Abstract
Twenty-six lactic acid bacterium strains isolated from European dairy products were identified as Streptococcus thermophilus and characterized by bacterial growth and exopolysaccharide (EPS)-producing capacity in milk and enriched milk medium. In addition, the acidification rates of the different strains were compared with their milk clotting behaviors. The majority of the strains grew better when yeast extract and peptone were added to the milk medium, although the presence of interfering glucomannans was shown, making this medium unsuitable for EPS screening. EPS production was found to be strain dependent, with the majority of the strains producing between 20 and 100 mg of polymer dry mass per liter of fermented milk medium. Furthermore, no straightforward relationship between the apparent viscosity and EPS production could be detected in fermented milk medium. An analysis of the molecular masses of the isolated EPS by gel permeation chromatography revealed a large variety, ranging from 10 to >2,000 kDa. A distinction could be made between high-molecular-mass EPS (>1,000 kDa) and low-molecular-mass EPS (<1,000 kDa). Based on the molecular size of the EPS, three groups of EPS-producing strains were distinguished. Monomer analysis of the EPS by high-performance anion-exchange chromatography with amperometric detection was demonstrated to be a fast and simple method. All of the EPS from the S. thermophilus strains tested were classified into six groups according to their monomer compositions. Apart from galactose and glucose, other monomers, such as (N-acetyl)galactosamine, (N-acetyl)glucosamine, and rhamnose, were also found as repeating unit constituents. Three strains were found to produce EPS containing (N-acetyl)glucosamine, which to our knowledge was never found before in an EPS from S. thermophilus. Furthermore, within each group, differences in monomer ratios were observed, indicating possible novel EPS structures. Finally, large differences between the consistencies of EPS solutions from five different strains were assigned to differences in their molecular masses and structures.
Lactic acid bacteria (LAB) are useful in the food industry, not only because of their ability to acidify and hence preserve food products from spoilage, but also for their contribution to the organoleptic properties of the final fermented food (75). LAB play an important role in the texture development of yogurts and other fermented milks, low-fat cheeses, and dairy desserts (13). Extracellularly secreted sugar polymers, or exopolysaccharides (EPS), are partly responsible for this (10, 16, 52). In general, the food industry is particularly interested in natural viscosifiers and texture enhancers, so-called biothickeners. These are mostly plant carbohydrates (e.g., starch, pectin, and guar gum), animal hydrocolloids (e.g., gelatin and casein), or bacterial biopolymers (e.g., xanthan and gellan) (62). Also, the EPS produced by LAB may have technological and health benefits in food products (12, 13, 52). The in situ use of these generally recognized as safe, food-grade bacteria as functional starter cultures in fermented dairy products is preferred to the addition of thickeners to the food product (11). However, little is known about the relationships between the amount and molecular characteristics of EPS and their functionality (13, 16, 53, 54).
EPS from LAB can be subdivided into two major groups: (i) the homopolysaccharides, composed of one type of monosaccharide, and (ii) the heteropolysaccharides, composed of a repeating unit that contains two or more different monosaccharides (12). Based on their structures, four groups of homopolysaccharides can be distinguished: α-d-glucans, β-d-glucans, β-d-fructans, and others, such as polygalactan (12, 38). Heteropolysaccharides are produced with more variety concerning the chemical composition, monomer ratio, and molecular structure of the repeating unit, as well as the molecular mass of the polymer (12). The repeating units most often contain a combination of d-glucose, d-galactose, and l-rhamnose, albeit in different ratios. In a few cases, l-fucose, acetylated amino sugars (e.g., N-acetyl-d-galactosamine), d-ribose, d-glucuronic acid, and d-nononic acid, as well as noncarbohydrate constituents such as glycerol, phosphate, pyruvyl, and acetyl groups, are present (15, 21, 26, 35, 41, 50, 51, 68, 69). The heteropolysaccharides are produced by mesophilic (e.g., Lactococcus lactis, Lactobacillus sakei, Lactobacillus rhamnosus, and Lactobacillus casei) and thermophilic (e.g., Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus helveticus, and Streptococcus thermophilus) LAB (13). Recently, an attempt was made to classify EPS from mesophilic LAB (72). According to their monomer compositions, three major groups have been distinguished: (i) EPS containing only galactose; (ii) EPS containing galactose and glucose; and (iii) EPS containing galactose, glucose, and rhamnose. A preliminary grouping has been suggested for the EPS from a limited number of thermophilic LAB strains in which each group corresponds with an EPS with a particular subunit structure (37).
The molecular masses of the EPS from LAB range from 10 kDa (71) and over 200 kDa (1) to >1,000 kDa (9, 26, 32, 41). Importantly, both structure and molecular mass influence the rheological properties of a polysaccharide (18, 61). Also, environmental factors (carbohydrate source, nitrogen source, and carbon/nitrogen ratio of the growth medium) can influence the production, monomer composition, and molecular mass of the EPS produced by a particular strain (6, 10, 23).
Rational screening for novel EPS from particular LAB strains that are characterized by a unique structure or molecular mass is of utmost importance regarding possible applications in the food industry (16, 77). Meanwhile, classical selection criteria for starter strains, such as a high growth and acidification rate (33, 59) and the presence of proteolytic properties (4, 42), in milk should not be overlooked for the exploitation of ropy, EPS-producing starter cultures in the manufacture of dairy products. A systematic approach to study these or other technological properties among EPS-producing S. thermophilus strains is scarce (39). In this study, a wide screening of S. thermophilus strains was carried out. Furthermore, an appropriate EPS isolation protocol to accurately determine molecular masses and monomer compositions was developed. The aim of this study was to characterize the fermentation properties of thermophilic LAB strains in milk-based media and to explore the biodiversity of their EPS to find possible correlations. In addition, an in-depth EPS characterization should permit the classification of these strains, which in turn can lead to the discovery of strains producing high levels of EPS, new EPS structures, or interesting structure-function relationships.
MATERIALS AND METHODS
Bacterial strains and media.
Twenty-six ropy strains of S. thermophilus were studied. They were previously isolated from different (fermented) dairy products (raw milk, yogurt, and cheese) and from several industrial starter cultures, most of them originating from Eastern European countries (Table 1). Strains were stored at −80°C in MRS medium (Oxoid, Basingstoke, United Kingdom) containing 25% (vol/vol) glycerol as a cryoprotectant. To obtain fresh cultures, we cultivated strains in MRS medium (Oxoid) at 42°C for 12 h.
TABLE 1.
S. thermophilus strains used throughout this study
| Strain(s) | Place of depositiona | Source |
|---|---|---|
| LY03 | Industrial starter culture, HUD, United Kingdom | Yogurt |
| Sfi20 | Industrial starter culture, Nestlé, Switzerland | Yogurt |
| BTC (IMDOST03) | Industrial starter culture, UN, France | Yogurt |
| 480 | Laboratory collection, AUA, Greece | Yogurt |
| T7, T149 | Laboratory collection, UWM, Poland | Yogurt |
| SY102 | Industrial starter culture, Rhodia Food, France | Yogurt |
| NCFB 2393 | Laboratory collection, HUD, United Kingdom | Yogurt |
| CH101, D22, 01/12, 5F/51 | Industrial starter culture, Biolacta, Poland | Yogurt |
| ST 33, ST 111, ST86, ST 110, ST 113, ST 114 | Laboratory collection, IFR, Romania | Yogurt |
| STD, ST 30 | Laboratory collection, IFR, Romania | Raw milk |
| S509 | Industrial starter culture, IFR, Romania | Yogurt |
| SR1 | Industrial starter culture, Sunrise, Romania | Yogurt |
| VUM ST01, ST 144, 534b | Industrial starter culture, Milcom Prague, Czech Republic | Cheese |
| CRL1190 | Laboratory collection, CERELA, Argentina | Yogurt |
HUD, Huddersfield University, Huddersfield, United Kingdom; UN, Université de Nancy, Nancy, France; AUA, Agricultural University of Athens, Athens, Greece; UWM, University of Warmia and Mazury, Olsztyn, Poland; IFR, Institute of Food Research, Bucharest, Romania; CERELA, Centro de Referencia para Lactobacilos, San Miguel de Tucumán, Argentina.
The media used for fermentations were semiskimmed milk medium (UHT milk; Campina N.V., Aalter, Belgium), milk medium (10.0% [wt/vol] skimmed milk powder; Dairy Industry Inco, Kallo, Belgium), enriched milk medium (10.0% [wt/vol] skimmed milk powder [Dairy Industry Inco], 1.0% [wt/vol] peptone [Oxoid], and 0.5% [wt/vol] yeast extract [VWR International, Darmstadt, Germany]), and milk medium supplemented with lactalbumine hydrolysate (1.6% [wt/vol]) (Oxoid). LAPTg (46) agar, composed of 1.0% (wt/vol) yeast extract (VWR International), 1.5% (wt/vol) peptone (Oxoid), 1.0% (wt/vol) tryptone (Oxoid), 1.0% (wt/vol) glucose, 0.1% (vol/vol) Tween 80, and 1.5% (wt/vol) agar (Oxoid), was used for cell enumeration.
SDS-PAGE of whole-cell proteins.
Cells were cultivated for 24 h as indicated above. Whole-cell protein extracts were prepared, and one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis was performed as described previously (44). A densitometric analysis, normalization and interpolation of the protein profiles, and a numerical analysis were performed by using the GelCompar software package, versions 3.1 and 4.2 (Applied Maths, Sint-Martens-Latem, Belgium).
Fermentation conditions.
Fresh cultures were propagated at 42°C for 12 h, first in MRS medium (Oxoid), followed by two subcultivations in the medium used later. The transfer inoculum was always 1.0% (vol/vol). Except for the fermentations with milk medium supplemented with lactalbumine hydrolysate (see below), the initial pH was always adjusted to pH 6.3 and incubations were performed under static conditions with a free pH at 42°C for 12 h. An inoculum of 1.0% (vol/vol) of a fresh culture was used.
To study the rheology of pure EPS solutions from S. thermophilus LY03, ST 111, CH101, ST 113, and STD, we set up fermentations in milk medium supplemented with lactalbumine hydrolysate to obtain EPS that were free of contaminating polysaccharides. After 12 h of fermentation at 42°C and a constant pH of 6.2 (controlled by the automatic addition of 10 N NaOH), EPS were isolated, further purified, and analyzed for molecular mass and apparent viscosity (see below).
Determination of cell growth, acidification rate, and milk-clotting ability.
