Abstract
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) plays an important role in glycolysis but also in non-metabolic processes, including transcription activation and apoptosis. We report the isolation of an hGAPDH (2-32) fragment peptide from human placental tissue exhibiting antimicrobial activity. The peptide was internalized by cells of the pathogenic yeast Candida albicans and initiated a rapid apoptotic mechanism, leading to killing of the fungus. Killing was dose-dependent, with 10 µg/ml (3.1 µM) and 100 µg/ml hGAPDH (2-32) depolarizing 45% and 90% of the fungal cells in a population, respectively. Experimental C. albicans infection induced epithelial hGAPDH (2-32) expression. Addition of the peptide significantly reduced the tissue damage as compared to untreated experimental infection. Secreted aspartic proteinases (Saps) activity of C. albicans was inhibited by the fragment at higher concentrations with an ED50 of 160 mg/l (50 μM) for Sap1p and 200 mg/l (63 μM) for Sap2p while Sap3 was not inhibited at all. Interestingly, hGAPDH (2-32) induced significant epithelial IL-8 and GM-CSF secretion and stimulated TLR4 expression at low concentrations independently of the presence of C. albicans without any toxic mucosal effects.
In the future, the combination of different antifungal strategies, e.g. a conventional fungicidal with immunomodulatory effects and the inhibition of fungal virulence factors might be a promising treatment option.
Keywords: GAPDH, antimicrobial peptide, secreted aspartic proteases, reconstituted human oral epithelium (RHE)
INTRODUCTION
Due to the increased number of immunosuppressed patients resulting from AIDS, organ transplants, and drug addiction, the frequency of fungal infections with the opportunistic yeast Candida albicans (C. albicans) has risen (Leroy et al., 2009). These infections, especially oropharyngeal candidiasis, are widely treated with fluconazole. The long-term treatment with azoles has led to the emergence of azole-resistant strains of C. albicans (Fera et al., 2009). Azoles inhibit the key enzyme of ergosterol biosynthesis, cytochrome P-450-dependent lanosterol-14α-demethylase. Known mechanisms of azole resistance are the decreased availability of lanosterol-14α-demethylase and efflux pumps (Peman et al., 2009; Sanglard et al., 2009).
Another therapeutic option is to affect fungal virulence factors, such as secreted hydrolytic enzymes, yeast-to-hyphal transition, adhesion factors, phenotypic switching, thigmotropism, and molecular mimicry. During the last decade, secreted aspartic proteinases (Sap) activity has been characterized as one of the major virulence factors of C. albicans (Schaller et al., 2005). Ten SAP have been identified in C. albicans. The corresponding proteinases are crucial for distinct steps in pathogenesis, like adhesion and penetration. The interest in Sap inhibitors started with the treatment of AIDS patients with highly active antiretroviral therapy (HAART), a combination of HIV aspartic proteinase and reverse transcriptase inhibitors. Some of the clinically used HIV proteinase inhibitors, e. g. saquinavir and indinavir, also have the ability to inhibit Sap activity and, therefore may prevent fungal infections or reduce their severity (Korting et al., 1999). This finding led to an enhanced effort of focusing on the research on specific Sap inhibitors by solving the X-ray crystal structures of these proteinases and by development of Sap-specific inhibitors (Borelli et al., 2008; Braga-Silva and Santos, 2011). Most HIV aspartic proteinase inhibitors and Sap inhibitors are short peptide-like ligands mimicking substrates for HIV proteinase (Gauwerky et al., 2009). Moreover, it has been shown that lysozyme (muramidase), an antimicrobial peptide effective against a wide range of bacteria, not only inhibited the growth of C. albicans, but also decreased the secretion of the isoenzyme Sap2 which is the predominant secreted isoenzyme (Wu et al., 1999).
In the present investigation we examined the effect of an antimicrobial peptide, purified from human placental tissue, on the growth of C. albicans, and on Sap activity. In addition, we analyzed the protective and immunomodulatory effects during epithelial infection.
