Abstract
Purpose
To present a new a measurement instrument, the Effectiveness of Treatment Apnea-Hypopnea Index (ET-AHI), which we have developed for the purpose of determining an estimate of the therapeutic control of obstructive sleep apnea (OSA).
Materials and Methods
We retrospectively evaluated a cohort of patients with OSA who initially attempted to use CPAP therapy and subsequently underwent maxillomandibular advancement surgery (MMA). We performed ET-AHI calculations to estimate the level of CPAP adherence that would be necessary to achieve equivalence with the MMA surgical result, and to reach an effective AHI of 5 and 15.
Results
The sample was composed of 37 adult (mean age = 44.2 ± 9.0 years, 73% males) patients with moderate to severe OSA (baseline AHI = 56.3 ± 22.6). CPAP therapy was highly efficacious (CPAP Titration AHI = 4.3 ± 5.9), but no patient could adequately adhere to treatment. MMA produced a significant reduction in OSA (Post-MMA AHI = 11.6 ± 7.4). ET-AHI calculations predicted that an 86% adherence rate with CPAP would be necessary to achieve equivalence with MMA, while adherence rates of 99% and 79% may be required to achieve an effective AHI of 5 and 15, respectively.
Conclusion
The ET-AHI has the potential to significantly impact the care of patients with OSA, as it may provide the treating clinician with a valuable tool to estimate the control of OSA for any therapeutic intervention. How closely the ET-AHI predicts the true effective AHI in the home setting requires further validation.
Keywords: Obstructive Sleep Apnea, Effectiveness of Treatment Apnea-Hypopnea Index (ET-AHI), Maxillomandibular Advancement, CPAP
Introduction
The effect that obstructive sleep apnea (OSA) has on general health and well-being has been well documented.[1] OSA is a common disorder affecting at least 2-4% of the adult population and is associated with hypertension,[2-4] diabetes,[5] and cardiovascular disease,[6] thereby making it a significant public health concern. The standard measure used to assess the presence and severity of OSA, is the apnea–hypopnea index (AHI), as determined by overnight polysomnography (Diagnostic PSG). The AHI is also the primary metric used to assess the efficacy and effectiveness of OSA treatment.
Nasal continuous positive airway pressure (CPAP) is the accepted first-line therapy for patients with OSA, and is presumed to be highly efficacious, virtually eliminating OSA.[7] The standard measure used to determine the efficacy of CPAP therapy is overnight polysomnography (CPAP Titration PSG), where the CPAP machine pressure is incrementally increased until the optimal control of OSA is achieved, as measured by a reduction in the AHI. As a guideline we aim for an AHI < 5 with CPAP therapy.
The major barrier to translating the efficacy of CPAP into clinical effectiveness is non-adherence with therapy which is highly variable across clinical practice. When CPAP adherence is defined as at least 4 hours of nightly use, 29-83% of patients with OSA have been reported to be non-adherent to treatment.[8] The most common measure used to assess the clinical effectiveness of CPAP is the percent adherence with therapy, which is routinely measured in clinical practice in the home setting by monitors built into the CPAP machine. However, percent adherence to CPAP therapy is an indirect, surrogate measure of the actual therapeutic control of disease—as it does not directly measure the effective change in AHI that occurs during use and non-use of CPAP in the home setting. Ravesloot and de Vries[9], as well as Stuck and coworkers,[10] have reported on the importance of using a mean AHI—which includes assessment of the AHI when CPAP is and is not being used—to determine the actual effectiveness of CPAP therapy.
Several short term observational studies indicate that maxillomandibular advancement surgery (MMA) may be a clinically effective alternative therapy for patients with OSA who are unable to adhere to CPAP therapy.[11-17] Although the level of evidence is low, substantial and consistent reductions in AHI have been observed following MMA.[11] One of the major challenges in determining the comparative effectiveness of surgical interventions and CPAP for treatment of OSA comes from the complexity of comparing a treatment that requires adherence for clinical effectiveness to one that does not. As a result, there is no universally agreed upon primary outcome for sleep-disordered breathing by which to measure treatment effectiveness, as measured by changes in AHI. This has contributed to the lack of rigorous data evaluating surgical modifications of the upper airway.