The bacterial growth of different strains was assessed after 12 h of fermentation in 1-liter flasks that contained milk medium or enriched milk medium. Both pH (acidifying properties) and cell count (after plating on LAPTg agar) measurements were performed. The number of viable cells was expressed as CFU per milliliter. Growth experiments were carried out in duplicate.
The maximum acidification rate (Vmax) was determined by online pH measurements in 10 liters of milk medium containing 105 CFU ml−1 as the starting population (with the corresponding pH designated pH0), carried out in a BiostatC fermentor (B. Braun Biotech International, Melsungen, Germany) at 42°C. The fermentor was continuously agitated at 100 rpm. The Vmax was calculated by linear regression (indicated by the correlation coefficient r2) from plots of ln pH/pH0 versus time. Also, the time needed to decrease the pH 0.5 units (t0.5) was calculated. Experiments were performed in duplicate; for one strain (S. thermophilus ST 111), the experiment was performed four times.
The milk-clotting abilities of the strains were determined on a relative basis by cultivating them at the suboptimal growth temperature of 30°C in glass tubes containing 10 ml of semiskimmed milk medium. This method is based on the phenotypic characterization of fast milk-coagulating strains of lactococci (79). All determinations were done in triplicate. The following groups were used to define the milk-clotting behaviors of the strains: (i) fast milk coagulation (FMC), when the milk was clotted between 0 and 12 h of incubation; (ii) intermediate milk coagulation (IMC), when the milk was clotted between 12 and 24 h of incubation; and (iii) slow milk coagulation (SMC), when clotting of the milk was observed later than 24 h of incubation.
Isolation and purification of EPS.
The isolation of EPS from milk medium, enriched milk medium, or milk medium supplemented with lactalbumine hydrolysate that was fermented for 12 h was performed with 1-liter samples according to a two-step precipitation protocol outlined previously (9). A distinction was made between floating and nonfloating EPS material (5). The floating EPS material could be collected after the first and/or second acetone precipitation step and was previously referred to as high-molecular-mass EPS (5). After the second acetone precipitation, the residual lactose content was determined and expressed in glucose equivalents (56). This repeated acetone precipitation step seemed to isolate EPS free from lactose, with a residual concentration of 0.01 glucose equivalents liter−1. EPS isolation was done in duplicate. Nonfermented media were used as a baseline. Total EPS yields from all S. thermophilus strains were determined after cultivation in fermented milk medium and enriched milk medium. These EPS yields were determined gravimetrically by measuring the polymer dry mass (PDM) after 48 h of drying at 42°C.
Further purification of the EPS was done by dissolving crude EPS in ultra-pure water adjusted to pH 7.0 and dialyzing it against distilled water at 4°C for 7 days, with water replacement twice a day. For dialysis, Spectra/Por membranes (VWR International) with a molecular mass cutoff of 3,500 Da were used. After dialysis, the EPS solutions were freeze-dried (Heto Drywinner; Heto-Holten, Allerød, Denmark). To have an idea of the purity of the EPS material, we measured both the residual lactose and protein contaminants in solutions of purified EPS. In pure EPS, no residual lactose could be detected by high-performance anion-exchange chromatography (HPAEC) with a CarboPac PA 10 column (Dionex, Sunnyvale, Calif.) with pulsed amperometry (Electrochemical Detection ED 40; Dionex). The protein concentrations of all EPS samples, determined with a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, Calif.), were always lower than 3% (wt/wt).
Molecular mass determination.
The molecular masses of all isolated and purified EPS were determined. Freeze-dried EPS were dissolved in potassium phosphate buffer (50 mM, pH 6.8) at a concentration of 20 to 40 mg ml−1. The molecular masses were determined by gel permeation chromatography (GPC). A Sephacryl S-400 gel (Amersham Biosciences AB, Uppsala, Sweden) was used, applying 50 mM potassium phosphate-NaOH buffer (pH 6.8) with 0.15 M NaCl as the eluent at a flow rate of 1 ml min−1. A dextran standard series (molecular masses of 80, 150, 270, 670, and 1,800 kDa) (Sigma, St. Louis, Mo.) was used to estimate the molecular masses of the purified EPS. The polysaccharide content was determined online with a Waters 2410 refractive index detector (Waters Corp., Milford, Mass.) at an internal temperature of 40°C. Experiments were performed in duplicate.
Monomer analysis.
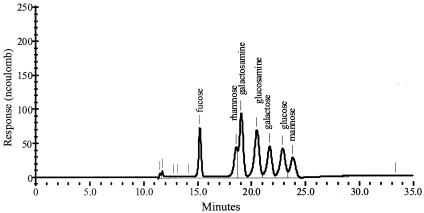
For monomer analysis, 10 mg of pure, freeze-dried EPS was dissolved in 0.5 ml of ultra-pure water. After the addition of an equal volume of 6 N trifluoroacetic acid, acid hydrolysis was performed at a temperature of 100°C for 3 h (9). The hydrolyzed EPS was freeze-dried and redissolved in ultra-pure water at a concentration of 100 μg ml−1. Monomer analysis was carried out by HPAEC with a CarboPac PA 10 column (Dionex). The monomers were eluted with 18 mM NaOH at a fixed flow rate of 1 ml min−1. Pulsed amperometry (Electrochemical Detection ED 40; Dionex) allowed detection simultaneously of the following sugars and sugar derivatives (ranked by increasing retention time): fucose, rhamnose, galactosamine, glucosamine, galactose, glucose, mannose, and fructose (Fig. 1). To check the influence of acid hydrolysis conditions on the elution behaviors of sugars, we subjected solutions of different monosaccharides (fucose, rhamnose, galactosamine, N-acetylgalactosamine, glucosamine, N-acetylglucosamine, galactose, glucose, mannose, and fructose) to similar acid hydrolysis conditions as the EPS, followed by HPAEC separation. For the quantification of the monomers in the EPS, a series of mixed standards containing the following sugars was used: fucose, 0.5 to 10 μg ml−1; rhamnose, 0.5 to 10 μg ml−1; galactosamine, 0.5 to 10 μg ml−1; glucosamine, 0.5 to 20 μg ml−1; galactose, 0.5 to 20 μg ml−1; glucose, 0.5 to 20 μg ml−1; and mannose, 0.5 to 20 μg ml−1. The confidence intervals for the standards were determined to be ±0.058, ±0.153, ±0.044, ±0.052, ±0.052, ±0.024, and ±0.063 μg ml−1, respectively.
FIG. 1.
Different monomers (standard solution of 10 ppm) were separated by HPAEC in a CarboPac PA10 column (Dionex) after elution at a fixed flow rate of 1 ml · min−1 with 18 mM NaOH. The response of the different monomers was measured by pulsed amperometry.
Evaluation of viscosity buildup and EPS production in milk fermentations.
Based on differences in EPS production and EPS characteristics, five strains, namely S. thermophilus LY03, ST 111, CH101, ST 113, and STD, were tested for their influence on viscosity buildup during milk fermentation (working volume of 5 liters) in a Biostat CT fermentor (B. Braun Biotech International). Agitation of the milk medium was programmed at 100 rpm to keep it homogeneous. The temperature was kept constant at 42°C. The pH decline was monitored online. For elimination of the influence of acidity on the apparent viscosity measurements, samples were taken at specific pH values (6.29, 5.75, 5.25, and 4.98) for all fermentations. Additional samples were taken after 12 and 24 h of fermentation. Samples were analyzed for EPS yield and apparent viscosity (see below).
Apparent viscosity measurements of fermented milk samples and pure EPS solutions.
Apparent viscosity measurements were performed on 0.5-ml samples by using a cone-plate Brookfield Digital Rheometer Model DV III (Brookfield Engineering Laboratories Inc., Stoughton, Mass.). The rheometer was equipped with a flat spindle (type CP 40; Brookfield Engineering Laboratories Inc.), which rotated in a sample-containing chamber connected to a temperature-controlled cryostat water bath (Thermomix; B. Braun Biotech International). The rheometer was controlled with the Brookfield Rheocalc software (Brookfield Engineering Laboratories Inc.).
Apparent viscosity measurements in samples from milk media fermented with S. thermophilus LY03, ST 111, CH101, ST 113, and STD were performed at 42°C. Spindle speeds of 0, 10, 15, 20, 30, 50, 70, 90, 100, and 120 rpm were applied for 90 s, provided that the torque to rotate the spindle in the fluid was between 15.0 and 85.0% of the maximum torque (0.0673 mNm). Values out of this range were not valid and resulted in misinterpretation of the data. Therefore, for comparisons of the samples between the different strains, a spindle speed of 50 or 10 rpm was chosen. These speeds corresponded with shear rates of 375 and 75 s−1, respectively. All apparent viscosity measurements were expressed in millipascal seconds, performed in triplicate, and averaged, and standard deviations were determined.
The consistencies of solutions of pure EPS from each of the five strains were determined for different concentrations, based on flow curve measurements. This resulted in a graphical presentation of the effect on viscosity of each EPS as a function of its concentration. Initially, a series of apparent viscosity measurements (for 90 s) was performed on solutions with various EPS concentrations (expressed as glucose equivalents) (56) to determine the range of possible programmable measurements for which the torque comprised between 15 and 85% of the maximum torque. Subsequently, for each EPS solution, flow curves were determined for a certain concentration range, depending on the EPS used. This was carried out by gradually increasing the velocity of the spindle with a constant step after a constant time interval (90 s). If possible, measurements were programmed to build flow curves composed of 10 to 20 experimental points. For comparison of the viscosities of solutions of EPS from the different strains, the flow properties of these EPS solutions were calculated based on the power law model (74), given by the equation τ = Kγν, for which τ is the applied shear stress (in pascals), K is the consistency index (in pascal seconds) that is typical for a certain liquid, γ is the velocity gradient or so-called shear rate (per second), and ν is an exponent that characterizes the shear-thinning (0 < ν < 1) or shear-thickening (ν > 1) behavior of a liquid. When ν = 1, the solution behaves as a Newtonian liquid. The different parameters of the power law equation are graphically obtained after linearization of the equation. The values of the parameters are independent of the applied shear rates and allow the comparison of different samples.
Guar gum (from the leguminous plant Cyamopsis tetragonolobus; molecular mass, approximately 220 kDa) (Sigma) was used as a reference polysaccharide during all experiments. For each polysaccharide solution, programmable measurements were performed in triplicate for at least two different EPS concentrations. The parameters were averaged, and standard deviations were calculated. All flow curves were measured at 25°C.
Statistics.
For the apparent viscosity measurements of the different fermented milks and for the molecular mass values of different EPS, levels of significance were determined by using Student's t test.
