Abstract
The development of oral drug delivery platforms for administering therapeutics in a safe and effective manner across the gastrointestinal epithelium is of much importance. A variety of delivery systems such as enterically coated tablets, capsules, particles, and liposomes have been developed to improve oral bioavailability of drugs. However, orally administered drugs suffer from poor localization and therapeutic efficacy due to various physiological conditions such as low pH, and high shear intestinal fluid flow. Novel platforms combining controlled release, improved adhesion, tissue penetration, and selective intestinal targeting may overcome these issues and potentially diminish the toxicity and high frequency of administration associated with conventional oral delivery. Microfabrication along with appropriate surface chemistry, provide a means to fabricate these platforms en masse with flexibility in tailoring the shape, size, reservoir volume, and surface characteristics of microdevices. Moreover, the same technology can be used to include integrated circuit technology and sensors for designing sophisticated autonomous drug delivery devices that promise to significantly improve point of care diagnostic and therapeutic medical applications. This review sheds light on some of the fabrication techniques and addresses a few of the microfabricated devices that can be effectively used for controlled oral drug delivery applications.
Keywords: Bioadhesive Devices, Unidirectional Release, Sustained Release, Pulsatile Release, Microfabrication, Photolithography, Gastrointestinal Targeting
1. Introduction
In the past two decades, micro- and nanotechnology has exerted a greater influence on how researchers understand and design analytical micro- and nanomaterials to unravel the mysteries of systems biology, thereby delivering novel devices for various biomedical applications. Specifically in the field of controlled- and/or sustained drug delivery, the translation of semiconductor technology into producing micro-electro-mechanical systems (MEMS) and microfluidic lab-on-chip biomedical systems has enabled the field of point of care medicine to grow leaps and bounds. More recently MEMS technology has been widely employed in generating platforms for use in tissue engineering, drug delivery, diagnostic, and therapeutic applications. This review addresses the progress and prospects of microfabricated technologies that can be used towards the development of novel oral drug delivery devices.
Among the various conventional drug delivery routes of administration (intravenous, intramuscular, etc), oral administration is the preferred route as it offers numerous advantages such as being less invasive, self-administrable leading to higher patient compliance, rapid availability, and lower cost of manufacture. Unlike intravenous and intramuscular administrations, wherein organ accumulation of the delivery vehicle is a common issue, the size of the oral delivery vehicle can be tuned to avoid endocytosis and still be eliminated by the gastrointestinal (GI) tract in a matter of hours. However, the human GI tract forms a formidable barrier for the absorption of active therapeutics such as proteins, peptides, and other large molecules until they are broken down into smaller molecules [1]. In addition to difficulties of permeating the thick mucus layers and intestinal epithelial tight junctions (figure 1a), the acidic environment of the stomach, combined with an array of intestinal enzymes and flow conditions reduce the oral bioavailability to values as low as 3% [2]. Although a variety of delivery paradigms including enteric-coated capsules, tablets, liposomes, use of bioadhesive agents, and permeation enhancers have been developed to raise this number, many drugs are currently administered with increased frequency on an extended schedule [3–11]. This approach is not practical for expensive biologics/proteins and toxic drugs, especially those used to treat cancer, wherein most of the therapeutic is often non-specifically delivered to the healthy tissue throughout the intestine leading to atrophy of healthy tissue [12, 13]. Other disadvantages include increased risk of side effects due to toxic concentrations of drug usage, absence of targeting strategies for the treatment of intestinal diseases such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and Crohn’s disease, and the need to administer multiple individual pills in order to deliver multiple drugs.
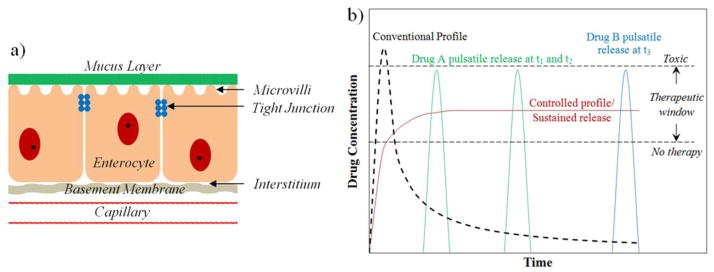
Figure 1.
(a) Schematic structure of the intestinal epithelium through which the oral drug has to pass across to reach the blood plasma. (b) The model concentration versus time profiles for conventional and controlled release drug delivery devices. Also shown are the model profiles demonstrating multiple pulsatile releases for different drugs that can be achieved using novel microfabricated multireservoir drug delivery devices.
1.1. The need for novel drug delivery systems
The route of drug delivery has a significant effect on the drug’s therapeutic efficacy, safety, and bioavailability [14]. The ideal aim of administration should be to deliver the drug at a required concentration within the therapeutic window at the right time to a specific target, in a safe and reproducible manner. Figure 1b. shows a model profile of a conventionally administered oral drug after its passage through the intestinal epithelium and entrance into the blood plasma. The initial concentration of the delivered drug has a sharp rise that may exceed a toxic level beyond the therapeutic window followed by a decrease over time to a sub-therapeutic level making the duration of therapy dependent on the frequency of administration and the half life of the drug. This inconsistency in maintaining the drug concentration within the therapeutic window along with the low bioavailability renders traditional oral administration ineffective. Therefore, there is an inherent need for alternate systems, an example being the controlled release system, which achieves a sustained drug release in the therapeutic window at a rate equivalent to the rate of drug degradation and elimination over an extended period of time (figure 1b). However, in certain cases such as hormone delivery, diabetic treatment, and others, the preferred method of drug delivery is in the form of pulses at variable time intervals as shown in figure 1b [15]. There are also instances such as the treatment of malaria, cancer, and others, wherein combination therapies involving multiple drugs to exploit the synergistic and additive potential of individual drugs are required [16]. All these requirements were initially achieved by employing polymers as drug carrying matrices, wherein their diffusive, degradability, and/or responsive properties to external stimuli controlled the sustained release of the drug [17–26].
The versatility of using polymers in combination with nanotechnology has led to several innovative ideas of miniaturization of oral drug delivery carriers for controlled release and targeting. Most of these miniaturized systems are based on polymer gels, vesicles, liposomes, or inorganic metallic and semiconducting microspheres or nanoparticles [27–31]. The common techniques used to synthesize the various particulate systems include emulsification or droplet extrusion, suspension and dispersion, solvent evaporation, nanoprecipitation, and spray drying techniques [32–34]. Although these techniques allow for the mass production of microspheres and nanoparticles, in the majority of cases, these particulates tend to be polydisperse and coagulated. Polydispersity is an issue for therapies requiring spatial and temporal targeting, wherein specific modes of cellular uptake to the nucleus are dependent on the size of the particle [35, 36]. Also, the ability to manufacture reproducible monodispersed particles in large numbers is critical for dosing, safety and regulatory reasons, achieving tighter release rate profiles, higher yields, simpler purification processes, and for scalability [37]. While size of the microspheres and nanoparticles as mentioned above is critical for various reasons, the symmetrical spherical shape of the particle causes a multidirectional drug release [1]. Instead of the drug being available only at the mucus-device interface, the multidirectional release in the surrounding GI fluid results in drug loss and thus may reduce the oral therapeutic efficacy. Considering the above issues with spherical particulates, there is an imminent need for the mass fabrication of easily reproducible, asymmetrical, unidirectional, mucoadhesive microdevices for achieving better oral administration efficacies.
