Abstract
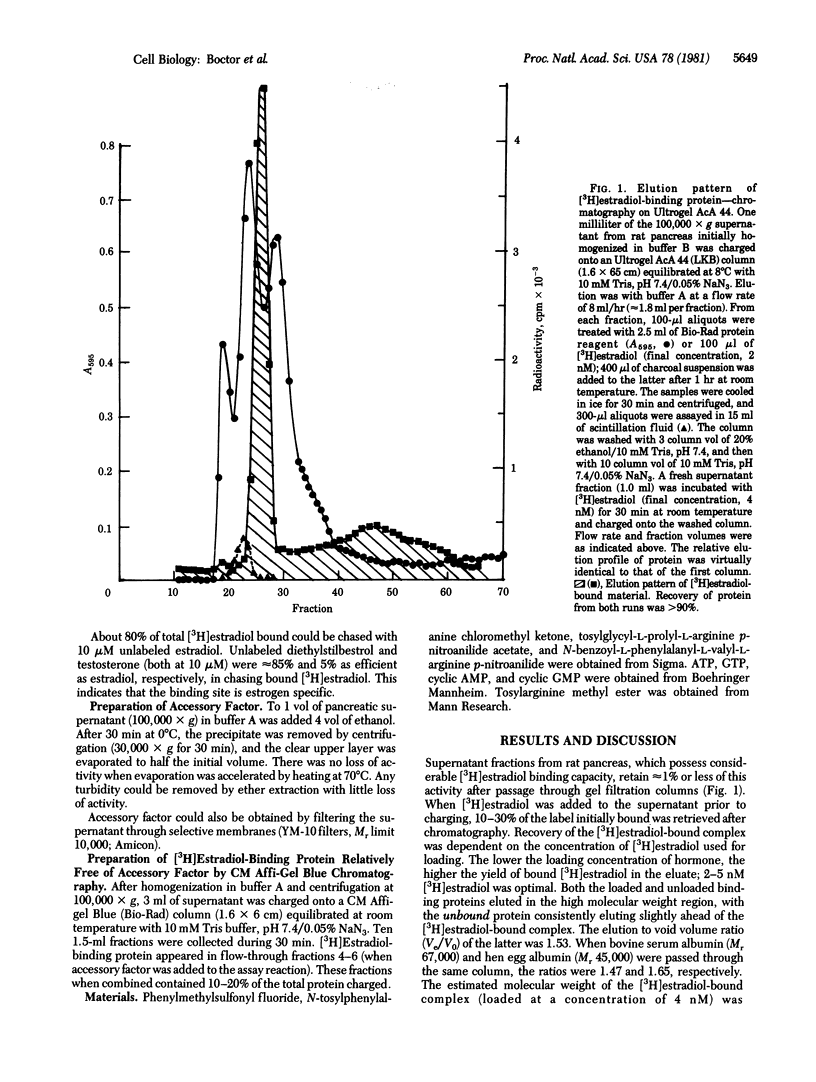
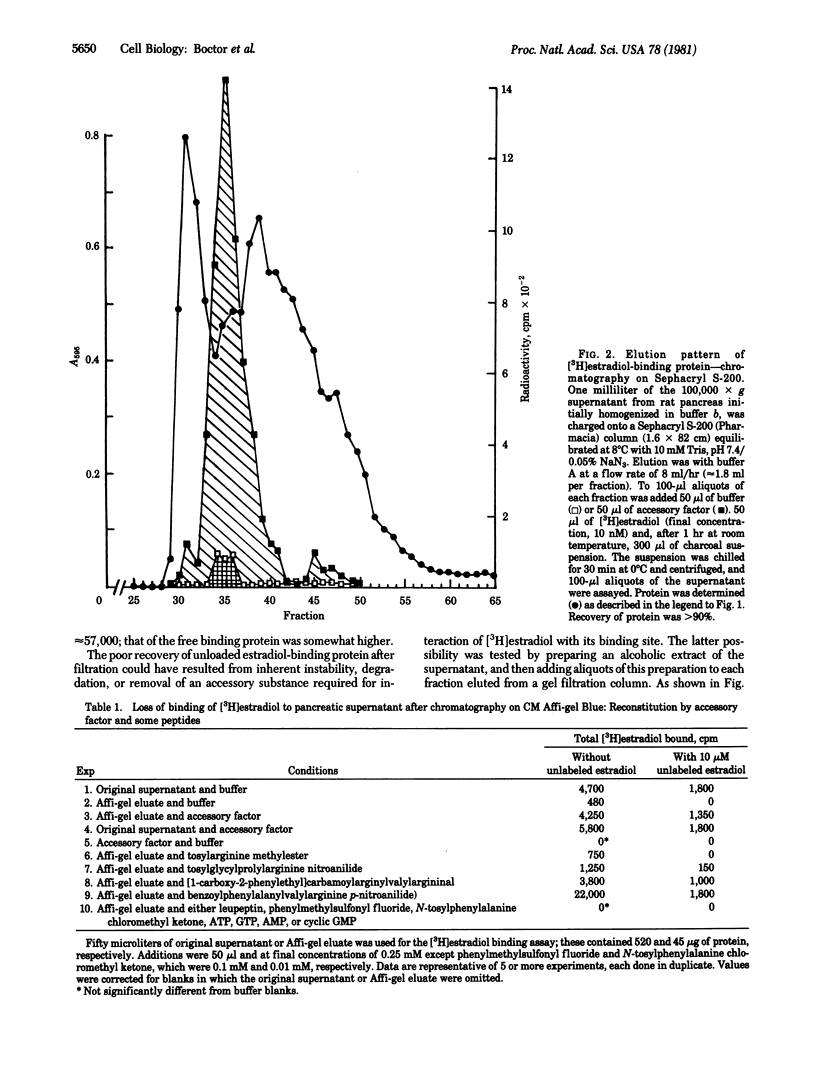
Supernatant fractions from rat pancreas bind approximately 300 fmol of [3H]estradiol per mg of protein when incubated with 5 nM [3H]estradiol for 1 hr at room temperature. Passage through gel filtration columns reduces binding in the eluate to approximately 1% of its initial activity. Extracts of the supernatant contain a factor that reactivates binding in gel filtrates. Addition of accessory factor to fractional eluates gives one sharp peak of activity. Since fractions that cannot be reactivated contain as much or more protein as fractions that can be reactivated, it is concluded that interaction of accessory factor and [3H]estradiol-binding protein is specific. Peptides such as antipain [(1-carboxy-2-phenylethyl)carbamoyl-L-arginyl-L-valyl-L-argininal] and, especially, N-benzoyl-L-phenylalanyl-L-valyl-L-arginine-p-nitroanilide also enhanced binding of [3H]estradiol. Accessory factor is water soluble, dialyzable, and heat stable. Although as currently purified, it contains several substances, the data suggest that at least one component of accessory factor is an oligopeptide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyagi T., Takeuchi T., Matsuzaki A., Kawamura K., Kondo S. Leupeptins, new protease inhibitors from Actinomycetes. J Antibiot (Tokyo) 1969 Jun;22(6):283–286. doi: 10.7164/antibiotics.22.283. