Abstract
Context
Cognitive impairment commonly affects cancer patients.
Objectives
To examine whether minor cognitive impairment in patients with advanced cancer is associated with the intensity of end-of-life (EOL) care or modifies the influence of patient and caregiver preferences on the intensiveness of EOL care.
Methods
Data were derived from structured interviews with 221 advanced cancer patient-caregiver dyads in the Coping with Cancer Study, a multi-site, longitudinal cohort study. Deficits in patients’ cognitive function were identified using the Short Portable Mental Status Questionnaire (SPMSQ). Patients and caregivers reported preferences regarding life-extending versus symptom-directed care. Information regarding EOL care was obtained from post-mortem interviews with caregivers. Logistic regression analyses modeled main and interactive effects of patients’ cognitive impairment and patients’ and caregivers’ treatment preferences on intensive EOL care.
Results
Cognitive impairment was associated with less intensive EOL care (OR=0.56; 95% CI, 0.34–0.91). Patients and caregivers had poor agreement regarding preferences for lifeextending versus symptom-directed care (Φ=0.10; χ2=2.32, df=1, P=0.13). Patient preference for life-extending care predicted intensive EOL care irrespective of cognitive status (AOR=2.11; 95% CI, 1.04–4.28). For patients with no errors on the SPMSQ, caregiver preference for life-extending care was unrelated to intensive EOL care (AOR=0.40; 95% CI, 0.09–1.77). However, the association between caregiver preference for life-extending care and intensive EOL care increased by nearly a factor of seven for every error on the SPMSQ (interaction AOR=6.90; 95% CI, 1.40–34.12).
Conclusion
Cognitive impairment in patients with advanced cancer is associated with less intensive EOL care. Caregivers’ influence on intensive EOL care dramatically increases with minor declines in patients’ cognitive function.
Keywords: Cancer, terminal illness, end of life, cognitive impairment, treatment preferences, caregiver
Introduction
Cognitive impairment refers to dysfunction in thought that may involve problems with memory, concentration, language, reasoning, the formulation of ideas, or judgment.1,2 Studies indicate that 25% to 50% of terminal cancer patients are cognitively impaired upon admission to hospitals and hospices, and up to 90% develop delirium in their final days.2–4 Cognitive impairment is an independent predictor of mortality in cancer patients5,6 and may complicate clinical decision making as patients lose their ability to communicate effectively with family and health care providers. When cognitive impairment hinders decision-making capacity, family members often make treatment decisions on the patient’s behalf.7 Research to date has focused on end-of-life (EOL) decision making in the setting of dementia and delirium.8,9 However, less severe cognitive impairment that frequently accompanies late-stage cancer often goes unrecognized by physicians.10 The impact of such common yet subtle degrees of cognitive impairment on decisions and care at the EOL has remained largely unexplored.
Specifically, little is known about the impact of cognitive impairment on the intensiveness of EOL care received by cancer patients. One study from the early 1990s found that cognitively impaired patients, a subset of whom were cancer patients, received less intensive medical interventions at the EOL.11 Consistent with those results, we expect that cognitively impaired cancer patients, their families, and physicians would recognize the patients’ deteriorating health and more readily appreciate the futility of life-prolonging efforts, resulting in less intensive EOL care.
Research also shows that patients’ preferences significantly influence EOL care,12–14 particularly when patients are cognitively aware of their terminal prognosis.15 However, EOL care decisions often involve patients who are cognitively impaired and caregivers who are surrogate decision makers.16 Little is known about how cognitive impairment, particularly minor deficits, affects the impact of patient and caregiver treatment preferences on EOL care. Advanced cancer patients with impaired cognition may communicate their preferences less effectively, and their preferences may be discounted by family or clinicians. Consequently, caregivers may be more assertive of their EOL care preferences even when the patient has subtle cognitive deficits.
The present study examines whether minor cognitive impairment in otherwise cognitively intact patients with advanced cancer is associated with the intensity of EOL care received. We also investigate the extent to which patient cognitive impairment modifies the influence of patient and caregiver preferences on the intensiveness of EOL care. We hypothesize that patients with more cognitive deficits would receive less intensive EOL care. We also hypothesize that with increasing levels of cognitive impairment, patients' preferences for life-extending care would be less predictive of intensive EOL care, and caregivers' preferences would become more influential.
Methods
Sample
Study participants were recruited as part of the Coping with Cancer (CwC) study, a prospective, multi-institutional cohort study of advanced cancer patients and their caregivers funded by the National Cancer Institute and the National Institute of Mental Health.17 Participants were recruited between September 2002 and February 2008 at six comprehensive cancer centers across the U.S.: Yale Cancer Center (New Haven, CT), Veterans Affairs Connecticut Healthcare System Comprehensive Cancer Clinics (West Haven, CT), Parkland Hospital (Dallas, TX), Simmons Comprehensive Cancer Center (Dallas, TX), Dana-Farber Cancer Institute (Boston, MA), and New Hampshire Oncology-Hematology (Hookset, NH). The review boards of all participating institutions approved the study procedures, and all participants provided written, informed consent. Criteria for patient eligibility included diagnosis of advanced cancer (presence of distant metastases and disease refractory to first-line chemotherapy); age 20 years or older; availability of an informal caregiver willing to participate in the study; adequate stamina to complete the interview; and fluency in English or Spanish. Patients with readily apparent cognitive impairment (e.g., dementia/delirium), based on the evaluations of trained interviewers and clinicians, were excluded from the CwC survey because their responses to the detailed self-report measures used in the survey were considered unlikely to be reliable or valid. Of 939 eligible patients, 661 (70.4%) participated in the survey. Common reasons for non-participation were “not interested” (n=106), “caregiver refuses” (n=32), and “too upset” (n=21). Patient participants and non-participants did not differ significantly in age, gender, race/ethnicity, and education.