RESULTS
Strain identification, bacterial growth, acidification rate, and milk-clotting ability.
The species identity of all LAB strains studied was confirmed to be S. thermophilus (Table 1), as revealed by SDS-PAGE analysis of whole-cell proteins. The growth of almost all strains was enhanced in enriched milk medium compared with that in milk medium; lower pH values and higher cell counts were obtained with the enriched milk medium (Table 2). Some strains, such as S. thermophilus NCFB 2393, D22, 01/12, 5F/51, ST 111, CRL 1190, and ST 144, displayed relatively high viable cell numbers in milk and/or enriched milk medium after 12 h of incubation (>109 CFU ml−1). The differences in final pH and the ratios of the cell counts between the duplicate assays were never larger than 0.4 pH units and 0.7 log units, respectively.
TABLE 2.
Growth characteristics and EPS production of S. thermophilus strains grown in milk medium and enriched milk medium
| Strain | Characteristic in milk mediuma
|
Characteristic in enriched milk mediumb
|
Milk-clotting abilityf | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Final pH | Cell count (CFU ml−1) | Total EPS yieldc (mg of PDM liter−1) | Vmaxd (r2) | t0.5e | Final pH | Cell count (CFU ml−1) | Total EPS yieldc (mg of PDM liter−1) | ||
| LY03 | 4.50 | 1.0 × 107 | 29 | 0.104 (0.999) | 145 | 4.10 | 3.3 × 108 | 32g | IMC |
| Sfi20 | 4.55 | 1.0 × 107 | 6 | 0.109 (0.997) | 126 | 4.20 | 3.5 × 108 | 151 | IMC |
| IMDOST03 | 4.65 | 2.9 × 108 | 6 | 0.065 (0.998) | 182 | 4.25 | 1.0 × 109 | 139 | SMC |
| 480 | 4.60 | 2.2 × 108 | 19 | 0.108 (0.999) | 138 | 4.43 | 3.7 × 108 | 186 | SMC |
| T7 | 4.50 | 7.4 × 108 | 14 | 0.085 (0.990) | 124 | 4.41 | 7.5 × 108 | 162 | IMC |
| T149 | 4.99 | 3.5 × 107 | 27 | 0.121 (0.995) | 126 | 4.48 | 3.5 × 108 | 162 | SMC |
| SY102 | 4.32 | 4.9 × 108 | 99 | 0.088 (0.996) | 165 | 4.31 | 7.4 × 108 | 286 | SMC |
| NCFB 2393 | 4.58 | 3.9 × 108 | 30 | 0.094 (0.992) | 143 | 4.16 | 1.2 × 109 | 138 | SMC |
| CH101 | 4.35 | 5.6 × 108 | 60 | 0.108 (0.994) | 136 | 4.45 | 6.3 × 108 | 185 | FMC |
| D22 | 4.29 | 2.5 × 108 | 58 | 0.115 (0.988) | 169 | 4.30 | 1.7 × 109 | 260 | FMC |
| 01/12 | 4.50 | 1.2 × 109 | 106 | 0.098 (0.999) | 143 | 4.35 | 1.1 × 109 | 290 | SMC |
| 5F/51 | 4.34 | 1.6 × 109 | 59 | 0.102 (0.999) | 132 | 4.30 | 1.8 × 109 | 140 | SMC |
| ST 33 | 4.45 | 6.0 × 107 | <5 | 0.126 (0.999) | 130 | 4.23 | 4.6 × 108 | 126 | SMC |
| ST 111 | 4.60 | 2.5 × 108 | 50 | 0.080 (0.997) | 158 | 4.40 | 4.7 × 109 | 173 | SMC |
| ST 86 | 4.50 | 9.0 × 107 | 0 | 0.103 (0.996) | 83 | 4.36 | 2.1 × 108 | 178 | SMC |
| ST 110 | 4.63 | 7.5 × 107 | 12 | 0.108 (0.999) | 139 | 4.40 | 5.0 × 108 | 170 | SMC |
| ST 113 | 4.64 | 3.0 × 107 | 23 | 0.086 (0.991) | 160 | 4.68 | 4.1 × 108 | 284 | SMC |
| ST 114 | 4.68 | 1.5 × 107 | 14 | 0.115 (0.999) | 116 | 4.30 | 6.0 × 107 | 230 | SMC |
| STD | 4.85 | 1.5 × 107 | <5 | 0.081 (0.999) | 178 | 4.50 | 1.5 × 108 | 144 | IMC |
| ST 30 | 4.60 | 2.5 × 107 | <5 | 0.121 (0.991) | 126 | 4.20 | 3.4 × 108 | 161 | SMC |
| S509 | 4.71 | 2.1 × 108 | 36 | 0.068 (0.993) | 171 | 4.50 | 8.3 × 108 | 178 | IMC |
| SR1 | 4.73 | 1.4 ×108 | 28 | 0.044 (0.979) | 210 | 4.30 | 4.0 × 108 | 162 | SMC |
| VUM ST01 | 4.46 | 3.4 × 108 | 133 | 0.125 (0.998) | 119 | 4.46 | 3.8 × 108 | 214 | FMC |
| ST 144 | 4.45 | 5.2 × 108 | 114 | 0.112 (0.999) | 132 | 4.38 | 2.0 × 109 | 236 | FMC |
| 534b | 4.52 | 6.4 × 108 | 125 | 0.094 (0.994) | 147 | 4.20 | 7.3 × 108 | 209 | IMC |
| CRL 1190 | 4.58 | 8.9 × 108 | 99 | 0.090 (0.996) | 90 | 4.37 | 1.1 × 109 | 180 | IMC |
Incubation in milk medium (10.0% [wt/vol] skimmed milk powder) at 42°C for 12 h.
Incubation in enriched milk medium (10.0% [wt/vol] skimmed milk powder, 1.0% [wt/vol] peptone, 0.5% [wt/vol] yeast extract) at 42°C for 12 h.
Determined as outlined previously (5).
The acidification rate (h−1) was determined by online pH measurements in a Biostat C fermentor filled with 10 liters of milk medium containing 105 CFU ml−1 as the starting population. It was calculated by linear regression from the plots of ln pH/pH0 versus time.
Time (in minutes) needed to decrease the pH of the milk (10.0% [wt/vol] skimmed milk powder) by 0.5 pH units.
FMC, clotting of the milk (semiskimmed) between 0 and 12 h of incubation; IMC, clotting of the milk between 12 and 24 h of incubation; and SMC, clotting of the milk later than 24 h of incubation.
Both floating and nonfloating EPS material appeared after the first and second precipitation step, but only the floating EPS material was measured.
With milk medium, significant differences in acidification rates were observed between the different S. thermophilus strains tested (Table 2). Their acidifying capacities varied from very slow (<0.100 h−1), such as that for S. thermophilus SR1 (Vmax = 0.044 h−1; r2 = 0.979), to fast (>0.100 h−1), such as that for S. thermophilus ST 33 (Vmax = 0.126 h−1; r2 = 0.999). The majority of the strains displayed acidification rates between 0.090 and 0.110 h−1. For all strains, the time needed to decrease the pH of the milk medium by 0.5 pH units (t0.5) was ≤3.5 h. However, differences between the strains were evident (Table 2). For one strain (S. thermophilus ST 111), the acidification experiment was performed four times, resulting in confidence intervals of ±0.008 h−1 for Vmax and ±24 min for t0.5.
Finally, 85% of the S. thermophilus strains caused intermediate to slow coagulation of the milk. Only four strains (S. thermophilus CH101, D22, ST 144, and VUM ST01) coagulated milk fast.
EPS isolation and production.
All S. thermophilus strains produced EPS in milk medium and in enriched milk medium, except for S. thermophilus ST 86, which did not produce any EPS in milk medium (Table 2). During EPS isolation from fermented milk medium, a distinction was made between strains producing only floating EPS material (3 strains), strains producing both floating and nonfloating EPS material (2 strains), and strains producing only nonfloating EPS material (20 strains). When EPS were isolated from fermented enriched milk medium, strains that produced only floating EPS material were not found. For instance, S. thermophilus ST 111 produced both floating and nonfloating EPS when the strain was grown in enriched milk medium and in milk medium supplemented with lactalbumine hydrolysate (see below), but it produced only floating EPS material when grown in milk medium. In contrast, both EPS fractions were produced by S. thermophilus LY03, independent of the medium used. No precipitate was found during EPS isolation from baseline medium, nonfermented milk medium, and milk medium supplemented with lactalbumine hydrolysate, indicating that no lactose or other medium components interfered with the EPS isolation. Consequently, no baseline amount of EPS needed to be considered when the strains were grown in milk medium.
EPS yields were determined for all S. thermophilus cultures in milk medium and enriched milk medium. Higher concentrations of EPS for all S. thermophilus cultures were observed in enriched milk medium than in milk medium (Table 2). However, although EPS isolation from a baseline, nonfermented, enriched milk medium yielded 100 to 120 mg of PDM liter−1, the addition of yeast extract and peptone stimulated growth, depending on the strain, and hence growth-associated EPS production, confirming earlier observations (10). Interestingly, in milk medium, i.e., in the absence of yeast extract and peptone, four S. thermophilus strains produced >100 mg of PDM liter−1. Twelve strains produced between 20 and 100 mg of PDM liter−1, while nine strains produced <20 mg of PDM liter−1. S. thermophilus VUM ST01 yielded the highest EPS concentration in milk medium (133 mg of PDM liter−1). Finally, EPS production with S. thermophilus ST 111 and S. thermophilus NCFB 2393 was variable. Based on repeated EPS isolations, large experimental errors of ±20% for EPS yields were found for different strains.
Molecular mass determination.
