it was noted that for nearly all subjects, the expression of basophil syk increased 3. This early non-redundant tyrosine kinase is essential for early signal transduction and is required for IgE-mediated histamine release as well as essentially all IgE-mediated functional outcomes in basophils. The increase in syk expression was accompanied by an increase in the response of basophils to the pan-stimulus, anti-IgE antibody (an aggregating anti-IgE antibody routinely used to assess the IgE-mediated response from human basophils). The increase in syk expression was unexpected unless certain properties of basophil maturation were taken into account 4, but even with this consideration, the behavior was surprising and clearly represented a possible confounding process towards the efficacy of treatments like omalizumab. Although results from cat allergy study suggested that there might be a mitigating factor related to the basophil’s intrinsic sensitivity, the current peanut study demonstrated that this effect could be a dominant aspect of the underlying biological response to omalizumab. This study will demonstrate how two biological processes, the specific-to-total IgE ratio and the omalizumab-induced increase in the basophil’s IgE-mediated response, compete to alter the basophil’s response to challenge with specific allergen, peanut in this case. In addition, this analysis of the peanut response improves upon the interpretation of the mechanisms underlying the clinical response presented in the clinical part of the study.
Methods
Study Design
The study was a 6 month, open label study of 14 subjects and is described in greater detail in a companion manuscript 2. Briefly, subjects were treated with omalizumab according to the package insert for a period of 6 months. Prior to the start of treatment, several weeks after the start of treatment and 6 months after the start of treatment blood was obtained and basophils tested for IgE-mediated responses, FcεRI and syk expression. Also prior to treatment subjects were tested with a double-blind oral peanut challenge (OFC). During the first weeks of treatment, subjects returned for blood sampling at week 2 and then weekly until week 6 to monitor peanut allergen induced basophil histamine release (BHR). When a subject’s peanut allergen induced BHR decreased to <20% of baseline values (assessed by area under the dose response curve to peanut allergen, peanut-BHR AUC), a second OFC (OFC 2) and skin prick test titration (peanut-SPT) were performed. If a subject’s peanut-BHR AUC had not declined to <20% of baseline values by week 6, OFC 2 was performed at week 8. Peanut-BHR AUC was followed monthly after OFC 2 in those subjects whose peanut-BHR AUC had not declined to <20% of baseline values. After 6 months of omalizumab, a final OFC (OFC 3), and peanut-skin prick test were completed.
Total and peanut specific IgE levels
Total and peanut allergen-specific IgE were measured by using the Immuno-CAP 250 (Phadia, Uppsala, Sweden).
Basophil Histamine Release
Venous blood was drawn into syringes containing PBS-EDTA and basophils were isolated using a single Percoll-based density-gradient with Accuspin separation tubes. Enriched basophils were enumerated and stimulated for BHR using anti-IgE (HP6061, Hybridoma Reagent Laboratory, 0.03–1 μg/mL) and peanut allergen (0.001–10,000 ng/mL) in duplicate for 45 minutes at 37°C using calcium-containing buffers. Automated fluorometry was used to measure BHR in cell free supernatants 5. Results for each stimulus are reported as a percentage of the total histamine content found in an aliquot of perchloric acid-lysed leukocytes after subtraction of spontaneous BHR from cells in buffer alone [(stimulated HR/lysed HR)-(spontaneous HR/lysed HR)]. In some analyses, the results are reported as the area under the basophil histamine release dose response curve at all 8 concentrations of peanut allergen (peanut-BHR AUC). Basophil histamine release was performed twice prior to treatment with omalizumab, at the screening during recruitment and on the first day (day 0 = baseline) of the study prior to drug administration. The ‘fold change’ in either the clinical response or the basophil response was calculated. For the basophil response, the AUC of the peanut basophil response at OFC2 vs. OFC1 was calculated and the fold change calculated as OFC2-AUC/OFC1-AUC. For the clinical response, the ratio of the amount of peanut ingested prior to stopping the challenge at OFC2 vs. OFC1 was calculated.
