Abstract and Perspective
This study aimed to: 1) examine trajectories of insomnia symptoms in adolescents with chronic pain compared to their healthy peers, 2) evaluate psychological and behavioral risk factors for longitudinal insomnia symptoms, and 3) evaluate insomnia as a predictor of quality of life, activity limitations, and healthcare utilization over 12 months. Participants included 61 adolescents with chronic pain and 60 youth without chronic pain (12–18 years; 72% female). Questionnaires were completed at enrollment, 6-months, and 12-months, and assessed pain intensity, insomnia symptoms, sleep hygiene, pre-sleep arousal, depression, pubertal status, activity limitations, quality of life, and healthcare utilization. Insomnia symptoms persisted for both groups, and remained higher at all time points for youth with chronic pain. GEE modeling identified three risk factors for longitudinal insomnia symptoms: having chronic pain, poorer sleep hygiene, and higher depressive symptoms. Insomnia symptoms also predicted poorer quality of life over time, and were associated with more frequent healthcare utilization. Findings suggest that sleep problems are persistent and associated with negative impact for youth with chronic pain. Treatment of insomnia symptoms in youth with chronic pain may lead to improvements in quality of life and reductions in healthcare costs.
Perspective
Insomnia symptoms are persistent over a 12 month period and are associated with negative impact for youth with chronic pain. These findings suggest that treatment of insomnia symptoms in youth with chronic pain may lead to improvements in quality of life and reductions in healthcare costs.
Keywords: chronic pain, insomnia, adolescents, longitudinal, quality of life
Introduction
Adolescents with chronic pain commonly report sleep problems 2, 3, 15, 16, 26, 31. In previous studies assessing insomnia symptoms (defined as difficulties falling or staying asleep), over 50% of youth with chronic pain report frequent problems with insomnia 11, 22. Comorbid insomnia symptoms are not only prevalent but also associated with adverse consequences including increased pain-related functional limitations and lower quality of life 5, 11, 22. In adults, insomnia related to chronic pain has been recognized among the most costly forms of illness in terms of prescription medications and lost work productivity 27, 29. The impact of insomnia symptoms on the health care utilization of children and adolescents with chronic pain has not yet been described.
Several behavioral and psychosocial factors are thought to be important in the development of sleep problems in adolescents, including sleep habits, behaviors and mood/affect. Poor sleep habits (e.g., erratic schedule, lack of bedtime routine), and high levels of somatic and cognitive arousal at bedtime (e.g., worries, physiological arousal) have predicted worse sleep problems in healthy and pain samples 10, 22. Moreover, depressive symptoms have predicted sleep problem severity and insomnia symptoms in youth with chronic pain 21.
In a previous paper 22 we examined a model of behavioral and psychosocial predictors of insomnia symptoms in adolescents with chronic pain and healthy adolescents using cross-sectional data. Our findings demonstrated that having chronic pain and higher levels of cognitive pre-sleep arousal (e.g., worries at bedtime) predicted higher rates of insomnia. One prevailing conceptual model classifies longitudinal etiologic factors involved in the development of insomnia as predisposing, precipitating, and perpetuating factors 28. While certain child factors (e.g., anxiety, high achievement orientation) may predispose adolescents to the development of insomnia, the experience of pain (e.g., high pain intensity, high levels of arousal) can precipitate insomnia, and poor sleep hygiene practices (e.g., too much time in bed, concern about daytime deficits) can perpetuate insomnia. In particular, arousal at bedtime can directly interfere with settling to sleep and maintaining sleep and lead to negative behavioral patterns that sustain sleep problems over time.
Therefore, we have completed longitudinal follow-up of our original sample 22 to examine insomnia symptoms over 12 months. Specifically, the primary aim of this paper was to examine trajectories of insomnia symptoms in adolescents with chronic pain compared to their healthy peers over 12 months. We hypothesized that insomnia symptoms would persist for both groups but remain higher at all time points for youth with chronic pain. We also hypothesized that membership in the chronic pain cohort, higher depressive symptoms, poorer sleep hygiene, and higher levels of pre-sleep arousal would predict severity of insomnia symptoms over time. Finally, we hypothesized that persistent insomnia symptoms would be associated with more activity limitations, lower quality of life, and greater health care utilization over the 12 month study period.
Materials and Methods
Participants
Participants were part of a longitudinal study examining sleep-wake disturbances in adolescents. Previous published reports from this sample were based on cross-sectional data from study enrollment only, and included a report of daily associations between pain and sleep 14, behavioral and psychosocial predictors of insomnia symptoms 22, and daytime and nighttime sleep patterns 12, 17. We have not reported longitudinal data on the course of insomnia symptoms in this sample. All procedures were approved by the Institutional Review Board where the study took place. Written informed consent from parents and guardians, and written assent from adolescents were obtained prior to participation in this study.
Participants with chronic pain were recruited from a multidisciplinary pediatric chronic pain clinic at an academic medical center in the Pacific Northwest via letter or in-person at a clinic appointment. Youth with chronic pain were included in the study if they met all of the following criteria: 1) between 12 and 18 years of age, 2) had pain present for three months or more, 3) pain occurred at least 3 days per week, 4) pain intensity equal to or above the midpoint on a visual analogue scale, 5) no known diagnosis of a developmental disability, and 6) fluent in English. Ninety-seven adolescents with chronic pain and their parents were contacted regarding participation in this study; 20 were excluded due to failure to meet inclusion criteria and 16 declined to participate, resulting in a total of 61 adolescents with chronic pain enrolled in the current study.