The sample for the present study was restricted to deceased patient participants with complete baseline assessments of the patient’s mental status, both the patient’s and caregiver’s preferences for life-extending versus symptom-directed care, and data on EOL care, i.e., to 221 (57.6%) of 384 patients deceased by the close of the study. Deceased patients included in the present study (n=221) did not differ significantly from those excluded (n=163) with respect to age, gender, race/ethnicity, and education.
Measures
Sociodemographic and Health Status Characteristics
Patient-caregiver dyads provided information regarding age (years), gender (male or female), race/ethnicity (White, Black, Asian or Pacific Islander, Hispanic, or Other), education (years), marital status (married – yes or no), primary cancer diagnosis, health insurance status (yes or no), and relationship with each other (spouse or partner, child, sibling, parent, other relative, friend, or other). Patient's functional status was determined by the Karnofsky Performance Status score as assessed by a trained interviewer or physician.18 The Charlson Comorbidity Index evaluated the number and severity of the patient’s comorbid illnesses.19 Patient survival represented the number of days between baseline interview and date of death.
Patient Cognitive Impairment
At the baseline interview, patients completed the Short Portable Mental Status Questionnaire (SPMSQ), a screen for cognitive impairment in elderly and cancer patients.20–22 The SPMSQ includes 10 questions such as “What is your telephone number?” and “What is the name of this place?” The SPMSQ was selected because it was designed to be easily administered by clinicians in clinical settings, has been validated among ethnically diverse samples of elderly subjects with a variety of health impairments, and provides scoring directions that take into account limited formal education.20 Specifically, one more error on the test is allowed in the scoring if a respondent has had a grade school education or less and one less error is allowed if the respondent has had education beyond the high school level. These were important considerations in our patient sample. The number of errors on the SPMSQ typically classifies patients as having no cognitive impairment (0–2 errors), mild impairment (3–4 errors), moderate impairment (5–7 errors), and severe impairment (8–10 errors). Because patients with obvious signs of cognitive impairment were excluded from the CwC study, the vast majority (95.5%) of patients in the present study had no more than two errors and would be considered cognitively intact. The remaining minority (4.5%) had 3–4 errors, indicating mild cognitive impairment (MCI). Mild cognitive impairment is a heterogeneous clinical syndrome with various proposed definitions, including amnestic and nonamnestic subtypes. A single consensus definition for MCI is, therefore, lacking, and our clinical judgment is that making more than one error on the relatively basic cognitive screening questions in the SPMSQ reflects subtle but meaningful deficits in awareness. In the present study, we used the number of SPMSQ errors to characterize the level of cognitive impairment into four groups (no errors, one error, two errors, and three to four errors).
Patient and Caregiver Preferences for Life-Extending Care
At the baseline interview, patients were administered a questionnaire14,23,24 that asked "If you could choose, would you prefer: 1) a course of treatment that focused on extending life as much as possible, even if it meant more pain and discomfort, or 2) a plan of care that focused on relieving pain and discomfort as much as possible, even if that meant not living as long?" Caregivers were asked this same question regarding the course of treatment that they would prefer for the patient. Participants were asked to respond with one of the following: “Extend life as much as possible,” “Relieve pain or discomfort as much as possible,” or “Don't know.” Responses were used to construct indicators of preference for life-extending care. There were no “don’t know” responses among patients and 11 “don’t know” responses among caregivers, which were coded as not wanting life-extending care.
Intensive End-of-Life Care
Information regarding the type and location of treatments during the last week of life, including treatments previously demonstrated as indicators of intensive care,25,26 were obtained via post-mortem chart reviews and interviews with caregivers within three weeks of the patient’s death. For this study, intensive EOL care was defined as care in an intensive care unit, or use of mechanical ventilation, chemotherapy, tube feeding, or cardiopulmonary resuscitation in the last week of life.
Statistical Analyses
Means, standard deviations (SDs), and frequencies were used to describe patient and caregiver baseline characteristics. Median survival in days also was used to assess survival time. Associations between participant characteristics and patient cognitive impairment were assessed in terms of odds ratios (ORs) derived from ordinal logistic regression. Chi-square (χ2) tests and phi coefficients (Φ) were used to determine the degree of association between patient and caregiver preferences for life-extending care. Associations between participant characteristics and patient and caregiver preferences for life-extending care and patient receipt of intensive EOL care were evaluated as ORs estimated using logistic regression. Main and interactive effects of patients’ level of cognitive impairment and patients’ and caregivers’ preferences for life-extending care on patient receipt of intensive EOL care were evaluated using logistic regression models. The interaction terms were estimated to determine whether the influence of patients’ and caregivers’ preferences regarding life-extending care on the level of intensive EOL care actually received by the patients varied by the cognitive status of the patient. Baseline factors that significantly predicted intensive EOL care were considered for inclusion in the adjusted analyses. However, given that no significant confounds emerged (i.e., variables P<0.05 associated with both independent and dependent variables), none were included for adjustment. All analyses were performed using the SAS statistical package, version 9.1 (SAS Institute Inc., Cary, NC).