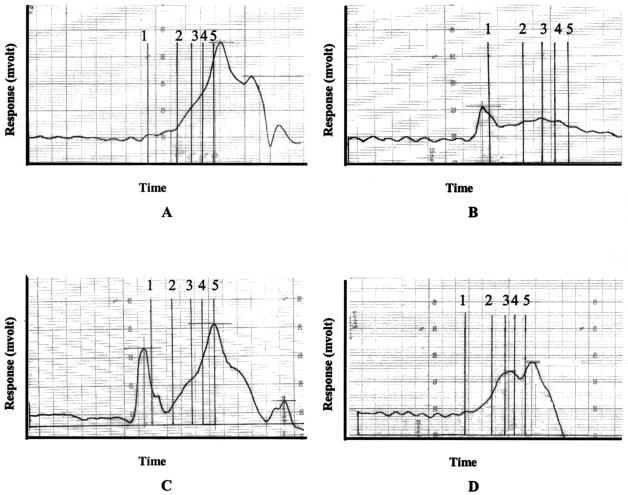
For molecular mass determination, six EPS samples, isolated from S. thermophilus ST 111, SR1, S509, ST 30, ST 114, and 534b, grown in enriched milk medium at 42°C for 12 h, were first analyzed by GPC. They all showed a similar peak around 75 kDa, as shown for S. thermophilus ST 111 (Fig. 2C). GPC of EPS from a baseline, nonfermented, enriched milk medium resulted in a very broad peak that covered a molecular mass range from 8 to 727 kDa (Fig. 2A). A maximum was observed at 75 kDa, indicating the presence of glucomannans (7). This indicates contamination of the EPS samples derived from fermented enriched milk medium with polysaccharide components isolated from the medium (present in yeast extract and/or peptone). This was also seen when the monomer compositions of these samples were analyzed (see below). Since this broad peak comprised the region in which low-molecular-mass EPS eluted, molecular mass determination for EPS samples derived from fermented enriched milk medium was only possible if a distinct high-molecular-mass EPS peak was present. However, when the peaks of the low-molecular-mass EPS and the contaminating polysaccharides could not be separated, as was observed for EPS produced by S. thermophilus ST 113 (Fig. 2D), an alternative strategy was needed. Therefore, by comparing the monomer compositions of different eluted fractions in the molecular mass range of 14 to 1,700 kDa with the monomer compositions of the EPS isolated from milk medium, the 210-kDa peak (Fig. 2D) could be allocated to the low-molecular-mass EPS produced by S. thermophilus ST 113. After extensive purification, the molecular masses of the EPS from 25 S. thermophilus strains, grown in milk medium, enriched milk medium, or milk medium supplemented with lactalbumine hydrolysate, were determined. A distinction could be made between high-molecular-mass EPS (>1,000 kDa) and low-molecular-mass EPS (<1,000 kDa). Based on the type(s) of molecular mass produced, the strains were divided into three groups (Table 3). Five S. thermophilus strains produced only high-molecular-mass EPS (group I), 10 strains produced only low-molecular-mass EPS (group II), and another 10 strains produced both types of EPS (group III). Ten strains produced EPS with molecular masses of >2,000 kDa, which exceeded the linear range of the GPC column. No correlation was seen between the molecular mass and the floating or nonfloating EPS fractions. Only for the floating and nonfloating EPS fractions from S. thermophilus LY03 and Sfi20 did molecular mass analysis reveal the presence of high-molecular-mass and low-molecular-mass EPS in the respective fractions when the strains were grown in milk medium. However, no difference in molecular mass between the two EPS fractions was observed when S. thermophilus LY03 was grown in milk medium supplemented with lactalbumine hydrolysate. In milk medium, S. thermophilus ST 111 produced a high-molecular-mass EPS of >2,000 kDa (Fig. 2B). The same molecular mass was found when this strain was grown in enriched milk medium or milk medium supplemented with lactalbumine hydrolysate. In contrast, the molecular masses of the EPS from other strains were influenced by the medium composition (Table 3). For instance, S. thermophilus ST 113 produced a low-molecular-mass EPS of 210 kDa when grown in enriched milk medium (see above), but when this strain was grown in milk medium supplemented with lactalbumine hydrolysate, it produced a high-molecular-mass EPS of >2,000 kDa. Its retention time (82 min) was significantly longer (P < 0.05) than those of the high-molecular-mass EPS (>2,000 kDa) from S. thermophilus LY03 (75 min) and S. thermophilus ST 111 (76 min), indicating the production of an EPS with a lower molecular mass by S. thermophilus ST 113. Similarly, growth of S. thermophilus 534b in enriched milk medium resulted in the production of a high-molecular-mass EPS (1,990 kDa), while growth of this strain in milk medium yielded only a low-molecular-mass EPS (59 kDa) (Table 3). Also, S. thermophilus CH101 produced an EPS with a higher molecular mass when grown in enriched milk medium than that of the EPS isolated from fermented milk medium supplemented with lactalbumine hydrolysate (850 and 310 kDa, respectively).
FIG. 2.
Gel permeation chromatograms from polysaccharides isolated from a baseline, nonfermented enriched milk medium (10.0% [wt/vol] skimmed milk powder, 1.0% [wt/vol] peptone, 0.5% [wt/vol] yeast extract) (A), fermented milk medium (10.0% [wt/vol] skimmed milk powder) (B), and fermented enriched milk medium (C and D). (B and C) Chromatograms of EPS from S. thermophilus ST 111; (D) chromatogram of EPS from S. thermophilus ST 113. Vertical lines represent the molecular mass markers as follows: 1, 1,800 kDa; 2, 670 kDa; 3, 270 kDa; 4, 150 kDa; and 5, 80 kDa.
TABLE 3.
Molecular masses of EPS produced by S. thermophilus strains grown in milk-based media
| Group | S. thermophilus strain | High-molecular-mass EPS (kDa) | Low-molecular-mass EPS (kDa) |
|---|---|---|---|
| I | 01/12 | 1,558a | None |
| ST 111 | >2,000a,b,c | None | |
| ST 110 | >2,000a | None | |
| ST 114 | >2,000a | None | |
| CRL1190 | 1,782a | None | |
| II | T7 | None | 30a |
| T149 | None | 336a | |
| SY102 | None | 10-180a,d | |
| CH101 | None | 850b-310c | |
| 5F/51 | None | 856a | |
| ST 33 | None | 13-197a,d | |
| STD | None | 4a,c,d | |
| SR1 | None | 18a | |
| ST 144 | None | 26a | |
| VUM ST01 | None | 19a | |
| III | LY03 | 1,800a->2,000c | 470a,d |
| Sfi20 | >2,000a | 4a,d | |
| IMDOST03 | >2,000a | 500a | |
| 480 | 1,200a | 4a,d | |
| NCFB 2393 | >2,000a | 35a | |
| D22 | 1,800a | 500a | |
| ST 113 | >2,000c | 210b | |
| ST 30 | >2,000a | 35-402a,d | |
| S509 | >2,000a | 469a | |
| 534b | 1,990b | 59a |
EPS isolated from fermented milk medium (10.0% [wt/vol] skimmed milk powder). Fermentations were done at 42°C and under non-pH-controlled conditions.
EPS isolated from fermented enriched milk medium (10.0% [wt/vol] skimmed milk powder, 1.0% [wt/vol] peptone, 0.5% [wt/vol] yeast extract). Fermentations were done at 42°C under non-pH-controlled conditions.
EPS isolated from fermented milk medium supplemented with lactalbumine hydrolysate (1.6% [wt/vol]). Fermentations were done at 42°C and a constant pH of 6.2.
Range of low-molecular-mass EPS fractions, possibly due to EPS degradation.
Monomer analysis of EPS.
Recently, it was shown that HPAEC with pulsed amperometric detection makes an accurate monomer analysis of pure EPS possible (7). However, in some cases the acid hydrolysis of the EPS influenced the elution behaviors of the sugars. In the cases of fructose, N-acetylglucosamine, and N-acetylgalactosamine, a decrease in retention time was observed when treated and nontreated monosaccharides were compared. Moreover, the acetylated amino sugars N-acetylglucosamine and N-acetylgalactosamine were converted into the amino sugars glucosamine and galactosamine, respectively. Likewise, it was not possible to determine whether glucosamine (or galactosamine) or its acetylated form was present in the EPS. In addition, N-acetylglucosamine and N-acetylgalactosamine showed the same retention times as mannose (Fig. 1).
EPS isolated from fermented enriched milk medium contained mannose and glucose in high concentrations due to the presence of glucomannans derived from the yeast extract and peptone in the medium. Subculturing of the strains in MRS medium before inoculation in milk medium was also responsible for the presence of traces of mannose during monomer analysis (results not shown). Therefore, monomer analysis of the EPS from the 25 S. thermophilus cultures mentioned above was carried out on purified EPS isolated from fermented milk medium only. Six different groups could be distinguished (Table 4). The largest group (six strains) produced EPS that contained only galactose and glucose (group I). A second group of EPS (two strains) consisted only of galactose and rhamnose (group II). Four strains produced EPS which contained galactose, glucose, and (N-acetyl)galactosamine (group III), whereas five strains produced EPS composed of galactose, glucose, and rhamnose (group IV). A fifth group consisted of five EPS which contained galactose, glucose, (N-acetyl)galactosamine, and rhamnose (group V). EPS belonging to group VI (three strains) contained galactose, glucose, (N-acetyl)glucosamine, and (N-acetyl)galactosamine (in the EPS of S. thermophilus ST 33) or rhamnose (in the EPS of S. thermophilus T7). In nine EPS, galactose was present in a higher amount than glucose, whereas the opposite was observed in another eight EPS. Within all groups, the monomer ratios of the different monosaccharides varied, although several strains in most of these groups (I, II, III, IV, and V) produced EPS composed of a similar monomer ratio.
TABLE 4.
Monomer compositions of EPS produced by S. thermophilus strains grown in milk mediuma
| Group | S. thermophilus strain | Ratio of monomer relative to other monomers in compound (on a molar basis)b
|
||||
|---|---|---|---|---|---|---|
| Gal | Glc | GalN(Ac) | GlcN(Ac) | Rha | ||
| I | SY102 | 1.0 | 1.0 | |||
| CH101 | 1.0 | 1.0 | ||||
| D22 | 1.0 | 1.0 | ||||
| 01/12 | 1.0 | 1.0 | ||||
| 5F/51 | 1.0 | 1.0 | ||||
| CRL1190 | 1.5 | 1.0 | ||||
| II | ST 111 | 2.5 | 1.0 | |||
| S509 | 3.0 | 1.0 | ||||
| III | LY03 | 3.4 | 1.4 | 1.0 | ||
| Sfi20 | 3.1 | 1.5 | 1.0 | |||
| IMDOST03 | 3.3 | 2.1 | 1.0 | |||
| ST 110 | 3.9 | 1.2 | 1.0 | |||
| IV | 480 | 1.7 | 4.5 | 1.0 | ||
| T149 | 3.3 | 2.5 | 1.0 | |||
| NCFB 2393 | 3.2 | 1.0 | 1.6 | |||
| ST 114 | 3.7 | 2.4 | 1.0 | |||
| SR1 | 1.6 | 1.0 | 1.0 | |||
| V | ST 113 | 1.7 | 3.9 | 1.0 | 1.5 | |
| STD | 3.5 | 6.2 | 1.0 | 1.2 | ||
| ST 144 | 1.4 | 2.3 | 1.0 | 1.0 | ||
| 534b | 2.5 | 3.3 | 1.0 | 3.2 | ||
| VUM ST01 | 1.5 | 2.8 | 1.1 | 1.0 | ||
| VI | T7 | 1.1 | 1.5 | 1.1 | 1.0 | |
| ST 33 | 2.3 | 3.6 | 1.1 | 1.0 | ||
| ST 30 | 1.0 | 1.1 | 1.0 | |||
Milk medium consisted of 10.0% (wt/vol) skimmed milk powder.