Participants in the healthy comparison group were recruited via advertisements posted in the local metropolitan area. Youth who responded to the advertisements were screened by phone or in person by the research team. Participants were included in the healthy comparison group if they met all of the following criteria: 1) between 12 and 18 years of age, 2) absence of chronic pain, defined as pain present for three months or more, occurring at least three days per week, with intensity above the midpoint on a visual analogue scale, 3) no known diagnosis of a serious chronic illness or developmental disability, and 4) fluent in English. Selective recruitment on age and sex was used to reduce the likelihood of significant differences between the two groups on these factors. One hundred seven adolescents were screened for inclusion in the healthy comparison group; 41 were excluded due to failure to meet inclusion criteria and 6 declined to participate, resulting in a total of 60 otherwise healthy adolescents enrolled in the current study.
Procedure
Participants completed questionnaires at three time points: at enrollment, 6-month follow-up, and 12-month follow-up. At enrollment, questionnaires were completed by parents and adolescents during a home visit by a member of the research team. At 6-month and 12-month follow-up, questionnaires were completed at participants’ homes and returned to study staff via mail. Parents completed a demographic questionnaire and reported on their adolescent’s health care utilization. Adolescents completed questionnaires about pain intensity, insomnia symptoms, sleep hygiene, pre-sleep arousal, depressive symptoms, pubertal status, activity limitations and health-related quality of life. Participants were compensated for their time with gift cards to local stores after each data collection point.
Measures
Demographics
Parents provided information regarding the teen’s age, sex, race, and socioeconomic status. Parents also reported on the teen’s prescription pain medication use at enrollment in three medication classes: antidepressants, anticonvulsants, and opioids.
Pain intensity
Adolescents rated usual pain intensity over the past month on an 11-point Numerical Rating Scale (NRS; 0 = no pain, 10 = worst pain possible). The NRS is a widely used assessment of pain intensity in children and has demonstrated good validity and reliability 34.
Insomnia symptoms
The 28-item Adolescent Sleep Wake Scale (ASWS) 13 was completed by adolescents to assess self-reported sleep quality and insomnia symptoms. Items assess the frequency of various sleep behaviors over the past month, with response options ranging from 1 = always to 6 = never. Higher scores represent better sleep quality. Consistent with our previous work 17, 22, two items from the ASWS were used to assess insomnia symptoms: difficulty falling asleep (i.e., “I have trouble going to sleep”) and difficulty maintaining sleep (i.e., “After waking up during the night, I have trouble going back to sleep”). Responses to these items were summed to create a continuous variable with scores ranging from 2 to 12.
Sleep hygiene
The 24-item Adolescent Sleep Hygiene Scale (ASHS) 13 was completed by adolescents to assess the frequency of sleep-facilitating and sleep-inhibiting behaviors in the previous month. Response options range from 1 = never to 6 = always, with higher scores indicating better sleep hygiene. The ASHS is comprised of six subscales including physiological, cognitive, emotional, sleep environment, substances, and sleep stability. A total sleep hygiene score was calculated by taking the mean of the six subscale scores. The ASHS has also demonstrated good internal consistency (α = .80) 13.
Pre-sleep arousal
Adolescents completed the 18-item Pre-Sleep Arousal Scale (PSAS) 18 to assess somatic and cognitive pre-sleep arousal. Somatic arousal was measured by items that assessed symptoms such as muscle tension, trouble breathing, and irregular heartbeat prior to falling asleep. Cognitive arousal is measured by items assessing symptoms such as worry about falling asleep, racing thoughts, and depressing or anxious thoughts while attempting to fall asleep. Items assess the intensity of these symptoms in a typical week. Responses range from 1 = not at all to 5 = extremely, with higher scores indicating greater arousal before falling asleep. The PSAS has demonstrated acceptable internal consistency and test-retest reliability in healthy children and adolescents 10, using slight wording modifications to make it developmentally appropriate. In the current study, we also used this slightly modified version which we have used in two previous studies of adolescents with and without chronic pain 21, 22.
Depressive symptoms
The 20-item Center for Epidemiological Studies Depression Scale (CES-D) was used to measure adolescents’ depressive symptoms 25. The CES-D is scored by summing all items to yield a total score, with higher scores indicating greater depressive symptoms. The validity of the CES-D is supported by comparisons with other measures of depression in youth 7. The CES-D has also demonstrated adequate one-week test-retest reliability25.
Pubertal development
The 8-item Pubertal Development Scale was used to measure adolescent self-report of pubertal development. This questionnaire is widely used and has acceptable reliability and validity 24. Responses are classified into one of 5 categories ranging from pre-pubertal to post-pubertal. Scores for boys and girls were calculated separately based on sex-specific scoring criteria. The resulting categories were then combined into one continuous variable for all participants, ranging from 1 (pre-pubertal) to 5 (post-pubertal).
Activity limitations
The 21-item Child Activity Limitations Interview was used to measure adolescent subjective report of activity limitations (CALI-21) 8, 20. The CALI-21 is designed to assess perceived difficulty in completing daily activities due to pain. On a 5-point rating scale ranging from 0 = not difficult to 4 = extremely difficult, adolescents reported pain-related difficulty in completing 21 daily activities over the past four weeks. A total score can be calculated by summing all items. Higher scores indicated greater activity limitations. The CALI has demonstrated good internal consistency and high cross-informant reliability 20.