RESULTS
Purification of hGAPDH (2-32) from human placental tissue
A peptide library from human placental tissue was initially screened for growth inhibition against Escherichia coli (E. coli) using a radial diffusion assay. Antimicrobial activity was detected in several HPLC fractions of pH pool 4. Of this pool, the fractions 19 and 20 which exhibited the highest growth inhibitory activity were selected for further purification (Fig. 1a). To isolate the active compounds, two subsequent HPLC steps were carried out, tracking the maximum antimicrobial activity within the resulting fractions. The antimicrobial active fractions were loaded onto an RP column. Elution of bound material was carried out by linearly increasing the amount of solvent B. Growth inhibitory activity was eluted over a broad range, and antibacterial fractions were selected for further purification. Since it is known that most antimicrobial peptides are of a cationic nature, a strong cation-exchange column was used for final purification. The resulting three HPLC fractions showing the strongest antimicrobial activity (Fig. 1b) were desalted and subsequently tested for further analyses (Fig. 1c). Analysis of HPLC fraction 23 by capillary zone electrophoresis revealed a compound of high purity (data not shown).
Figure 1. Purification of the antimicrobial peptide hGAPDH (2-32) from human placental tissue.
Each purification step was monitored by radial diffusion assay for detection of antimicrobial activity. The bars show the diameters of inhibition zones indicating the antimicrobial activity against E. coli. Fractions 19 and 20, corresponding to the maximum growth inhibition, were selected for further purification (a). Fractions 19 and 20 were pooled and fractionated by RP chromatography (b). Final purification of the antimicrobial peptide was performed, separating fraction 23 (b) using a strong cation-exchange column (c). MALDI-MS analysis of the purified peptide revealed a molecular mass of 3188 Da (d). Sequence analysis led to the identification of an N-terminal fragment of GAPDH. Amino acid sequence is shown in the single letter code (e).
The molecular mass of the purified peptide was determined to be 3188 Da measured by MALDI mass spectrometry (Fig. 1d).
Edman degradation yielded the following peptide sequence of 31 amino acid residues: GKVKVGVNGFGRIGRLVTRAAFNSGKVDIVA. Comparison of the amino acid sequence obtained with the SwissProt Database showed 100% identity with the N-terminal fragment of the human protein glyceraldehyde-3-phosphate dehydrogenase (Fig. 1e).
Functional characterization of the synthetic antimicrobial peptide
Antimicrobial activity
To analyze the identity and biological properties of the isolated peptide, hGAPDH (2-32) was chemically synthesized. To specify the spectrum of activity of the peptide, its antimicrobial activity was determined by a flow cytometric antimicrobial killing assay (Nuding et al., 2006). This assay demonstrated a potent antibacterial activity of this peptide against E. coli (Fig. 2a) and confirmed the results of the radial diffusion assay which was originally used to screen the HPLC fractions for growth inhibitory activity against this gram-negative bacterium (Fig. 1c). Two of the four tested C. albicans strains were similar highly susceptible to the hGAPDH (2-32) fragment with 10 μg/ml depolarizing approximately 50% of the fungi in a population, while two other strains showed a slightly lower sensitivity (Fig. 2a). We compared the activity spectrum of our peptide with that of LL-37 and hBD-3. The antifungal activities of all three peptides against C. albicans were not significantly different, while hGAPDH (2-32) was less active against E. coli. The antifungal effect of h(GAPDH) 2-32 was dose dependent as shown for C. albicans SC5314 (Fig. 2b)
Figure 2. Antimicrobial killing assays and electron microscopy.
Flow cytometric antimicrobial killing assay of E. coli and C. albicans incubated with 10 μg/ml hGAPDH (2-32), LL-37 or hBD-3 (a). Dose dependent effect of hGAPDH (2-32). Suspensions of C. albicans were incubated with hGAPDH (2-32) for 90 min. The antimicrobial activity is shown as percentage of depolarized microorganisms (b). The data are means of one representative experiment in triplicate. Electron microscopy of C. albicans SC5314 cells grown without (c) and with (d) 125 μg/ml hGAPDH (2-32) for 24 h. Cells grown without hGAPDH (2-32) with a regular morphology (c). C. albicans grown under the influence of hGAPDH (2-32) shows enlargement of cytoplasmic vacuoles and disorganization of the internal organelles (d). Bar = 500 nm.