1.2. Developing drug delivery systems using microfabrication technologies
Microfabrication, defined as the collection of techniques developed to fabricate micron sized features is best suited to develop the aforementioned novel drug delivery microdevices. In addition to the increased cellular integration, adhesion, proliferation, differentiation, and signaling observed with the use of micro- and nanomaterials, the size and shape of the device plays a significant role in determining receptor mediated phagocytosis [38–42]. Using microfabrication, the size of the devices can be fabricated small enough to allow better contact with the undulations of the intestinal wall and yet large enough to avoid device endocytosis. Unlike spherical particles, the microdevice shape can be engineered using computer aided software prior to microfabrication. They can be made flat, thin, and disc-shaped to maximize the contact surface area for better ligand and intestinal cell presentation and minimize the shear stress experienced by the device side areas due to the continuous liquid flow in the intestines [1, 43]. Unlike microspheres and nanoparticles, wherein surface functionalization results in particle agglomeration (via charge neutralization, hydrogen bonding, etc.), the surface chemistry of devices can be uniformly or selectively modified during on-wafer fabrication with ease. Modifications can include bioadhesive targeting agents such as lectins that recognize and bind to intestinal mucosa and/or enzyme inhibitors to protect the drug from degradation by the colonic enzymes [44, 45]. The other vital advantage is that the microdevice can be precisely machined to include multiple reservoirs of desired size to contain multiple drugs/biomolecules of interest [46, 47]. The unidirectional drug release from these reservoirs also achieves a highly localized concentration of the drug in close proximity to the targeted cells. Finally, the facile incorporation of integrated circuit technology to make microelectromechanical systems (MEMS) and nanoelectromechanical systems (NEMS), facilitating closed loop feedback controlled drug release may enable therapy that will be responsive to the patient needs, thereby significantly improving the future of medical care.
MEMS technology is a powerful platform for delivering potent therapeutic agents whose temporal administration is vital to the drugs therapeutic efficacy. The ease of en masse fabrication with consistency, along with the device portability, and a potential for multi-functioning single-use application make them applicable in both biosensing and therapeutic applications. MEMS technology has been used to fabricate microreservoirs, micropumps, nanoporous membranes, microvalves, microfluidic channels, and sensors for various modes of drug administration [48–51]. Such devices are typically fabricated using silicon substrates [52], but alternative materials such as glass, gold, metal thin films, and metal oxides have also been used to improve reliability and design flexibility, and to decrease cost [51, 53]. The relatively low cost and versatility in modifying/tuning the various physicochemical properties such as responsive behavior, degradability, and biocompatibility using simple chemistry make polymers (e.g. polymethylmethacrylate (PMMA), polyethyleneglycol (PEG), polylactic acid (PLA), polyglycolic acid (PGA), poly(DL-lactide-co-glycolide) (PLGA), poly(caprolactone) (PCL), poly(glycerol-sebacate) (PGS)) as alternatives to silicon for bioMEMS based applications [54, 55]. A variety of the MEMS based techniques as applied to fabricate devices for therapeutic delivery will be highlighted as a general overview in the following section followed by a few exemplary devices that can be effectively used as such or modified for achieving effective oral drug administration.
2. Microfabrication techniques
Developed as the workhorse of the microelectronics industry, lithographic microfabrication provides a mature set of tools for the en masse fabrication of devices for computation, memory storage, wireless communication, remote sensing, and novel biomedical diagnostic and therapeutic applications [37, 51]. They have developed tremendously from the traditional use of light-projection techniques to maskless projection of laser light, electrons, ions, or molecules to patterning onto substrates for fabricating features ranging from a few nanometers to several microns [56]. These techniques have led to features with high aspect ratios that are known to alter cell phenotype, proliferation, and differentiation [51, 57–59]. Some of the lithographic techniques widely used in the biomedical world for optimizing drug release kinetics [60, 61], binding molecule functionalization [41, 42], surface fouling characteristics [62], and others are highlighted below.
2.1. Conventional photolithography
Optical or photolithography is the most successful technology in fabricating MEMS/NEMS devices, microarrays, lab on a chip, and other microdevices. The process involves the photopolymerization of a thin resist film through the localization of light using a photomask that defines the pattern shape. By using alternating steps of masked exposure and thin film application, multi-layered resists can be formulated to control the size and aspect ratio of the microfeature [51]. The incorporation of micromachining processes such as chemical etching and surface micromachining with photolithography has resulted in the development of a variety of biomedical microdevices including Beebe’s microactuator [63], Peppas group’s microcantilevers [64, 65], Baldi’s micropumps and microvalves [66], and Madou’s microactuators [67]. The localization of micromachining processes is controlled by the selection of suitable photoresists, such as SU-8 epoxy resins, PMMA, and phenol-formaldehyde mixtures during the photolithography process. Photolithographic patterning of other polymers in the presence of a photoinitiator proves useful to tailor specific material properties such as hydrophobicity, biodegradability, and biocompatibility that play a role in drug release kinetics, cellular interaction, and immunogenicity. These properties can also be modified by varying the chemical structure/functionality of the monomer used, its molecular weight, and/or crosslinking density [68–71].
2.2. High energy lithography
Since many of the scales encountered in the field of biology and medicine lie in the sub-nanometer range, fabricating features at this size scale is necessary. As the desired feature size decreases, an illuminating source with a shorter wavelength and/or a smaller numerical aperture is required. This led to the development of high energy microfabrication techniques including X-ray LIGA (lithography, electroforming, and molding), e-beam lithography, and ion-beam lithography. In X-ray LIGA, a synchrotron X-ray source in combination with electro-deposition is used to fabricate high aspect ratio nanofeatures that can either be used directly or for further molding and embossing steps [72]. Modification of the aforementioned process using an inexpensive UV light (UV-LIGA) source to expose SU-8 has emerged as a more readily available technique and results in microstructures with aspect ratios greater than 50:1 [73–75]. Electron beam (or ‘e-beam’) lithography rastors a beam of electrons across an electron-irradiation sensitive substrate to create a pattern that is subsequently developed into required features [76]. An alternate method used on a wide variety of materials for achieving nanometer scale resolution is the focused ion beam (FIB) lithography, which involves sputtering heavy ions onto a substrate to modify the surface [77]. A novel approach that reduces the number of lithographic steps involved in the fabrication of 3D patterned features relies on freeing the substrate from its conventional orthogonal exposure with respect to the projection source. In this case, X-ray and UV sources have been used for patterning on a substrate stage that is freely rotated along three axes allowing multiple exposures from different angles prior to a single combined development process to obtain the feature with fewer steps [78–81]. Although conventional photolithography and high energy lithographic techniques are successful for microfabrication in the semiconductor industry, disadvantages including the need for a better illuminating source, higher working costs, and difficulty of en masse production, maintenance, and safety considerations limit their viability for most biomedical device applications. In response to these issues, the field of soft lithography has been developed to fabricate high quality micro- and nanostructures, without having the continuous need for the laborious photolithographic steps.
2.3. Soft lithography
Soft lithography has found numerous applications in the field of biology and biochemistry. Soft lithography is well suited to control the composition, topography, and properties of surfaces [82], patterning of hydrophilic or hydrophobic molecules or polymers [83], polysaccharides [84], stimuli sensitive and responsive materials [85], and proteins or growth factors [86] over a wide variety of surfaces. In soft lithography, the key element is an elastomeric stamp or mold, which transfers the pattern to the substrate. The same mold can be used multiple times due to its rigidity, and this enables rapid prototyping of similar patterns. Another advantage is the ability to pattern non-planar surfaces due to the flexibility of molds [87]. Further, the amalgamation of various soft lithography techniques with self assembled monolayers (SAMs) has led to the easy patterning of various functionalities over surfaces [88].
The most commonly employed soft lithography techniques for fabricating high quality micro- and nanostructures include replica molding (REM), microtransfer molding (μTM), micromolding in capillaries (MIMIC), solvent assisted micromolding (SAMIM), and nanoimprint lithography [82, 87–90]. Polydimethylsiloxane (PDMS), the widely used soft lithography stamp is usually prepared by replica molding (REM). The advantages of using PDMS for soft lithography are that it is chemically inert, adheres reversibly to most surfaces, homogenous, isotropic, and optically transparent down to about 300 nm allowing UV crosslinking of moldable prepolymers [87]. Other materials that can be used as stamps are polyurethanes, polyimides, and crosslinked Novolac resins [91]. In μTM, a drop of the prepolymer solution is applied to a PDMS mold. Then the mold is placed in contact with the substrate and irradiated or heated to cure into a solid. The mold is finally peeled away to leave the patterned microstructure on the surface of the contacted substrate [92]. In MIMIC, the PDMS mold is made to come into contact with the substrate surface. A low viscosity prepolymer solution is then placed at the open ends of the mold’s channel features, wherein by capillary action, the solution fills up the insides of the channels. After thermal or optical curing is complete, the PDMS is peeled away and a network of polymer features remains on the substrate [93]. In addition to making quasi-three-dimensional structures, SAMIM can be used to modify surface morphologies of polymers [94]. Herein, a PDMS mold is wetted with a solvent that is known to be good for the polymer. It is then brought into contact with the surface of the polymer substrate, which is dissolved or swollen in a gel-like feature that conforms to the surface topology of the PDMS mold. With time, the polymer solidifies and results in a pattern complementary to that of the mold. Nanoimprint lithography is a two step process, wherein the first step involves the pressing of a mold with nanostructured features into a thin resist cast on a substrate, followed by its removal. During this step, the resist is heated above its glass transition temperature into a viscous flowing liquid that can be deformed and shaped. The second step is the final pattern transfer where an anisotropic etching process such as reactive ion etching is carried out to remove any residual resist that escaped the compression step [95, 96].
