Abstract
Objectives
Test the association between coronary heart disease (CHD) risk scores and neighborhood socioeconomic status (NSES) in a US nationally-representative sample and describe whether any association varies by gender and race/ethnicity.
Study Design
Cross-sectional study
Methods
We use Health and Nutrition Examination Survey (NHANES) data from 1999 to 2004 linked with Census tract data. Multivariable regression models and propensity score adjusted models are employed to test the association between NSES and 10-year risk of CHD based on the Framingham Risk Score (FRS), adjusting for individual-level characteristics.
Results
An individual living in a neighborhood at the 75th percentile of NSES (high NSES) has, on average, a 10-year CHD risk that is 0.16 percentage points lower (95% Confidence Interval 0.16, 0.17) than a similar person residing in a neighborhood at the 25th percentile of NSES (low NSES). Race/ethnicity and gender were found to significantly modify the association between NSES on CHD risk: the association is larger in men than women and in whites than minorities. Propensity score models showed findings on the main effects of NSES were robust to self-selection into neighborhoods. Similar results were observed between NSES and risk of cardiovascular disease events.
Conclusions
NSES is significantly associated with CHD risk, and the relationship varies by gender and race/ethnicity.
Keywords: Coronary Disease, Residence Characteristics, Socioeconomic Factors, Social Class
INTRODUCTION
Substantial evidence to date links neighborhood characteristics, such as neighborhood socioeconomic status (NSES), with coronary heart disease (CHD) risk factors, including smoking, hypertension and cholesterol.1-3 Further, neighborhood characteristics influence the incidence of CHD even after accounting for individual-level socioeconomic status (SES) and traditional CHD risk factors.4, 5
In clinical guidelines and practice, many individual-level CHD risk factors comprise what is known as the Framingham Risk Score (FRS).6 FRS has been widely used to estimate an individual’s 10-year risk of CHD events and select an appropriate risk-reduction strategy. For example, FRS is used when determining the appropriate levels at which to treat high blood pressure,7 use aspirin for primary prevention,8, 9 and initiate lipid lowering medications.10 FRS is typically calculated using a point system based on an individual’s gender, age, total cholesterol, high density lipoprotein (HDL) cholesterol, systolic blood pressure, current tobacco smoking, and whether they are on treatment for hypertension.10 Adding SES—at either an individual- or neighborhood-level—improves accuracy of the FRS in predicting CHD outcomes among low income populations in the United States and the United Kingdom.11-14
In clinical settings, clinicians commonly use FRS to stratify individuals by level of cardiac risk when choosing treatment options. Better estimation of the association between NSES and FRS may help inform the appropriate individual- and population-level risk reduction strategies. It is unclear, however, to what extent neighborhood characteristics such as NSES are linked with FRS. To our knowledge, only one study has examined the association between NSES and predicted cardiac risk scores.15 After controlling for individual SES, Cubbin and colleagues did not find a consistent or significant pattern of association between a cardiac risk score and neighborhood deprivation in four California cities. The present study extends previous work by examining the association between NSES and 10-year CHD risk, based on FRS, using a nationally-representative sample and propensity score modeling. We further test whether any association between NSES and CHD risk varies by gender and race/ethnicity and whether results remain consistent when examining 10-year risk of cardiovascular disease (CVD) events.
The study builds on prior research which suggests a combination of social and biologic pathways through which NSES may impact CHD risk.1 For example, living in a low SES neighborhood may affect an individual’s exposure to particular stressors and constrain opportunities to pursue positive health behaviors (e.g. through decreased access to healthy foods and safe places to exercise), and it may do so in ways that have differential effects by gender and race/ethnicity.16 Repeated and chronic activation of the stress response over time is hypothesized to lead to physiologic dysregulation17,18 embodied in the concept of allostatic load which has been shown to lead to excess morbidity and mortality.19-21
METHODS
The study sample included 31,126 participants in the National Health and Nutrition Examination Survey (NHANES) (1999-2004), a nationally-representative cross-sectional sample of the U.S. population. We included subjects who participated in the interview, clinical examination, and laboratory components. NHANES data were geocoded to a census tract based on either participant’s home address or closest street intersection and then merged with Census data. The study was approved by the RAND Corporation’s Human Subject Protection Committee.
Exclusion criteria
We excluded individuals who could not be geocoded (N=24), people younger than 30 and those over age 75 (those beyond the age range for which FRS has been validated) (N=20,845), pregnant women (N=239), and those who did not undergo the physical and laboratory examination (N=566). We further excluded people with established CHD (as defined by the participant reporting having been previously told that he/she had CHD, angina/angina pectoralis, a heart attack, or a stroke, N=926), individuals who did not self-identify their race/ethnicity as either non-Hispanic white, non-Hispanic black, or Hispanic (N=293) and those who were missing control variables (N=110) other than income (see below).
We also excluded individuals with diabetes, which is considered a CHD equivalent in the 2001 National Cholesterol Education Program (NCEP).10 We classified individuals as having diabetes if they either (a) report having been told by a medical professional that they have diabetes (N = 946), or (b) have a hemoglobin A1c above 6.5% on laboratory testing (N = 351).22,23 Finally, we excluded individuals missing any of the data necessary to calculate a FRS (N=342).
Our final sample included 6484 adults (unweighted). After applying appropriate sample weights, the final sample represented 11,946 adults.