Flow cytometry
Flow cytometry methods relevant to a study of omalizumab treatment have been described in detail in a previous publication 3 and are included in the online repository.
Statistics
Figures show average data ± standard error of the mean with relevant comparisons using the Student’s T-test. Pearson’s correlations and linear regression are used for relationships. For curve fitting, data for the dose response curves shown in the online repository figure E2 were fit to a Gaussian function from which 50% of maximum and peak position were extracted.
Results
As previously reported 2, 6/14 patients’ basophil response to peanut allergen stimulation (AUC of the full dose response curve) decreased while the 8 remaining subjects showed an increase that averaged 2.9 fold (95% C.I.; 2.29–3.41). In our previous study of cat allergics 1, only 2/12 of patients showed an increase in the basophil response to cat allergen and only one that was significantly greater than a ratio of 1.0 (post-treatment/baseline). The difference in the two studies was unexpected, quite marked and puzzling since omalizumab is largely expected to reduce the function of basophils and mast cells. The difference may derive from two sources of biological variation that now appear to be relevant to the function of basophils.
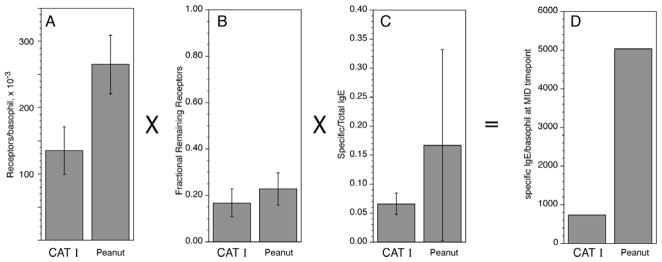
The density of cell surface peanut-specific IgE was considerably greater during treatment than the density of cat-specific IgE observed in the cat study. Figure 1 summarizes the comparison. The calculation is based on several pieces of information. First, the starting FcεRI density was greater in the peanut allergic patients (panel 1A). Second, the ratio of treatment FcεRI density/baseline FcεRI density was slightly higher in the peanut study (panel 1B). Third, the average peanut specific/total ratio was higher in the peanut allergic patients (panel 1C). With these 3 factors and one additional factor, the ratio of IgE/FcεRI for different IgE concentrations (for peanut and cat studies, a factor of 0.5, see online supplement, figure E1), the average cell surface specific IgE density was approximately 7 fold higher in the peanut study than the cat study (5040/cell vs. 740/cell, respectively). This difference is important since several studies have suggested that basophils respond maximally to stimulation with only 5000 antigen-specific IgE/cell 6. Note also, that this number represents the average for the study subjects and that the heterogeneity is important for understanding the detailed response (see below).
Figure 1.
Summary of factors needed to calculate the cell surface allergen-specific IgE density after treatment with omalizumab: comparison of two studies, Cat and Peanut (the current study). Panels A–C; mean ± standard deviations. Panel D; calculated allergen-specific IgE density which is a product of the panels A–C and a fourth factor that is similar for the two studies (see online repository).