Gal, galactose; Glc, glucose; GalN(Ac), (N-acetyl)galactosamine; GlcN(Ac), (N-acetyl)glucosamine; Rha, rhamnose.
Viscosity buildup and EPS production during milk fermentations.
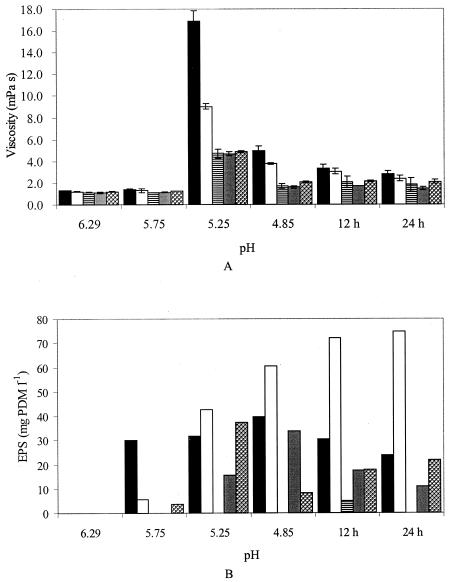
Based on differences in EPS production, molecular masses of the EPS, and EPS structures, five strains (S. thermophilus LY03, ST 111, CH101, ST 113, and STD) were selected for determination of the apparent viscosities and corresponding EPS yields during milk fermentations (Fig. 3) to link EPS characteristics with viscosity buildup. In the cases of S. thermophilus LY03 and CH101, the apparent viscosities of samples at pH 5.25 could only be measured at a spindle speed of 10 rpm because of their high torque values. All other apparent viscosities were measured at a spindle speed of 50 rpm. For all strains, the apparent viscosity increased when the pH dropped below 5.75 during the fermentation (Fig. 3A). At pH 5.25, the highest apparent viscosity was observed, but viscosities decreased upon further acidification. For S. thermophilus LY03 and CH101, the apparent viscosities of the fermented milks were significantly higher (P < 0.05) than with the other three strains. Although this difference was still significant (P < 0.05) after 12 h of fermentation, it was not significant after 24 h of fermentation (Fig. 3A).
FIG. 3.
Apparent viscosity (A) and EPS measurements (B) during fermentations of S. thermophilus strains in milk medium (10.0% [wt/vol] skimmed milk powder) at 42°C. Samples were taken at different pH values and after 12 and 24 h of fermentation. Black bars, S. thermophilus LY03; white bars, S. thermophilus CH101; striped bars, S. thermophilus STD; gray bars, S. thermophilus ST 113; dotted bars, S. thermophilus ST 111.
Large differences in EPS production between the five different strains were observed during milk fermentations (Fig. 3B). While S. thermophilus LY03 produced high EPS amounts (on average, 31 mg of PDM liter−1), the highest EPS concentration was observed for S. thermophilus CH101 (75 mg of PDM liter−1 after 24 h of fermentation), whereas almost no EPS was produced by S. thermophilus STD throughout the fermentation (<5 mg of PDM liter−1 after 12 h of fermentation). Except for the last strain, EPS could be isolated at any time during the 24-h fermentations.
Apparent viscosity measurements of pure EPS solutions.
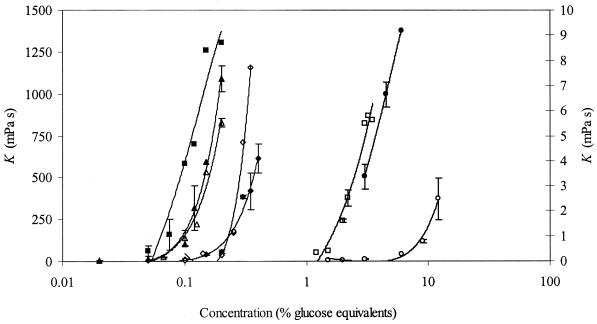
Pure EPS solutions from the five strains mentioned above were analyzed for their effect on viscosity. Based on apparent viscosity measurements of solutions with various EPS concentrations, it turned out that the five different EPS solutions could not be compared at a common concentration because the torque exceeded the range of 15 to 85% of the maximum torque. Consequently, flow curves were determined with different concentrations of pure EPS. The effect of EPS concentration on the consistency (K, in millipascal seconds) of the different EPS solutions was determined (Fig. 4). In general, K increased with increasing EPS concentrations. However, large differences in the viscosifying effects of the different EPS were determined (Fig. 4). The highest consistencies were found for guar gum and EPS from S. thermophilus LY03. A 10-fold increase in concentration resulted in a similar range of K for EPS solutions from S. thermophilus ST 113. A high concentration of EPS from S. thermophilus STD displayed a very low consistency (9 mPa · s). No differences were observed between floating and nonfloating EPS materials of S. thermophilus LY03. In contrast, the nonfloating EPS material from S. thermophilus ST 111 showed a lower K than the floating EPS material. All of the different EPS discussed here displayed a shear-thinning character (0 < ν < 1).
FIG. 4.
Influence of different EPS concentrations on the consistencies (K) of the EPS solutions. Strains were grown in milk medium (10.0% [wt/vol] skimmed milk powder) supplemented with lactalbumine hydrolysate (1.6% [wt/vol]) at 42°C for 12 h and at a constant pH of 6.2. ▪, guar gum; ▴, nonfloating EPS of S. thermophilus LY03; ▵, floating EPS of S. thermophilus LY03; ⧫, nonfloating EPS of S. thermophilus ST 111; ◊, floating EPS of S. thermophilus ST 111; □, nonfloating EPS of S. thermophilus ST 113; •, nonfloating EPS of S. thermophilus STD (second y axis); ○, nonfloating EPS of S. thermophilus CH101.
DISCUSSION
The fermentation characteristics of heteropolysaccharide-producing strains of S. thermophilus grown in milk medium differ considerably. First of all, EPS production yields in milk medium fermented with S. thermophilus are low and strain dependent. The majority of the S. thermophilus strains tested produced between 20 and 100 mg of PDM liter−1. EPS production displays primary metabolite kinetics and can be enhanced under optimal physical and chemical conditions (5, 6, 9). The availability of a complex nitrogen source such as yeast extract and peptone (consisting of several peptides and amino acids) is of utmost importance for both cell synthesis and EPS production (5, 9, 47). The limited proteolytic character of most S. thermophilus strains is well known, and their capacities to grow in milk vary between pure and mixed cultures (4, 42, 58). In addition, it has been shown that vitamins present in yeast extract play an important role in growth and EPS production (24). However, yeast extract and peptone cannot be used in yogurt manufacture, while EPS production by the starter culture is desired to improve the organoleptic properties of yogurt (16). This is particularly problematic for growth of S. thermophilus strains that produce low levels of EPS in milk, and hence a rational selection of overproducers is desirable. On the other hand, yeast extract and peptone contain glucomannans that interfere with EPS isolation, purification, and structural characterization (34, 64), which hampers a detailed structural study of EPS.
Screening for heteropolysaccharide biosynthesis by LAB has been carried out mostly on agar media with relatively low glucose or lactose concentrations, and selection has been based on colony ropiness (29, 70). Alternatively, liquid media containing high sugar concentrations can be used to select EPS-positive strains (71). Therefore, as shown in this paper, milk is an appropriate medium to screen for EPS-producing LAB strains, since it contains a high amount of lactose and no polysaccharides that interfere with EPS isolation. Meanwhile, it gives an idea of the milk adaptation of the strain, which is necessary for successful industrial implementation in dairy products.
In this study, no clear correlation was found between the acidification rates and milk-clotting abilities of the S. thermophilus strains or between the acidification rates and EPS production. However, their low t0.5 values indicate the adaptation of the strains to a milk medium. All slow acidifying strains caused intermediate to slow coagulation of milk. All fast milk-coagulating strains resulted in fast acidification. Alternatively, strong acidifiers could be intermediate to slow milk-coagulating strains. The clotting abilities of these strains can give an idea about their proteolytic character in milk (79). Some studies reported a correlation between the occurrence of cell-wall-associated proteinase (PrtS) activity of particular S. thermophilus strains and a high acidification rate in milk (58), which is an extra indication of the adaptation of these strains to a milk medium. PrtS is essential for the growth of S. thermophilus in milk medium (4), although the growth rate of this species is not limited by the PrtS activity, but by the transport rate of the proteolysis products (33).
A combination of fast acidification and EPS production, as observed in this study for several strains (e.g., S. thermophilus LY03 and CH101), is desirable in milk fermentations. A high acidification rate (and hence a high growth rate) may result in a high level of EPS production because of the growth-associated character of EPS production by S. thermophilus strains (5, 6, 9). Also, Pailin et al. (42) showed growth-associated EPS production on a solid milk medium, based on the size of the EPS capsule and colonies. In yogurt, the EPS improve the viscosity, body, texture, and mouth feel of the end product (16, 55, 57). In low-fat mozzarella cheese, the EPS contribute to an increased moisture level (2, 35, 43).
In this study, it was shown that S. thermophilus STD, a strain that produced almost no EPS during milk fermentation, could contribute to the same extent to the apparent viscosity of the fermented milk as the EPS-producing strains S. thermophilus ST 111 and ST 113, which both produced EPS with high molecular masses. All three strains displayed a comparable acidification rate and hence a similar growth rate. Consequently, the lower level of EPS production of S. thermophilus STD was probably compensated for by other factors, such as its stronger milk-clotting ability. Moreover, complex interactions exist between bacterial cells (all or not surrounded by EPS, encapsulated or not), milk proteins (degree of denaturation, colloid diameter, protein network, and interactions), the microstructure (gel network, pH, and ionic strength), and EPS (molecular mass, monomer composition, degree of branching, concentration, and stiffness), resulting in the formation of a more rigid network (16, 54, 63). In contrast, the use of a high-level EPS-producing strain displaying a stronger milk-clotting ability, such as S. thermophilus CH101 compared with S. thermophilus LY03, did not necessarily result in a higher apparent viscosity of the fermented milk. As both strains also showed a similar acidification rate, it can be concluded that other factors, such as the EPS characteristics (molecular mass or EPS structure) or the EPS-protein interactions, are important for texture buildup (16, 53, 54). The relationship between viscosity and EPS production was not always straightforward, as a further increase in EPS production below pH 5.25 did not result in a further apparent viscosity increase of the milk fermented with S. thermophilus CH101. At lower pH values, differences between the apparent viscosity measurements for the different fermented milks became smaller, which was in agreement with the results obtained for viscosity measurements of ropy cultures, capsule-forming nonropy cultures, or noncapsule-forming nonropy cultures (25).