Neighborhood Socioeconomic Status (NSES)
We employed an index of NSES based on Census-tract level Census data. As described in previous studies,24-26 we used factor analysis to select six Census variables which were then summarized into an index: percentage of households with children headed only by a female caregiver, percent of male population ages 16 and older that is unemployed, percent of households receiving public assistance, percent of households with income below the poverty threshold, median household income, and percent of adults older than 25 with a high school diploma or higher education. The NSES index ranges from 0 (low) to 100 (high) for all U.S. census tracts. NSES was calculated using interpolated 1990 and 2000 Census data and the 2000 standardized census tract boundaries. Estimates were derived for intervening years using geometric or linear interpolations using the 1990/2000 Census Tract Relationship File to facilitate the interpolation.27
Ten-Year Risk of Coronary Heart Disease based on Framingham Risk Score
We calculated 10-year risk of CHD using the 2001 Framingham point system as described by the NCEP guidelines.10 Risk factors included gender, age, total cholesterol, HDL cholesterol, systolic blood pressure, tobacco smoking, and treatment for hypertension. Smoking was measured as a categorical variable indicating whether the individual had smoked more than 100 cigarettes in their lifetime and whether they currently smoked every day or some days. Blood pressure was averaged over three to four readings on a single day and treatment was defined as reporting taking antihypertensive medications. Total points based on the NCEP algorithm were summed (<0 to ≥17 among men; <9 to ≥25 among women) and converted into a gender-specific 10-year risk of CHD ranging from <1% to 30% or more.
Ten-Year Risk of Coronary Vascular Disease events
Similarly, we calculated 10-year risk of CVD events (for example, coronary heart disease, cerebrovascular disease, peripheral vascular disease, and heart failure) using the revised FRS.28 For CVD risk, patients with diabetes are retained in the analytic sample. .
Individual-level Control Variables
All regression models controlled for the following individual-level variables: age, age squared (to allow for non-linearities), gender, race/ethnicity (non-Hispanic white, non-Hispanic black, and Hispanic), nativity (whether or not an individual was US-born), marital status (married or partnered, never married, or widowed, divorced, separated or other), educational attainment (less than high school, high school graduate, or more than high school) and income to poverty ratio (IPR; categories of <1 (poorest), 1 to <2, 2 to <3, 3 to <4, 3 to <4, 4 to <5, and ≥5 (richest)). For IPR, household income was divided by the federal poverty threshold, adjusted for family size. IPR was categorized to allow for potential non-linearities in the relationship between IPR and FRS. To address missing data, we imputed IPR using a single-imputation method for all subjects with complete data on age, gender, race/ethnicity, U.S. birth, education, marital status, employment status, and neighborhood characteristics.
Statistical Analyses
We assessed the association between NSES and 10-year risk of CHD events using hierarchical linear models with tract and county random effects. We report three models: model 1 includes individual-level sociodemographic characteristics (gender, age, age squared, race/ethnicity, nativity, marital status, education, and IPR); model 2 adds NSES; and model 3 tests whether the relationship between NSES and CHD risk varies by gender and race/ethnicity by adding NSES by gender, NSES by race/ethnicity, and gender by race/ethnicity interaction terms. While age and gender are included as part of the formula for the outcome, they were controlled for in our regression models in order to examine (1) whether NSES moderated the impact of these variables on 10 year risk of CHD and (2) to increase precision of the estimated effect of NSES. We note our models also included an age-squared term since it was found in preliminary model building steps that the effect of age was not linearly related to 10 year risk of CHD. When selecting model 3, we explored all possible two-way interactions from model 2 as candidates for model 3 and only those that were found to be statistically significant were included in the final version of model 3. All results are appropriately adjusted for NHANES sample weights. All models were fit in SAS using PROC MIXED. Similar hierarchical linear models were used to examine 10-year risk of CVD events.
We assessed the sensitivity of our findings by employing propensity-score stratification methods developed for continuous treatments to account for potential selection effects on the observed individual-level control variables on NSES in our models.29 Given individuals living in different neighborhoods are likely to differ systematically by individual level control variables, it is critical to utilize robust methods for removing bias from observables on the estimated relationship between NSES and 10 year risk of CHD. Specifically, we assigned each individual in our analytic sample an estimated propensity score that equaled his/her predicted value of NSES from a linear regression model fit to NSES that controlled for gender, age, age squared, race/ethnicity, nativity, marital status, education, and IPR (i.e, the same core controls for Models 2 and 3). We then re-fit our models within three approximately equal size subclasses, defined by the predicted value of NSES and combined these results to produce our propensity score adjusted results (see Table 3). We tried a range of values for the number of subclasses (2, 3, 5, 10) to be utilized in this propensity score adjustment approach; three subclasses were selected for reporting the final results because this produced the optimal balance on the control variables within the subclasses for models 1 and 2. Despite our large sample, sufficient balance within gender and race/ethnicity categories for each of the three subclasses was not obtainable, therefore propensity score adjustment of model 3 was not warranted.
RESULTS
Table 1 presents the sociodemographic characteristics of the sample and CHD risk factors. Women composed approximately half the sample (51.6%) and the mean age was 47 years old. The median predicted risk of CHD events was 4.1%.
Table 1.