Health-related quality of life
The Pediatric Quality of Life Inventory Version 4.0 (PedsQL) 32 was used to assess health-related quality of life (HRQOL). Respondents rate the frequency of experiencing problems over the past month on a 5-point rating scale ranging from 0 = never to 4 = almost always. Psychosocial and physical summary scores, as well as a total scale score were calculated. Scores are transformed on a scale from 0 to 100 with higher scores representing better quality of life. The PedsQL is widely used and has demonstrated excellent internal consistency and construct validity 32.
Health care utilization
Parents from both groups reported on whether (yes or no) and how many appointments their adolescent had with a primary or specialty care physician (e.g., gastroenterologist, neurologist) over the prior 6 month period for the treatment of their adolescent’s pain symptoms. This questionnaire has been used in previous research examining patterns of health care utilization in youth with and without chronic pain 30. A composite health care utilization variable was created by summing the total number of visits across primary care and specialty care providers over an 18 month period.
Data Analysis Plan
Analyses were performed with Stata software version 10.1. Statistical significance levels for analyses were set at 0.05 and two-sided tests were used. Descriptive analyses were conducted and the two cohorts (chronic pain vs healthy) were compared with respect to demographic characteristics (age, sex, race and family income), as well as pubertal development, depressive symptoms, sleep hygiene behaviors, cognitive and somatic pre-sleep arousal, pain intensity, insomnia symptoms, health-related quality of life, activity limitations and health care utilization at enrollment. Means and standard deviations for continuous variables (age, study measures) and percentages for categorical variables (sex, race, family income) were calculated. The means of continuous variables were compared using Student’s t-tests; categorical variables were compared using Pearson’s chi-square tests. For longitudinally-measured variables, paired t-tests were used to compare differences between variables at enrollment and the 12-month follow-up within each group.
Within subject correlation structures for all longitudinally-measured variables were examined in order to determine the optimal longitudinal analysis method. Generalized estimating equations (GEE) cross-sectional time-series, using Stata’s xtgee command, were used to model the longitudinal relationship between insomnia symptoms and independent variables of group status, pubertal status, depressive symptoms, sleep hygiene, cognitive and somatic presleep arousal, and pain intensity over 12 months. GEE analysis employs a population-averaged model, a marginal model, which includes all three time points measured in the study. Further longitudinal modeling, using cross-sectional time-series population-averaged GEE, was conducted to examine the association of insomnia symptoms with quality of life and activity limitations, controlling for group status, depressive symptoms and pain intensity. In all GEE analyses identity link function, exchangeable correlation structure, and normally-distributed errors were specified. GEE analyses were conducted using robust standard errors (sandwich). Finally, multiple regression analysis was used to examine the association between persistent insomnia symptoms (defined as reporting insomnia symptoms at least 2 of the 3 time points) and healthcare use (defined as the total number of visits to primary and specialty care providers across the three time points) after controlling for group status (pain vs. healthy).
Missing data was minimal and was handled in longitudinal GEE analysis by including all available data points for each participant. Thus, participants were excluded from analyses only if data were missing at all three time points for a given variable. Ninety-three percent (n=113) of participants provided data on insomnia symptoms at all three time points, 5% (n=6) had data at two time points, and the remaining 2% (n=2) had data at one time point. Similar patterns of missing data were observed for the other study variables.
Results
Descriptive Statistics
One hundred twenty-one adolescent participants were included in the study; 60 were in the healthy cohort and 61 in the chronic pain cohort. Baseline age range was 12–18 years, with a mean of 15 years (SD 1.7). A majority of participants were female (72%) and Caucasian (83%). Demographic characteristics for the two groups at enrollment are compared in Table 1. No statistically significant differences were found between the two groups at enrollment in age, sex, race, or income. Table 1 also summarizes baseline study measures of prescription pain medication use, pubertal development, depressive symptoms, sleep hygiene practices, cognitive and somatic pre-sleep arousal, pain intensity, insomnia symptoms, health-related quality of life and activity limitations. At enrollment, the chronic pain and healthy cohorts differed significantly on these measures (as expected) with the exception of sleep hygiene behaviors which were similar between groups.
Table 1.
Demographic characteristics and study measures at enrollment by study group.