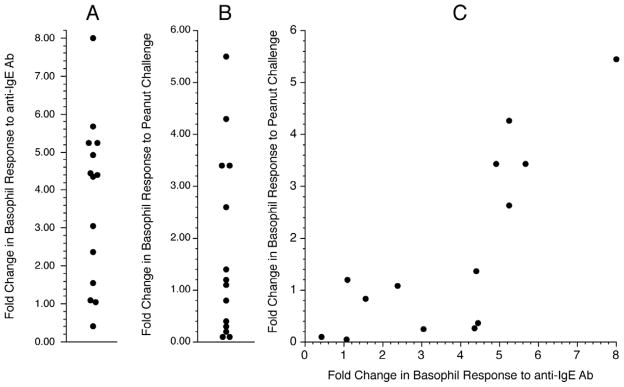
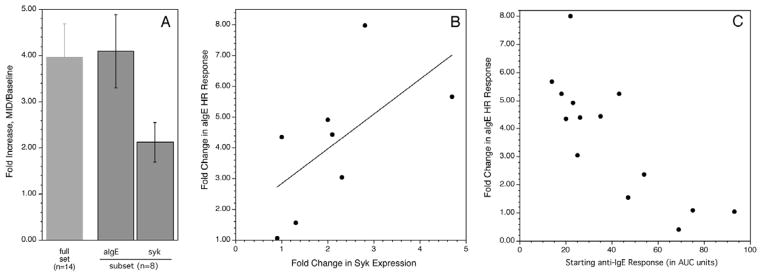
An unexpected observation from the cat allergy study was that most subjects on omalizumab demonstrated an increase in the basophil response to the pan-stimulus anti-IgE Ab (an aggregating anti-IgE Ab) 3. This was accompanied by an increase in syk expression in basophils 3. A similar observation was made in the peanut study, demonstrating that in food allergy, omalizumab also alters intrinsic responsiveness. In this study, the AUC for the anti-IgE dose response curve was used as a metric of the change for comparison purposes to the peanut dose response curve. The average increase in the response was 3.9 fold (figure 2A) and there was a correlation, r = 0.729, between the increase in the response to anti-IgE Ab and the change in response to peanut allergen (figure 2C, figure 2B also shows the distribution in peanut response). In a subset of subjects (those for whom a syk measurements was made), n=8, syk expression was measured in basophils by flow cytometry. As found in our previous cat study, syk expression increased about 2 fold in these subjects and the extent of syk expression correlated with the increase in the anti-IgE response (figure 3A & 3B, also see the online supplement for a discussion of the syk assay). There was also an inverse correlation between the starting anti-IgE response and the fold increase in the anti-IgE response (figure 3C).
Figure 2.
Distribution of responses obtained from the Peanut I study. Panel A: Distribution of the in vitro basophil response to anti-IgE Ab (area-under-the curve, AUC); fold change between the pretreatment response (BSL) and the MID point response. Panel B: Distribution in the in vitro basophil response to peanut allergen (AUC). Panel C: Relationship between the distributions shown in panels A and B.
Figure 3.
Changes in syk expression during treatment and relationship of starting to final anti- IgE response. Panel A: far left column shows the average fold change in the basophil response to anti-IgE antibody while the right two columns show the average fold change in the anti-IgE Ab response or syk expression for the subset of 8 subjects where syk expression was measured. Panel B; correlation between the fold up-regulation of the anti-IgE Ab response vs. change in syk expression. Panel C; relationship between the starting anti-IgE Ab response and the fold change in this response at the MID time point during treatment with omalizumab.
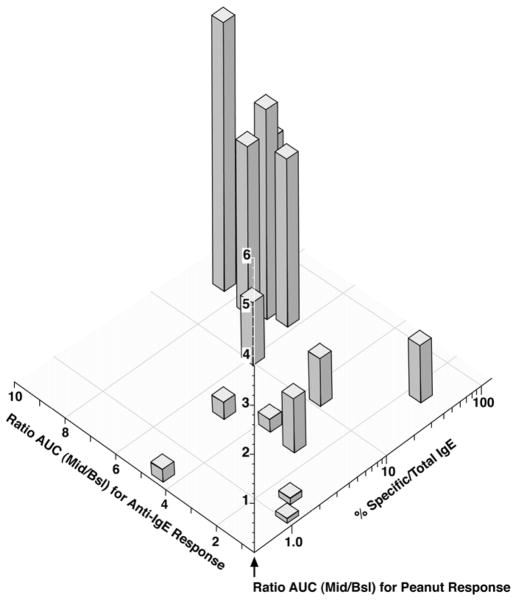
The combined observations of high remaining peanut specific IgE and increase in the cell’s responsiveness offers some insight into events occurring during treatment. The results suggest that two parameters determine the final response to peanut allergen; the specific-to-total ratio and the increase in cellular responsiveness as measured by the basophil response to stimulation with the pan-crosslinking stimulus, anti-IgE. Figure 4 shows the 3-dimensional relationship. It is evident that there is interplay between suppression of the allergen-specific IgE on the cell surface and increased cellular sensitivity. In the worst cases, high specific/total ratio and increased cellular responsiveness, the peanut dose response curves are markedly enhanced. An alternative perspective on this analysis is to categorize the specific/total ratio and increased cellular responsiveness and average the peanut dose response curves for the 4 groups generating by the category divisions. Figure E2 in the online respository shows the results analyzed this way. The thresholds used to make the categories are suggestive; specific/total ratios less than 4% and changes in cellular responsiveness less than 1.5 fold (to anti-IgE) result in a basophil response to peanut allergen that is markedly suppressed. Specific/total ratios considerably greater than 4% and an increased cellular response greater than 4 fold result in the increased response to peanut allergen.