Characteristics of the study population,* NHANES 1999-2004
| N (%) | |
|---|---|
| Total | 11,946 (100) |
| Female (%) | 6,160 (51.6) |
| Age (yr), mean (SE) | 47.0 (0.2) |
| Race/ethnicity (%) | |
| Non-Hispanic White | 9,315 (78.0) |
| Non-Hispanic Black | 1,182 (9.9) |
| Hispanic | 1,448 (12.1) |
| Nativity (%) | |
| US-born | 10,390 (87.0) |
| Born outside US | 1,556 (13.0) |
| Marital status (%) | |
| Married or partner | 8,293 (69.4) |
| Never Married | 1,090 (9.1) |
| Widowed, divorced, separated or other | 2,563 (21.5) |
| Education (%) | |
| Less than High School | 2,018 (16.9) |
| High School Graduate or GED | 3,077 (25.8) |
| More than High School | 6,851 (57.3) |
| Income to Poverty Ratio (IPR) | 3.9 (0.1) |
| ratio less than 1 | 1,223 (10.2) |
| ratio >=1, < 2 | 1,966 (16.5) |
| ratio >=2, < 3 | 1,904 (15.9) |
| ratio >=3, < 4 | 1,752 (14.7) |
| ratio >=4, < 5 | 1,535 (12.9) |
| ratio >=5 | 3,566 (29.8) |
| Total cholesterol, mean (SE) | 207.5 (0.9) |
| HDL, mean (SE) | 53.0 (0.3) |
| LDL, mean (SE) | 125.7 (1.0) |
| Hypertension Control | 2,249 (18.8) |
| Systolic Blood Pressure, mean (SE) | 122.4 (0.4) |
| Diastolic Blood Pressure, mean (SE) | 74.0 (0.2) |
| Current smoker | 2,922 (24.5) |
| NSES, mean (SE) | 71.5 (0.5) |
|
Framingham Risk Score 2001, mean
(SE) |
4.1 (0.1) |
Results are weighted
Table 2 presents the results from the multivariable regression models. In model 1, having less than a high school education, lower IPR, and older age were associated with higher CHD risk than their respective baseline categories. Being Hispanic was associated with a 0.36 (95%CI 0.07, 0.65) percentage points lower CHD risk versus non-Hispanic whites, and being born outside the US was associated with lower CHD risk than those born in the U.S. On average, men had a 5.09 (95%CI 4.83, 5.35) percentage point higher 10-year risk of CHD than did women.
Table 2.
Hierarchical linear regression models, adjusted for county- and tract-level effects, with the percentage point difference in coronary heart disease risk calculated using the Framingham Risk Score as the outcome variable, NHANES 1999-2004
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Characteristic | Coef | 95% CI | Coef | 95% CI | Coef | 95% CI |
| Gender | ||||||
| Male | 5.09 | (4.83, 5.35) | 5.09 | (4.83, 5.35) | 6.81 | (5.46, 8.16) |
| Female | Ref | ref | Ref | |||
| Age | 0.19 | (0.13, 0.26) | 0.19 | (0.13, 0.26) | 0.19 | (0.13, 0.25) |
| Age squared | 0.0006 | (−0.0001, 0.001) | 0.0006 | (−0.0001, 0.001) | 0.0006 | (−0.00004, 0.001) |
| Race/ethnicity | ||||||
| Non-Hispanic White | Ref | Ref | Ref | |||
| Non-Hispanic Black | −0.17 | (−0.40, 0.05) | −0.33 | (−0.57, −0.09) | −1.52 | (−2.51, −0.53) |
| Hispanic | −0.36 | (−0.65, −0.07) | −0.44 | (−0.75, −0.14) | −1.41 | (−2.74, −0.09) |
| Nativity | ||||||
| US-born | Ref | Ref | Ref | |||
| Born outside US | −0.43 | (−0.72, −0.14) | −0.43 | (−0.72, −0.14) | −0.41 | (−0.70, −0.13) |
| Marital status | ||||||
| Married or partner | Ref | Ref | Ref | |||
| Widowed, divorced, separated |
−0.006 | (−0.22, 0.21) | -0.01 | (−0.23, 0.20) | −0.01 | (−0.22, 0.20) |
| Never Married | −0.12 | (−0.43, 0.20) | −0.14 | (−0.46, 0.17) | −0.14 | (−0.46, 0.17) |
| Education | ||||||
| Less than High School | 0.81 | (0.46, 1.17) | 0.76 | (0.40, 1.12) | 0.77 | (0.41, 1.14) |
| High School Graduate or GED |
0.44 | (0.13, 0.75) | 0.42 | (0.12, 0.73) | 0.41 | (0.11, 0.72) |
| More than High School | Ref | Ref | Ref | |||
|
Income to Poverty Ratio
(IPR) |
||||||
| ratio less than 1 | 1.23 | (0.89, 1.55) | 1.11 | (0.78, 1.44) | 1.07 | (0.73, 1.40) |
| ratio >=1, < 2 | 0.84 | (0.41, 1.27) | 0.76 | (0.32, 1.20) | 0.73 | (0.29, 1.17) |
| ratio >=2, < 3 | 0.56 | (0.25, 0.86) | 0.51 | (0.19, 0.83) | 0.48 | (0.15 0.81) |
| ratio >=3, < 4 | 0.53 | (0.14, 0.92) | 0.49 | (0.11, 0.87) | 0.46 | (0.08, 0.84) |
| ratio >=4, < 5 | 0.48 | (0.15, 0.82) | 0.45 | (0.12, 0.79) | 0.44 | (0.10, 0.78) |
| ratio >=5 | Ref | Ref | Ref | |||
| NSES | - | −0.01 | (−0.02, −0.005) | −0.01 | (−0.03, −0.001) | |
| Gender*Race interaction | - | - | ||||
| Male, Hispanic | - | - | −0.90 | (−1.53, −0.27) | ||
| Male, Black | - | - | −1.02 | (−1.53, −0.50) | ||