Results
Participant Characteristics
Baseline characteristics for patients and caregivers are presented in Tables 1 and 2, respectively. Baseline evaluations were conducted a median of 104 (range 1–868; mean [SD] 159 [161] days prior to the patient’s death. A majority (62.9%) of patients committed no errors on the SPMSQ, 24.0% committed one error, 8.6% committed two errors, and 4.5% had three to four errors and would be considered mildly cognitively impaired. Fifty-seven (25.8%) patients and 20 (9.1%) caregivers indicated a preference for life-extending care. Only 3.6% of patient-caregiver dyads shared a preference for life-extending care. Patients and caregivers showed low rates of agreement over preferences for life-extending versus symptom-directed care (Φ=0.10; χ2=2.32, df=1, P=0.13). Fifty (22.6%) patients ultimately received intensive EOL care, including 18 (31.6%) of the 57 patients who indicated a preference for life-extending care.
Table 1.
Patient Characteristics (N=221)
| Patient Characteristic | n | % |
|---|---|---|
| Age in years, mean (SD)a | 59.5 | (12.4) |
| Gender, male | 119 | (53.9) |
| Race/ethnicity | ||
| White | 138 | (62.4) |
| Black | 46 | (20.8) |
| Hispanic | 33 | (14.9) |
| Other | 4 | (1.8) |
| Education in years, mean (SD) | 12.4 | (3.8) |
| Marital Status, Married | 118 | (53.9) |
| Health Insurance | 122 | (56.5) |
| Recruitment Site | ||
| Yale Cancer Center | 23 | (10.5) |
| West Haven VA Cancer Center | 4 | (1.8) |
| Simmons Comprehensive Cancer Center | 30 | (13.6) |
| Parkland Hospital | 96 | (43.6) |
| Partners (DFCI, MGH) Cancer Centers | 6 | (2.7) |
| New Hampshire Oncology-Hematology | 61 | (27.7) |
| Cancer Diagnosis | ||
| Lung | 51 | (23.3) |
| Colon | 28 | (12.8) |
| Pancreatic | 16 | (7.3) |
| Other Gastrointestinal | 33 | (15.1) |
| Breast | 28 | (12.8) |
| Other | 63 | (28.8) |
| Karnofsky Performance Status score, mean (SD) | 63.7 | (14.0) |
| Charlson Comorbidity Index, mean (SD) | 8.7 | (2.8) |
| Survival in days, median (range) | 104 | (1 – 868) |
| Cognitive Impairment | ||
| No errors | 139 | (62.9) |
| 1 error | 53 | (24.0) |
| 2 errors | 19 | (8.6) |
| 3–4 errors (mild cognitive impairment) | 10 | (4.5) |
| Preference for life-extending EOL care | 57 | (25.8) |
| Intensive EOL care | 50 | (22.6) |
Variables with missing data: marital status (n=2), health insurance (n=5), recruitment site (n=1), cancer diagnosis (n=2), Karnofsky (n=2), Charlson (n=4), survival (n=6).
Patients’ age range: 23 to 93 years; education range: 0 to 21 years; Karnofsky Performance Score range: 20 to 100; Charlson Comorbidity Index range: 2 to 17.
Table 2.
Caregiver Characteristics (N=221)
| Caregiver Characteristic | n | % |
|---|---|---|
| Age in years, mean (SD) | 51.4 | (14.1) |
| Gender, male | 54 | (24.4) |
| Race/ethnicity | ||
| White | 134 | (61.2) |
| Black | 46 | (21.0) |
| Hispanic | 33 | (15.1) |
| Other | 6 | (2.7) |
| Education in years, mean (SD) | 13.4 | (3.2) |
| Relationship to Patient | ||
| Spouse/partner | 98 | (47.8) |
| Child | 54 | (26.3) |
| Other | 53 | (25.9) |
| Preference for life-extending EOL care | 20 | (9.1) |
Variables with missing data: race/ethnicity (n=2), relationship to patient (n=16). Caregivers’ age range: 20 to 83 years; education range: 2 to 21 years.
Table 3 presents bivariate associations between patient and caregiver baseline characteristics and the primary study variables – patient cognitive impairment, patient and caregiver preferences for life-extending care, and intensive EOL care. Greater cognitive impairment was associated with patients who were older, racial/ethnic minorities, less educated, unmarried, uninsured, recruited at Parkland Hospital, and who had non-White caregivers, and caregivers who were not a spouse/partner. Patients who preferred life-extending care were more likely to be younger, male, recruited at Yale Cancer Center/West Haven VA Cancer Center, and more likely to have caregivers who were Black. Caregivers who preferred life-extending care were more likely to be racial/ethnic minorities other than Black or Hispanic and more likely to care for patients who were younger, racial/ethnic minorities other than Black or Hispanic, and less burdened by co-morbidities. Patients’ level of cognitive impairment was unrelated to patients’ survival from baseline and patients’ or caregivers’ preferences for life-extending care. Overall, increased levels of cognitive impairment (i.e., increasing errors on the SPMSQ) were associated with less intensive EOL care (OR=0.56; 95% confidence interval [CI], 0.34–0.91; P=0.02).
Table 3.