Figure 4.
In vitro basophil response to peanut allergen stimulation (AUC of the full peanut dose response curve) vs. 2 parameters of basophil function, % specific/total IgE and fold increase in the basophil response to stimulation with the pan-stimulus, anti-IgE Ab. 362 Fold changes refer to the responses at the mid-visit vs. the response before (Bsl) treatment with omalizumab (i.e., Mid/Bsl). Each vertical bar represents the in vitro peanut response (AUC-DRC) for each patient.
Parenthetically, it is apparent from the character of the dose response curves shown in figure 4 that peanut allergens are complex enough to generate some unusual patterns in these dose response curves, notably a double maximum in some cases. In addition, as has been pointed out in the companion manuscript for these studies 2, a general behavior is for the curve to shift rightward during treatment, or, using the nomenclature proposed by Nopp et al. 7, CDsens shifts rightward. For simple antigens and monospecific antibodies, aggregation theory predicts a stable optimum for secretion throughout all densities of cell surface IgE 8–10. However, peanut antigens are complex and the IgE bound to the cell surface is composed of many epitope specificities, so the consequences of decreasing densities is less well defined. Supplement figure 2E shows one experimental multivalent antigen dose response curve at two widely different densities and demonstrates that the optimum does indeed shift rightward with lower densities of antigen-specific IgE (see discussion).
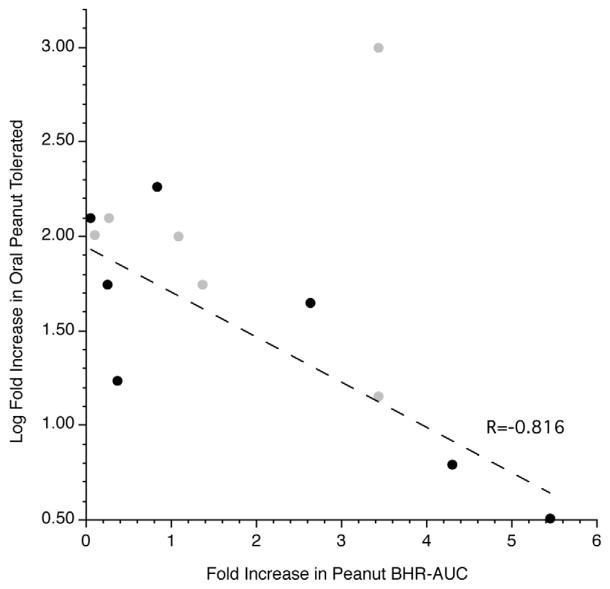
The range of basophil responses led to the analysis shown in figure 5. On the abscissa is plotted the fold increase in the AUC for the peanut dose response curve (when comparing the second challenge to baseline) and on the ordinate the log-fold change in the peanut tolerated in the OFC for these same two time points. The plot presents two groups of subjects, those for whom the ratio in OFC could be directly calculated because the 10 gram stopping rule was not needed (n=7, solid block symbols) and those for whom the ratio is not explicit because the stopping rule was imposed (so the numerator of the ratio is necessarily fixed to 10 grams)(n=6, light gray symbols). The regression/correlation was analyzed using only the explicit ratio data (black symbols). The plot suggests that the more enhanced the basophil response, the more poorly the subject responds to omalizumab.