| Gender*NSES interaction | - | - | ||||
| Male*NSES | - | - | −0.02 | (−0.04, −0.004) | ||
| Race*NSES interaction | - | - | ||||
| NSES*Hispanic | - | - | 0.02 | (0.002, 0.04) | ||
| NSES*Black | - | - | 0.03 | (0.01, 0.04) | ||
As shown in model 2 (Table 2), even after controlling for these individual-level covariates, NSES was significantly associated with lower CHD risk (percentage point estimate for a 10-unit increase in NSES was −0.1 (95%CI −0.2, −0.05). To better illustrate the effect of NSES on FRS, we compare the estimated difference in 10-year risk of CHD based on FRS for two hypothetical people living in neighborhoods with low and high NSES: Anacostia, in the southeast region of the District of Columbia (NSES = 62.3), which was within the bottom NSES quartile of U.S. census tracts, and Northwest DC (NSES = 87.0) which was within the top NSES quartile of U.S. census tracts. The two neighborhoods differed by 24.7 points on the NSES index, which is double the interquartile range for the index across all U.S. census tracts. Based on the results of model 2, a resident of Anacostia would have had a predicted 10-year risk of CHD events on was on average 0.32 percentage points higher (95% CI 0.31, 0.33) than a similar person living in Northwest DC. While not as large as the effect of an individual-level factor such as gender, the magnitude of the effect of NSES on FRS is on par with being non-Hispanic white versus Hispanic and having a high school diploma versus more than a high school diploma.
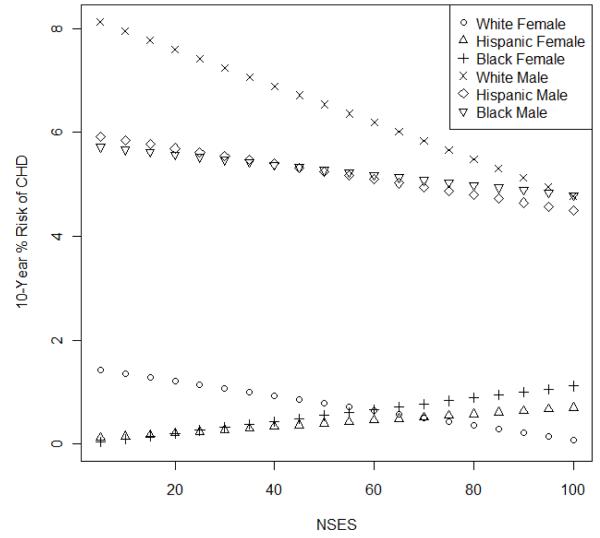
Our final model (model 3 in Table 2) shows a statistically significant interaction between race/ethnicity and gender as well as between NSES and gender and NSES and race/ethnicity. As shown, for the race/ethnicity by gender interaction, we see that the magnitude of the difference in CHD risk that exists between non-Hispanic white males and females (namely, 6.81 points with a 95% CI of 5.46, 8.16) is reduced on average by 0.90 (95% CI 0.27, 1.53) and 1.02 (95% CI 0.50, 1.53) percentage points among Hispanics and non-Hispanic blacks, respectively. Figure 1 illustrates these relationships and the interactions between NSES and gender and NSES and race/ethnicity with a predicted 10-year CHD risk as a function NSES for each race/ethnicity and gender group. The difference in the slopes of the lines indicates that the inverse association of NSES with 10-year risk of CHD is larger for men than women and for whites than Hispanics and non-Hispanic blacks. The effect of NSES on 10-year risk of CHD was not statistically significant in black and Hispanic men and women.
Fig. 1.
Estimated 10 year risk of coronary heart disease (CHD) vs neighbourhood socioeconomic status (NSES) for each gender and race/ethnicity group from Model 3, setting all other covariates in the model equal to the mean (continuous variables) or mode (categorical variables) in the data.
Using the 2008 Framingham risk score for CVD events revealed similar findings to those for CHD events (Appendix Table 1). Specifically, we observe a statistically significant association between NSES and CVD risk (main effect) as well as effect modification interaction between race/ethnicity and gender, NSES and gender, and NSES and race/ethnicity. In the above example, a resident of Anacostia would have had a predicted 10-year risk of CVD events on was on average 0.63 percentage points higher (95% CI 0.61, 0.63) than a similar person living in Northwest DC.
In sensitivity analyses, the propensity-score stratified regression model shown in Table 3 yielded a point estimate of the effect of NSES on predicted CHD risk similar that observed in Table 2, model 2. Namely, after propensity score adjustment, higher NSES continues to be associated with lower CHD risk. A 10-unit higher NSES is associated with an approximately 0.1 point lower FRS. While the result is not statistically significant due to the increased variability that comes from fitting a propensity score model, the effect size is the same as in the multivariate models. Similarly, the association between NSES and CVD risk remained robust in propensity-score adjusted models (Appendix Table 2).
Table 3.