Bivariate Associations Between Patient and Caregiver Characteristics and Primary Study Variables (N=221) a
| Patient Cognitive Impairment |
Patient Preference for Life-Extending Care |
Caregiver Preference for Life-Extending Care |
Intensive EOL Care | |||||
|---|---|---|---|---|---|---|---|---|
| Patient Characteristic | OR | P | OR | P | OR | P | OR | P |
| Age in years | 1.04 | 0.001 | 0.98 | 0.04 | 0.94 | 0.001 | 0.99 | 0.41 |
| Gender, male | 1.35 | 0.28 | 2.28 | 0.01 | 1.05 | 0.91 | 1.12 | 0.73 |
| Race/ethnicity | ||||||||
| White | ref. | ref. | ref. | ref. | ||||
| Black | 2.15 | 0.03 | 2.02 | 0.06 | 1.22 | 0.75 | 1.17 | 0.69 |
| Hispanic | 6.67 | <0.001 | 1.11 | 0.83 | 1.77 | 0.36 | 0.59 | 0.32 |
| Other | 5.41 | 0.05 | 1.15 | 0.91 | 12.80 | 0.02 | 1.10 | 0.93 |
| Education in years | 0.81 | <0.001 | 0.99 | 0.78 | 1.02 | 0.80 | 1.04 | 0.40 |
| Married | 0.55 | 0.03 | 1.03 | 0.93 | 1.66 | 0.30 | 1.38 | 0.32 |
| Health Insurance | 0.33 | <0.001 | 1.34 | 0.36 | 0.94 | 0.89 | 1.15 | 0.67 |
| Recruitment Site | ||||||||
| Yale CC/West Haven VA CC | 0.41 | 0.05 | 2.54 | 0.04 | 2.46 | 0.12 | 1.16 | 0.78 |
| Simmons Comprehensive CC | 0.19 | 0.002 | 1.15 | 0.76 | 0.61 | 0.55 | 1.23 | 0.68 |
| Parkland Hospital | ref. | ref. | ref. | ref. | ||||
| Partners & NHOH | 0.46 | 0.02 | 0.84 | 0.65 | 0.27 | 0.09 | 1.49 | 0.29 |
| Cancer Diagnosis | ||||||||
| Lung | 1.03 | 0.93 | 1.46 | 0.37 | 0.43 | 0.23 | 1.32 | 0.54 |
| Colon | 0.36 | 0.07 | 1.07 | 0.90 | 0.26 | 0.21 | 1.54 | 0.41 |
| Pancreatic | 1.11 | 0.85 | 0.74 | 0.67 | 0.98 | 0.98 | 0.89 | 0.87 |
| Other Gastrointestinal | 1.37 | 0.46 | 1.83 | 0.20 | 0.69 | 0.60 | 0.69 | 0.52 |
| Breast | 0.55 | 0.23 | 0.53 | 0.31 | 0.83 | 0.79 | 1.54 | 0.41 |
| Other | ref. | ref. | ref. | ref. | ||||
| Karnofsky Performance Status score | 0.98 | 0.11 | 0.99 | 0.52 | 1.00 | 0.96 | 0.99 | 0.62 |
| Charlson Comorbidity Index | 1.04 | 0.47 | 0.96 | 0.50 | 0.83 | 0.05 | 0.99 | 0.80 |
| Survival in days | 1.00 | 0.60 | 1.00 | 0.27 | 1.00 | 0.18 | 1.00 | 0.15 |
| Patient Cognitive Impairment | ref. | ref. | 0.99 | 0.97 | 0.84 | 0.58 | 0.56 | 0.02 |
| Age in years | 1.00 | 0.94 | 0.99 | 0.41 | 0.97 | 0.07 | 1.02 | 0.12 |
| Gender, male | 1.49 | 0.22 | 1.13 | 0.74 | 0.97 | 0.95 | 1.19 | 0.65 |
| Race/ethnicity | ||||||||
| White | ref. | ref. | ref. | ref. | ||||
| Black | 2.06 | 0.04 | 2.43 | 0.02 | 1.32 | 0.66 | 1.37 | 0.42 |
| Hispanic | 6.31 | <0.001 | 1.21 | 0.68 | 1.92 | 0.31 | 0.62 | 0.36 |
| Other | 1.44 | 0.68 | 3.79 | 0.12 | 13.89 | 0.003 | 1.73 | 0.54 |
| Education in years | 0.88 | 0.003 | 1.01 | 0.82 | 0.90 | 0.15 | 1.01 | 0.87 |
| Relationship to Patient | ||||||||
| Spouse/partner | ref. | ref. | ref. | ref. | ||||
| Child | 2.04 | 0.04 | 0.71 | 0.40 | 0.38 | 0.23 | 0.57 | 0.21 |
| Other (Sibling, other relative, or friend) | 1.90 | 0.06 | 0.99 | 0.99 | 1.26 | 0.68 | 1.17 | 0.69 |
All P-values are based on the Wald Chi-square statistic with 1 degree of freedom.
Effects of Cognitive Impairment and Treatment Preferences on Intensive EOL Care
Table 4 presents the results of two logistic regression models estimating the main and interactive effects of patients’ cognitive impairment and patients’ and caregivers’ preferences for life-extending care on patients’ receipt of intensive EOL care. Model 1 includes interactions between patients’ cognitive impairment and patients’ and caregivers’ preferences for life-extending care. Here, patients’ cognitive impairment modifies the association between caregivers’ preference for life-extending care and patients’ receipt of intensive EOL care (OR=6.55; 95% CI, 1.32–32.50; P=0.02) but does not modify that of patients’ preference for life-extending care and patients’ receipt of intensive EOL care (OR=0.40; 95% CI, 0.09–1.75; P=0.22). Based on this result, and to facilitate data interpretation, Model 2 was fit without an interaction between patients’ cognitive impairment and patients’ preference for life-extending care.