2.4. Other novel techniques
Direct-write techniques including fused deposition modeling, stereolithography, inkjet printing, and scanning probe lithography are some of the maskless approaches towards the fabrication of microdevices. Similar to the aforementioned techniques, maskless fabrication of the various three dimensional features are based on the concept of assembling a series of two dimensional cross sectional areas [37]. Scanning probe lithography involves the writing of a pattern by rastering a sharp tip over the substrate. This method achieves a fine level of control and can even achieve precise arrangement of atoms over the substrate [97]. When the tip is exposed to a high energy as in atomic force microscopy anodization or local oxidation nanolithography, it results in physical, chemical, or electronic deformation/subtraction of the substrate resulting in high resolution precise features. Dip pen nanolithography, an additive probe lithography technique can be used to pattern sub-100 nanometer features using self assembling molecules over various substrates [98]. A series of these probes has been used in parallel for en masse fabrication [99, 100].
3. Microfabricated implants and devices for oral drug delivery
In the past two decades, research on the use of micro- and nanotechnology for biology and medicine has had an overall impact over a broad range of therapies and tissue applications. Although a multitude of MEMS/NEMS platform based implants have been developed for various controlled/sustained drug delivery applications, not much work has been done on fabricating microdevices for oral administration. A few of these microfabricated devices are highlighted as follows.
3.1. Electronically actuated devices
The earliest research on novel oral drug delivery platforms included the GI microcapsule and the ChipRx smart pill. The microcapsule carries out real-time drug release and gastrointestinal fluid sampling, while passing through the GI tract [101]. This remote controlled capsule made of polycarbonate measures about 1 cm in diameter and 3 cm in length and has five components - a location monitoring unit, a receiver, a driving device, a reservoir for drug and sampling, and a battery. The key component of the capsule, the driving device is a stretchable component and MEMS calorific elements trigger that works as a piston. With the wireless activation of the stretchable component, a built-in piston is driven to push the drug out of the reservoir. Liquid sampling via vacuum sorption takes place simultaneously making this capsule a vital therapeutic sensor in addition to drug delivery. The ChipRx smart pill is a matchstick sized, implantable, single reservoir device equipped with an external sensor that detects changes in the surrounding environment [53]. After the sensor’s signal is processed by a built-in chip, a drug releasing signal is sent, actuating a polymer membrane that covers the reservoir. The controlled actuation of the polymer results in the release of a measured amount of drug. Even though, the ChipRx device was not intended for oral administration, this and most of the following implantable microdevices can be modified for oral drug delivery applications. A similar device with multiple drug reservoirs gated with electronically actuated smart polymers can be used to dispense multiple drugs at different instances in the GI tract, thereby making it widely applicable for patients with a range of physiological conditions.
3.2. Responsive hydrogel coated microdevices
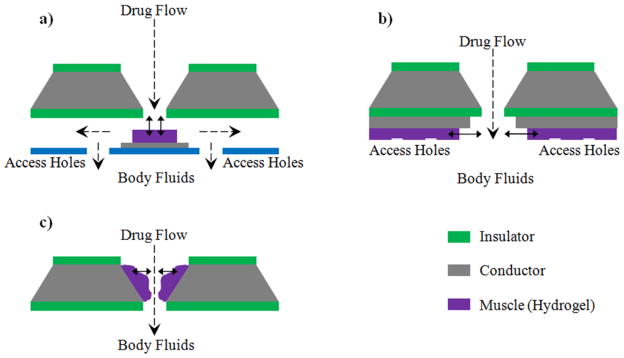
One class of smart polymers that can be effectively used for pulsatile/controlled drug delivery applications are responsive hydrogels. The ability of hydrogels to reversibly respond to variations in their environment and the corresponding rubbery nature makes them viable for widespread applications in the biological and medical fields [102]. Beebe et al. [63, 103] have lithographically fabricated microfluidic actuating systems, in which the hydrogel present at the junction of the microchannel actuates in response to the changes in environmental conditions, thereby gating the microchannel accordingly. Baldi and his group [66] have synthesized micropumps and microvalves using a micro-structured silicon membrane with entrapped hydrogels for environmentally sensitive fluid gating. The stimuli sensitive hydrogel posts inside the orifice were incorporated using thermal polymerization. Madou et al. [67] have introduced a responsive controlled drug release system, where the controlled release is achieved by actuating a polymeric valve system also called an ‘artificial muscle’ (synthesized using a blend of redox polymer and hydrogel). Several configurations for the ‘artificial muscle’ valves have also been proposed for use in biomedical micro- and nanodevices (figure 2). Dorski et al. and Alarco’n et al. have fabricated microdevices with pH responsive hydrogels encompassing enzyme glucose oxidase for insulin delivery applications [104, 105]. When blood glucose level rises, the enzyme converts glucose to gluconic acid. Presence of gluconic acid reduces the surrounding pH thereby actuating the hydrogel gate for blood glucose concentration dependent insulin release.
Figure 2.
Schematic designs for opening and closing holes in a drug reservoir using the hydrogel based artificial muscle concept. (a) Plunger configuration, (b) Tube configuration, and (c) Sphincter configuration [67].
3.3. Asymmetric bioadhesive microdevices
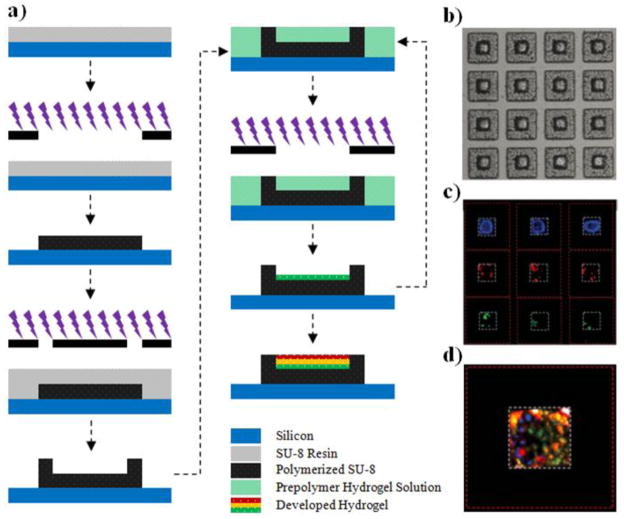
The short retention time of various drugs and recently developed novel particulate systems (and aforementioned devices) in the GI tract deter the progress of oral drug delivery from its conventional methods of administration. This is mostly due to the shear stress exhibited by the particulates during the constant flow of GI fluids. Drug retention can be enhanced by extending the time period of the drug carrier at the site of release, which in turn can be enhanced by introducing bioadhesive properties to the carrier. For nearly a decade, the Desai Lab has been developing novel bioadhesive microdevices to overcome this and other GI delivery related issues [106–110]. Using a sequential layer by layer photolithographic process (Figure 3), we have successfully fabricated asymmetric, flat, single reservoir microdevices that result in unidirectional drug release for enhanced GI delivery by increasing the localized concentration of drug across the epithelium [1, 106]. In these devices, multiple drugs can be easily loaded and released sequentially or simultaneously by employing a suitable drug encompassing hydrogel. The planar shape of the particle reduces the side area of the device susceptible to shear stress, making them superior to spherical particles for drug delivery. The bioadhesive property of the devices can be enhanced by introducing adhesive proteins such as lectins that specifically target the glycosaminoglycan sugar molecules available on the surface of human colonic cells. Ainslie et al. demonstrated the incorporation of mucoadhesive lectins with the drug encompassing hydrogel matrix in the device reservoir to show enhanced bioadhesive property under physiologically relevant flow conditions [107].