For these reasons, it is important to have a true quantitative measure of the therapeutic control of OSA. It is therefore important that outcome measures be developed to directly and precisely assess the clinical effectiveness of a specific treatment for OSA. Ideally, the outcome measure would allow comparison of different forms of therapy to provide a method to assist in the selection of the most effective treatment of OSA for an individual patient. The outcome measure should also have the ability to assess the effectiveness of treatment over a designated period of time—as patterns of adherence to therapy, response to surgery and changes in physical status (e.g. weight) may vary over time and consequently impact the therapeutic control of OSA.
We have developed a measurement instrument, the Effectiveness of Treatment Apnea-Hypopnea Index (ET-AHI) for the purpose of determining a mathematical estimate of the true therapeutic control of OSA, as measured by changes in AHI. The ET-AHI was designed to examine the effectiveness of any therapeutic intervention, including those that require patient compliance (e.g. CPAP and oral appliances) and those that do not (e.g. surgery). The ET-AHI is a weighted, composite measurement of respiratory events that occur both during use and non-use of therapy. ET-AHI = (Treatment AHI x % Adherence to Therapy) + (Non-Treatment AHI x % Non-adherence to Therapy).
If validated, use of the ET-AHI could have a significant impact on the care of patients with OSA, as it would provide the treating clinician with a valuable tool to estimate the true therapeutic control of OSA for any therapeutic intervention. The purpose of this study was to demonstrate the potential clinical application of the ET-AHI by performing ET-AHI calculations for a cohort of patients with OSA who were unable to adhere to CPAP therapy and subsequently underwent surgical treatment by MMA. The development of the ET-AHI is illustrated and some representative examples of its potential use are shown.
Methods
To determine the ET-AHI for a particular therapy, treatment specific AHI values—derived from quantitative measurements obtained during routine clinical care and home monitoring of adherence—are used to calculate the ET-AHI. One underlying assumption of the ET-AHI is that there is a linear dose response relationship between % adherence to treatment and change in AHI, without any threshold or ceiling effect. A second assumption is the Non-Treatment AHI—when therapy is not being used in the home setting—is equivalent to the PSG derived Diagnostic AHI which is calculated during the time no therapy is being used in the laboratory setting. Finally, a third assumption was made that when CPAP is being used in the home setting the Treatment AHI is equivalent to the laboratory based CPAP Titration AHI—which is the standard measure of the efficacy of CPAP treatment. For CPAP therapy: ET-AHI CPAP = (CPAP Titration AHI x % Adherence to Therapy) + (Diagnostic AHI x %Non-adherence to Therapy). In developing the surgical ET-AHI we assumed that the AHI in the home setting—following surgical treatment—is equivalent to the laboratory based AHI derived from the PSG performed after surgical treatment. Since there is 100% adherence with surgical treatment the non-adherence component of the ET-AHI formula is zero and drops out of the equation. So for MMA: ET-AHIMMA = Post-MMA AHI.
To demonstrate the potential clinical impact of elucidating the dose response relationship between percent adherence to CPAP and changes in the effective AHI, we performed ET-AHICPAP calculations for the study sample, using different levels of adherence to CPAP therapy. It is apparent from examination of the ET-AHICPAP equation that the severity of OSA at baseline may have a significant impact on the control of disease for specific levels of adherence with CPAP. To demonstrate this principle, we completed ET-AHICPAP calculations for a variety of baseline levels of OSA severity ranging from an AHI of 30-60. For each designated level of disease severity, we then determined the % adherence to CPAP that would be necessary to achieve an effective AHI (ET-AHICPAP) of 5 and 15. We used these AHI levels because an AHI of 5 or less is considered normal, and an AHI of 15 or less—without symptoms or significant comorbid medical conditions—may be considered sufficiently mild OSA that does not require treatment.[18] We then calculated, for the MMA Cohort, the level of CPAP adherence that would be necessary to achieve equivalence with the MMA surgical result (ET-AHIMMA), as well as adherence levels that would reduce the effective AHI to 5 and 15. We then performed ET-AHI calculations for two individual patients from the MMA Cohort, one patient with moderate OSA and a second with severe OSA, to demonstrate how the ET-AHI could be applied to the clinical care of individual patients.
This research protocol was approved by the Vanderbilt University IRB (Human Research Protection Program) and ethical standards were used to conduct this research.