The antimicrobial activity was also confirmed by the broth microdilution method (MIC 100%) against several bacterial strains and C. albicans. The identified peptide exhibited a broad spectrum of activity, inhibiting the growth of Gram-negative bacteria (E. coli BL21, P. aeruginosa PAO, P. aeruginosa clinical isolate) and C. albicans in micromolar concentrations (data not shown).
Ultrastructural changes of C. albicans morphology induced by hGAPDH (2-32)
Whereas untreated C. albicans SC5314 cells were in a uniform physiological state (Fig. 2c), incubation of yeasts with hGAPDH (2-32) in a concentration of 125 μg/ml for 24 h resulted in distinct changes of the cell wall, plasma membrane and the cytoplasm (Fig. 2d). Morphological alterations included enlargement of the fungal cytoplasmic vacuoles, disorganization of the internal organelles and the appearance of yeasts with an empty cytoplasm resembling necrotic ghost cells.
Inhibition of Sap activity
Addressing the question whether hGAPDH (2-32) has an inhibitory activity against C. albicans Sap, we tested inhibition of the recombinant proteins Sap1, 2 and 3. The Sap-specific test showed an ED50 of 160 μg/ml (50 μM) for Sap1p and 200 μg/ml (63 μM) for Sap2p while Sap3 was not inhibited (Fig. 3).
Figure 3. Human glyceraldehyde-3-phosphate dehydrogenase (hGAPDH) (2-32) inhibits activity of secreted aspartic proteinases (Saps), a virulence attributes of C. albicans.
Specific inhibition of Sap1-3 showed an ED50 of 160 μg/ml (50 μM) for Sap1p and 200 μg/ml (63 μM) for Sap2p while Sap3p was not inhibited. Error bars indicate range of duplicates. Each duplicate consists of three background-normalized measures.
Experimental C. albicans infection
We used epithelial monolayers to analyze the antimicrobial effect of hGAPDH (2-32). LDH values 12 h and 24 h after infection with C. albicans SC5314 were significantly decreased even in the presence of the lowest hGAPDH (2-32) concentration of 5 μg/ml as compared to untreated controls (Fig. 4a). The protective effect of hGAPDH (2-32) was similar to that of LL-37. Treatment of uninfected epithelial cells with hGAPDH (2-32) also demonstrated that this peptide has low toxicity on mammalian cells (Fig. 4a). Analyses of standard LDH samples in the absence and presence of 5 and 125 μg/ml hGAPDH (2-32) excluded inhibition of the LDH enzymatic assay by the antimicrobial peptide. We also could demonstrate that LDH is exclusively secreted by the epithelial and not by C. albicans cells (data not shown).
Figure 4. Experimental C. albicans infection.
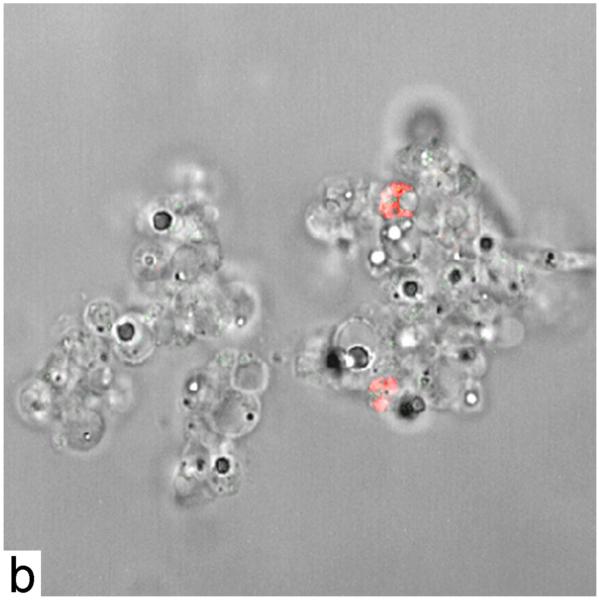
Release of LDH by monolayer epithelial cells 12 h after infection (or not) with C. albicans in the presence and absence of 5 μg/ml LL-37 or hGAPDH (2-32). Multiplicity of infection (MOI) 0.1. Epithelial cells were preincubated with the peptide for 1 h (a). Statistical significance was determined using the 2-tailed paired Student’s t test (n = 6). Light micrographs of reconstituted human oral epithelium (RHE) 18 h after infection with C. albicans SC5314 in the absence and presence of hGAPDH (2-32). Invasion by C. albicans of all epithelial layers with extensive edema and vacuolization (b) in the absence of hGAPDH (2-32). Strongly reduced virulence phenotype resulting in a protective effect in the presence of hGAPDH (2-32). Decreased number of C. albicans cells (c). Confocal laser microscopy of oral RHE after 12 h in the presence and absence of C. albicans and 5 μg/ml hGAPDH (2-32) (cell nuclei, green; hGAPDH (2-32), red; C. albicans, blue). No evidence for hGAPDH (2-32) in the uninfected and untreated oral RHE (d). Presence of hGAPDH (2-32) on the superficial layers of the uninfected RHE after external addition (e). Increased expression in the C. albicans infected but untreated oral RHE (f). Strong affinity of the peptide to the C. albicans cells ( f, g) after external addition of hGAPDH (2-32). Higher-magnification image demonstrating direct contact of the peptide with C. albicans cells (short arrow) and internalization by the fungal cells (long arrow). Bar = 30 μm.