Figure 5.
Relationship between the fold change in the in vitro basophil response to peanut allergen (AUC) and the log fold change in the tolerated peanut ingestion during oral food challenge (MID/BSL). Points rendered in gray (
 ) were those subjects who reached the stopping rule for ingestion of peanut at the MID OFC while points rendered in black (●) were those subjects from what a ratio could be calculated. The linear regression was fit to the non-capped data points (●).
) were those subjects who reached the stopping rule for ingestion of peanut at the MID OFC while points rendered in black (●) were those subjects from what a ratio could be calculated. The linear regression was fit to the non-capped data points (●).
Discussion
Recent studies exploring the immunological changes that accompany treatment with omalizumab have provided some interesting insights into the behavior of basophils and their role in the allergic disease under study. By combining our experiences obtained from a cat allergy study 1 and the current peanut study 2, it is possible to perceive a developing pattern. One of the most obvious patterns is the relationship of the basophil response to the ratio of specific IgE/total IgE. This is a logical link because this ratio determines how readily one can decrease basophil antigen-specific IgE densities during treatment to reach levels that are known to be suboptimal for IgE-mediated stimulation 6–11. The basophil is quite sensitive and the sensitivity among individuals’ basophils is quite heterogeneous. In the general population, the average density of antigen-specific IgE that is required for a 50% of maximal response is approximately 2000 molecules/cell but this value varies by nearly 30 fold among individuals 6,11,12. There is a correlation between the maximum response induced by anti-IgE Ab and this intrinsic sensitivity 6 and from an as yet unpublished ongoing study of omalizumab effects we now know that the intrinsic sensitivity increases considerably (meaning fewer IgE/cell needed for a response). The full basis for this change is not yet known although the increases in syk expression are consistent with a more sensitive basophil 6.
The observation that the response to anti-IgE antibody generally increases, as well as the observation that syk expression increases, has now been replicated in this peanut study. This appears to be a general property of basophils during treatment with omalizumab although the cause of the change is not known. But this property works against the effects of decreasing the antigen-specific IgE density on the basophil cell surface. The results presented in figure 4 highlight the competition between the two effects. It is notable that a marked increase in basophil response is possible (up to 5 fold when taking the dose response curve as a whole). Coupling both a marked increase in cellular sensitivity and a very high specific/total IgE ratio (see also panel D in figure E2, resulting in ≈9000 peanut-specific IgE/cell, or the points furthest from the origin in figure 4) produces such as increase. In contrast, when the intrinsic sensitivity doesn’t increase (see below), and the density of peanut-specific IgE is very low after treatment (panel A in figure E2, approx. 750 molecules/basophil or the points nearest the origin in figure 4), the peanut response is completely suppressed. Based on the results in panels B & C, it appears that the tipping point for suppression is around >5 fold changes in the basophil responsiveness or >10% specific/total ratio of peanut specific IgE, with some compensatory exchange between these two factors to produce a cell that is not suppressed (i.e., a ratio of specific/total of 10% may require only a modest increase in responsiveness, 1.5–2.0 fold, while a ratio of specific/total of 40% requires only a small change in responsiveness).
It was also useful to note that the unexpected characteristic of omalizumab treatment to increase in syk expression and IgE-mediated release was similar in both aeroallergen and food allergic conditions. One possible distinguishing aspect of these two types of allergy would relate to the avoidance that is possible in food allergics. Provided that the individual has no non-food allergies, the immune system isn’t being contstantly exposed to allergen which might alter the character of the basophil response to omalizumab as has been suggested in our previous study for cat or other perennial allergens. Surveys of the frequency of coincident allergies 13 suggest that food-only allergies are likely rare so that it is likely that even food allergics that are avoiding specific foods continue to be exposed to non-food allergens, indoor or outdoor.