The molecular masses of the EPS from the S. thermophilus strains tested ranged from 4 to >2,000 kDa, indicating a great biodiversity of EPS with respect to this property. Further, molecular mass displays the potential relevance of these EPS in several industrial applications. It is an important factor determining the intrinsic viscosity (18, 65-67) and hence the functional properties of EPS (18, 53). In this study, a distinction was made between two types of EPS, high-molecular-mass EPS (>1,000 kDa) and low-molecular-mass EPS (<1,000 kDa). Based on the molecular mass of the EPS produced, the 25 S. thermophilus strains tested were divided into three groups. Some strains produced only one type of EPS, either high-molecular-mass or low-molecular-mass EPS (groups I and II, respectively), while other strains produced both types of EPS in milk medium (group III). Several authors already reported the existence of LAB strains producing more than one size of EPS, depending on the culture conditions (5, 23, 36, 47). For example, for S. thermophilus LY03 and Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772, the proportions of the EPS produced are dependent on the nature of the nitrogen and carbohydrate source, respectively (5, 23). In the present work, we showed that the nitrogen source (yeast extract, peptone, or lactalbumine hydrolysate) may influence the molecular mass of the EPS. The presence of floating or nonfloating EPS during isolation was not directly related to the occurrence of high-molecular-mass or low-molecular-mass EPS, as described previously for S. thermophilus LY03 (5). However, based on the differences in consistency between floating and nonfloating EPS, it is possible that S. thermophilus ST 111 produced two high-molecular-mass EPS in milk supplemented with lactalbumine hydrolysate. Unfortunately, these EPS could not be differentiated from each other because of the limited linear range of the GPC column. Both the medium composition and the amount of EPS produced influenced EPS isolation and recovery.
An analysis of the EPS monomer composition also reflected the biodiversity of EPS produced by S. thermophilus strains. The EPS of the 25 strains used in this study could be classified into six groups according to monomer composition. The majority of the EPS (from 20 strains) belonged to four groups (groups I, III, IV, and V) containing the classical monosaccharide constituents of EPS produced by LAB, namely galactose, glucose, rhamnose, and/or (N-acetyl)galactosamine, but in different ratios. S. thermophilus strains producing EPS belonging to each of these groups have been reported in the literature (for groups I and IV, see reference 32; for group III, see reference 15; and for group V, see reference 1). This biodiversity may be ascribed to the sugar nucleotide biosynthesis routes and genetic potential of these strains (12). Several authors have reported a correlation between the presence of certain monomers in the EPS repeating units and the intracellular activity of both glycosyltransferases (28, 60, 72) and enzymes that are necessary for the supply of sugar nucleotides, the precursor molecules of heteropolysaccharide biosynthesis (7, 17, 40). Concomitantly, different eps operons have been discovered in the genomes of S. thermophilus strains (27). Group II consisted of EPS containing only galactose and rhamnose (two strains). Such EPS were also found previously in S. thermophilus (3, 20). Most of the S. thermophilus strains belonging to group V contained EPS with a lower molar amount of galactose in the EPS subunit than of glucose. Although this has been observed for other thermophilic LAB (19, 49), this low galactose-to-glucose ratio has never been detected in EPS from S. thermophilus. Interestingly, one EPS group (VI) encompassed EPS that contained (N-acetyl)glucosamine. To our knowledge, this has never been found in EPS from S. thermophilus until now. EPS from Lactobacillus helveticus TY1-2 (76), Lactobacillus acidophilus LMG 9433 (50), and Streptococcus macedonicus Sc 136 (73) have been reported to contain the same monomers (galactose, glucose, and N-acetylglucosamine) as the EPS from S. thermophilus ST 30 (this study), albeit in different ratios. The EPS from Lactobacillus rhamnosus GG (30) is composed of N-acetylglucosamine, galactose, and rhamnose. Rhamnose was also found in the N-acetylglucosamine-containing EPS from S. thermophilus T7 (this study). However, the nature of the monomers of the EPS of strains belonging to group VI, as well as their low EPS yields, may indicate that these EPS are located on the surface and hence more tightly bound to the cells (8). Consequently, these EPS are more difficult to recover by the EPS isolation method applied in this study. Heat treatment of samples before EPS isolation can increase the recovery of these EPS (48). Moreover, glucose, galactose, rhamnose, and N-acetylglucosamine are also constituents of cell wall polysaccharides, which can be involved in phage adsorption (22, 45).
Some of the structures of the EPS classified during this study have been previously revealed by nuclear magnetic resonance (NMR) spectroscopy, including the EPS from S. thermophilus SY102 (37), S. thermophilus LY03 (7), S. thermophilus Sfi20 (7), and S. thermophilus IMDOST03 (37). Also, a structure analysis has been performed on the EPS from S. thermophilus ST 111, CH101, and STD (14). For some of these EPS, differences were noticed between the monomer analysis after acid hydrolysis and the NMR structure elucidation. Monomer analysis after acid hydrolysis of the EPS from S. thermophilus IMDOST03 predicted a lower amount of acetylated amino sugars than did its previously determined structure (37). These results confirm earlier comparisons between monomer compositions after acid hydrolysis and NMR structure analyses of EPS from S. thermophilus LY03 and Sfi20 (7). Also, monomer analysis of the EPS from S. thermophilus STD revealed the presence of galactose, glucose, galactosamine, and rhamnose in this study, whereas NMR analysis indicated the presence of a tetrasaccharide containing only galactose and glucose in an equimolar ratio (14). This may also be due to the occurrence of more than one EPS. Since NMR analysis can only detect soluble EPS and because higher concentrations of EPS are needed for structure analysis, monomer analysis as performed in this study is useful, for instance, in cases of low-level EPS-producing strains (e.g., S. thermophilus STD), whereby the EPS isolation is often hindered by the simultaneous extraction of peptidoglycan-associated cell wall polymers. Moreover, monomer analysis after acid hydrolysis could be used to select EPS for further NMR structure analysis. For instance, as the monomer ratio within one group of EPS often differs, NMR analysis will certainly elucidate novel EPS structures from the S. thermophilus strains studied.
Structural and molecular mass analysis of different EPS is necessary to elucidate structure-function relationships (26, 31, 53). In the present study, it was shown that molecular mass positively influences the consistency of EPS solutions. However, no clear-cut relationship was observed between the amount of EPS produced and the medium viscosity when different strains were compared. In particular, it was shown that similar consistencies were obtained with solutions that are composed of EPS with a higher molecular mass at a low concentration or of EPS with a lower molecular mass at a high concentration. Furthermore, the molecular structure of the EPS also seemed to determine the consistency of the solution, in particular for EPS with comparable molecular masses, e.g., the high-molecular-mass EPS from S. thermophilus ST 111 and LY03. In contrast to the backbone of EPS from S. thermophilus ST 111, which is solely composed of α(1→2) and α(1→3) linkages (F. Vaningelgem, M. Zamfir, T. Adriany, A. P. Laws, and L. De Vuyst, unpublished results), the backbone of S. thermophilus LY03 is composed of both α(1→3) and β(1→3) linkages (7). The β-linkages may contribute to stiffer chains (31) and hence a higher consistency of EPS solutions. Similarly, the backbone of the EPS from Lactococcus lactis subsp. cremoris B39 consists only of α(1→4) linkages, resulting in a very flexible polymer compared with the EPS from Lactococcus lactis subsp. cremoris B40, which only contains β(1→4) linkages (53). In addition, the complexity of the primary structure (size, monomer composition, side groups, and branching) will influence the viscosifying effects of the EPS solutions (67, 78).
In conclusion, although S. thermophilus strains generally display low EPS yields, a large biodiversity with respect to their yields, monomer compositions, and molecular masses, as well as functionalities, is seen. No clear-cut relationship between the amount of EPS produced and the medium viscosity occurs when different strains are compared. This is of utmost importance for a rational screening of high-level EPS-producing strains to be implemented in the food industry. Based on properties such as the molecular mass and the monomer composition of the EPS produced by these strains, classifications can be made and EPS databases can be built. These may in turn be useful for further research on structure-function relationships and to predict the influence of the EPS on texture formation, e.g., in yogurt production.
Acknowledgments
We acknowledge the financial support of the European Commission (grants FAIR-CT-98-4267 and INCO Copernicus IC15-CT98-0905), the Flemish Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT), the Fund for Scientific Research (FWO-Flanders), the International Scientific and Technological Cooperation between Flanders, Belgium, and Romania from the Administration of Science and Innovation in Flanders (AWI-BIL01/52), the LINK 2000 and 2002 Actions of the Brussels Capital Region, and the Research Council of the Vrije Universiteit Brussel. F.V. is a recipient of an IWT fellowship.
The support from Bartosz Brzozowski (University of Warmia and Mazury, Olsztyn, Poland), Helen Dunn (The University of Huddersfield, Huddersfield, United Kingdom), Piotr Kolakowski (Biolacta-Texel, Olsztyn, Poland), and Sŏna Jamrichová (Dairy Research Institute, Zilina, Slovakia) is very much appreciated.