Histological examination of reconstituted human oral epithelium (RHE) which consists of differentiated multilayers of the TR146 cell line taken 18 h after infection with C. albicans SC5314 demonstrated clusters of fungal cells on the superficial keratinocytes and prominent lesions with edema and vacuolization of the keratinocytes and enlarged intercellular spaces as a sign of spongiosis. C. albicans effectively invaded all keratinocyte layers of the oral epithelium affecting the tissue with a high number of microorganisms (Fig. 4b). In contrast, histological examination of infected samples treated with 125 μg/ml hGAPDH (2-32) demonstrated far less marked morphological alterations. Spongiosis and invasion of keratinocytes by yeast cells were inhibited by hGAPDH (2-32), only the formation of mild edema in the uppermost keratinocyte layers resulted from Candida infection. In accordance with the minor tissue lesions observed, the number of yeast cells on the mucosal surface was decreased (Fig. 4c).
We used an anti-hGAPDH (2-32) antiserum raised in rabbits to carry out immunofluorescence in our RHE model by confocal laser microspcopy. The specificity of the generated antibody against hGAPDH (2-32) was verified by ELISA demonstrating a signal against the hGAPDH (2-32) peptide but no signal against the hGAPDH protein. The specificity of our antibody was further confirmed in our RHE experiments by staining of the peptide only in the hGAPDH (2-32) treated (Fig. 4e, g) and/or C. albicans infected samples (Fig. 4f, g) but not in the untreated and uninfected RHE (Fig. 4d). Confocal laser scanning microscopy showed increased endogenous expression of hGAPDH (2-32) during RHE infection (Fig. 4f) while no evidence for staining was seen in the uninfected PBS control (Fig. 4d). In addition we were able to demonstrate a strong affinity of the external but also of the endogenous peptide to C. albicans and internalization by the fungal cells (Fig. 4f, g higher magnifications).
Apoptosis and necrosis of C. albicans in the presence of hGAPDH (2-32)
Several reports about apoptotic cell death induced by antimicrobial peptides prompted us to investigate whether an apoptosis-like process occurs in hGAPDH (2-32) treated C. albicans cells. Cells dying under these conditions display several markers characteristic of apoptosis. These include the rapid exposure of phosphatidylserine (PS) at the outer cell membrane by annexin V-FITC staining. In our assay, apoptotic and necrotic cells were distinguished by double staining for annexin V-FITC (green) and propidium iodide (PI), which is a membrane-impermeant DNA fluorescent stain (Fig. 5). Cells exposed to 5 μg/ml hGAPDH (2-32) for 1 h showed peripheral fluorescence in 31% of the protoplasts (Fig. 5c, e). C. albicans cells treated for a longer time with this concentration or at higher doses of these compound showed cellular changes characteristic of necrosis (Fig. 2d). Annexin V-FITC-stained cells were only observed rarely in control assays performed without peptide (Fig. 5a, b).