Figure 3.
Asymmetric, unidirectional releasing microdevices. (a) Schematic flow diagram depicting the sequential layer by layer photolithographic process, (b) Optical micrograph of drug-hydrogel loaded devices, (c) Fluorescent micrograph showing multiple model drugs (DNP-BSA; FITC-BSA; Texas red-BSA) loaded via layer by layer lithography in the same reservoir, and (d) Fluorescent micrograph of combined filters showing the three BSAs loaded in the same microdevice [106].
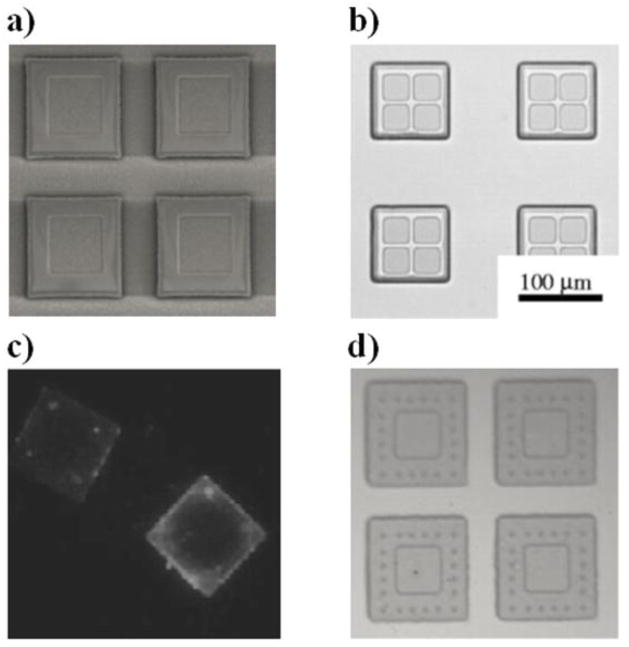
An alternate way of introducing mucoadhesives to the device involves the surface functionalization of the carrier instead of the hydrogel to covalently conjugate the proteins. These SU-8 microdevices can be modified chemically using traditional silane chemistry or acid catalyzed epoxy ring opening to incorporate various functional groups. Tao et al. used N-lithioethylenediamine to introduce amine groups on the surface of biocompatible PMMA microdevices that were pre-fabricated using a series of photolithography and reactive ion etching steps (figure 4) [43, 108, 109]. Using simple carbodiimide chemistry of the amine groups, mucoadhesive lectins were introduced on the surface of the PMMA carriers for increasing device bioadhesion. The reactive ion etching method as performed by Tao et al. is used to fabricate multireservoir containing microdevices with ease. Unlike multidrug loaded single reservoir devices (as shown in figure 3d), wherein the release profile of each drug is dependent on the property of all hydrogel layers, the release profile of multireservoir microdevice loaded with individual drugs in individual reservoirs is dependent solely on the property of the respective encompassing polymer/hydrogel matrix. These devices can be effectively used to treat diseases such as cancer that require a combination of different drugs acting therapeutically in different therapeutic windows. The Desai lab has also fabricated microdevices with microposts instead of targeting ligands to enhance the bioadhesive property [110].
Figure 4.
Bioadhesive PMMA microdevices fabricated using photolithography and reactive ion etching. Optical micrograph of (a) single [43] and (b) multi-reservoir fabricated microdevices [108], (c) fluorescent micrograph showing the presence of covalently bound avidin-FITC as a model for introducing targeting proteins to device surfaces [109], and (d) microdevices containing microposts for the physical introduction of bioadhesive property [110].
3.4. Microdevices with degradable polymers
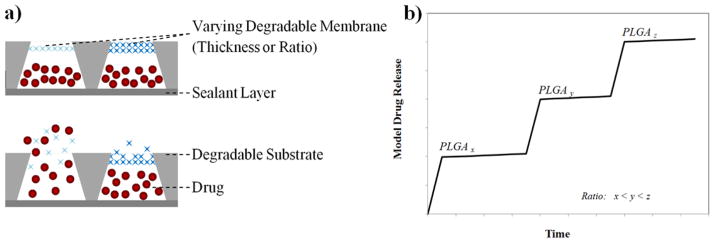
Unlike responsive hydrogels, whose release profile is dependent on the rate of achieving equilibrium swelling ratio, the rate of degradation in biodegradable polymer networks can be harnessed for precise time-dependent drug release. In addition to the size and shape of the reservoir controlling the release profile, the functionality and molecular weight of a degradable polymer can be tuned to control the degradation rate for achieving pulsatile release. A single biodegradable polymer-based microchip made up of an array of microreservoirs capped with resorbable membranes of different thicknesses or chemical compositions was fabricated by Grayson et al. [111, 112]. As shown in the schematic device (Figure 5a), the body of the device consisting of the reservoir holding substrate is fabricated of a degradable polymer, thereby eliminating the need for a surgery to remove the implant chip after its intended delivery usage. The absence of any release electronics is an added advantage to utilize most of the device volume for drug loading. The reservoirs holding the drugs are sandwiched between a sealant layer and the degradable resorbable membrane and were fabricated by compression molding of PLA. The degradable PLGA membranes are synthesized using various ratios of lactic acid to glycolic acid and different molecular weights to control release (Figure 5b). This microchip release design proves useful for complex treatment profiles requiring synchronous or serial delivery of multiple therapeutic drugs.
Figure 5.
(a) A design of a degradable polymer micro-chip device made up of conical reservoirs coated with degradable membranes, each loaded with a specific drug. The top and bottom images show the device in its pre-release and release state respectively. (b) Schematic cumulative release of radio labeled model molecules from devices coated with different PLGA membrane types (x, y, z are ratios of lactic acid to glycolic acid).
3.5. Microdevices with dissolving thin films
Both the Desai Lab microdevices and the degradable polymer microchip are ‘passively-smart’ systems that deliver drug depending on the conditions of the surrounding environment. An increased patient compliance can be introduced by including electronic components in the various devices for patient specific programmed drug delivery. MicroCHIPS Inc. developed a programmable, microdevice implant as a replacement for the tedious conventional long-term injection based intra-venous or intra-muscular administration. Although this device was originally developed for sub-dermal delivery, the device can be easily modified to be adapted for oral administration. This device can be pre-programmed by the doctor to release drug doses based on a schedule from any of the 100 pyramid shaped 300 nL microreservoirs. Each reservoir top is 50 μm in diameter and covered by a thin metal film, usually gold that serves as an anode in an electrochemical reaction [113]. The anode material is chosen in a way that it serves as an impermeable material to the overlying electrolyte, but can be easily dissolved when an electrochemical potential is applied to it. The reservoir is filled with the drug of interest using inkjet printing followed by sealant using a solder material. A cathode is also made available on the device using a suitable conducting material. The devices are then programmed by radiofrequency telemetry to selectively open specific reservoirs, thereby initiating drug release. A wireless signal from outside the body causes oxidation of the anode material forming a soluble complex with the electrolyte ions over a specific reservoir. The dissolution of the complex in the electrolyte releases the drug into the surrounding tissue via simple diffusion. Using this device, both in vitro and in vivo pulsatile release of both model and therapeutic drugs has been demonstrated [114–117]. Although these array of microreservoir devices including the biodegradable chip can be effectively used as implantable chips for pulsatile/sustained drug delivery applications, the centimeter size of these chips makes them patient non-compliant for oral drug administration. Therefore, individual drug carrying devices with volumes similar to the above mentioned microreservoirs are required to achieve efficient oral drug delivery.