Results
We retrospectively evaluated a group (MMA Cohort) of 37 adult patients (mean age = 44.2 ± 9.0 years; 73% males) with moderate to severe OSA (Diagnostic AHI = 56.3 ± 22.6), as determined by a Diagnostic PSG. Each patient had a CPAP Titration PSG which showed that CPAP therapy was highly efficacious (CPAP Titration AHI = 4.3 ± 5.9). No patient in the study sample was adherent to CPAP therapy, and all individuals subsequently underwent MMA for treatment of OSA. MMA produced a significant reduction in AHI as measured by PSG 3-6 months after MMA (Post-MMA AHI = 11.6 ± 7.4). No significant changes in BMI were observed following MMA (baseline BMI = 29.8 ± 3.8 vs. post-MMA BMI=29.9 ± 3.3; p=0.2300).
Figure 1 demonstrates the effect that % adherence to CPAP may have on the reduction in AHI for the MMA Cohort. It is predicted that CPAP adherence rates of 25%, 50% and 75% would produce reductions in AHI to 43.3, 30.3 and 17.3 respectively.
Figure 1.
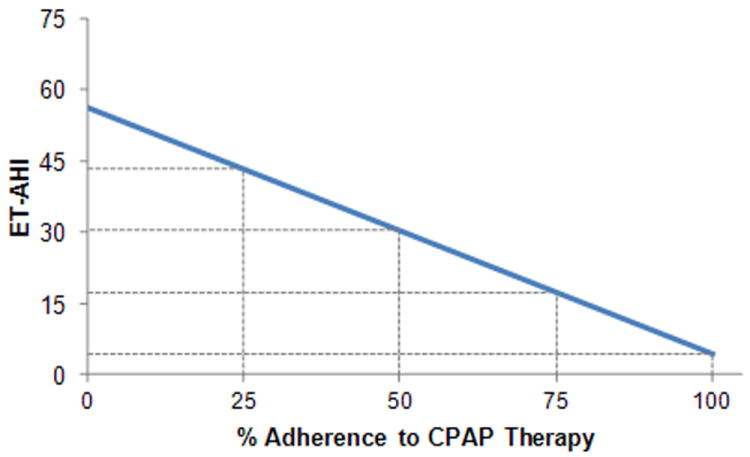
Predicted ET-AHI for varying levels of adherence to CPAP.
Figure 2 shows the significant impact that the baseline severity of OSA may have on the ET-AHICPAP. For patients ranging in baseline Diagnostic AHI from 30-60, it is predicted that very high levels of adherence (exceeding 90%) may be necessary to completely control disease at an effective AHI (ET-AHICPAP) of 5, while more reasonable levels of compliance (58-81%) would result in control at an effective AHI of 15.
Figure 2.
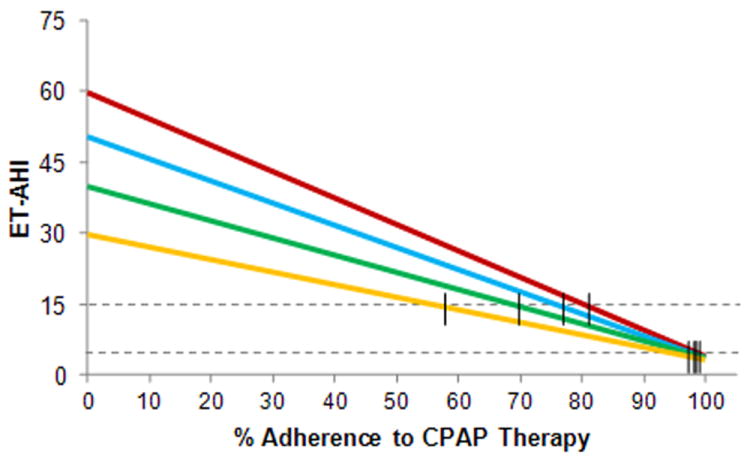
Relationship of % adherence to CPAP therapy and control of OSA with varying levels of disease severity at baseline.
Figure 3 shows that for the MMA Cohort, it is predicted that an 86% adherence rate with CPAP would be necessary to achieve equivalence with the effective AHI for the MMA Cohort (ET-AHI MMA=11.6). It is predicted that a CPAP adherence rate of about 99% would be required to achieve an ET-AHICPAP of 5 and a rate of 79% to reach an ET-AHICPAP of 15.
Figure 3.