The underlying mechanisms that cause a change in cellular responsiveness remain unknown but in both the predecessor cat study 3 and this study, there is an excellent inverse correl ation between the starting responsiveness and the increase that occurs during treatment. The system is behaving as if low starting response is under the control of an IgE-dependent mechanism because omalizumab relieves the suppression that may be inherent in these individuals. In vitro studies of basophil maturation have suggested that chronic stimulation may generate a basophil with normal granule and receptor expression but suppressed syk expression 4. Therefore, relief of chronic aggregation would be expected to reverse suppression but with undetectable changes in receptor density or granule expression because these are not altered by chronic aggregation. If this were an explanation, it would follow that some subjects experience a natural form of aggregation (that has yet to be identified) that is reversed by omalizumab.
From both the cat and peanut studies, it has also become apparent that, on average, the dose response curve for the antigen shifts rightward, both the peak response and 50% point for the response (so-called CDsens 7,14,15). Both theoretical and experimental studies of simple bivalent haptens and monoclonal or polyclonal IgE antibodies specific for the haptens have shown that cell surface density of hapten-specific IgE does not shift the concentration for optimal histamine release due to the nature of the crosslinking reaction that occurs with bivalent haptens 8–15. However, normal antigens have multiple epitopes that bind to complex mixtures of epitope-specific IgE antibodies on the cell surface and theoretical studies are less specific about a prediction for the behavior of the dose response curve with changing cell surface density. We examined a multivalent antigen with a polyclonal IgE (albeit probably close to monoclonal in its target epitope, penicillin) and found that with changing densities of penicillin-specific IgE, the optimum and CDsens are shifted rightward with decreasing densities. Therefore, the rightward shift observed during treatment with omalizumab is likely to be a simple reflection of the decreasing densities of either cat or peanut-specific IgE. Whether the same rightward shift that occurs in tissue responses results from the same mechanisms is not yet clear.
In this peanut study, all subjects experienced an improvement in their tolerance for orally ingested peanut. The fact that 60% of subjects’ basophils showed no suppression, indeed, a marked increase in basophil response (when considering the entirety of the dose response curve), gives the impression that the basophil response is not particularly relevant to the clinical response. The analysis shown in figure 5 suggests that the clinical response may be mitigated by a poor improvement in the basophil response. Approximately 25% of the subjects showed poor improvement in the oral food challenge when the basophil response was markedly enhanced. If this perspective is correct, the peanut study indirectly suggests the possibility that the mast cell response is largely corrected by treatment but that the basophil response might interfere with optimal improvement. There are indications from the cat study that a similar trend occurs (unpublished results); poorer improvement associates with less suppression of the basophil response. In the companion manuscript describing the clinical outcomes of this study, it was noted that both the basophil and oral food challenge dose-response curves shift rightward. From this perspective, the change in basophil response is consistent with the change in the oral food challenge response, albeit with changes that are not as great as observed in the oral food challenge. This interpretation is consistent with a role for the basophil in the clinical response. But this interpretation ignores the entirety of peanut dose response curve and it can not be known how much of the dose-response curve the basophil experiences in vivo. An alternative interpretation, based on the available data and the analysis presented in figure 5, continues to suggest a role for the basophil but one that modulates a suppressed intestinal mast cell response, sometimes opposing the apparent omalizumab-mediated suppression of this cell. These studies were not designed to distinguish between these two possible interpretations.
In summary, these studies highlight two competing factors that determine the nature of the antigen-specific IgE response in human basophils. Variability in the cell density of antigen-specific IgE (peanut in this study) as a result of variable specific-to-total IgE ratios provides one determinant of the response. The second determinant, which opposes the effect of decreasing antigen-specific IgE, is an increased sensitivity of basophils to IgE-mediated stimulation.
Key Messages.