Table 4.
Effects of Patient Cognitive Impairment and Patient and Caregiver Treatment Preferences on Intensive EOL Care (N=221)a
| Model 1 |
Model 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Regressor | β | SE | OR | P | β | SE | OR | P |
| Patient Cognitive Impairment (CI) | −0.69 | 0.35 | 0.50 | 0.05 | −0.81 | 0.30 | 0.45 | 0.007 |
| Patient preference for life extending EOL care | 0.86 | 0.41 | 2.36 | 0.04 | 0.74 | 0.36 | 2.11 | 0.04 |
| CI × patient preference interaction | −0.92 | 0.76 | 0.40 | 0.22 | --- | --- | --- | --- |
| Caregiver preference for life extending EOL care | −0.36 | 0.622 | 0.70 | 0.56 | −0.92 | 0.76 | 0.40 | 0.23 |
| CI × caregiver preference interaction | 1.88 | 0.82 | 6.55 | 0.02 | 1.93 | 0.82 | 6.90 | 0.02 |
All P-values are based on the Wald Chi-square statistic with 1 degree of freedom.
Model 2 indicates that increasing cognitive impairment was associated with less intensive EOL care when caregivers did not prefer life-extending care (adjusted OR [AOR]=0.45; 95% CI, 0.25–0.80; P=0.007). Patient preference for life-extending care predicted intensive EOL care regardless of their cognitive status (AOR=2.11; 95% CI, 1.04–4.28; P=0.04). Caregiver preference for life-extending care was not associated with intensive EOL care in patients who had no errors on the SPMSQ (AOR=0.40; 95% CI, 0.09–1.77; P=0.23). However, the association between caregiver preference for life-extending care and intensive EOL care increased by nearly a factor of seven for each additional error on the SPMSQ (interaction AOR=6.90; 95% CI, 1.40–34.12; P=0.02). Importantly, caregiver preference for life-extending care significantly predicted intensive EOL care for patients with two or more errors (AOR=19.05; 95% CI, 1.52–238.13; P=0.02).
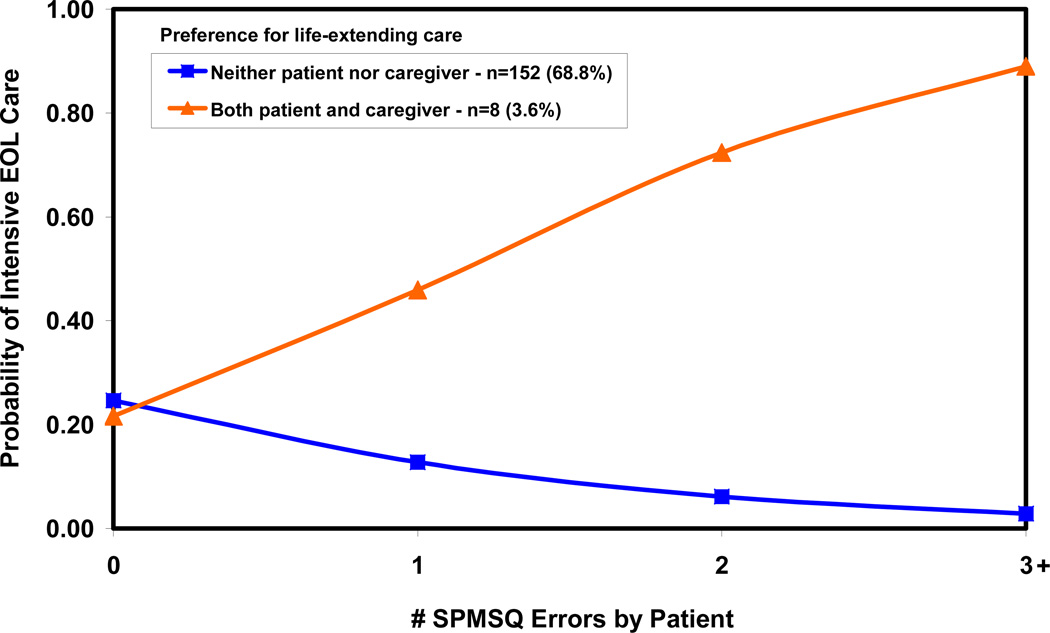
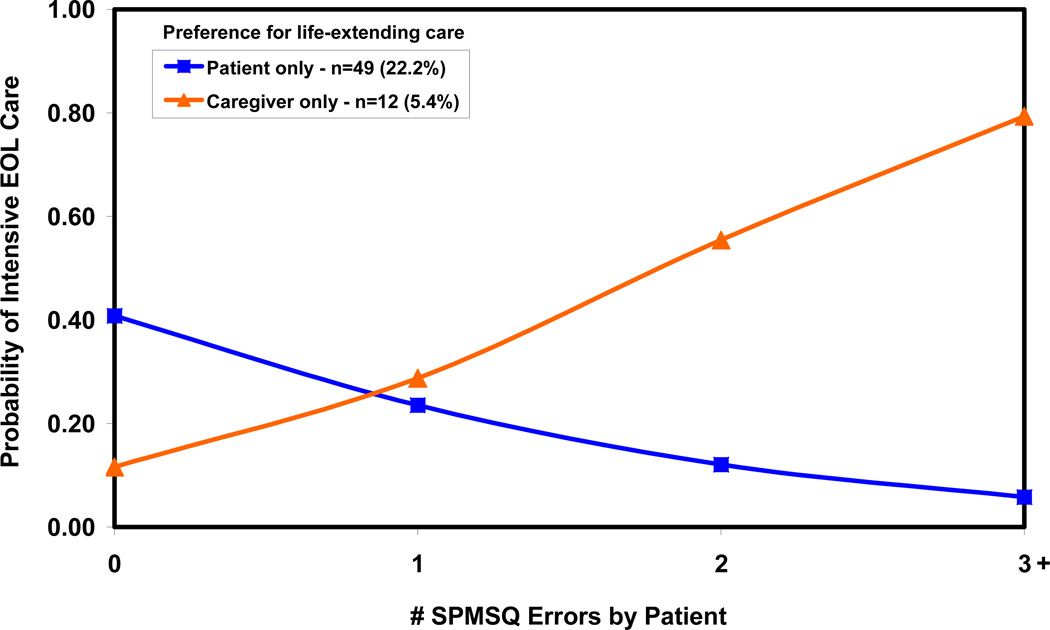
Figures 1 and 2 illustrate the effects of patient cognitive impairment and patient and caregiver preferences for life-extending care on intensive EOL care based on Model 2. When patient and caregiver preferences agree (Fig. 1), common preference for either life-extending or symptom-directed care has little effect on the intensity of EOL care when patients have zero/no cognitive impairment, but matters increasingly more with increasing cognitive impairment. In a majority (68.8%) of cases when neither patients nor caregivers prefer life-extending care, patients with higher levels of cognitive impairment are less likely to receive intensive EOL care. In a small minority (3.6%) of cases when patients and caregivers both prefer life-extending care, patients with higher levels of cognitive impairment are more likely to receive intensive EOL care. When patient-caregiver dyads disagree on preferences for life-extending care (Fig. 2), increasing cognitive impairment is associated with less intensive EOL care in the minority (22.2%) of patients who prefer life-extending care. However, increasing cognitive impairment is associated with more intensive EOL care in the smaller minority (5.4%) of patients who preferred symptom-directed care, but whose caregivers preferred life-extending care.
Figure 1.
Probability of intensive EOL care: patient and caregiver agreement on preferences.
Figure 2.
Probability of intensive EOL care: patient and caregiver disagreement on preferences
Discussion