| Characteristic | Mean/Proportion Healthy Group (n=60) |
Mean/Proportion Pain Group (n=61) |
p-value |
|---|---|---|---|
| Age (years) | 14.8 | 15.1 | 0.378 |
| Gender | |||
| Female (%) | 72.1 | 71.7 | 0.955 |
| Race (%) | 0.122 | ||
| Caucasian | 78.3 | 86.9 | |
| African American | 11.7 | 0 | |
| Am. Indian/Alaska Native | 1.7 | 1.6 | |
| Asian | 1.7 | 3.3 | |
| Other/biracial | 6.7 | 5.6 | |
| Missing data | 0 | 1.6 | |
| Family income (annual) | 0.248 | ||
| $30,000 or less (%) | 10.0 | 8.2 | |
| $30,000-$69,000 | 33.3 | 31.2 | |
| $70,000 or more | 56.7 | 54.1 | |
| Missing | 0 | 6.6 | |
| Prescription Medications (%) | 0.045 | ||
| Anticonvulsants | 0 | 45.0 | |
| Antidepressants | 0 | 38.0 | |
| Opioids | 0 | 33.0 | |
| Pubertal Development | 3.4 | 3.8 | 0.045 |
| Depressive Symptoms | 8.3 | 14.4 | <0.001 |
| Sleep Hygiene | 4.9 | 4.8 | 0.259 |
| Cognitive Pre-sleep Arousal | 15.1 | 17.0 | 0.045 |
| Somatic Pre-sleep Arousal | 10.8 | 13.4 | <0.001 |
| Pain Intensity | 3.1 | 6.4 | <0.001 |
| Insomnia symptoms | 4.4 | 6.5 | <0.001 |
| PedsQL total score | 82.8 | 66.2 | <0.001 |
| Psychosocial subscale | 80.4 | 72.6 | 0.003 |
| Physical subscale | 87.5 | 53.4 | <0.001 |
| Activity Limitations | 5.9 | 29.7 | <0.001 |
| Outpatient medical visits | 0.14 | 6.10 | <0.001 |
At enrollment, insomnia symptoms were more prevalent (52% vs. 22%, p < 0.001), and depressive symptoms were more pronounced (CES-D score of 14.4 vs. 8.3, p < 0.001; See Table 1) in adolescents with chronic pain compared to their healthy peers. Adolescents with chronic pain also reported greater activity limitations and poorer quality of life than the healthy group at enrollment. In addition, health service utilization differed significantly (p < 0.001) where youth with chronic pain had more than thirty times the number of primary and specialty care visits over the 6 months prior to enrollment compared to healthy youth. None of the adolescents in the healthy group were prescribed antidepressants, anticonvulsants, or opioids for pain management whereas 75% (n = 46) of youth with chronic pain were prescribed one or more of these medications to treat their pain problem (p < 0.001).
Persistence of Insomnia Symptoms over Time
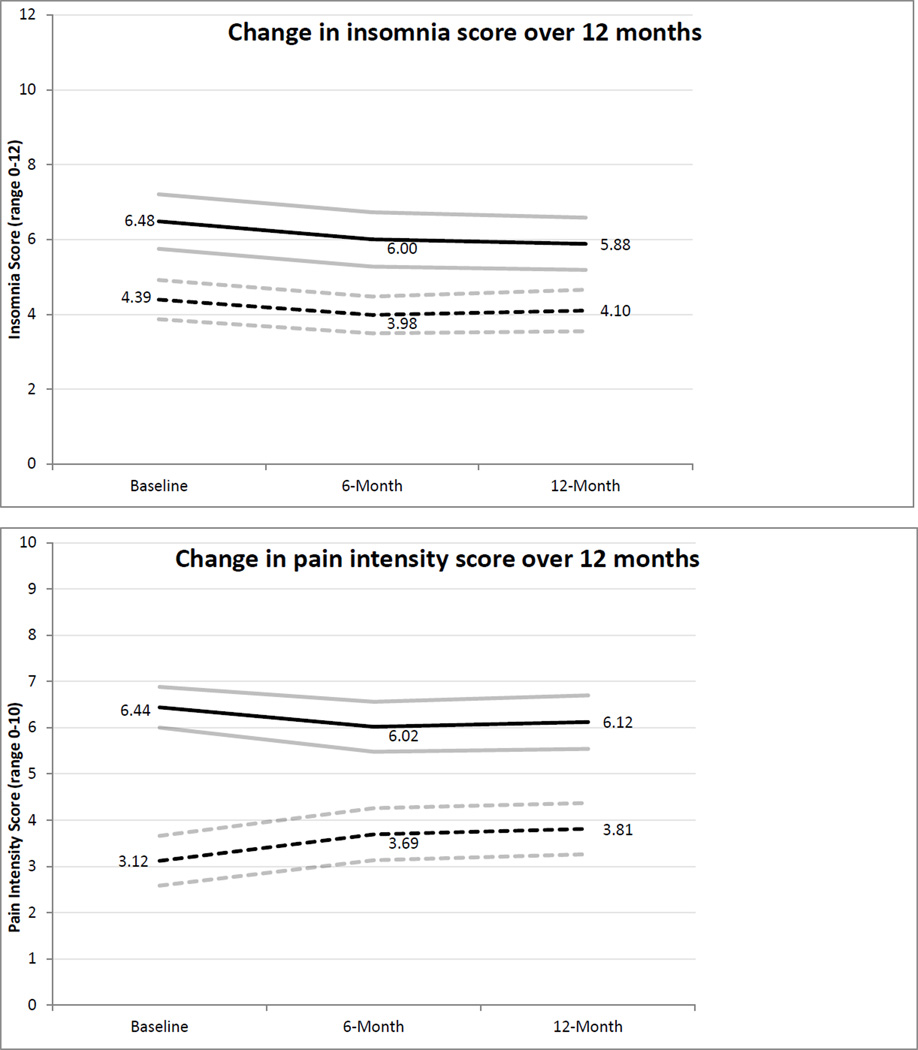
To test our hypothesis that insomnia symptoms would persist over time, we used additional cross-sectional time-series GEE modeling to examine temporal trends over the 12-month follow-up for each study group separately and across the entire sample. In these analyses, the models were adjusted for baseline insomnia symptoms and study time point was entered as an independent variable. In addition, analyses using the entire sample were adjusted for group status (pain vs. healthy). There were no significant time trends in either group (pain group p = 0.125, healthy group p = 0.314) or when the two groups were combined (p = 0.067), indicating stability in insomnia symptoms over the year. The pain group had higher insomnia symptoms across the study period compared to the healthy group (p = 0.017). Severity of insomnia symptoms at each time point between groups was also compared using independent-samples t-tests, indicating that adolescents with chronic pain had more severe insomnia symptoms at each time point compared to healthy adolescents (p’s < 0.001).