Figure 5. Apoptotic marker induced by 5 μg/ml hGAPDH (2-32) on C. albicans cells.
Representative micrographs showing cells stained with FITC-annexin V (green) and propidium iodide (PI, red) to detect apoptosis (phosphatidylserine externalization) and necrosis, respectively. The cells were untreated (a, b) or previously treated (c, d) with hGAPDH (2-32) for 1 h. Subpanels b and d are phase-contrast micrographs. Subpanels a and b show annexin and PI staining. (e) Percentage of 300 fungal cells that are classified as apoptotic [annexin (+) PI(−); green bars] and necrotic [annexin(+/−) PI(+); red bars] after treatment with hGAPDH (2-32) and H2O2. Bar=10 μm.
Immunomodulatory activity of hGAPDH (2-32)
We investigated the role of hGAPDH (2-32) in initiating an epithelial chemokine response. Protein secretion and gene expression were quantified in the presence and absence of C. albicans SC5314 and 5 μg/ml hGAPDH (2-32) by use of ELISA and real-time RT-PCR. In response to C. albicans, epithelial cells secreted significantly increased concentrations of IL-8 and GM-CSF (Fig. 6a) which is in line with our previous studies (Weindl et al., 2007). Surprisingly, hGAPDH (2-32) also induced increased secretion of GM-CSF and IL-8 of uninfected epithelial cells. Secretion of IL-8 was even increased after C. albicans infection (Fig. 6a, b). A similar stimulation pattern was also observed for epithelial TLR4 mRNA expression (Fig. 6c).
Figure. 6. Expression of cytokines and TLR4 by hGAPFH (2-32).
IL-8, GM-CSF secretion (a) and TLR expression (b) of epithelial cells 12 h after infection (or not) with C. albicans SC5314 in the presence and absence of 5 μg/ml hGAPDH (2-32) (n = 6). Data are expressed as means±SD from duplicate assays of three different experiments. Statistical significance was determined using the 2-tailed paired Student’s t test. A p value of 0.01 or less was considered significant (n = 6).
Extensive studies ruled out any effects of possible contamination with LPS (< 100 pg/ml). The data thus indicate that hGAPDH (2-32) possesses immune stimulatory activity which might contribute to the protective effect.
DISCUSSION
With the aim of identifying naturally occurring antimicrobial active peptides in humans, we established a peptide library from human placental tissue as a known source for low molecular mass antimicrobial components. Purification procedures led to the isolation of a GAPDH-derived peptide with antibacterial and anticandidal properties. While its glycolytic function, the conversion of D-glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate is well-known, recent evidence suggests that this highly versatile molecule plays several diverse roles in living systems. Mammalian GAPDH is involved in membrane fusion (Morero et al., 1985), vesicle transport (Tisdale, 2001), microtubule bundling (Sirover, 1999), phosphotransferase activity, nuclear RNA export (Singh and Green, 1993), prostate cancer progression, programmed cell death, DNA replication, and DNA repair (Sirover, 1999, 2011). This came as a surprise to researchers but it makes evolutionary sense to re-use and adapt an existing protein instead of evolving a novel protein from scratch. It also make sense that a GAPDH fragment acts as an antimicrobial peptide because GAPDH is widely expressed in a multitude of tissues (Barber et al., 2005) and is found not only intra- but also extracellularly (Yamaji et al., 2005). Cell injury by invading pathogens might even enlarge this extracellular fraction and proteolytic degradation of GAPDH by the pathogen or the host might lead to the generation of smaller peptides with antimicrobial activity. Most recently, a role of microbial GAPDH in virulence has been postulated (Dumke et al., 2011; Egea et al., 2007). Moreover, evidence has been shown that GAPDH is relevant for the functioning of cationic host defense peptides as a mononuclear cell receptor for human cathelicidin LL-37 and immunomodulatory IDR-1 (Mookherjee et al., 2009).