Doubly robust propensity score–weighted regression models with Framingham Risk Score as the outcome, NHANES 1999-2004
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Characteristic | Coef | 95% CI | Coef | 95% CI |
| Gender | ||||
| Male | 5.14 | (4.76, 5.51) | 5.13 | (4.76, 5.50) |
| Female | ||||
| Age | 0.21 | (0.09, 0.33) | 0.21 | (0.09, 0.33) |
| Age squared | 0.0005 | (−0.001, 0.002) | 0.0005 | (−0.001, 0.002) |
| Race/ethnicity | ||||
| Non-Hispanic White | Ref | Ref | ||
| Non-Hispanic Black | −0.34 | (−0.77, 0.09) | −0.35 | (−0.78, 0.08) |
| Hispanic | −0.43 | (−1.00, 0.14) | −0.43 | (−1.00, 0.135) |
| Other | ||||
| Nativity | ||||
| US-born | Ref | Ref | ||
| Born outside US | −0.44 | (−1.01, 0.14) | −0.43 | (−1.01, 0.14) |
| Marital status | ||||
| Married or partner | Ref | Ref | ||
| Widowed, divorced, or separated | −0.03 | (−0.42, 0.36) | −0.02 | (−0.41, 0.37) |
| Never Married | −0.13 | (−0.70, 0.43) | −0.14 | (−0.70, 0.43) |
| Education | ||||
| Less than High School | 0.83 | (0.16, 1.49) | 0.81 | (0.14, 1.48) |
| High School Graduate or GED |
0.42 | (−0.10, 0.95) | 0.41 | (−0.11, 0.93) |
| More than High School | Ref | Ref | ||
|
Income to Poverty Ratio
(IPR) |
||||
| ratio less than 1 | 1.15 | (0.37, 1.94) | 1.13 | (0.34, 1.92) |
| ratio >=1, < 2 | 0.75 | (−0.005, 1.51) | 0.73 | (−0.03, 1.49) |
| ratio >=2, < 3 | 0.45 | (−0.19, 1.09) | 0.43 | (−0.21, 1.07) |
| ratio >=3, < 4 | 0.45 | (−0.21, 1.11) | 0.43 | (−0.22, 1.09) |
| ratio >=4, < 5 | 0.42 | (−0.28, 1.12) | 0.40 | (−0.30, 1.10) |
| ratio >=5 | Ref | Ref | ||
| NSES | −0.01 | (−0.05, 0.03) | ||
DISCUSSION
Using a nationally-representative sample, we find evidence that NSES is significantly associated with 10-year risk of CHD events predicted using FRS. These results persist after adjustment for individual-level sociodemographic factors including marital status, income, and education, was similar in models that examined risk of CVD events, and remain consistent in propensity score-adjusted models. The relationship between NSES and CHD was stronger for men compared to women and were not significant among blacks and Hispanics. The effect size of NSES on predicted risk are relatively small compared to other indicators of SES; however, the difference becomes both meaningful when considering the differences in CHD risk across populations and the need to address such disparities through prevention and treatment.
Findings of a small but robust association between NSES and predicted risk are consistent with prior studies linking neighborhood factors to individual-level CHD risk factors. NSES has been linked with smoking, physical inactivity, dietary patterns and obesity in local and national samples.1, 2 Individuals living in a poorer neighborhood may, for example, have less access to healthy and affordable food,24, 30 and/or resources for recreational activities which should be taken into consideration. Differences in the populations studied and in calculating NSES, CHD risk, may help account for the contrasting findings with the present study and those of Cubbin and Winkleby who did not find a significant association.15
The association between suboptimal neighborhood factors such as low SES has also been linked with higher prevalence, incidence, and mortality from CHD, even after accounting for other SES measures and traditional CHD risk factors.4, 5 Because FRS tends to underestimate CHD risk among populations living in low-income neighborhoods,11, 13, 31 NSES may not only affect CHD risk through traditional risk factors as described in the present study but there may exist alternative pathways linking NSES with CHD outcomes. The pathways and mechanisms remain uncertain and may stem from residual selection effects, neighborhood exposures, and social environments that influence biological pathways and contribute to poorer health trajectories for residents of low SES neighborhoods. For example, living in a low NSES neighborhood may also expose individuals to neighborhood hazards, disorder, and violence that result in increased levels of stress, which are not captured by FRS but could amplify its effects. Chronic inflammation may also mediate the relationship between NSES and CHD,26, 32 yet in a study by Deans and colleagues, inflammatory biomarkers did not appear to mediate the association between neighborhood deprivation and carotid atherosclerosis.33 NSES effects on CHD outcomes may also operate through a difference in illness trajectories.
We found significant differences in the relationship between NSES and FRS among men and women of different races and ethnicities. Men and women may tend to have varying levels of exposure to neighborhood environments,34 and women’s preventive behaviors and health may be less related to their environment.16 Although our results show no statistically significant evidence of an effect of NSES on FRS for Hispanics and blacks, it is not clear whether this result is driven by power issues or a true null effect. Racial/ethnic variation may stem from high levels of segregation such that people of different races/ethnicities are frequently living in different neighborhoods;35 the same level of NSES may reflect dissimilar neighborhood environments. Though not significant, the association between NSES and CHD risk among black and Hispanic women warrants careful scrutiny to investigate potential scenarios and mechanisms through which higher SES neighborhoods might be less protective for these women.