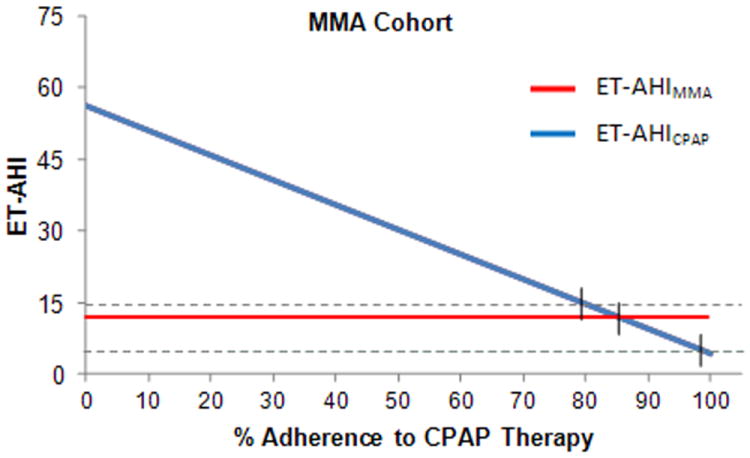
Group comparison of reduction in AHI between MMA and different levels of adherence to CPAP therapy.
Figure 4 demonstrates the potential clinical application of the ET-AHI for an individual patient (Patient #1) with moderate OSA at baseline (Diagnostic AHI=23.5), where CPAP was highly efficacious (CPAP Titration AHI=0). For Patient #1, it is predicted that a 77% adherence rate with CPAP would be necessary to achieve equivalence with the effective AHI for MMA (ET-AHI MMA = 5.5). It is predicted that a CPAP adherence rate of 79% would be necessary to achieve an ET-AHICPAP of 5 and a rate of 36% to reach an ET-AHICPAP of 15.
Figure 4.
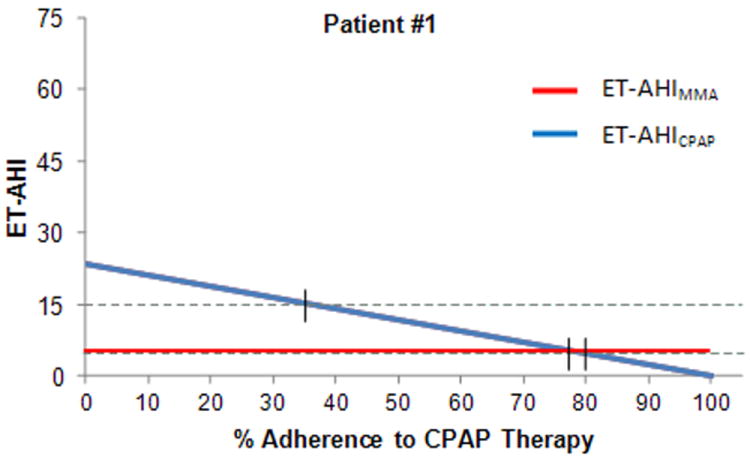
Individual comparison of reduction in AHI between MMA and different levels of adherence to CPAP for a patient with moderate OSA at baseline.
Figure 5 shows the individual data for a patient (Patient #2) with very severe OSA at baseline (Diagnostic AHI = 74), where CPAP was highly efficacious (CPAP Titration AHI=0). For Patient #2, it is predicted that a 93% adherence rate with CPAP would be necessary to achieve equivalence with the effective AHI for MMA (ET-AHI MMA = 5.1), and a CPAP adherence rate of 80% would be necessary to achieve an ET-AHICPAP of 15. This is a clinical situation that would favor MMA over CPAP because very high levels of adherence with CPAP would be necessary to achieve an equivalency with MMA.
Figure 5.
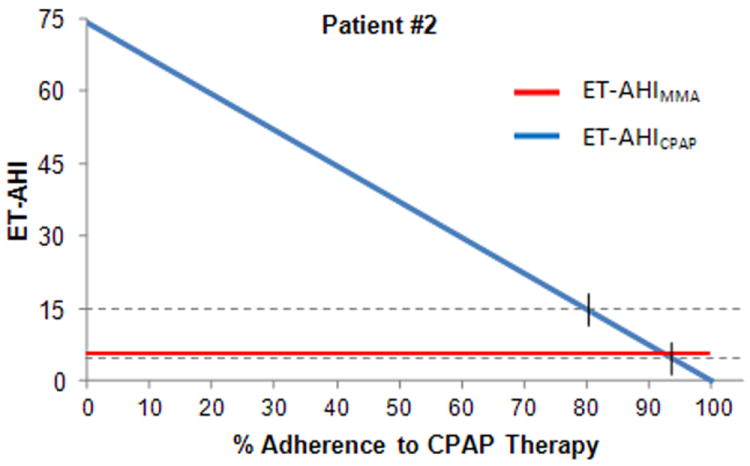
Individual comparison of reduction in AHI between MMA and different levels of adherence to CPAP for a patient with severe OSA at baseline.