REFERENCES
- 1.Almirón-Roig, E., F. Mulholland, M. J. Gasson, and A. M. Griffin. 2000. The complete cps gene cluster from Streptococcus thermophilus NCFB 2393 involved in the biosynthesis of a new exopolysaccharide. Microbiology 146:2793-2802. [DOI] [PubMed] [Google Scholar]
- 2.Broadbent, J. R., D. J. McMahon, C. J. Oberg, and D. L. Walker. 2001. Use of exopolysaccharide-producing cultures to improve the functionality of low fat cheese. Int. Dairy J. 11:433-439. [Google Scholar]
- 3.Bubb, W. A., T. Urashima, R. Fujiwara, T. Shinnai, and H. Ariga. 1997. Structural characterisation of the exocellular polysaccharide produced by Streptococcus thermophilus OR901. Carbohydr. Res. 301:41-50. [DOI] [PubMed] [Google Scholar]
- 4.Courtin, P., V. Monnet, and F. Rul. 2002. Cell-wall proteinases PrtS and PrtB have a different role in Streptococcus thermophilus/Lactobacillus bulgaricus mixed cultures in milk. Microbiology 148:3413-3421. [DOI] [PubMed] [Google Scholar]
- 5.Degeest, B., and L. De Vuyst. 1999. Indication that the nitrogen source influences both amount and size of exopolysaccharides produced by Streptococcus thermophilus LY03 and modelling of the bacterial growth and exopolysaccharide production in a complex medium. Appl. Environ. Microbiol. 65:2863-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degeest, B., F. Vaningelgem, and L. De Vuyst. 2001. Microbial physiology, fermentation kinetics and process engineering of heteropolysaccharide production by lactic acid bacteria. Int. Dairy J. 11:747-757. [Google Scholar]
- 7.Degeest, B., F. Vaningelgem, A. Laws, and L. De Vuyst. 2001. UDP-N-acetylglucosamine 4-epimerase activity indicates the presence of N-acetylgalactosamine in exopolysaccharides of Streptococcus thermophilus strains. Appl. Environ. Microbiol. 67:3976-3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delcour, J., T. Ferain, M. Deghorain, E. Palumbo, and P. Hols. 1999. The biosynthesis and functionality of the cell-wall of lactic acid bacteria. Antonie Leeuwenhoek 76:159-184. [PubMed] [Google Scholar]
- 9.De Vuyst, L., F. Vanderveken, S. Van de Ven, and B. Degeest. 1998. Production by and isolation of exopolysaccharides from Streptococcus thermophilus grown in milk medium and evidence for their growth-associated biosynthesis. J. Appl. Microbiol. 84:1059-1068. [DOI] [PubMed] [Google Scholar]
- 10.De Vuyst, L., and B. Degeest. 1999. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol. Rev. 23:157-177. [DOI] [PubMed] [Google Scholar]
- 11.De Vuyst, L. 2000. Technology aspects related to the application of functional starter cultures. Food Technol. Biotechnol. 38:105-112. [Google Scholar]
- 12.De Vuyst, L., F. De Vin, F. Vaningelgem, and B. Degeest. 2001. Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int. Dairy J. 11:687-707. [Google Scholar]
- 13.De Vuyst, L., and F. Vaningelgem. 2003. Developing new polysaccharides, p. 275-320. In B. M. McKenna (ed.), Texture in food, vol. 2. Semi-solid foods. Woodhead Publishing Ltd., Cambridge, United Kingdom.
- 14.De Vuyst, L., M. Zamfir, F. Mozzi, T. Adriany, V. Marshall, B. Degeest, and F. Vaningelgem. 2003. Exopolysaccharide-producing Streptococcus thermophilus strains as functional starter cultures in the production of fermented milks. Int. Dairy J. 13:707-717. [Google Scholar]
- 15.Doco, T., J. M. Wieruszeski, B. Fournet, D. Carcano, P. Ramos, and A. Loones. 1990. Structure of an exopolysaccharide produced by Streptococcus thermophilus. Carbohydr. Res. 198:313-321. [DOI] [PubMed] [Google Scholar]
- 16.Duboc, P., and B. Mollet. 2001. Applications of exopolysaccharides in the dairy industry. Int. Dairy J. 11:759-768. [Google Scholar]
- 17.Escalante, A., J. Villegas, C. Wacher, M. García-Garibay, and A. Farrés. 2002. Activity of the enzymes involved in the synthesis of exopolysaccharide precursors in an overproducing mutant ropy strain of Streptococcus thermophilus. FEMS Microbiol. Lett. 209:289-293. [DOI] [PubMed] [Google Scholar]
- 18.Faber, E. J., P. Zoon, J. P. Kamerling, and J. F. G. Vliegenthart. 1998. The exopolysaccharides produced by Streptococcus thermophilus Rs and Sts have the same repeating unit but differ in viscosity of their milk cultures. Carbohydr. Res. 310:269-276. [DOI] [PubMed] [Google Scholar]
- 19.Faber, E. J., J. P. Kamerling, and J. F. G. Vliegenthart. 2001. Structure of the extracellular polysaccharide produced by Lactobacillus delbrueckii subsp. bulgaricus 291. Carbohydr. Res. 331:183-194. [DOI] [PubMed] [Google Scholar]
- 20.Faber, E. J., M. J. van den Haak, J. P. Kamerling, and J. F. G. Vliegenthart. 2001. Structure of the exopolysaccharide produced by Streptococcus thermophilus S3. Carbohydr. Res. 331:173-182. [DOI] [PubMed] [Google Scholar]
- 21.Faber, E. J., D. J. van Haaster, J. P. Kamerling, and J. F. G. Vliegenthart. 2002. Characterization of the exopolysaccharide produced by Streptococcus thermophilus 8S containing an open chain nononic acid. Eur. J. Biochem. 269:5590-5598. [DOI] [PubMed] [Google Scholar]
- 22.Gopal, P. K., and V. L. Crow. 1993. Characterization of loosely associated material from the cell surface of Lactococcus lactis subsp. cremoris E8 and its phage-resistant variant strain. Int. Dairy J. 5:1095-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grobben, G. J., W. H. M. van Casteren, H. A. Schols, A. Oosterveld, G. Sala, M. R. Smith, J. Sikkema, and J. A. M. de Bont. 1997. Analysis of the exopolysaccharides produced by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 grown in continuous culture on glucose and fructose. Appl. Microbiol. Biotechnol. 48:516-521. [Google Scholar]
- 24.Grobben, G. J., I. Chin-Joe, V. A. Kitzen, I. C. Boels, F. Boer, J. Sikkema, M. R. Smith, and J. A. M de Bont. 1998. Enhancement of exopolysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 with a simplified defined medium. Appl. Environ. Microbiol. 64:1333-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan, A. N., M. Corredig, and J. F. Frank. 2002. Capsule formation by nonropy starter cultures affects the viscoelastic properties of yoghurt during structure formation. J. Dairy Sci. 85:716-720. [DOI] [PubMed] [Google Scholar]
- 26.Higashimura, M., B. W. Mulder-Bosman, R. Reich, T. Iwasaki, and G. W. Robijn. 2000. Solution properties of Viilian, the exopolysaccharide from Lactococcus lactis subsp. cremoris SBT 0495. Biopolymers 54:143-158. [DOI] [PubMed] [Google Scholar]
- 27.Jolly, L., and F. Stingele. 2001. Molecular organisation and functionality of exopolysaccharide gene clusters in lactic acid bacteria. Int. Dairy J. 11:733-745. [Google Scholar]
- 28.Jolly, L., J. Newell, I. Porcelli, S. J. F. Vincent, and F. Stingele. 2002. Lactobacillus helveticus glycosyltransferases: from genes to carbohydrate synthesis. Glycobiology 12:319-327. [DOI] [PubMed] [Google Scholar]
- 29.Knoshaug, E. P., J. A. Ahlgren, and J. E. Trempy. 2000. Growth associated exopolysaccharide expression in Lactococcus lactis subspecies cremoris Ropy352. J. Dairy Sci. 83:633-640. [DOI] [PubMed] [Google Scholar]
- 30.Landersjö, C., Z. Yang, E. Huttunen, and G. Widmalm. 2002. Structural studies of the exopolysaccharide produced by Lactobacillus rhamnosus strain GG (ATCC 53103). Biomacromolecules 3:880-884. [DOI] [PubMed] [Google Scholar]
- 31.Laws, A. P., and V. M. Marshall. 2001. The relevance of exopolysaccharides to the rheological properties in milk fermented with ropy strains of lactic acid bacteria. Int. Dairy J. 11:709-721. [Google Scholar]
- 32.Lemoine, J., F. Chirat, J. M. Wieruszeksi, G. Strecker, N. Favre, and J.-R. Neeser. 1997. Structural characterisation of the exocellular polysaccharides produced by Streptococcus thermophilus Sfi39 and Sfi12. Appl. Environ. Microbiol. 63:3512-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letort, C., M. Nardi, P. Garault, V. Monnet, and V. Juillard. 2002. Casein utilization by Streptococcus thermophilus results in a diauxic growth in milk. Appl. Environ. Microbiol. 68:3162-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levander, F., M. Svensson, and P. Rådström. 26. September 2001, posting date. Small-scale analysis of exopolysaccharides from Streptococcus thermophilus grown in a semi-defined medium. BMC Microbiol. 1:23. [Online.] http://www.biomedcentral.com/1471-2180/1/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Low, D., J. A. Ahlgren, D. Horne, D. J. McMahon, C. J. Oberg, and J. R. Broadbent. 1998. Role of Streptococcus thermophilus MR-1C capsular exopolysaccharide in cheese moisture retention. Appl. Environ. Microbiol. 64:2147-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall, V. M., E. N. Cowie, and R. S. Moreton. 1995. Analysis and production of two exopolysaccharides from Lactococcus lactis subsp. cremoris LC330. J. Dairy Res. 62:621-628. [Google Scholar]
- 37.Marshall, V. M., A. P. Laws, Y. Gu, F. Levander, P. Rådström, L. De Vuyst, B. Degeest, F. Vaningelgem, H. Dunn, and M. Elvin. 2001. Exopolysaccharide-producing strains of thermophilic lactic acid bacteria cluster into groups according to their EPS structure. Lett. Appl. Microbiol. 32:433-437. [DOI] [PubMed] [Google Scholar]
- 38.Monsan, P., S. Bozonnet, C. Albenne, G. Joucla, R.-M. Willemot, and M. Remaud-Siméon. 2001. Homopolysaccharides from lactic acid bacteria. Int. Dairy J. 11:675-685. [Google Scholar]
- 39.Mora, D., M. G. Fortina, C. Parini, G. Ricci, M. Gatti, G. Giraffa, and P. L. Manachini. 2002. Genetic diversity and technological properties of Streptococcus thermophilus strains isolated from dairy products. J. Appl. Microbiol. 93:278-287. [DOI] [PubMed] [Google Scholar]
- 40.Mozzi, F., G. Savoy de Giori, and G. Font de Valdez. 2003. UDP-galactose 4-epimerase: a key enzyme in exopolysaccharide formation by Lactobacillus casei CRL 87 in controlled pH batch cultures. J. Appl. Microbiol. 94:175-183. [DOI] [PubMed] [Google Scholar]
- 41.Navarini, L., A. Abatangelo, B. Claudia, E. Conti, M. Bosco, and F. Picotti. 2001. Isolation and characterization of the exopolysaccharide produced by Streptococcus thermophilus Sfi20. Int. J. Biol. Macromol. 28:219-226. [DOI] [PubMed] [Google Scholar]
- 42.Pailin, T., D. H. Kang, K. Schmidt, and D. Y. C. Fung. 2001. Detection of extracellular proteinase in EPS-producing lactic acid bacteria cultures on skim milk agar. Lett. Appl. Microbiol. 33:44-49. [DOI] [PubMed] [Google Scholar]
- 43.Petersen, B. L., R. I. Dave, D. J. McMahon, C. J. Oberg, and J. R. Broadbent. 2000. Influence of capsular and ropy exopolysaccharide-producing Streptococcus thermophilus on mozzarella cheese and cheese whey. J. Dairy Sci. 83:1952-1956. [DOI] [PubMed] [Google Scholar]
- 44.Pot, B., P. Vandamme, and K. Kersters. 1994. Analysis of electrophoretic whole-organism protein fingerprints, p. 493-521. In M. Goodfellow and A. G. O'Donell (ed.), Chemical methods in prokaryotic systematics. J. Wiley and Sons, Chichester, United Kingdom.