The analyses have several limitations. First, FRS was developed for adults 30 to 74 years old and validated in diverse populations. However, there are recognized limitations in the ability of FRS to accurately predict risk among certain populations, including Hispanic men.36, 37 Though using the point-based FRS may misclassify patients compared with the original FRS model,38 the point-based FRS model is widely used in clinical practice. Second, people with a history of CHD were excluded based on self-report which may be prone to recall bias. Third, consistent with previous studies on neighborhood-level effects, we use census-tracts to define neighborhoods while recognizing that census tracts may not capture residents’ perceptions of their neighborhoods. We are also unable to determine length of neighborhood exposure. Fourth, our NSES data was derived from 1990 to 2000 and did not perfectly align with the dates of the NHANES data (1999 to 2004). To the extent that NSES may have changed in subsequent years, this may bias our findings. Our measure of NSES has been used in prior studies24-26 and is consistent with approaches used previously.4 However, we were unable to capture contextual factors (e.g. substandard housing, lack of supermarkets) that may be associated with CHD. Further, using NSES data from after 2000 along with more recent NHANES data is important in continuing to assess the relationship between NSES and CHD risk. Finally, because FRS tends to underestimate the true coronary artery risk11, 13 and stroke prediction39 among individuals with low SES and those living in deprived areas, the observed associations between FRS and NSES provide a lower bound of this association. Our results on 10 year CHD risk are therefore conservative and generalizable exclusively to individuals without overt CHD. However, finding similar results with the FRS model for CVD, which included individuals with diabetes, strengthens our findings.
The results suggest that in addition to individual-level factors, 10-year CHD risk is independently associated with the socioeconomic environment within which individuals live further augmenting the disadvantages of low income for those in low SES neighborhoods and the advantages of high income for those in high SES neighborhoods. Together with findings that FRS underestimates risk among low SES11, 13 and higher rates of CHD outcomes occur in low SES neighborhoods,4, 5 the study adds to the body of research linking neighborhood environments and CHD risk. Determining what factors in the neighborhood socioeconomic environment may contribute to CHD risk and why the association is differential between men and women and among people of different races and ethnicities is critical in determining what interventions will best reduce CHD risk and disparities in CHD incidence and disease trajectories.
ACKNOWLEDGMENTS
We would like to thank Ricardo Basurto, Nataliya Kravets, Karen Davis, Snatosh Gambhir, and Vijay Gambhir for their assistance with the study. This work does not reflect the opinions of the CDC.
This work was supported by the National Institute of Environmental Health Services (Grant number P50ES012383-05) and by the National Institute Heart, Lung and Blood Institute (Grant number R21HL095028). Dr. Pollack’s salary was supported by a career development award from the NIH National Cancer Institute and Office of Behavioral and Social Sciences Research (K07CA151910).
Appendix
Appendix Table 1.
Hierarchical linear regression models, adjusted for county- and tract-level effects, with the percentage point difference in cardiovascular disease risk calculated using the 2008 version of the Framingham Risk score, NHANES 1999-2004
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Characteristic | Coef | 95% CI | Coef | 95% CI | Coef | 95% CI |
| Gender | ||||||
| Male | 5.45 | (5.10 , 5.81) | 5.45 | (5.09 , 5.81) | 8.2 | (6.54 , 9.85) |
| Female | ref | ref | ref | |||
| Age | 0.2 | (0.10 , 0.30) | 0.19 | (0.09 , 0.29) | 0.19 | (0.09 , 0.29) |
| Age squared | 0.003 | (0.002 , 0.004) | 0.003 | (0.002 , 0.004) | 0.003 | (0.002 , 0.004) |
| Race/ethnicity | ||||||
| Non-Hispanic White | ref | ref | ref | |||
| Non-Hispanic Black | 0.7 | (0.38 , 1.01) | 0.4 | (0.07 , 0.73) | −1.57 | (−2.91 , −0.24) |
| Hispanic | −0.11 | (−0.47 , 0.25) | −0.28 | (−0.64 , 0.07) | −2.95 | (−4.54 , −1.35) |
| Nativity | ||||||
| US-born | ref | ref | ref | |||
| Born outside US | −0.63 | (−1.00 , −0.26) | −0.63 | (−0.99 , −0.26) | −0.6 | (−0.96 , −0.24) |
| Marital status | ||||||
| Married or partner | ref | ref | ref | |||
| Widowed, divorced, or separated |
0.05 | (−0.22 , 0.33) | 0.04 | (−0.23 , 0.31) | 0.04 | (−0.23 , 0.30) |
| Never Married | 0.18 | (−0.26 , 0.63) | 0.14 | (−0.30 , 0.57) | 0.14 | (−0.31 , 0.58) |
| Education | ||||||
| Less than High School |
1.18 | (0.78 , 1.58) | 1.08 | (0.68 , 1.48) | 1.11 | (0.71 , 1.51) |
| High School Graduate or GED |
0.53 | (0.07 , 0.98) | 0.5 | (0.04 , 0.95) | 0.49 | (0.03 , 0.94) |
| More than High School |
ref | ref | ref | |||
| Income to Poverty Ratio (IPR) |
||||||
| ratio less than 1 | 1.5 | (0.92 , 2.09) | 1.32 | (0.71 , 1.92) | 1.24 | (0.64 , 1.83) |
| ratio >=1, < 2 | 1.35 | (0.72 , 1.99) | 1.21 | (0.56 , 1.86) | 1.15 | (0.52 , 1.78) |
| ratio >=2, < 3 | 0.71 | (0.26 , 1.17) | 0.62 | (0.16 , 1.09) | 0.55 | (0.09 , 1.02) |
| ratio >=3, < 4 | 0.88 | (0.43 , 1.34) | 0.81 | (0.35 , 1.26) | 0.74 | (0.29 , 1.19) |
| ratio >=4, < 5 | 1 | (0.63 , 1.36) | 0.94 | (0.57 , 1.31) | 0.91 | (0.54 , 1.28) |
| ratio >=5 | ref | ref | ref | |||
| NSES | - | −0.03 | (−0.03 , −0.02) | −0.03 | (−0.04 , −0.02) | |
| Gender*Race interaction |
- | - | ||||
| Male, Hispanic | - | - | −1.1 | (−1.89 , −0.3) | ||
| Male, Black | - | - | −1.43 | (−2 , −0.85) | ||
| Gender*NSES interaction |
- | - | ||||
| Male*NSES | - | - | −0.03 | (−0.06 , −0.01) | ||
| Race*NSES interaction |
- | - | ||||
| NSES*Hispanic | - | - | 0.05 | (0.02 , 0.07) | ||
| NSES*Black | - | - | 0.04 | (0.02 , 0.06) | ||
Appendix Table 2.