Time trends in pain intensity were also examined with GEE modeling, adjusting for baseline pain intensity, finding that pain intensity was stable in the chronic pain group (p = 0.166) but increased significantly in the healthy group over the 12-month follow-up period (p < 0.008). Pain intensity was stable in the moderate to severe range for youth with chronic pain (M’s = 6.44 at enrollment to 6.11 at 12-month follow-up). For healthy youth, pain intensity increased over time but remained in the mild range (M’s = 3.11 at enrollment to 3.81 at 12-month follow-up). Figure 1 shows the time trends in insomnia and pain intensity over the 12 month study period by group.
Figure 1.
Insomnia symptoms and pain intensity over 12 months by group.

Predictors of Insomnia Symptoms over 12 months
Predictors of insomnia symptoms over the 12 month period were examined inlongitudinal multi-variable GEE analysis, using data from all three time points (baseline, 6-month follow-up, 12-month follow-up). As shown in Table 2, we used a model of predictor variables which included group status (pain vs. healthy), pubertal development, depressive symptoms (CES-D), sleep hygiene, cognitive and somatic pre-sleep arousal, and pain intensity. As expected, the strongest predictor of insomnia symptoms in this model was group status. Adolescents with chronic pain had on average a 1.5-point increase on the insomnia symptom score over the healthy adolescents (range 2–12), p < 0.001. Sleep hygiene was another strong predictor of insomnia. Every point decrease in the sleep hygiene score, indicating poorer sleep hygiene practices (range 2–6), corresponded with a one-point increase in the insomnia symptom score (range 2–12), p = 0.003. Greater depressive symptoms were also a significant predictor of insomnia although the effect size was relatively small where every point increase in depressive symptoms score (range 0–48) corresponded to a 0.05-point increase in the insomnia symptom score, p = 0.031. The remaining variables were not statistically significant in predicting longitudinal insomnia symptoms.
Table 2.
Predictors of longitudinal insomnia symptoms using Generalized Estimating Equation (GEE) models
| Coefficient | 95% CI | p-value | |
|---|---|---|---|
| Pain Group | 1.52 | 0.90, 0.21 | <0.001 |
| Pubertal Development | −0.15 | −0.41, 0.11 | 0.262 |
| Depressive Symptoms | 0.05 | 0.01, 0.10 | 0.031 |
| Sleep Hygiene | −0.98 | −1.62, −0.34 | 0.003 |
| Cognitive Pre-sleep Arousal | 0.03 | −0.03, 0.10 | 0.327 |
| Somatic Pre-sleep Arousal | −0.02 | −0.11, 0.07 | 0.644 |
| Pain Intensity | 0.08 | −0.05, 0.21 | 0.208 |
Longitudinal Association of Insomnia Symptoms with Health-Related Quality of Life (HRQOL) and Activity Limitations
The relationship of insomnia symptoms with HRQOL and activity limitations was examined longitudinally in GEE models, using data from all three time points. Dependent variables included the psychosocial, physical, and total PedsQL scores, and the total CALI score. Results are summarized in Table 3. Independent variables included group status (pain vs. healthy), pain intensity, depressive symptoms and insomnia symptoms. As hypothesized, increased insomnia symptoms (p < 0.05 in all models) predicted poorer HRQOL outcomes (even after controlling for group status, pain intensity, and depressive symptoms). However, insomnia symptoms did not predict activity limitations after adjusting for groups status, pain intensity, and depressive symptoms (p-value=0.098). Greater depressive symptoms (p < 0.001 in all models) were a significant predictor of more activity limitations and poorer HRQOL. Membership in the chronic pain cohort and higher pain intensity emerged as significant predictors of Total HRQOL, Physical HRQOL, and activity limitations but not Psychosocial HRQOL.
Table 3.
GEE models testing associations between insomnia symptoms, health-related quality of life and activity limitations over 12 months after controlling for group status (pain vs. healthy), pain intensity, and depressive symptoms.