Discussion
We have introduced a new measurement instrument, the Effectiveness of Treatment Apnea-Hypopnea Index (ET-AHI) that was developed for the purpose of determining a quantitative estimate of the effective therapeutic control of OSA for a specific treatment, as measured by changes in AHI. We have presented ET-AHI calculations derived from actual patient data, to show how the ET-AHI may be used clinically to determine: 1) the effective AHI for a specific therapy, 2) the level of CPAP adherence necessary to achieve a specific level of control of OSA, and 3) the comparative clinical effectiveness of CPAP and MMA surgical treatment for individual patients. It is to be emphasized that the ET-AHI is only an estimate of the actual effective AHI. How completely the ET-AHI predictions reflect the true effective AHI in the home setting requires validation of the assumptions set forth in the ET-AHI equation.
It is important, from a clinical perspective, to differentiate between treatment efficacy and effectiveness. Efficacy is defined as the ability of an intervention to produce the optimal treatment effect or desired beneficial effect in highly controlled circumstances in selected clinical settings. Whereas, effectiveness is defined as the ability of an intervention to produce the desired beneficial effect in actual practice or usage (e.g. home use of CPAP). Currently, the CPAP Titration PSG is the gold standard for determining the efficacy of CPAP therapy for an individual patient and is also commonly used as the measure of effectiveness of CPAP therapy. However, to accurately determine the effective AHI for CPAP therapy, it is necessary to have an accurate measure of the AHI while the patient is actually using CPAP in a home setting. For non-compliance based therapy, such as surgery, treatment efficacy should be essentially equivalent to treatment effectiveness.
Most commonly the success of treatment for OSA has been assessed in binary format. Was treatment a success or failure? A variety of definitions of successful treatment have been used such as an AHI of ≤ 5,[19] or a post-surgical AHI <20 and a ≥ 50% reduction from baseline.[12] Furthermore, most of these definitions of successful treatment do not discriminate between the efficacy and effectiveness of therapy. This approach has stimulated significant controversy and discussion focused on what constitutes successful treatment. Using criteria for OSA treatment effectiveness set in a binary outcome format (e.g. presence or absence of disease) as the primary or exclusive outcome measure may be considered inappropriate because there is a spectrum of severity of disease and OSA is a chronic disease that likely requires lifelong management.[18]
Chronic disease outcomes are not typically expressed in binary format, rather in levels of control of disease (e.g. well controlled vs. poorly controlled). Therefore, OSA is analogous to other common chronic diseases such as hypertension and diabetes, and treatment benefit in reducing risk of poor health outcomes likely occurs in incremental fashion. The value given to different levels of control of disease is typically based upon anticipated or expected health outcomes. For example, for every 20 mm Hg systolic or 10 mm Hg diastolic increase in blood pressure, there is a doubling of the mortality from both ischemic heart disease and stroke.[20] Therefore, it appears that assessment of clinically effective treatment of OSA should be approached from a perspective of levels of control of disease and the associated improvement or impact on important health outcomes rather than the all or none phenomenon (binary outcome—success or failure of treatment). Currently, a therapy for OSA—especially surgical treatment—may be erroneously rejected or not endorsed as effective because it does not routinely reach a designated level of “successful treatment” or cure—as expressed in the binary format. For example, if a patient with severe OSA is non-adherent to CPAP therapy, the patient would remain untreated and presumably at much higher risk for poor health outcomes, if surgery (e.g. MMA) was not considered as a legitimate treatment option because it did not consistently provide a “successful” result as defined by an AHI of ≤ 5 (see Patient #2 Figure 5).
One of the major goals of developing the ET-AHI was to overcome the problems associated with binary outcomes by providing an outcome measure that quantitatively assesses the actual control of disease as a measure of the clinical effectiveness of treatment. Furthermore, the potential quantitative precision of the ET-AHI could be used to elucidate the predictive value of quantitative reductions in AHI for improvement in important outcomes such as sleepiness, health-related quality of life, neurocognitive function, blood pressure and reduction in the risk of cardiovascular events, including stroke and death. For example, what is the clinical significance of reducing the effective AHI (ET-AHI) to specific levels (e.g. 5 or 15) on the likelihood of having a fatal or non-fatal cardiovascular event?