- 45.Quiberoni, A., J. I. Stiefel, and J. A. Reinheimer. 2000. Characterization of phage receptors in Streptococcus thermophilus using purified cell walls obtained by a simple protocol. J. Appl. Microbiol. 89:1059-1065. [DOI] [PubMed] [Google Scholar]
- 46.Raibaud, P., M. Caulet, J. V. Galpin, and G. Mocquot. 1961. Studies on the bacterial flora of the alimentary tract of pigs. II. Streptococci: selective enumeration and differentiation of the dominant group. J. Appl. Bacteriol. 24:285-306. [Google Scholar]
- 47.Ricciardi, A., E. Parente, M. A. Crudele, F. Zanetti, G. Scolari, and I. Mannazzu. 2002. Exopolysaccharide production by Streptococcus thermophilus SY: production and preliminary characterization of the polymer. J. Appl. Microbiol. 92:297-306. [DOI] [PubMed] [Google Scholar]
- 48.Rimada, P. S., and A. G. Abraham. 2003. Comparative study of different methodologies to determine the exopolysaccharide produced by kefir grains in milk and whey. Lait 83:79-87. [Google Scholar]
- 49.Robijn, G. W., J. R. Thomas, H. Haas, D. J. C. van den Berg, J. P. Kamerling, and J. F. G. Vliegenthart. 1995. The structure of the exopolysaccharide produced by Lactobacillus helveticus 766. Carbohydr. Res. 276:137-154. [DOI] [PubMed] [Google Scholar]
- 50.Robijn, G. W., R. G. Gallego, D. J. C. van den Berg, H. Haas, J. P. Kamerling, and J. F. G. Vliegenthart. 1996. Structural characterisation of the exopolysaccharide produced by Lactobacillus acidophilus LMG9433. Carbohydr. Res. 288:203-218. [DOI] [PubMed] [Google Scholar]
- 51.Robijn, G. W., H. J. L. Wienk, D. J. C. van den Berg, H. Haas, J. P. Kamerling, and J. F. G. Vliegenthart. 1996. Structural studies of the exopolysaccharide produced by Lactobacillus paracasei 34-1. Carbohydr. Res. 285:129-139. [DOI] [PubMed] [Google Scholar]
- 52.Ruas-Madiedo, P., J. Hugenholtz, and P. Zoon. 2002. An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int. Dairy J. 12:163-171. [Google Scholar]
- 53.Ruas-Madiedo, P., R. Tuinier, M. Kanning, and P. Zoon. 2002. Role of exopolysaccharides produced by Lactococcus lactis subsp. cremoris on the viscosity of fermented milks. Int. Dairy J. 12:689-695. [Google Scholar]
- 54.Ruas-Madiedo, P., and P. Zoon. 2003. Effect of exopolysaccharide-producing Lactococcus lactis strains and temperature on the permeability of skim milk gels. Colloids Surf. A 213:245-253. [Google Scholar]
- 55.Schellhaass, S. M., and H. A. Morris. 1985. Rheological and scanning electron microscopic examination of skim milk gels obtained by fermenting with ropy and non-ropy strains of lactic acid bacteria. Food Microstruct. 4:279-287. [Google Scholar]
- 56.Scott, T. A., and E. H. Melvin. 1953. Determination of dextran with anthrone. Anal. Chem. 25:1656-1661. [Google Scholar]
- 57.Sebastiani, H., and G. Zelger. 1998. Texture formation by thermophilic lactic acid bacteria. Milchwissenschaft 53:15-17. [Google Scholar]
- 58.Shabhal, S., D. Hemme, and M. Desmazeaud. 1991. High cell wall-associated proteinase activity of some Streptococcus thermophilus strains (H strains) correlated with high acidification rate in milk. Lait 71:351-357. [Google Scholar]
- 59.Sodini, I., E. Latrille, and G. Corrieu. 2000. Identification of interacting mixed cultures of lactic acid bacteria by their exclusion from a model predicting the acidifying activity of non-interacting mixed cultures. Appl. Microbiol. Biotechnol. 54:715-718. [DOI] [PubMed] [Google Scholar]
- 60.Stingele, F., J.-R. Neeser, and B. Mollet. 1996. Identification and characterization of the eps (exopolysaccharide) gene cluster from Streptococcus thermophilus Sfi6. J. Bacteriol. 178:1680-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sutherland, I. 1996. Extracellular polysaccharides, p. 615-652. In M. Roehr, J. Rhem, and G. Reed (ed.), Bio/Technology, vol. 6, 2nd ed. VCH Publishers, Weinheim, Germany.
- 62.Sutherland, I. 2001. Microbial polysaccharides from gram-negative bacteria. Int. Dairy J. 11:663-674. [Google Scholar]
- 63.Teggatz, J. A., and H. A. Morris. 1990. Changes in the rheology and microstructure of ropy yoghurt during shearing. Food Struct. 9:133-138. [Google Scholar]
- 64.Torino, M. I., F. Sesma, and G. Font de Valdez. 2000. Semi-defined media for exopolysaccharide (EPS) production by Lactobacillus helveticus ATCC 15807 and evaluation of the components interfering with quantification. Milchwissenschaft 55:204-206. [Google Scholar]
- 65.Tuinier, R., P. Zoon, C. Olieman, M. A. Cohen-Stuart, G. J. Fleer, and C. G. De Kruif. 1999. Isolation and physical characterization of an exocellular polysaccharide. Biopolymers 49:1-9. [DOI] [PubMed] [Google Scholar]
- 66.Tuinier, R., P. Zoon, M. A. Cohen-Stuart, G. J. Fleer, and C. G. De Kruif. 1999. Concentration and shear rate dependence of the viscosity of an exocellular polysaccharide. Biopolymers 50:641-646. [DOI] [PubMed] [Google Scholar]
- 67.Tuinier, R., W. H. M. van Casteren, P. J. Looijesteijn, H. A. Schols, A. G. J. Voragen, and P. Zoon. 2001. Effects of structural modifications on some physical characteristics of exopolysaccharides from Lactococcus lactis. Biopolymers 59:160-166. [DOI] [PubMed] [Google Scholar]
- 68.Van Calsteren, M. R., C. Pau-Roblet, A. Bégin, and D. Roy. 2002. Structure determination of the exopolysaccharide produced by Lactobacillus rhamnosus strains RW-9595M and R. Biochem. J. 363:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Casteren, W. H. M., P. de Waard, C. Dijkema, H. A. Schols, and A. G. J. Voragen. 2000. Structural characterisation and enzymic modification of the exopolysaccharide produced by Lactococcus lactis subsp. cremoris B891. Carbohydr. Res. 327:411-422. [DOI] [PubMed] [Google Scholar]
- 70.van den Berg, D. J. C., A. Smits, B. Pot, A. M. Ledeboer, K. Kersters, J. M. A. Verbakel, and C. T. Verrips. 1993. Isolation, screening and identification of lactic acid bacteria from traditional food fermentation processes and culture conditions. Food Microbiol. 7:189-205. [Google Scholar]
- 71.van Geel-Schutten, G. H., F. Flesch, B. ten Brink, M. R. Smith, and L. Dijkhuizen. 1998. Screening and characterisation of Lactobacillus strains producing large amounts of exopolysaccharides. Appl. Microbiol. Biotechnol. 50:697-703. [Google Scholar]
- 72.van Kranenburg, R., H. R. Vos, I. I. van Swam, M. Kleerebezem, and W. M. de Vos. 1999. Functional analysis of glycosyltransferase genes from Lactococcus lactis and other gram-positive cocci: complementation, expression, and diversity. J. Bacteriol. 181:6347-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vincent, S. J., E. J. Faber, J.-R. Neeser, F. Stingele, and J. P. Kamerling. 2001. Structure and properties of the exopolysaccharide produced by Streptococcus macedonicus Sc 136. Glycobiology 11:131-139. [DOI] [PubMed] [Google Scholar]
- 74.Walter, R. H. 1998. Concentration regimes and mathematical modeling, p. 71-100. In R. H. Walter (ed.), Polysaccharide dispersions: chemistry and technology in food. Academic Press, London, United Kingdom.
- 75.Wood, B. J. B. 1997. Microbiology of fermented foods. Blackie Academic & Professional, London, United Kingdom.
- 76.Yamamoto, Y., S. Murosaki, R. Yamauchi, K. Kato, and Y. Sone. 1994. Structural study on an exocellular polysaccharide produced by Lactobacillus helveticus TY1-2. Carbohydr. Res. 261:67-78. [DOI] [PubMed] [Google Scholar]
- 77.Yang, Z., E. Huttunen, M. Staaf, G. Widmalm, and H. Tenhu. 1999. Separation, purification and characterisation of extracellular polysaccharides produced by slime-forming Lactococcus lactis subsp. cremoris strains. Int. Dairy J. 9:631-638. [Google Scholar]
- 78.Yang, Z., M. Staaf, E. Huttunen, and G. Widmalm. 2000. Structure of a viscous exopolysaccharide produced by Lactobacillus helveticus K16. Carbohydr. Res. 329:465-469. [DOI] [PubMed] [Google Scholar]
- 79.Yu, W., K. Gillies, J. K. Kondo, J. R. Broadbent, and L. L. McKay. 1996. Loss of plasmid-mediated oligopeptide transport system in lactococci: another reason for slow milk coagulation. Plasmid 35:145-155. [DOI] [PubMed] [Google Scholar]