| Coefficient | 95% CI | p-value | ||
|---|---|---|---|---|
| PedsQL, Total | ||||
| Insomnia sx | −0.81 | (−1.33, −0.29) | 0.002 | |
| Pain group | −5.26 | (−8.74, −1.78) | 0.003 | |
| Pain intensity | −1.08 | (−1.67, −0.49) | <0.001 | |
| Depressive sx | −0.86 | (−1.01,−0.71) | <0.001 | |
| PedsQL, Psychosocial | ||||
| Insomnia sx | −0.69 | (−1.26, −0.12) | 0.017 | |
| Pain group | 2.03 | (−1.48, 5.55) | 0.257 | |
| Pain intensity | 0.51 | (−1.15, 0.13) | 0.118 | |
| Depressive sx | −1.03 | (−1.20, −0.86) | <0.001 | |
| PedsQL, Physical | ||||
| Insomnia sx | −1.10 | (−2.13, −0.08) | 0.035 | |
| Pain group | −19.69 | (−25.96, −13.43) | <0.001 | |
| Pain intensity | −2.15 | (−3.22, −1.09) | <0.001 | |
| Depressive sx | −0.55 | (−0.82, −0.28) | <0.001 | |
| Activity Limitations | ||||
| Insomnia sx | 0.58 | (−0.11, 1.27) | 0.098 | |
| Pain group | 13.62 | (9.90, 17.33) | <0.001 | |
| Pain intensity | 2.03 | (1.39, 2.68) | <0.001 | |
| Depressive sx | 0.34 | (0.18, 0.49) | <0.001 | |
Longitudinal Association of Insomnia Symptoms with Health Care Utilization
Adolescents with chronic pain had significantly more medical visits at baseline compared to their healthy counterparts (p < 0.001). This pattern persisted throughout the 12-month follow-up period. The association between persistent insomnia symptoms (defined as reporting insomnia symptoms at least 2 of the 3 time points) with the total number of primary and specialty care visits across the three time points was examined. In univariate regression analysis, persistent insomnia symptoms were associated with a greater number of medical visits (p < 0.001). As hypothesized, in multiple regression analysis controlling for group status (pain vs. healthy), insomnia symptoms approached statistical significance as a predictor of the number of medical visits (p=0.055), and group status (chronic pain cohort) was a significant predictor (p < 0.001).
Discussion
This is the first longitudinal study of insomnia symptoms in a pediatric pain population. Our findings demonstrated that insomnia persisted over a one year period for youth with and without chronic pain. Adolescents with chronic pain endorsed more problems with insomnia than healthy adolescents. Specifically, about half of the adolescents with chronic pain reported significant problems with falling asleep or staying asleep that continued over the 12 month period in contrast to about 20% of the healthy cohort. This high rate of insomnia symptoms is similar to that reported in cross-sectional studies of youth with chronic pain 11, 22, highlighting the importance of understanding the potential health impact of insomnia on youth with chronic pain given the frequency of this problem. It is important to note that insomnia symptoms also persisted among healthy youth in our sample who experienced everyday aches and pains, which is consistent with known associations between advancing pubertal development and increased risk for sleep problems during adolescence 6.
In our analysis of the potential adverse health outcomes of insomnia, significant associations were found between persistent insomnia symptoms and poorer health-related quality of life over 12 months. This extends findings of studies that have shown that sleep difficulties (and particularly insomnia symptoms) at a single time point are associated with concurrent physical function limitations, poor quality of life, and greater depressive symptoms 11, 19, 22. In addition, the analysis of outpatient medical service use showed that membership in the chronic pain cohort and persistent insomnia symptoms were related to a higher rate of utilization of outpatient medical services. Our study is the first to examine health service use in youth with chronic pain and comorbid insomnia. This finding extends similar work done in adults with chronic pain and insomnia that has also found a relationship to higher health service use including high costs of prescription medications 27, 29. Together, our findings suggest that insomnia symptoms not only persist over time but contribute to a pattern of poor adolescent quality of life. Uninterrupted, this pattern may continue to impact quality of life and function into adulthood.
Using a model that we previously tested with cross-sectional survey data, we examined behavioral factors that may contribute to longitudinal insomnia symptoms. We found that insomnia symptoms over time were best predicted by behavioral factors (e.g., pre-sleep arousal, sleep hygiene) rather than by pain intensity, which is consistent with the conceptualization of perpetuating factors for the maintenance of insomnia. Although pain intensity may serve to precipitate difficulties with falling or staying asleep, over time, behaviors surrounding sleep habits and behaviors present at bedtime are likely to sustain problems with insomnia symptoms. For example, a high level of cognitive or somatic arousal at bedtime (e.g., worries about sleep, physiological hyperarousal) may directly interfere with falling asleep quickly and lead to negative sleep habits (e.g., taking long naps during the day) that sustain problems with insomnia.
There are several limitations of the study that should be kept in mind when interpreting findings. There is not currently consensus on the ideal measure to assess insomnia symptoms in children or adolescents. This is dissimilar to assessment of insomnia symptoms in adults where one measure, the Insomnia Severity Index 1, is the gold standard. Thus, the ability to make comparisons across pediatric studies is more limited due to the differing methods that have been reported. Nonetheless, similar rates of sleep problems and insomnia symptoms have been found across pediatric studies. We are the first group to report on the association of insomnia symptoms with health care utilization of children and adolescents with chronic pain. This analysis should be considered somewhat preliminary given that we collected parent report data on service use only rather than cost data specifically. Further research is needed to replicate these findings using objective measures of healthcare utilization such as medical chart review and billing records.
There are several factors that could influence differences in longitudinal trajectories of insomnia symptoms between youth with chronic pain and healthy youth which are beyond the scope of this manuscript. For example, prescription pain medication use may impact daytime and nighttime sleep patterns and contribute to the persistence of insomnia symptoms over time. Research is needed to examine longitudinal associations between prescription pain medication use and insomnia symptoms. The impact of school schedules on sleep patterns in youth with chronic pain is also unknown, and future research could examine differences in insomnia symptoms by the setting where the adolescent receives schooling (e.g., home school vs. public or private school). Differences in longitudinal trajectories of insomnia symptoms between youth with chronic pain and youth with other types of chronic illness are also unknown.