3.6. Three dimensional patterned microcontainers
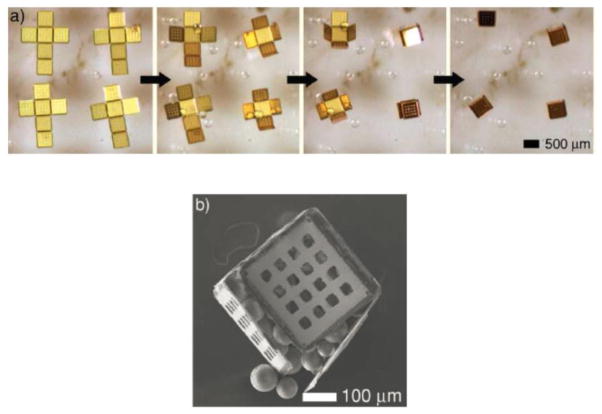
The Gracias lab uses a hybrid approach of photolithography in combination with self assembly to build complex three dimensional structures with great precision, which in general require a considerable amount of time and lithographic steps during the fabrication. They have fabricated various polyhedral shaped, self-loading, mobile, microcontainers, ranging from tens of microns to a few centimeters in an efficient and en masse parallel fashion [118, 119]. Since it is compatible with well-established lithographic fabrication techniques and equipment, this technique has an inherent advantage of producing monodispersed containers at high yields. Figure 6 shows the sequence of self assembly that converts a 2D patterned feature into a respective 3D microcontainer. Briefly, the sides of the microcontainers are patterned as 2D cruciform template using nickel and gold over a water soluble sacrificial polymer. The areas between the face edges that comprise of the folding hinges are patterned using a thermally responsive trilayer of chromium, copper, and polymer. The metal portion of the layer enables the rotation of the assembling faces, while the polymer aids in sealing the container after assembly. The dissolution of the underlying sacrificial layer in water releases the 2D cruciform templates. Heating the solution over 40 °C results in the spontaneous assembly of the template into their three dimensional counterparts. The temperature actuation is controlled effectively to enable self-loading of the microcontainers with both biologics and non living components [120, 121]. Containers containing pores for drug diffusion can also be fabricated by using a suitable lithographic mask [37, 122, 123]. Although it is a recent technology, the Gracias lab technique provides a possibility for conjugating the gold-nickel microcontainers with targeting proteins (using thiol chemistry) into drug carrying, targetable, microdevices that would serve to treat the various diseases of the intestine and oral drug delivery across the colon.
Figure 6.
(a) Time lapse image sequence of 500 μm self assembling microcontainers being loaded with glass beads. (b) A self-loaded microcontainer overfilled with 150 μm beads [121].
4. Conclusion
The development and implementation of cost-effective, en masse, microfabricated devices continue to be addressed in order to facilitate further progress in the field of controlled/sustained drug delivery. Particularly, the use of novel MEMS and NEMS technology for fabricating oral drug delivery microdevices offers potential advantages such as patient compliance, rapid availability, and low cost over other conventional administration routes. The utilization of various polymer degradation, swelling, and biocompatibility properties in combination with microfabrication techniques allow us to achieve both sustained and pulsatile delivery within the therapeutic window. The presence of multiple reservoirs loaded with multiple drugs proves to be effective in treating a wide range of diseases that require simultaneous drug delivery. Harnessing the advances in the field of surface chemistry and self assembly can facilitate the selective targeting of devices to the various diseased regions of the GI tract. In addition to the increased localized drug concentration caused by unidirectional release across the epithelium, the targeting property will also make the devices bioadhesive under the extreme physiological flow conditions, thereby extending the device residence time and subsequently enhancing the oral bioavailability of the drugs. Moreover, by incorporating evolving tools of integrated circuit technology and information sciences including wireless signaling and feedback loops between biosensors and drug release controllers, widespread applications may be obtained with regard to personalized medicine and autonomous delivery. As with other miniaturized drug delivery systems, these devices are mostly tested with in vitro or ex vivo models. Future developments would involve considering the optimum topography, and material biodegradability to understand how these devices behave, especially in vivo, during failure and component clearance. Considering that the application of MEMS and NEMS technology for medicine is only at its relative inception, the exponential development of novel implants and microdevices as seen so far allows us to foresee a future, filled with improved point of care diagnostic and therapeutic devices.
Acknowledgments
This work was supported by the NIH, the T. Gary and Kathleen Rogers Family Foundation, and Z Cube Zambon Research Venture. The authors also thank Kimberly Kam, Jennifer Wade, Drs. Julien Schweicher, and Rob Tucker for their helpful comments during preparation of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ainslie KM, Desai TA. Microfabricated implants for applications in therapeutic delivery, tissue engineering, and biosensing. Lab Chip. 2008;8:1864–1878. doi: 10.1039/b806446f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eaimtrakarn S, Itoh Y, Kishimoto JI, Yoshikawa Y, Shibata N, Takada K. Retention and transit of intestinal mucoadhesive films in rat small intestine. Int J Pharm. 2001;224:61–67. doi: 10.1016/s0378-5173(01)00738-4. [DOI] [PubMed] [Google Scholar]
- 3.Okumu FW, Cleland JL. Implants and injectables. In: Rathbone MJ, Hadgraft J, Roberts MS, editors. Modified release drug delivery technology. Marcel Dekker; New York: 2003. pp. 633–638. [Google Scholar]
- 4.Saffran M, Kumar GS, Neekers DC, Pena J, Jones RH, Field JB. Biodegradable azopolymer coating for oral delivery of peptide drugs. Biochem Soc Trans. 1990;18:752–754. doi: 10.1042/bst0180752. [DOI] [PubMed] [Google Scholar]
- 5.Iwanaga K, Ono S, Narioka K, Kakemi M, Morimoto K, Yamashita S, Namba Y, Oku N. Application of surface coated liposomes for oral delivery of peptide: effects of coating the liposomes surface on the GI transit of insulin. J Pharm Sci. 1999;88:248–252. doi: 10.1021/js980235x. [DOI] [PubMed] [Google Scholar]
- 6.Ponchel P, Irache JM. Specific and non specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv Drug Deliv Rev. 1998;34:191–219. doi: 10.1016/s0169-409x(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 7.Arangoa MA, Ponchel G, Orecchioni AM, Renedo MJ, Duchene D, Irache JM. Bioadhesive potential of gliadin nanoparticulate systems. Eur J Pharm Sci. 2000;11:333–341. doi: 10.1016/s0928-0987(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 8.Lehr CM. Lectin mediated drug delivery. J Control Release. 2000;65:19–29. doi: 10.1016/s0168-3659(99)00228-x. [DOI] [PubMed] [Google Scholar]
- 9.Fasano A, Uzzau S. Modulation of intestinal tight junctions by Zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J Clin Invest. 1997;99:1158–1164. doi: 10.1172/JCI119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasano A. Innovative strategies for the oral delivery of drugs and peptides. Trends Biotechnol. 1998;16:152–157. doi: 10.1016/s0167-7799(97)01170-0. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz UI, Gramatte T, Krappweis J, Oertel R, Kirch W. P-glycoprotein inhibitor erythromycin increases oral bioavailability of talinolol in human. Int J Clin Pharmacol Ther. 2000;38:161–167. doi: 10.5414/cpp38161. [DOI] [PubMed] [Google Scholar]