The ET-AHI was designed to be a dynamic outcome metric that accounts for several important variables that likely define the actual control of OSA with therapy. Each of these variables may contribute individually but the interrelationship of the variables likely defines the actual control of disease and the effectiveness of a specific therapeutic intervention for an individual patient. These variables include: 1) the effective AHI when therapy is being used, 2) the effective AHI when therapy is not being used, 3) the proportion of time that therapy is being used during sleep, and 4) the dose response characteristics of therapy. The ability to measure changes in the effective AHI over time is a particularly important feature of the ET-AHI. For example, the level of adherence to CPAP may vary over time and the ET-AHI could be calculated for specific time intervals and then compared to ascertain the magnitude of impact variation in adherence has on the overall control of OSA.
The ET-AHI is based upon a number of reasonable assumptions, so an important component of validating the ET-AHI will be to test the accuracy of the four stated assumptions: 1) the AHI when treatment is not being used in the home setting is equivalent to the laboratory based Diagnostic AHI, 2) the AHI when CPAP is being used in the home setting is equivalent to the laboratory based CPAP Titration AHI, 3) there is a linear dose response relationship between % adherence to CPAP therapy and change in AHI, without any threshold or ceiling effect and 4) the AHI in the home setting after surgical treatment is equivalent to the laboratory based post-surgical AHI.
The AHI calculated from the Diagnostic PSG should theoretically provide an accurate measure of the effective AHI when CPAP is not being used in the home setting. Particularly, since it appears that there are no lasting effects of CPAP when it is not being used, as CPAP withdrawal leads to a rapid recurrence of OSA, a return of subjective sleepiness and is associated with impaired endothelial function, increased urinary catecholamines, increased blood pressure and heart rate.[21] Therefore, we have used the Diagnostic AHI as the untreated AHI component of the ET-AHI equation. In this study, we showed how changes in the level of adherence to therapy and differences in the baseline severity of disease may significantly impact the control of disease. As the severity of OSA increases, the patient would need proportionally greater levels of % adherence to CPAP therapy to maintain an equivalent control of OSA. Others have also reported on the potential importance that the baseline severity of disease has in determining the therapeutic control of OSA by CPAP using slightly different, and in one instance more complex, mathematical equations than the ET-AHI to determine the effective AHI for CPAP.[9, 10] ENREF 24 These investigators have also used mathematical formulas to demonstrate the importance of measuring the mean or effective AHI, particularly when assessing the comparative effectiveness of CPAP and surgery.
The CPAP Titration AHI defines the efficacy of CPAP therapy, so it should theoretically be representative of the effective AHI that is achieved while the patient is using CPAP in a home setting. However, in the laboratory setting, the technician is closely monitoring the patient and making adjustments in CPAP pressure and mask fit to optimize the reduction in AHI. In a home setting this level of supervision is not present and the patient may experience problems with mask fit and other issues that diminish the capability of achieving the desired reduction in AHI that was accomplished in the controlled laboratory setting during the CPAP Titration PSG. So the CPAP Titration AHI may be an accurate measure of treatment efficacy but not necessarily an accurate measure of CPAP treatment effectiveness, as measured in the home setting.
Little information exists about the dose-response relationship between CPAP adherence and reduction in AHI. The results from the only published report indicate that there may be a linear dose-response relationship—but the study was limited to patients fully compliant with CPAP—so it is unknown if the strength of the relationship exists over the full spectrum of adherence to CPAP.[22] Weaver and coworkers[23] showed that a linear dose-response relationship exists between increased use of CPAP and achieving normal levels of objective and subjective daytime sleepiness. Therefore, it seems plausible to assume that there may be a linear dose response relationship between % adherence to CPAP and changes in AHI.
The ET-AHI may provide a more accurate estimate of the true control of OSA than either the CPAP Titration AHI or rate of adherence to therapy because it accounts for the effective AHI when the patient is adherent and non-adherent to therapy. However, prior to using the ET-AHI in a patient care setting, we need to validate the ET-AHI by showing that the predicted ET-AHI is equivalent to the observed effective AHI, as measured in the home setting. If validated, the ET-AHI could be used as a quantitative instrument to measure the effectiveness of treatment for OSA, as measured by changes in AHI, and could, therefore, have a significant impact on the care and health of patients with OSA, as it would provide the treating clinician with a very valuable tool to precisely assess the therapeutic control of OSA for any treatment intervention.