Doubly robust propensity score–weighted regression models with cardiovascular disease risk using the 2008 Framingham Risk Score as the outcome, NHANES 1999-2004
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Characteristic | Coef | 95% CI | Coef | 95% CI |
| Gender | ||||
| Male | 5.52 | (5.01 , 6.03) | 5.51 | (5.00 , 6.02) |
| Female | ref | ref | ||
| Age | 0.22 | (0.05 , 0.39) | 0.22 | (0.05 , 0.39) |
| Age squared | 0.00 | (0.001 , 0.004) | 0.00 | (0.001 , 0.005) |
| Race/ethnicity | ||||
| Non-Hispanic White | ref | ref | ||
| Non-Hispanic Black | 0.38 | (−0.18 , 0.93) | 0.36 | (−0.20 , 0.91) |
| Hispanic | −0.25 | (−0.91 , 0.41) | −0.26 | (−0.92 , 0.39) |
| Nativity | ||||
| US-born | ref | ref | ||
| Born outside US | −0.62 | (−1.25 , 0.02) | −0.60 | (−1.24 , 0.04) |
| Marital status | ||||
| Married or partner | ref | ref | ||
| Widowed, divorced, or separated |
−0.05 | (−0.60 , 0.49) | −0.05 | (−0.59 , 0.50) |
| Never Married | 0.12 | (−0.58 , 0.82) | 0.11 | (−0.59 , 0.81) |
| Education | ||||
| Less than High School | 1.09 | (0.37 , 1.81) | 1.06 | (0.33 , 1.79) |
| High School Graduate or GED |
0.47 | (−0.16 , 1.10) | 0.44 | (−0.19 , 1.07) |
| More than High School | ref | ref | ||
| Income to Poverty Ratio (IPR) |
||||
| ratio less than 1 | 1.43 | (0.40 , 2.45) | 1.37 | (0.33 , 2.40) |
| ratio >=1, < 2 | 1.17 | (0.24 , 2.11) | 1.12 | (0.171 , 2.06) |
| ratio >=2, < 3 | 0.59 | (−0.23 , 1.41) | 0.54 | (−0.29 , 1.37) |
| ratio >=3, < 4 | 0.81 | (−0.01 , 1.63) | 0.76 | (−0.06 , 1.59) |
| ratio >=4, < 5 | 0.75 | (−0.03 , 1.54) | 0.71 | (−0.08 , 1.50) |
| ratio >=5 | ref | Ref | ||
| NSES | −0.03 | (−0.07 , 0.01) | ||
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report.
REFERENCES
- 1.Chaix B. Geographic life environments and coronary heart disease: A literature review, theoretical contributions, methodological updates, and a research agenda. Annu Rev Public Health. 2009;30(1):81–105. doi: 10.1146/annurev.publhealth.031308.100158. [DOI] [PubMed] [Google Scholar]
- 2.Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: A critical review. J Epidemiol Community Health. 2001;55(2):111–22. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diez Roux A. Residential environments and cardiovascular risk. J Urban Health. 2003;80(4):569–89. doi: 10.1093/jurban/jtg065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roux AVD, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 5.Diez-Roux AV, Nieto FJ, Muntaner C, Tyroler HA, Comstock GW, Shahar E, et al. Neighborhood environments and coronary heart disease: A multilevel analysis. Am J Epidemiol. 1997;146(1):48–63. doi: 10.1093/oxfordjournals.aje.a009191. [DOI] [PubMed] [Google Scholar]
- 6.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 8.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: Consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation. 2002;106(3):388–91. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 9.Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136(2):161–72. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 10.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 11.Brindle PM, McConnachie A, Upton MN, Hart CL, Davey SMith G, Watt GCM. The accuracy of the Framingham Risk-Score in different socioeconomic groups: A prospective study. Br J Gen Pract. 2005;55(520):838–45. [PMC free article] [PubMed] [Google Scholar]
- 12.Fiscella K, Tancredi D. Socioeconomic status and coronary heart disease risk prediction. JAMA. 2008;300(22):2666–8. doi: 10.1001/jama.2008.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiscella K, Tancredi D, Franks P. Adding socioeconomic status to Framingham scoring to reduce disparities in coronary risk assessment. Am Heart J. 2009;157(6):988–94. doi: 10.1016/j.ahj.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Franks P, Tancredi DJ, Winters P, Fiscella K. Including socioeconomic status in coronary heart disease risk estimation. Ann Fam Med. 2010;8(5):447–53. doi: 10.1370/afm.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cubbin C, Winkleby MA. Protective and harmful effects of neighborhood-level deprivation on individual-level health knowledge, behavior changes, and risk of coronary heart disease. Am J Epidemiol. 2005;162(6):559–68. doi: 10.1093/aje/kwi250. [DOI] [PubMed] [Google Scholar]
- 16.Bird CE, Rieker PP. The effects of constrained choice and social policies. Cambridge University Press; London and New York: 2008. Gender and health. [Google Scholar]