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Grossman A., Boctor A. M., Lane B. Dependence of pancreatic integrity on adrenal and ovarian secretions. Endocrinology. 1969 Nov;85(5):956–959. doi: 10.1210/endo-85-5-956. [DOI] [PubMed] [Google Scholar]
- Korenman S. G. Radio-ligand binding assay of specific estrogens using a soluble uterine macromolecule. J Clin Endocrinol Metab. 1968 Jan;28(1):127–130. doi: 10.1210/jcem-28-1-127. [DOI] [PubMed] [Google Scholar]
- Sandberg A. A., Rosenthal H. E. Steroid receptors in exocrine glands: the pancreas and prostate. J Steroid Biochem. 1979 Jul;11(1A):293–299. doi: 10.1016/0022-4731(79)90311-x. [DOI] [PubMed] [Google Scholar]
- Suda H., Aoyagi T., Hamada M., Takeuchi T., Umezawa H. Antipain, a new protease inhibitor isolated from actinomycetes. J Antibiot (Tokyo) 1972 Apr;25(4):263–266. doi: 10.7164/antibiotics.25.263. [DOI] [PubMed] [Google Scholar]
- Thampan T. N., Clark J. H. An oestrogen receptor activator protein in rat uterine cytosol. Nature. 1981 Mar 12;290(5802):152–154. doi: 10.1038/290152a0. [DOI] [PubMed] [Google Scholar]