Acknowledgments
This investigation was supported in part by a Research Support Grant Award from the Oral and Maxillofacial Surgery Foundation and in part by the Vanderbilt CTSA grant UL1 RR024975 from the National Center for Research Resources, National Institutes of Health. The authors thank David Adkins for his contributions to data collection and figure construction. The authors also wish to thank Beth A. Malow, MD, MS and Kent Moore, DDS, MD, for their valuable comments on earlier versions of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, Skatrud J. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746. [PubMed] [Google Scholar]
- 5.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 6.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O’Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 7.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD001106.pub3. CD001106. [DOI] [PubMed] [Google Scholar]
- 8.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravesloot MJ, de Vries N. Reliable calculation of the efficacy of non-surgical and surgical treatment of obstructive sleep apnea revisited. Sleep. 2011;34:105. doi: 10.1093/sleep/34.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuck BA, Leitzbach S, Maurer JT. Effects of continuous positive airway pressure on apnea-hypopnea index in obstructive sleep apnea based on long-term compliance. Sleep & breathing = Schlaf & Atmung. 2011 doi: 10.1007/s11325-011-0527-8. [DOI] [PubMed] [Google Scholar]
- 11.Caples SM, Rowley JA, Prinsell JR, Pallanch JF, Elamin MB, Katz SG, Harwick JD. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396. doi: 10.1093/sleep/33.10.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holty JE, Guilleminault C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2010;14:287. doi: 10.1016/j.smrv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Bettega G, Pepin JL, Veale D, Deschaux C, Raphael B, Levy P. Obstructive sleep apnea syndrome. fifty-one consecutive patients treated by maxillofacial surgery. Am J Respir Crit Care Med. 2000;162:641. doi: 10.1164/ajrccm.162.2.9904058. [DOI] [PubMed] [Google Scholar]
- 14.Conradt R, Hochban W, Heitmann J, Brandenburg U, Cassel W, Penzel T, Peter JH. Sleep fragmentation and daytime vigilance in patients with OSA treated by surgical maxillomandibular advancement compared to CPAP therapy. J Sleep Res. 1998;7:217. doi: 10.1046/j.1365-2869.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- 15.Prinsell JR. Maxillomandibular advancement surgery in a site-specific treatment approach for obstructive sleep apnea in 50 consecutive patients. Chest. 1999;116:1519. doi: 10.1378/chest.116.6.1519. [DOI] [PubMed] [Google Scholar]
- 16.Riley RW, Powell NB, Guilleminault C. Maxillary, mandibular, and hyoid advancement for treatment of obstructive sleep apnea: a review of 40 patients. J Oral Maxillofac Surg. 1990;48:20. doi: 10.1016/0278-2391(90)90174-z. [DOI] [PubMed] [Google Scholar]
- 17.Waite PD, Wooten V, Lachner J, Guyette RF. Maxillomandibular advancement surgery in 23 patients with obstructive sleep apnea syndrome. J Oral Maxillofac Surg. 1989;47:1256. doi: 10.1016/0278-2391(89)90719-2. [DOI] [PubMed] [Google Scholar]
- 18.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, Weinstein MD. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263. [PMC free article] [PubMed] [Google Scholar]
- 19.Elshaug AG, Moss JR, Southcott AM, Hiller JE. Redefining success in airway surgery for obstructive sleep apnea: a meta analysis and synthesis of the evidence. Sleep. 2007;30:461. doi: 10.1093/sleep/30.4.461. [DOI] [PubMed] [Google Scholar]
- 20.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 21.Kohler M, Stoewhas AC, Ayers L, Senn O, Bloch KE, Russi EW, Stradling JR. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011;184:1192. doi: 10.1164/rccm.201106-0964OC. [DOI] [PubMed] [Google Scholar]
- 22.Stepnowsky CJ, Dimsdale JE. Dose-response relationship between CPAP compliance and measures of sleep apnea severity. Sleep Med. 2002;3:329. doi: 10.1016/s1389-9457(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 23.Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, Kader G, Mahowald M, Younger J, Pack AI. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]