- 12.Fahr A, Liu X. Drug delivery strategies for poorly soluble drugs. Exp Opin Drug Delivery. 2007;4:403–416. doi: 10.1517/17425247.4.4.403. [DOI] [PubMed] [Google Scholar]
- 13.Mustata G, Dinh SM. Approaches to oral drug delivery for challenging molecules. Crit Rev Ther Drug. 2006;23:111–135. doi: 10.1615/critrevtherdrugcarriersyst.v23.i2.20. [DOI] [PubMed] [Google Scholar]
- 14.Santini JT, Jr, Richards AC, Scheidt R, Cima MJ, Langer R. Microchips as controlled drug-delivery devices. Angew Chem Int Ed. 2000;39:2396–2407. [PubMed] [Google Scholar]
- 15.Kuret JA, Murad F. In: Goodman and Gilman’s the pharmacological basis of therapeutics. Gilman AG, Rall TW, Nies AS, Taylor P, editors. Pergamon; New York: 1990. pp. 1334–1360. [Google Scholar]
- 16.White NJ. Mini review: assessment of the pharmacodynamic properties of anti-malarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilt JZ, Peppas NA. Microfabricated drug delivery devices. Int J Pharm. 2005;306:15–23. doi: 10.1016/j.ijpharm.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Al Malyan M, Becchi C, Boncinelli S, Ashammakhi N. Novel drug delivery systems in pain therapy. Minerva Anestesiol. 2007;73:173–179. [PubMed] [Google Scholar]
- 19.Saylor DM, Kim CS, Patwardhan DV, Warren JA. Diffuse interface theory for structure formation and release behavior in controlled drug release systems. Acta Biomat. 2007;3:851–864. doi: 10.1016/j.actbio.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Angelos S, Yang YW, Patel K, Stoddart JF, Zink JI. pH – Responsive supramolecular nanovalves based on cucurbit[6]uril pseudorotaxanes. Angew Chem Int Ed. 2008;47:2222–2226. doi: 10.1002/anie.200705211. [DOI] [PubMed] [Google Scholar]
- 21.Kwon IC, Bae YH, Kim SW. Electrically credible polymer gel for controlled release of drugs. Nature. 1991;354:291–293. doi: 10.1038/354291a0. [DOI] [PubMed] [Google Scholar]
- 22.Schwendeman SF, Amidon GL, Levy RJ. Determinants of the modulated release of antiarrhythmic drugs by iontophoresis through polymer membranes. Macromol. 1993;26:2264–2272. [Google Scholar]
- 23.Edelman ER, Kost J, Bobeck H, Langer R. Regulation of drug release from polymer matrices by oscillating magnetic fields. J Biomed Mater Res. 1985;19:67–83. doi: 10.1002/jbm.820190107. [DOI] [PubMed] [Google Scholar]
- 24.Kost J, Leong K, Langer R. Ultrasound enhanced polymer degradation and release of incorporated substances. Proc Natl Acad Sci. 1989;86:7663–7666. doi: 10.1073/pnas.86.20.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghodsian FF, Brown L, Mathiowitz E, Brandenburg D, Langer R. Enzymatically controlled drug delivery. Proc Natl Acad Sci. 1988;85:2403–2406. doi: 10.1073/pnas.85.7.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutowska A, Bark JS, Kwon IC, Bae YH, Cha Y, Kim SW. Squeezing hydrogels for controlled drug delivery. J Control Rel. 1997;48:141–148. [Google Scholar]
- 27.Langer R. Drug delivery. Drugs Target Sci. 2001;293:58–59. doi: 10.1126/science.1063273. [DOI] [PubMed] [Google Scholar]
- 28.Pirollo KF, Rait A, Zhou Q, Hwang SH, Dagata JA, Zon G, Hogrefe RI, Palchik G, Chang EH. Materializing the potential of small interfering RNA via a tumor targeting nanodelivery system. Cancer Res. 2007;67:2938–2943. doi: 10.1158/0008-5472.CAN-06-4535. [DOI] [PubMed] [Google Scholar]
- 29.Gobin AM, Lee MH, Halas MJ, James WD, Drezek RA, West JL. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007;7:1929–1934. doi: 10.1021/nl070610y. [DOI] [PubMed] [Google Scholar]
- 30.Muller-Schulte D, Schmitz-Rode T. Thermosensitive magnetic polymer particles as contactless controllable drug carreris. J Magn Magn Mater. 2006;302:267–271. [Google Scholar]
- 31.Jain D, Panda AK, Majumdar DK. Eudragit S100 entrapped insulin microspheres for oral delivery. AAPS Pharm Sci Tech. 2005;6:E100–E107. doi: 10.1208/pt060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bugarski B, Li QL, Goosen MFA, Poncelet D, Neufeld RJ, Vunjakg A. Electrostatic droplet generation – mechanism of polymer droplet formation. AIChE J. 1994;40:1026–1031. [Google Scholar]
- 33.Wolters GHJ, Fritschy WM, Gerrits D, Vanschilfagaarde R. A versatile alginate droplet generator applicable for microencapsulation of pancreatic islets. J Appl Biomater. 1992;3:281–286. doi: 10.1002/jab.770030407. [DOI] [PubMed] [Google Scholar]
- 34.Leach WT, Simpson DT, Val TN, Yu ZS, Lim KT, Park EJ, Williams RO, Johnston KP. Encapsulation of protein nanoparticles into uniform sized microspheres formed in a spinning oil film. AAPS Pharm Sci Tech. 2005;6:E605–E617. doi: 10.1208/pt060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai SK, Hida K, Man ST, Chen C, Machamer C, Schroer TA, Hanes J. Privilged delivery of polymer nanoparticles to the perinuclear region of live cells via a non clathri, non degradative pathway. Biomaterials. 2007;28:2876–2884. doi: 10.1016/j.biomaterials.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 36.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size dependent internalization of particles via the pathways of clathrin and caveolae mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randall CL, Leong TG, Bassik N, Gracias DH. 3D lithographically fabricated nanoliter containers for drug delivery. Adv Drug Deliv Rev. 2007;59:1547–1561. doi: 10.1016/j.addr.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 38.Lim JY, Donahue HJ. Cell sensing and response to micro and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng. 2007;13:1879–1891. doi: 10.1089/ten.2006.0154. [DOI] [PubMed] [Google Scholar]
- 39.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung DR, Kapur R, Adams T, Giuliano KA, Mrksich M, Craighead HG, Taylor DL. Crit. Topographical and physicochemical modification of material surface to enable patterning of living cells. Rev Biotechnol. 2001;21:111–154. doi: 10.1080/20013891081700. [DOI] [PubMed] [Google Scholar]
- 41.Champion JA, Katare YK, Mitragotri S. Particle shape: A new design parameter for micro and nanoscale drug delivery carriers. J Control Release. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed A, Bonner C, Desai TA. Bioadhesive microdevices with multiple reservoirs: a new platform for oral drug delivery. J Control Rel. 2002;81:291–306. doi: 10.1016/s0168-3659(02)00074-3. [DOI] [PubMed] [Google Scholar]
- 44.Irrache JM, Durrer C, Duchene D, Ponchel G. Bioadhesion of lectin latex conjugates to rat intestinal mucosa. Parm Res. 1996;13:1716–1719. doi: 10.1023/a:1016405126656. [DOI] [PubMed] [Google Scholar]
- 45.Hussain N, Jani PU, Florence AT. Enhanced oral uptake of tomato lectin conjugated nanoparticles in the rat. Pharm Res. 1997;14:613–618. doi: 10.1023/a:1012153011884. [DOI] [PubMed] [Google Scholar]
- 46.Tao SL, Desai TA. Microfabricated drug delivery systems: from particles to pores. Adv Drug Delivery Rev. 2003;55:315–328. doi: 10.1016/s0169-409x(02)00227-2. [DOI] [PubMed] [Google Scholar]
- 47.Tao SL, Desai TA. Microfabrication of multilayer, asymmetric, polymeric devices for drug delivery. Adv Mater. 2005;17:1625–1630. [Google Scholar]
- 48.Amer S, Badawy W. An integrated platform for bioanalysis and drug delivery. Curr Pharm Biotech. 2005;6:57–64. doi: 10.2174/1389201053167220. [DOI] [PubMed] [Google Scholar]
- 49.Grayson ACR, Shawgo RS, Johnson AM, Flynn NT, Li T, Cima MJ, Langer R. A bioMEMS review: MEMS technology for physiologically integrated devices. Proc IEEE. 2004;92:6–21. [Google Scholar]