The in vitro IgE-mediated basophil response during omalizumab treatment increases for some patients and the variability in whether suppression or enhancement occurs results from two factors; the specific-to-total ratio and changes in intrinsic basophil sensitivity.
The variability in the basophil IgE-mediated response may predict the clinical of omalizumab.
Acknowledgments
The authors would like to thank John-Paul Courneya and Valerie Alexander for technical assistance. This work was supported by NIH grants AI20253 and AI070345 to DWM.
Abbreviations
- OFC
oral food challenge
- AUC
area under the curve
- BHR
basophil histamine release
- PBS
phosphate buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eckman JA, Sterba PM, Kelly D, Alexander V, Liu MC, Bochner BS, et al. Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge. J Allergy Clin Immunol. 2010;125:889–95. e7. doi: 10.1016/j.jaci.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savage J, MacGlashan DW, Jr, Saini S, Wood R. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. 2012. manuscript 379 submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaidi AK, Saini SS, Macglashan DW., Jr Regulation of Syk kinase and FcRbeta expression in human basophils during treatment with omalizumab. J Allergy Clin Immunol. 2010;125:902–8. e7. doi: 10.1016/j.jaci.2009.12.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishmael SS, MacGlashan DW., Jr Syk expression in peripheral blood leukocytes, CD34+ progenitors, and CD34-derived basophils. J Leukoc Biol. 2010;87:291–300. doi: 10.1189/jlb.0509336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siraganian RP. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974;57:383–94. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 6.MacGlashan DW., Jr Relationship Between Syk and SHIP Expression and Secretion from Human Basophils in the General Population. J Allergy Clin Immunol. 2007;119:626–33. doi: 10.1016/j.jaci.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 7.Nopp A, Johansson SG, Ankerst J, Bylin G, Cardell LO, Gronneberg R, et al. Basophil allergen threshold sensitivity: a useful approach to anti-IgE treatment efficacy evaluation. Allergy. 2006;61:298–302. doi: 10.1111/j.1398-9995.2006.00987.x. [DOI] [PubMed] [Google Scholar]
- 8.Dembo M, Goldstein B, Sobotka AK, Lichtenstein LM. Histamine release due to bivalent penicilloyl haptens: Control by the number of cross-linked IgE antibodies on the basophil plasma membrane. J Immunol. 1978;121:354–8. [PubMed] [Google Scholar]
- 9.Goldstein B, Dembo M, Sobotka AK, Lichtenstein LM. Some invariant 395 properties of IgE396 mediated basophil activation and desensitization. J Immunol. 1979;123:1873–82. [PubMed] [Google Scholar]
- 10.MacGlashan DW, Jr, Dembo M, Goldstein B. Test of a theory relating to the cross-linking of IgE antibody on the surface of human basophils. J Immunol. 1985;135:4129–34. [PubMed] [Google Scholar]
- 11.Ishmael S, MacGlashan D., Jr Early signal protein expression profiles in basophils: a population study. J Leukoc Biol. 2009;86:313–25. doi: 10.1189/jlb.1208724. [DOI] [PubMed] [Google Scholar]
- 12.MacGlashan DW., Jr Releasability of human basophils: Cellular sensitivity and maximal histamine release are independent variables. J Allergy Clin Immunol. 1993;91:605–15. doi: 10.1016/0091-6749(93)90266-i. [DOI] [PubMed] [Google Scholar]
- 13.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–83. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Nopp A, Johansson SG, Ankerst J, Palmqvist M, Oman H. CD-sens and clinical changes during withdrawal of Xolair after 6 years of treatment. Allergy. 2007;62:1175–81. doi: 10.1111/j.1398-9995.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- 15.Nopp A, Cardell LO, Johansson SG, Oman H. CD-sens: a biological measure of immunological changes stimulated by ASIT. Allergy. 2009;64:811–4. doi: 10.1111/j.1398-9995.2008.01900.x. [DOI] [PubMed] [Google Scholar]