Given the high rate of insomnia symptoms experienced by adolescents with chronic pain and their relationship to physical and psychosocial quality of life, there is a clear need to develop and test sleep interventions in this population. Focused strategies aimed directly at targeting dysfunctional thoughts and behavioral patterns around sleep, such as stimulus control strategies and sleep restriction that are components of cognitive-behavioral therapy for insomnia, may lead to improvements in sleep behaviors.
Cognitive-behavioral therapy (CBT) has been recommended by the American Academy of Sleep Medicine (AASM) for the treatment of adult insomnia and has proven effective in diverse patient populations with comorbid medical conditions 23. The primary treatment strategies of CBT for insomnia (CBT-I) include cognitive strategies to reduce dysfunctional thoughts about sleep, relaxation strategies to facilitate sleep onset, and stimulus control and sleep restriction to strengthen the association between sleep and the stimulus conditions under which sleep typically occurs. CBT-I has already been used effectively in adults with chronic pain and insomnia. For example, Vitiello and colleagues 33 demonstrated that not only do insomnia symptoms improve but that pain is also reduced in older adults receiving CBT-I.
CBT-I may also have substantial benefits for adolescents with chronic pain but this has not yet been evaluated. In fact, there is an extremely limited literature on treatment of sleep problems in children or adolescents with chronic pain. Only a few studies have evaluated any type of sleep intervention in pediatric pain populations. In one study of children with migraine 4, children randomized to receive sleep hygiene education had reduced migraines compared to children in a standard care control group. In a study of youth with fibromyalgia 9, sleep interventions were a component of a cognitive-behavioral intervention that was evaluated in an uncontrolled trial. Although both of these studies concluded that sleep intervention had positive effects on children’s pain, both trials were small, had limited assessment of sleep either at screening/enrollment or assessment of changes in sleep patterns or quality with treatment. Therefore, the efficacy of sleep interventions in pediatric pain populations is currently unknown. Well-designed trials of specific sleep interventions (e.g., CBT-I) are needed in children and adolescents with chronic pain.
Because of the adverse effects of inadequate sleep and sleep problems on children’s health, mood, and quality of life, assessment and treatment of sleep disturbances and disorders are an important part of the clinical care of children and adolescents with chronic pain.
Acknowledgments
We wish to thank the teens and families who participated in this study. We also acknowledge Caitlin Murray, Lexa Murphy, Gabrielle Tai, and Gabriel Dawson who provided invaluable research assistance.
This study was supported by the National Institute of Child Health and Human Development grant number R01HD053431 awarded to the first author (T.M.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no financial or other relationship that might lead to a conflict of interest.
References
- 1.Bastien CH, Vallieres A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 2.Bloom BJ, Owens JA, McGuinn M, Nobile C, Schaeffer L, Alario AJ. Sleep and its relationship to pain, dysfunction, and disease activity in juvenile rheumatoid arthritis. J Rheumatol. 2002;29:169–173. [PubMed] [Google Scholar]
- 3.Bruni O, Fabrizi P, Ottaviano S, Cortesi F, Giannotti F, Guidetti V. Prevalence of sleep disorders in childhood and adolescence with headache: A case-control study. Cephalalgia. 1997;17:492–498. doi: 10.1046/j.1468-2982.1997.1704492.x. [DOI] [PubMed] [Google Scholar]
- 4.Bruni O, Galli F, Guidetti V. Sleep hygiene and migraine in children and adolescents. Cephalalgia. 1999;19(Supp 25):57–59. doi: 10.1177/0333102499019s2516. [DOI] [PubMed] [Google Scholar]
- 5.Butbul Aviel Y, Stremler R, Benseler SM, Cameron B, Laxer RM, Ota S, Schneider R, Spiegel L, Stinson JN, Tse SM, Feldman BM. Sleep and fatigue and the relationship to pain, disease activity and quality of life in juvenile idiopathic arthritis and juvenile dermatomyositis. Rheumatology (Oxford) 2011;50:2051–2060. doi: 10.1093/rheumatology/ker256. [DOI] [PubMed] [Google Scholar]
- 6.Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17:5–12. [PubMed] [Google Scholar]
- 7.Chorpita BF, Yim L, Moffitt C, Umemoto LA, Francis SE. Assessment of symptoms of dsm-iv anxiety and depression in children: A revised child anxiety and depression scale. Behaviour Research & Therapy. 2000;38:835–855. doi: 10.1016/s0005-7967(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 8.Cohen LL, Lemanek K, Blount RL, Dahlquist LM, Lim CS, Palermo TM, McKenna KD, Weiss KE. Evidence-based assessment of pediatric pain. J Pediatr Psychol. 2008;33:939–955. doi: 10.1093/jpepsy/jsm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degotardi PJ, Klass ES, Rosenberg BS, Fox DG, Gallelli KA, Gottlieb BS. Development and evaluation of a cognitive-behavioral intervention for juvenile fibromyalgia. J Pediatr Psychol. 2006;31:714–723. doi: 10.1093/jpepsy/jsj064. [DOI] [PubMed] [Google Scholar]