Decision making at the EOL often poses a tremendous challenge for patients and their families, particularly when patients develop cognitive impairment with the progression of terminal illness. The results of this study suggest that subtle levels of cognitive impairment months prior to death may shift the influence of EOL decision making away from patients and toward caregivers. Consistent with previous studies,12 patient preference for life-extending care predicted intensive EOL care regardless of cognitive status. In contrast, caregiver influence on EOL care increased dramatically with only minor deterioration in patients’ cognitive status, increasing by nearly a factor of seven for each additional SPMSQ error made by the patient. Importantly, caregivers’ preferences significantly predicted the intensiveness of EOL care in patients with two or more errors. Therefore, whereas only patients’ preferences predict EOL care when they are cognitively intact, both patients’ and caregivers’ preferences may influence the care of patients with mild levels of cognitive impairment.
Overall, patients with greater degrees of cognitive impairment also received less aggressive care. This likely reflects our finding that EOL care of cognitively impaired patients is strongly influenced by caregivers, over 90% of who preferred symptom-directed care. Caregivers of cognitively impaired patients with advanced cancer may be more able to comprehend the patient’s poor prognosis and recognize the futility of intensive EOL care. Similarly, although we did not assess for delirium at the EOL, it is likely that caregivers also would recognize EOL delirium as an indicator of the patient’s poor prognosis and failing health and, therefore, more readily appreciate the futility of attempts to prolong the life of a patient in terminal decline. Cognitive impairment may be a side effect of treatment (e.g., pain medication) or a symptom of other forms of physical dysfunction or disease aside from global physical functional status, which we have not accounted for in our models, but may nevertheless predispose caregivers to want comfort care for the patient.
Despite receiving less intensive EOL care, patients with greater cognitive impairment at baseline had similar survival times. This contrasts with prior studies that suggest cognitive impairment is associated with increased mortality in cancer patients.5,6 However, the prior studies examined patients with more severe cognitive impairment than that noted in the relatively intact sample studied here.
The present findings extend prior research showing that surrogate decision makers often inaccurately predict patients' EOL treatment preferences and have a tendency to project their own preferences upon patients.27–31 According to a systematic review, surrogates incorrectly predicted patients' treatment preferences in 32% of overall cases and were least accurate in cases of dementia.32 Our results show similar levels of disagreement in treatment preferences between patients and their caregivers, with caregivers significantly influencing EOL care when patients show just subtle signs of cognitive impairment.
Oddly, we found that when patient-caregiver dyads agree on preferences for EOL care and patients have no indication of cognitive impairment, intensive EOL care is equally likely whether patients and caregivers jointly prefer life-extending care or not. Receipt of intensive EOL care is increasingly more consistent with their common preferences as the level of patients’ cognitive impairment increases (Fig. 1). One plausible explanation may be that patients and/or caregivers are more likely to communicate their shared treatment preferences to the health care team when patients are more cognitively impaired.