The isolated peptide exhibits antimicrobial activity against E. coli and C. albicans in micromolar concentrations. It is very difficult to compare the antifungal activity of hGAPDH (2-32) in our experiments with those of the peptides described before in the literature, because it is well known that their activity is highly reliant on the conditions used to test antifungal activity. We therefore used the depolarization assay described in this paper to compare the antifungal activity of hGAPDH (2-32) with those of LL-37 and HBD3. The antifungal activities of all three peptides against C. albicans were not significantly different, while hGAPDH (2-32) was less active against E. coli. The relevance of our peptide as an antifungal peptide is confirmed by the observation that antifungal activity was also demonstrated in different media by the radial diffusion test and microdilution broth method. In addition, we found a protective effect during C. albicans infection of epithelial mono- and multilayers comparable to the effect of LL-37 but independently from the culture media conditions.
The morphological changes observed in hGAPDH (2-32)-treated C. albicans cells are related mainly to cytoplasm and the organelles suggesting internalization of the peptide. We were able to confirm cellular uptake after binding of the peptide to the cell wall of the fungi by immunofluorescence microscopy. The induction of apoptosis by hGAPDH (2-32) was characterized as a major killing mechanism by the exposure of phosphatidylserine from the inner to outer surface of the membrane as a representative hallmark of early stage apoptosis. The ongoing spread of antimycotic resistance is seriously undermining the present alternatives for therapeutic intervention against human candidiasis. Therefore, it becomes important to search and develop targets for antimicrobial therapy. Over the last few decades, the search for drugs and drug targets has prompted an interest in antimicrobial peptides. These are important components of the innate immune system, used by the host to protect itself from different types of pathogenic microorganism. Besides their direct effects on microbial cells, there is a growing body of evidence that mammalian antimicrobial peptides directly influence host cells, thereby inducing several mechanisms of the inflammatory processes and an immune response to the invading pathogens (Schittek et al., 2008). In this study, we suggest a novel role of antimicrobial peptides during host-pathogen interaction by inhibition of aspartic proteinases secreted by C. albicans. The production of these secreted aspartic proteinases (Sap) by C. albicans is a putative virulence factor of these opportunistic yeasts (Zhu and Filler, 2010). Preincubation of epithelial cells with the proteinase inhibitor pepstatin A prior to C. albicans infection significantly reduced invasion compared to untreated cells (Dalle et al., 2010). Experiments using triple mutants, lacking Sap1-3, confirmed the role of these isoenzymes for induced endocytosis (Dalle et al., 2010). An ED50 of 160 μg/ml or 50 μM hGAPDH (2-32) for Sap1p and 200 μg/ml or 63 μM for Sap2p suggests that inhibition of these important virulence factors only partially contribute to epithelial protection in our experiments. A more significant role of this peptide as a Sap inhibitor in vivo can not be excluded as GAPDH is widely expressed and abundantly present in a multitude of tissues, especially after epithelial injury during mucosal infection.
The outlook for the effectiveness of therapeutic approaches combining inhibitory effects on the growth and against virulence factors of C. albicans appears quite favorable. Lysozyme is an antimicrobial protein ubiquitous in human mucosal secretions such as saliva and effective against a wide range of bacteria. In a previous study it has been shown that lysozyme has a bimodal action on C. albicans, killing the organism at higher concentrations and decreasing the production of the important virulence factor Sap at lower concentrations (Wu et al., 1999).
The peptide hGAPDH (2-32) is secreted by the oral epithelial cells during C. albicans infection suggesting that this antimicrobial peptide serves as a host defense substance also in vivo.
We also determined the effects of hGAPDH (2-32) on chemokine secretion and TLR4 expression of oral epithelial cells. In previous studies, it has been shown that neutrophil defensins increased the secretion of IL-8 by pneumocyte-like A549 and primary bronchial epithelial cells (Sakamoto et al., 2005; van Wetering et al., 2002). The increased epithelial expression of IL-8, GM-CSF and TLR4 of infected epithelial cells induced by hGAPDH (2-32) in the present study supports the important role of human antimicrobial peptides for mucosal immunity by recruitment of activated leukocytes and lymphocytes to the site of infection. In addition, we could demonstrate increased secretion of both cytokines and mRNA TLR4 expression by hGAPDH (2-32) also of uninfected epithelial cells compared to the PBS treated controls. Recently, we demonstrated the important role of a PMN-mediated upregulation of epithelial TLR4 for protecting the mucosal surface from fungal invasion and cell injury (Weindl et al., 2007). Upregulation of TLR4 also in the absence of PMNs suggests an immune conditioning mechanism by this peptide for host defense. An important role of hGAPDH (2-32) induced immunomodulatory effects for tissue protection is suggested by the observation that epithelial damage in our infection experiments was significantly reduced in the presence of 5 μg/ml hGAPDH (2-32), while the same concentration was not very effective in our killing assay.