- 10.Gregory AM, Willis TA, Wiggs L, Harvey AG. Presleep arousal and sleep disturbances in children. Sleep. 2008;31:1745–1747. doi: 10.1093/sleep/31.12.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaPlant MM, Adams BS, Haftel HM, Chervin RD. Insomnia and quality of life in children referred for limb pain. J Rheumatol. 2007;34:2486–2490. [PubMed] [Google Scholar]
- 12.Law EF, Dufton L, Palermo TM. Brief report: Daytime and nighttime sleep patterns in adolescents with and without chronic pain. Health Psychology. 2011 doi: 10.1037/a0026485. advance online publication doi: 10.1037/a0026485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeBourgeois MK, Giannotti F, Cortesi F, Wolfson AR, Harsh J. The relationship between reported sleep quality and sleep hygiene in italian and american adolescents. Pediatrics. 2005;115:257–265. doi: 10.1542/peds.2004-0815H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewandowski AS, Palermo TM, De la Motte S, Fu R. Temporal daily associations between pain and sleep in adolescents with chronic pain versus healthy adolescents. Pain. 2010;151:220–225. doi: 10.1016/j.pain.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meltzer LJ, Logan DE, Mindell JA. Sleep patterns in female adolescents with chronic musculoskeletal pain. Behav Sleep Med. 2005;3:193–208. doi: 10.1207/s15402010bsm0304_2. [DOI] [PubMed] [Google Scholar]
- 16.Miller VA, Palermo TM, Powers SW, Scher MS, Hershey AD. Migraine headaches and sleep disturbances in children. Headache. 2003;43:362–368. doi: 10.1046/j.1526-4610.2003.03071.x. [DOI] [PubMed] [Google Scholar]
- 17.Murray CB, Murphy LK, Palermo TM, Clarke GM. Brief report: Pain and sleep-wake disturbances in adolescents with depressive disorders. Journal of Clinical Child and Adolescent Psychology. 2012;41:482–490. doi: 10.1080/15374416.2012.658613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: The development of the pre-sleep arousal scale. Behaviour Research & Therapy. 1985;23:263–271. doi: 10.1016/0005-7967(85)90004-x. [DOI] [PubMed] [Google Scholar]
- 19.Palermo TM, Fonareva I, Janosy NR. Sleep quality and efficiency in adolescents with chronic pain: Relationship with activity limitations and health-related quality of life. Behav Sleep Med. 2008;6:234–250. doi: 10.1080/15402000802371353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palermo TM, Lewandowski AS, Long AC, Burant CJ. Validation of a self-report questionnaire version of the child activity limitations interview (cali): The cali-21. Pain. 2008;139:644–652. doi: 10.1016/j.pain.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palermo TM, Toliver-Sokol M, Fonareva I, Koh JL. Objective and subjective assessment of sleep in adolescents with chronic pain compared to healthy adolescents. Clinical Journal of Pain. 2007;23:812–820. doi: 10.1097/AJP.0b013e318156ca63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palermo TM, Wilson AC, Lewandowski AS, Toliver-Sokol M, Murray CB. Behavioral and psychosocial factors associated with insomnia in adolescents with chronic pain. Pain. 2011;152:89–94. doi: 10.1016/j.pain.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perlis ML, Junquist C, Smith MT, Posner D. Cognitive behavioral treatment of insomnia. New York: Springer; 2005. [Google Scholar]
- 24.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 25.Radloff LS. The ces-d scale: A self-report depression measure for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 26.Roth-Isigkeit A, Thyen U, Stoven H, Schwarzenberger J, Schmucker P. Pain among children and adolescents: Restrictions in daily living and triggering factors. Pediatrics. 2005;115:152–162. doi: 10.1542/peds.2004-0682. [DOI] [PubMed] [Google Scholar]
- 27.Smith MD, McGhan WF. Insomnia: Costs to lose sleep over. Business & Health. 1997;15:57–58. 60. [PubMed] [Google Scholar]
- 28.Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatric Clinics of North America. 1987;10:541–553. [PubMed] [Google Scholar]
- 29.Stoller MK. Economic effects of insomnia. Clinical Therapeutics. 1994;16:873–897. discussion 854. [PubMed] [Google Scholar]
- 30.Toliver-Sokol M, Murray CB, Wilson AC, Lewandowski A, Palermo TM. Patterns and predictors of health service utilization in adolescents with pain: Comparison between a community and a clinical pain sample. J Pain. 2011 doi: 10.1016/j.jpain.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valrie CR, Gil KM, Redding-Lallinger R, Daeschner C. Brief report: Sleep in children with sickle cell disease: An analysis of daily diaries utilizing multilevel models. J Pediatr Psychol. 2007;32:857–861. doi: 10.1093/jpepsy/jsm016. [DOI] [PubMed] [Google Scholar]
- 32.Varni JW, Burwinkle TM, Szer IS. The pedsql multidimensional fatigue scale in pediatric rheumatology: Reliability and validity. Journal of Rheumatology. 2004;31:2494–2500. [PubMed] [Google Scholar]
- 33.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. Journal of Clinical Sleep Medicine. 2009;5:355–362. [PMC free article] [PubMed] [Google Scholar]
- 34.von Baeyer CL. Numerical rating scale for self-report of pain intensity in children and adolescents: Recent progress and further questions. European Journal of Pain. 2009;13:1005–1007. doi: 10.1016/j.ejpain.2009.08.006. [DOI] [PubMed] [Google Scholar]