We note a number of limitations to our study and acknowledge that our findings should be considered preliminary as a result. First, in order to ensure that patients provided reliable responses to the detailed self-report measures of the parent survey, those who met criteria for dementia or delirium at baseline were excluded from participation. Therefore, the study examines the effects of relatively minor levels of cognitive impairment in a subset of patients who are otherwise cognitively intact at screening and prevents an analysis of the effects of more severe levels of cognitive impairment, including dementia and delirium. Second, prior studies show that deficits in memory and executive function correlate with impaired decisional capacity.33,34 Because the SPMSQ does not assess verbal memory or executive function and is traditionally used to screen for more substantial levels of cognitive impairment, our measure of impairment may be a crude indication of more specific and subtle deficits. Despite the subtlety of these deficits, we nonetheless found the influence of caregivers’ preferences increased significantly with each error on the patient’s SPMSQ, highlighting the important impact of such minor deficits. However, we recognize the limitations to our assessment of cognitive status in a sample of otherwise cognitively intact patients. Future investigators should employ more extensive assessments of cognitive status with measures that capture finer gradations and other forms of cognitive impairment and also assess whether physicians, caregivers, or the patients themselves were cognizant of any cognitive impairment. Future research is needed to confirm our findings in samples that include a broader spectrum of cognitive impairment.
Another limitation involves our dichotomized measure of EOL preferences, which allowed for only one of two opposing options – pain relief versus life-extending care. This measure was designed to provide a simplified assessment of palliative versus intensive care within the greater schema of the CwC study. Nevertheless, we recognize that EOL preferences involve numerous facets of care beyond pain relief and extension of life, and that the two are not mutually exclusive. Additional work may utilize a more detailed assessment of EOL preferences, including preferences regarding specific interventions.
Information regarding cognitive status and treatment preferences were determined during a single baseline interview conducted a median of 104 days prior to death. This study thus lacks the longitudinal data to characterize changes in cognitive status and treatment preferences with progression of illness. Previous research shows that cognitive impairment in terminal cancer patients tends to worsen with time, albeit a small proportion of patients improved.3 Similarly, prior studies have identified small but significant changes in patients' treatment preferences as their health declined.35 Longitudinal data are needed to examine how changes in patient cognitive status during the course of illness affect treatment preferences and care. Finally, participants reported a single category for race/ethnicity, and specific distinctions between race and ethnicity were not recorded.
The present study nevertheless has numerous strengths, including recruitment from multiple sites in the Northeast and Southwest U.S., an ethnically/racially diverse sample of patients and caregivers, use of formal assessments of cognitive status and EOL treatment preferences, and prospective data regarding intensive EOL care. To our knowledge, this is the first study to examine advanced cancer patients’ cognitive impairment and both patients’ and caregivers’ EOL treatment preferences as predictors of the intensiveness of EOL care.
Cognitive impairment is extremely common among patients with advanced cancer, and minor impairment may be easily missed or dismissed by physicians. Our findings suggest that mild forms of cognitive impairment may alter the dynamics of EOL decision making by promoting the role of caregivers. As a result, physicians should be more aware of, sensitive to, and probe for possible cognitive impairment in advanced cancer patients, even in cases when cognitive impairment is not obvious, and strive to ensure that all patients’ treatment preferences are sought and respected. Overall, these results suggest that patients, families, and health care providers may want to discuss and document the patient’s EOL treatment preferences early in the disease trajectory, before cognitive impairment complicates decision making. Communication with patients earlier in the course of illness will help to ensure that patients’ EOL treatment preferences are acknowledged and honored.
Acknowledgments
This research was supported in part by the following grants to Dr. Prigerson: MH63892 from the National Institute of Mental Health and CA106370 and CA156732 from the National Cancer Institute; and the Center for Psycho-Oncology and Palliative Care Research, Dana-Farber Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no potential conflicts of interest or financial disclosures. All authors have read and approved the manuscript.
References
- 1.Massie MJ, Holland J, Glass E. Delirium in terminally ill cancer patients. Am J Psychiatry. 1983;140:1048–1050. doi: 10.1176/ajp.140.8.1048. [DOI] [PubMed] [Google Scholar]
- 2.Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000;160:786–794. doi: 10.1001/archinte.160.6.786. [DOI] [PubMed] [Google Scholar]
- 3.Pereira J, Hanson J, Bruera E. The frequency and clinical course of cognitive impairment in patients with terminal cancer. Cancer. 1997;79:835–842. [PubMed] [Google Scholar]
- 4.Centeno C, Sanz A, Bruera E. Delirium in advanced cancer patients. Palliat Med. 2004;18:184–194. doi: 10.1191/0269216304pm879oa. [DOI] [PubMed] [Google Scholar]
- 5.Klein M, Postma TJ, Taphoorn MJ, et al. The prognostic value of cognitive functioning in the survival of patients with high-grade glioma. Neurology. 2003;61:1796–1798. doi: 10.1212/01.wnl.0000098892.33018.4c. [DOI] [PubMed] [Google Scholar]