In this study, we demonstrate that the antimicrobial peptide hGAPDH (2-32) is secreted by epithelial cells during mucosal candidiasis. The peptide has a high binding affinity to the fungal cell wall and induces apoptosis after internalization. It partially antagonizes Sap activity, modulates the immune response, and causes profound changes in the yeast ultrastructure in vitro. The therapeutic relevance of these effects during C. albicans infection is confirmed by a strong protection against tissue damage in an established model of oral candidiasis. These data imply a protective role for the antimicrobial peptide hGAPDH (2-32) during experimental oral candidiasis.
In summary, antimicrobial agents not only acting against microorganisms in a classical way by fungicidal mechanisms but also interfering as apoptotic, antivirulence und immunomodulating drugs are a promising approach to antimicrobial therapy.
MATERIALS AND METHODS
Experimental strategy to find antimicrobial peptides
To detect antimicrobial peptides, a peptide library from human placental tissue was prepared. The collected fractions were initially screened for growth inhibitory activity against Escherichia coli (E. coli) as described previously (Liepke et al., 2003). A fraction demonstrating antibacterial activity was selected for further purification and analyzed until the active compound was identified. The synthesized peptide was used for further testing of antimicrobial activity against C. albicans. In addition, we tested the inhibitory activity against secreted aspartic proteinases (Sap) and the therapeutic and immunomodulatory effects after infection of epithelial monolayers and in an in vitro model of oral candidiasis.
Preparation of the peptide library
Human placental tissue was obtained from healthy individuals in a maternity ward of a local hospital after informed written consent. The design of the work has been approved by the local ethical committee (ethics committee of Hannover Medical School) and the study was conducted according to Declaration of Helsinki principles. The tissue was processed immediately after delivery and extracted in ice-cold 0.5 M acetic acid containing 10 mM ascorbate and 0.5 mM ethylendiamintetraacetate (EDTA). The extract was homogenized filtered and subsequently ultrafiltered as described previously (Liepke et al., 2003). After cation-exchange chromatography further subfractioning of each pH pool was done by reversed-phase (RP) chromatography (Liepke et al., 2003). By this procedure 42 fractions of each pool were lyophilized and stored at −20°C, designated the peptide library.
Purification of antimicrobial peptides from human placental tissue
Peptides were analyzed by electrospray mass spectrometry and by capillary zone electrophoresis. Amino-acid sequencing was performed by Edman degradation (Liepke et al., 2003). Each purification step was monitored by the radial diffusion assay for detection of antimicrobial activity.
Peptide synthesis
The identified peptide hGAPDH (2-32) was synthesized using Fmoc solid-phase chemistry on a preloaded TentaGel F-PHB histidine TRT resin (Rapp Polymere, Tübingen, Germany) and were purified by reversed-phase HPLC (Vydac C18, 10 μm, 300 Å, gradient: 10 - 70% B in 30 min, eluent A: 0.07 % TFA/water, eluent B: 0.05 % TFA in acetonitrile/ water 4:1, flow rate: 0.8 ml / min, UV detection: 215/230 nm). Purity and identity of synthesized peptides were checked by analytical HPLC, mass spectrometry, and sequence analysis.
Microorganisms
For our antimicrobial experiments we used E. coli ATCC 25922 and the clinical C. albicans isolate SC5314 (Gillum et al., 1984). C. albicans strains 143 and 526 were isolated from faeces, strain 277 from tracheal secretions and provided by the Institute of Laboratory Medicine, Klinik am Eichert (Göppingen, Germany).