- 50.LaVan DA, McGuire T, Langer R. Small scale systems for in vivo drug delivery. Nat Biotechnol. 2003;21:1184–1191. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 51.Madou MJ. Fundamentals of microfabrication. 2. CRC Press; Boca Raton: 2002. [Google Scholar]
- 52.Petersen KE. Silicon as a mechanical material. Proc IEEE. 1982;70:420–457. [Google Scholar]
- 53.Staples M, Daniel K, Cima MJ, Langer R. Application of micro and nanoelectromechanical devices to drug delivery. Pharm Res. 2006;23:847–863. doi: 10.1007/s11095-006-9906-4. [DOI] [PubMed] [Google Scholar]
- 54.Buhsan B, Burton Z. Adhesion and friction properties of polymers in microfluidic devices. Nanotech. 2005;16:467–478. [Google Scholar]
- 55.Lu Y, Chen SC. Micro and nanofabrication of biodegradable polymers for drug delivery. Adv Drug Delivery Rev. 2004;56:1621–1633. doi: 10.1016/j.addr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Leong TG, Zarafshar AM, Gracias DH. Three-dimensional fabrication at small size scales. Small. 2010;6:792–806. doi: 10.1002/smll.200901704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vora KD, Peele AG, Shew BY, Harvey EC, Hayes JP. Sidewall slopes of SU-8 HARMST using deep x-ray lithography. Microsyst Technol. 2007;13:487–493. [Google Scholar]
- 58.Lopez CA, Fleischman AJ, Roy S, Desai TA. Evaluation of silicon nanoporous membranes and ECM-based microenvironments for neurosecretory cells. Biomaterials. 2006;27:3075–3083. doi: 10.1016/j.biomaterials.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 59.Saha K, Pollock JF, Schaffer DV, Healy KE. Designing synthetic materials to control stem cell phenotype. Curr Opin Chem Biol. 2007;11:381–387. doi: 10.1016/j.cbpa.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin F, Walczak R, Bioraski A, Cohen M, West T, Cosentino C, Shapiro J, Ferrari M. Tailoring width of microfabricated nanochannels to solute size can be used to control diffusion kinetics. J Control Release. 2005;102:183–189. doi: 10.1016/j.jconrel.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 61.Desai TA, West T, Cohen M, Boiarski T, Rampersaud A. A nanoporous microsystem for islet cell replacement. Adv Drug Delivery Rev. 2004;56:1661–1673. doi: 10.1016/j.addr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Ainslie KM, Sharma G, Dyer MA, Grimes CA, Pishko MV. Attenuation of protein adsorption on static and oscillating magnetostrictive nanowires. Nano Lett. 2005;5:1852–1856. doi: 10.1021/nl051117u. [DOI] [PubMed] [Google Scholar]
- 63.Beebe DJ, Moore JS, Bauer JM, Yu Q, Liu RH, Devadoss C, Jo B. Funtional hydrogel structures for autonomous flow control inside microfluidic channels. Nature. 2000;404:588–590. doi: 10.1038/35007047. [DOI] [PubMed] [Google Scholar]
- 64.Hilt JZ, Gupta AK, Bashir R, Peppas NA. Ultrasensitive biomems sensors based on microcantilevers patterned with environmentally responsive hydrogels. Biomed Microdevices. 2003;5:177–184. [Google Scholar]
- 65.Bashir R, Hilt JZ, Elibol O, Gupta AK, Peppas NA. Micromechanical cantilever as an ultrasensitive pH microsensor. App Phys Lett. 2002;81:3091–3093. [Google Scholar]
- 66.Baldi A, Lei M, Gu Y, Siegel RA, Ziaie B. A microstructured silicon membrane with entrapped hydrogels for environmentally sensitive fluid gating. Sens Actuators B. 2006;114:9–18. [Google Scholar]
- 67.Low LM, Seetharaman S, Madou MJ. Microactuators toward microvalves for responsive controlled drug delivery. Sens Actuators B. 2000;67:149–160. [Google Scholar]
- 68.Ito Y, Hasuda H, Morimatsu M, Takagi N, Hirai Y. A microfabrication method of a biodegradable polymer chip for a controlled release system. J Biomater Sci Polym Ed. 2005;16:949–955. doi: 10.1163/1568562054414621. [DOI] [PubMed] [Google Scholar]
- 69.He H, Cao X, Lee LJ. Design of a novel hydrogel based intelligent system for controlled drug release. J Control Release. 2004;95:391–402. doi: 10.1016/j.jconrel.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 70.Guan J, He H, Lee LJ, Hansford DJ. Fabrication of particulate reservoir containing, capsule like and self-folding polymer microstructures for drug delivery. Small. 2007;3:412–418. doi: 10.1002/smll.200600240. [DOI] [PubMed] [Google Scholar]
- 71.He H, Guan J, Lee J. An oral delivery device based on self-folding hydrogels. J Control Release. 2006;110:339–346. doi: 10.1016/j.jconrel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 72.Ehrfeld W, Schmidt A. Recent development in deep x-ray lithography. J Vac, Sci Technol B. 1998;16:3526–3534. [Google Scholar]
- 73.Fu C, Huang H. Different methods for the fabrication of UV-LIGA molds using SU-8 with tapered de-molding angles. Microsyst Technol. 2007;13:293–298. [Google Scholar]
- 74.Qu WM, Wenzel C, Jahn A. One-mask procedure for the fabrication of movable high aspect ratio 3D microstructures. J Micromech Microeng. 1998;8:279–284. [Google Scholar]
- 75.Laermer F, Urban A. Challenges, developments and applications of silicon deep reactive ion etching. Microelectron Eng. 2003;349:67–68. [Google Scholar]
- 76.Hu W, Sarveswaran K, Lieberman M, Bernstein GH. Su-10 nm e-beam lithography using cold development of PMMA. J Vac Sci Technol B. 2004;22:1711–1716. [Google Scholar]
- 77.Enkrich C, Perez-Willard F, Gerthsen D, Zhou JF, Koschny T, Soukoulis CM, Wegener M, Linden S. Focused ion beam fabrication of near infrared magnetic metamaterials. Adv Mater. 2005;17:2547–2549. [Google Scholar]
- 78.Feiertag G, Ehrfeld W, Freimuth H, Lehr H, Schmidt M, Sigalas MM, Soukoulis CM, Kiriakidis G, Pedersen T, Kuhl J, Koenig W. Fabrication of photonic crystals by deep X-ray lithography. Appl Phys Lett. 1997;71:1441–1443. [Google Scholar]
- 79.Yoon YK, Park JH, Allen MG. Multidirectional UV lithography for complex 3D MEMS structures. J Microelectromech, Syst. 2006;15:1121–1130. [Google Scholar]
- 80.Han M, Lee W, Lee SK, Lee SS. 3D microfabrication with inclined/rotated UV lithography. Sens Actuators A. 2004;111:14–20. [Google Scholar]
- 81.Yu H, Li B, Zhang X. Flexible fabrication of 3D multilayered microstructures using a scanning laser system. Sens Actuators A. 2006;125:553–564. [Google Scholar]
- 82.Xia Y, Whitesides GM. Extending Microcontact Printing as a Microlithographicv Technique. Langmuir. 1997;13:2059–2067. [Google Scholar]
- 83.Tan J, Tien J, Chen C. Microcontact printing of proteins on mixed self-assembled monolayers. Langmuir. 2002;18:519–523. [Google Scholar]
- 84.Bos GW, Scharenborg NM, Engbers GHM. Proliferation of endothelial cells on surface-immobilized albumin-heparin conjugate loaded with basic fibroblast growth factor. J Biomed Mater Res. 1999;44:330–340. doi: 10.1002/(sici)1097-4636(19990305)44:3<330::aid-jbm12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 85.Yamato M, Konno C, Utsumi M, Kikichi A. Thermally responsive polymer-grafted surfaces facilitate patterned cell seeding and co-culture. Biomaterials. 2003;23:561–567. doi: 10.1016/s0142-9612(01)00138-7. [DOI] [PubMed] [Google Scholar]
- 86.Blawas AS, Reichert WM. Protein patterning. Biomaterials. 1998;19:595–609. doi: 10.1016/s0142-9612(97)00218-4. [DOI] [PubMed] [Google Scholar]
- 87.Xia Y, Whitesides GM. Soft Lithography. Annu Rev Mater Sci. 1998;28:153–184. [Google Scholar]
- 88.Whitesides GM, Labinis PE. Wet chemical approaches to the characterization of organic surfaces: self-assembled monolayers, wetting, and the physical-organic chemistry of the solid-liquid interface. Langmuir. 1990;6:87–96. [Google Scholar]