- 17.McEwen BS. Stress, Adaptation, and Disease. Allostasis and Allostatic Load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 18.Seeman TE, McEwen BS. Impact of social environment characteristics on neuroendocrine function. Psychosom Med. 1996;58(5):459–471. doi: 10.1097/00006842-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics -- 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 20.Seeman TE, Crimmins E, Singer B, et al. Cumulative Biological Risk and SocioEconomic Differences in Mortality: MacArthur Studies of Successful Aging. Soc Sci Med. 2004;58(10):1985–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 21.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic Load as a Marker of Cumulative Biological Risk: MacArthur Studies of Successful Aging. Proc Natl Acad Sci USA. 2001;98(8):4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buell C, Kermah D, Davidson MB. Utility of A1C for diabetes screening in the 1999–2004 NHANES population. Diabetes Care. 2007;30(9):2233–5. doi: 10.2337/dc07-0585. [DOI] [PubMed] [Google Scholar]
- 23.Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008;93(7):2447–53. doi: 10.1210/jc.2007-2174. [DOI] [PubMed] [Google Scholar]
- 24.Dubowitz T, Heron M, Bird CE, Lurie N, Finch BK, Basurto-Dávila R, et al. Neighborhood socioeconomic status and fruit and vegetable intake among whites, blacks, and Mexican Americans in the United States. Am J Clin Nutr. 2008;87(6):1883–91. doi: 10.1093/ajcn/87.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bird CE, Seeman TE, Escarce JJ, Basurto-Davila R, Finch BK, Dubowitz T, et al. Neighborhood socioeconomic status and biological “wear & tear” in a nationally representative sample of U.S. adults. J Epidemiol Community Health. 2010;64:860–5. doi: 10.1136/jech.2008.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merkin SS, Basurto-Dávila R, Karlamangla A, Bird CE, Lurie N, Escarce JJ, et al. Neighborhoods and cumulative biological risk profiles by race/ethnicity in a national sample of U.S. adults: NHANES III. Ann Epidemiol. 2009;19(3):194–201. doi: 10.1016/j.annepidem.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Census Bureau Census tract relationship files. 2001 Available from: http://www.census.gov/geo/www/relate/rel_tract.html.
- 28.D’Agostino RB, Vasan RW, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General Cardiovascular Risk Profile for Use in Primary Care: The Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 29.Imai K, van Dyk DA. Causal inference with general treatment regimes. JASA. 2004;99(467):854–66. [Google Scholar]
- 30.Dubowitz T, Ghosh-Dastidar M, Eibner C, Slaughter ME, Fernandes M, Whitsel EA, et al. The Women’s Health Initiative: The food environment, neighborhood socioeconomic status, BMI, and blood pressure. Obesity. 2011 doi: 10.1038/oby.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brindle P, Jonathan E, Lampe F, Walker M, Whincup P, Fahey T, et al. Predictive accuracy of the Framingham coronary risk score in British men: Prospective cohort study. Br Med J. 2003;327(7426):1267. doi: 10.1136/bmj.327.7426.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen KL, Bleil ME, McCaffery J, Mackey RH, Sutton-Tyrrell K, Muldoon MF, et al. Community socioeconomic status is associated with carotid artery atherosclerosis in untreated, hypertensive men[ast] Am J Hypertens. 2006;19(6):560–6. doi: 10.1016/j.amjhyper.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Deans KA, Bezlyak V, Ford I, Batty GD, Burns H, Cavanagh J, et al. Differences in atherosclerosis according to area level socioeconomic deprivation: Cross sectional, population based study. Br Med J. 2009:339. doi: 10.1136/bmj.b4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inagami S, Cohen DA, Finch BK. Non-residential neighborhood exposures suppress neighborhood effects on self-rated health. Soc Sci Med. 2007;65(8):1779–91. doi: 10.1016/j.socscimed.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 35.Williams DR, Collins C. Racial residential segregation: A fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–16. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurley LP, Dickinson LM, Estacio RO, Steiner JF, Havranek EP. Prediction of cardiovascular death in racial/ethnic minorities using Framingham risk factors. Circ Cardiovasc Qual Outcomes. 2010;3:181–7. doi: 10.1161/CIRCOUTCOMES.108.831073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Agostino S, Ralph B, Grundy S, Sullivan LM, Wilson P, for the CHD Risk Prediction Group Validation of the Framingham coronary heart disease prediction scores: Results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 38.Gordon W, Polansky J, John Boscardin W, Fung K, Steinman M. Coronary risk assessment by point-based vs. equation-based Framingham models: Significant implications for clinical care. J Gen Intern Med. 2010;25(11):1145–51. doi: 10.1007/s11606-010-1454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jolobe OMP. Adding socioeconomic status to Framingham scoring might also improve stroke risk evaluation in young adults with hypertension. Am Heart J. 2009;158(3):e35–e. doi: 10.1016/j.ahj.2009.06.014. [DOI] [PubMed] [Google Scholar]