- 6.Robb C, Boulware D, Overcash J, Extermann M. Patterns of care and survival in cancer patients with cognitive impairment. Crit Rev Oncol Hematol. 2010;74:218–224. doi: 10.1016/j.critrevonc.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Emanuel EJ, Emanuel LL. Proxy decision making for incompetent patients. An ethical and empirical analysis. JAMA. 1992;267:2067–2071. [PubMed] [Google Scholar]
- 8.Potkins D, Bradley S, Shrimanker J, et al. End of life treatment decisions in people with dementia: carers' views and the factors which influence them. Int J Geriatr Psychiatry. 2000;15:1005–1008. doi: 10.1002/1099-1166(200011)15:11<1005::aid-gps223>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Livingston G, Leavey G, Manela M, et al. Making decisions for people with dementia who lack capacity: qualitative study of family carers in UK. BMJ. 2010;341:c4184. doi: 10.1136/bmj.c4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chodosh J, Petitti DB, Elliott M, et al. Physician recognition of cognitive impairment: evaluating the need for improvement. J Am Geriatr Soc. 2004;52:1051–1059. doi: 10.1111/j.1532-5415.2004.52301.x. [DOI] [PubMed] [Google Scholar]
- 11.Hanson LC, Danis M, Mutran E, Keenan NL. Impact of patient incompetence on decisions to use or withhold life-sustaining treatment. Am J Med. 1994;97:235–241. doi: 10.1016/0002-9343(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 12.Wright AA, Mack JW, Kritek PA, et al. Influence of patients' preferences and treatment site on cancer patients' end-of-life care. Cancer. 2010;116:4656–4663. doi: 10.1002/cncr.25217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weeks JC, Cook EF, O'Day SJ, et al. Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709–1714. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 14.Danis M, Mutran E, Garrett JM, et al. A prospective study of the impact of patient preferences on life-sustaining treatment and hospital cost. Crit Care Med. 1996;24:1811–1817. doi: 10.1097/00003246-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Mack JW, Paulk ME, Viswanath K, Prigerson HG. Racial disparities in the outcomes of communication on medical care received near death. Arch Intern Med. 2010;170:1533–1540. doi: 10.1001/archinternmed.2010.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berger JT, DeRenzo EG, Schwartz J. Surrogate decision making: reconciling ethical theory and clinical practice. Ann Intern Med. 2008;149:48–53. doi: 10.7326/0003-4819-149-1-200807010-00010. [DOI] [PubMed] [Google Scholar]
- 17.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karnofsky DA. Determining the extent of the cancer and clinical planning for cure. Cancer. 1968;22:730–734. doi: 10.1002/1097-0142(196810)22:4<730::aid-cncr2820220407>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 21.Allen RS, Haley WE, Small BJ, McMillan SC. Pain reports by older hospice cancer patients and family caregivers: the role of cognitive functioning. Gerontologist. 2002;42:507–514. doi: 10.1093/geront/42.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Angelo C, Mirijello A, Leggio L, et al. State and trait anxiety and depression in patients with primary brain tumors before and after surgery: 1-year longitudinal study. J Neurosurg. 2008;108:281–286. doi: 10.3171/JNS/2008/108/2/0281. [DOI] [PubMed] [Google Scholar]
- 23.Fried TR, Bradley EH, Towle VR. Assessment of patient preferences: integrating treatments and outcomes. J Gerontol B Psychol Sci Soc Sci. 2002;57:S348–S354. doi: 10.1093/geronb/57.6.s348. [DOI] [PubMed] [Google Scholar]
- 24.The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT) JAMA. 1995;274:1591–1598. [PubMed] [Google Scholar]
- 25.Mebane EW, Oman RF, Kroonen LT, Goldstein MK. The influence of physician race, age, and gender on physician attitudes toward advance care directives and preferences for end-of-life decision-making. J Am Geriatr Soc. 1999;47:579–591. doi: 10.1111/j.1532-5415.1999.tb02573.x. [DOI] [PubMed] [Google Scholar]
- 26.Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22:315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 27.Moorman SM, Hauser RM, Carr D. Do older adults know their spouses' end-of-life treatment preferences? Res Aging. 2009;31:463–491. doi: 10.1177/0164027509333683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Layde PM, Beam CA, Broste SK, et al. Surrogates' predictions of seriously ill patients' resuscitation preferences. Arch Fam Med. 1995;4:518–523. doi: 10.1001/archfami.4.6.518. [DOI] [PubMed] [Google Scholar]
- 29.Fagerlin A, Ditto PH, Danks JH, Houts RM, Smucker WD. Projection in surrogate decisions about life-sustaining medical treatments. Health Psychol. 2001;20:166–175. [PubMed] [Google Scholar]
- 30.Marks MA, Arkes HR. Patient and surrogate disagreement in end-of-life decisions: can surrogates accurately predict patients' preferences? Med Decis Making. 2008;28:524–531. doi: 10.1177/0272989X08315244. [DOI] [PubMed] [Google Scholar]
- 31.Cosgriff JA, Pisani M, Bradley EH, O'Leary JR, Fried TR. The association between treatment preferences and trajectories of care at the end-of-life. J Gen Intern Med. 2007;22:1566–1571. doi: 10.1007/s11606-007-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shalowitz DI, Garrett-Mayer E, Wendler D. The accuracy of surrogate decision makers: a systematic review. Arch Intern Med. 2006;166:493–497. doi: 10.1001/archinte.166.5.493. [DOI] [PubMed] [Google Scholar]
- 33.Moye J, Karel MJ, Gurrera RJ, Azar AR. Neuropsychological predictors of decision-making capacity over 9 months in mild-to-moderate dementia. J Gen Intern Med. 2006;21:78–83. doi: 10.1111/j.1525-1497.2005.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okonkwo OC, Griffith HR, Copeland JN, et al. Medical decision-making capacity in mild cognitive impairment: a 3-year longitudinal study. Neurology. 2008;71:1474–1480. doi: 10.1212/01.wnl.0000334301.32358.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fried TR, Van Ness PH, Byers AL, et al. Changes in preferences for life-sustaining treatment among older persons with advanced illness. J Gen Intern Med. 2007;22:495–501. doi: 10.1007/s11606-007-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]