- 89.Whitesides GM. The right size in nanobiotechnology. Nat Biotechnol. 2003;21:1161–1165. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 90.Truskett VN, Watts MPC. Trends in imprint lithography for biological applications. Trends Biotech. 2006;24:312–317. doi: 10.1016/j.tibtech.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 91.Kumar A, Whitesides GM. Features of gold having micrometer to centimeter dimensions can be formed through a combination of stamping with an elastomeric stamp and an alkanethiol “ink” followed by chemical etching. Appl Phys Lett. 1993;63:2002–2004. [Google Scholar]
- 92.Zhao XM, Xia Y, Whitesides GM. Fabrication of 3D microstructures: Microtransfer molding. Adv Mater. 1996;8:837–840. [Google Scholar]
- 93.Kim E, Xia Y, Whitesides GM. Polymer microstructures formed by moulding in capillaries. Nature. 1995;376:581–584. [Google Scholar]
- 94.Kim E, Xia Y, Zhao XM, Whitesides GM. Fabrication of 3D microstructures: Microtransfer molding. Adv Mater. 1997;9:651–654. [Google Scholar]
- 95.Chou SY, Krauss PR, Renstrom PJ. Nanoimprint lithography. J Vac Sci Technol B. 1996;14:4129–4133. [Google Scholar]
- 96.Zankovych S, Hoffmann T, Seekamp J, Bruch JU, Torres CMS. Nanoimprint technology, prospects and challenges. Nanotechnology. 2001;12:91–95. [Google Scholar]
- 97.Crommie MF, Lutz CP, Eigler DM. Confinement of electrons to quantum corrals on a metal surface. Science. 1993;262:218–220. doi: 10.1126/science.262.5131.218. [DOI] [PubMed] [Google Scholar]
- 98.Piner RD, Zhu J, Xu F, Hong S, Mirkin CA. Dip pen nanolithography. Science. 1999;283:661–663. doi: 10.1126/science.283.5402.661. [DOI] [PubMed] [Google Scholar]
- 99.Hong S, Zhu J, Mirkin CA. Multiple ink lithography: toward a multiple pen nanoplotter. Science. 1999;286:523–525. doi: 10.1126/science.286.5439.523. [DOI] [PubMed] [Google Scholar]
- 100.Zhang M, Bullen D, Chung SW, Hong S, Ryu S, Fan Z, Mirkin CA, Liu C. A MEMS nanoplotter with high density parallel dip pen nanolithography probe arrays. Nanotechnol. 2002;13:212–217. [Google Scholar]
- 101.Cui J, Zheng X, Hou W, Zhuang YP, Pi X, Yang J. The study of a remote controlled gastrointestinal drug delivery and sampling system. Telemed E-Health. 2008;14:715–719. doi: 10.1089/tmj.2007.0118. [DOI] [PubMed] [Google Scholar]
- 102.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to biotechnology. Adv Mater. 2006;18:1345–1360. [Google Scholar]
- 103.Sehershen S, Mensing G, Beebe D, West J. Independent optical control of microfluidic valves from optomechanically responsive nanocomposite hydrogels. Adv Mater. 2005;17:1366–1368. doi: 10.1002/adma.200401239. [DOI] [PubMed] [Google Scholar]
- 104.Alarcon CD, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chem Soc Rev. 2004;34:276–285. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- 105.Dorski CM, Doyle FJ, Peppas NA. Preparation and characterization of glucose sensitive p(MAA-g-EG) hydrogels. Polym Mater Sci Eng Proceed. 1997;76:281–282. [Google Scholar]
- 106.Ainslie KM, Desai TA. Microfabricated implants for applications in therapeutic delivery, tissue engineering, and biosensing. Lab Chip. 2008;8:1864–1878. doi: 10.1039/b806446f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ainslie KM, Lowe RD, Beaudette TT, Petty L, Bachelder EM, Desai TA. Microfabricated devices for enhanced bioadhesive drug delivery: attachment to and small molecule release through a cell monolayer under flow. Small. 2009;5:2857–2863. doi: 10.1002/smll.200901254. [DOI] [PubMed] [Google Scholar]
- 108.Tao SL, Lubeley MW, Desai TA. Bioadhesive poly(methyl methacrylate) microdevices for controlled drug delivery. J Control Release. 2003;88:215–228. doi: 10.1016/s0168-3659(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 109.Tao SL, Desai TA. Micromachined devices: The impact of controlled geometry from cell targeting to bioavailability. J Control Release. 2005;109:127–138. doi: 10.1016/j.jconrel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 110.Tao SL, Popat KP, Desai TA. Off wafer fabrication and surface modification of asymmetric 3D SU-8 microparticles. Nat Protocols. 2006;1:3153–3158. doi: 10.1038/nprot.2006.451. [DOI] [PubMed] [Google Scholar]
- 111.Grayson ACR, Choi IS, Tyler BM, Wang PP, Brem H, Cima MJ, Langer R. Multi pulse drug delivery from a resorbable polymeric microchip device. Nat Mater. 2003;2:767–772. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 112.Grayson ACR, Cima MJ, Langer R. Molecular release from a polymeric microreservoir device: influence of chemistry, polymer swelling, and loading on device performance. J Biomed Mater Res Part A. 2004;69A:502–512. doi: 10.1002/jbm.a.30019. [DOI] [PubMed] [Google Scholar]
- 113.Santini JT, Jr, Cima MJ, Langer R. A controlled release microchip. Nature. 1999;397:335–338. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 114.Li Y, Shawgo RS, Tyler B, Henderson PT, Vogel JS, Rosenberg A, Storm PB, Langer R, Brem H, Cima MJ. In vivo release from a drug delivery MEMS device. J Control Release. 2004;100:211–219. doi: 10.1016/j.jconrel.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 115.Li Y, Due HLH, Tyler B, Williams T, Tupper M, Langer R, Brem H, Cima MJ. In vivo delivery of BCNU from a MEMS device to a tumor model. J Control Release. 2005;106:138–145. doi: 10.1016/j.jconrel.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 116.Maloney JM, Uhland SA, Polito BF, Sheppard NF, Jr, Pelta CM, Santini JT., Jr Electrothermally activated microchips for implantable drug delivery and biosensing. J Control Release. 2005;109:138–145. doi: 10.1016/j.jconrel.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 117.Prescott JH, Lipka S, Baldwin S, Sheppard NF, Jr, Maloney JM, Coppeta J, Yomtov B, Staples MA, Santini JT., Jr Chronic, programmed polypeptide delivery from an implanted, multireservoir microchip device. Nat Biotech. 2006;24:437–438. doi: 10.1038/nbt1199. [DOI] [PubMed] [Google Scholar]
- 118.Gracias DH, Tien J, Breen TL, Hsu C, Whitesides GM. Forming electrical networks in 3D by self assembly. Science. 2000;289:1170–1172. doi: 10.1126/science.289.5482.1170. [DOI] [PubMed] [Google Scholar]
- 119.Boncheva M, Gracias DH, Jacobs HO, Whitesides GM. Biomimetic self-assembly of a functional asymmetrical electronic device. Proc Natl Acad Sci. 2002;99:4937–4940. doi: 10.1073/pnas.032667599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Breen TL, Tien J, Oliver SRJ, Hadzic T, Whitesides GM. Design and self assembly of open, 3D, mesostructures. Science. 1999;284:948–951. doi: 10.1126/science.284.5416.948. [DOI] [PubMed] [Google Scholar]
- 121.Leong TG, Randall CL, Benson BR, Zarafshar AM, Gracias DH. Self-loading lithographically structured microcontainers: 3D patterned, mobile microwells. Lab Chip. 2008;8:1621–1624. doi: 10.1039/b809098j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leong T, Gu Z, Koh T, Gracias DH. Spatially controlled chemistry using remotely guided nanoliter scale containers. J Am Chem Soc. 2006;128:11336–11337. doi: 10.1021/ja063100z. [DOI] [PubMed] [Google Scholar]
- 123.Desai TA, Chu WH, Tu JK, Beattle GM, Hayek A, Ferrari M. Microfabricated immunoisolating biocapsules. Biotechnol Bioeng. 1998;57:118–120. doi: 10.1002/(sici)1097-0290(19980105)57:1<118::aid-bit14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]