Antimicrobial assays
Flow cytometric antimicrobial assay measuring membrane depolarization of E. coli ATCC 25922 and fungi was carried out as described elsewhere (Nuding et al., 2006; Schroeder et al., 2011). Briefly, 1.5×106 cells of E. coli, and the C. albicans strains 143, 277, 526, and SC5314 were incubated in 1:6 diluted Schaedler broth at 37°C with hGAPDH (2-32) in a final volume of 50 μl. The antimicrobial peptides hGAPDH (2-32), hBD-3 (Peptide Institute, Osaka, Japan) or LL-37 (Innovagen, Lund, Sweden) were dissolved in 0.01% acetic acid and added to bacterial and fungal suspensions at 10 μg/ml. To determine concentration dependence of microbial killing, hGAPDH (2-32) was added in concentrations of 5, 10, 50, 100 and 125 μg/ml. Microbial suspensions incubated with solvent (0.01% acetic acid) served as controls for viability. After 90 min the suspensions were incubated for 10 min with 1 mg/ml of the membrane potential sensitive dye DiBAC4(3) (Invitrogen, Karlsruhe, Germany). Suspensions were centrifuged for 10 min at 4,500 g and the sediments were resuspended in 300 μl phosphate-buffered saline. The percentage of depolarized fluorescent bacteria or fungi in suspension was determined by a FACSCalibur flow cytometer (BD, CA, USA) using Cell Quest software (BD) for 10 000 events per sample.
Electron microscopy
Approximately 106 C. albicans SC5314 cells/ml were treated with hGAPDH (2-32) peptide in a concentration of 125 μg/ml and incubated for 24 h at 37 °C. Treated and untreated yeast cells as a control were centrifuged, and the pellet was fixed in a phosphate-buffered solution (0.05 M, pH 7.3) with 2.5% glutaraldehyde and 2% formaldehyde following standard methods. Postfixation was carried out with 1% osmium tetroxide in 0.1 M phosphate buffer at pH 7.3 at room temperature, the specimens were embedded in glycid ether. Ultra-thin sections, 20-30 nm thick, were mounted on uncoated copper grids and stained in 2% uranyl acetate for 3 min and examined using a Zeiss LIBRA transmission electron microscope.
Sap inhibition assay
We used recombinant Sap1, Sap2 and Sap3 proteins expressed in P. pastoris to evaluate the inhibitory activity of hGAPDH (2-32) against single Sap isoenzymes. We incubated 0.25 μg of the indicated Sap protein with the increasing hGAPDH (2-32) peptide at non-permissive pH 7.4 for 1h at room temperature in 40 μl reaction volume. The Sap proteolytic reaction was then initiated by addition of substrate (BSA, 8 mg/ml) and pH shift to permissive pH 3.2 in a final volume of 100 μl (peptide/antibody concentration was calculated relative to the 100 μl final volume). The reaction was incubated for 30 minutes at 37°C and stopped by addition of TCA. TCA-soluble peptides were extracted and quantified using the BCA protein quantification kit from Thermo Scientific (Bonn, Germany) according to the manufacturer’s indications. Error bars indicate range of duplicates. Each duplicate consists of 3 background-normalized measures.
Generation and affinity purification of the hGAPDH-peptide antibody
The antigenic peptide GKVKVGVNGFGRIGRLVTRAAFNSGKVDIVA hGAPDH (2-32) was synthesized on a multiple peptide synthesizer, Syro II (MultiSynTech, Witten, Germany) using the Fmoc-chemistry. The peptide was purified using RP-HPLC to >90% purity and the identity was confirmed using MALDI-MS-TOF analysis. The peptide was coupled to key hole limpet hemocycanin (KLH) using the glutardialdehyde method. The obtained antiserum was further purified by affinity chromatography on a CH-activated Sepharose 4B column (GE healthcare) containing the peptide immobilized via a stable peptide bond (Zaidi et al., 2007). After concentration on a 20-kDa membrane the resulting antibody was tested by ELISA on a microplate coated with GAPDH or the peptide hGAPDH (2-32). For control, the preimmunserum was used.
Analyses of apoptotic and necrotic marker
C. albicans were grown overnight in YPD (yeast extract/peptone/dextrose) by shaking at 37°C. 1 × 107 cells in 250 μl PBS were treated with 5 μg/ml hGAPDH (2-32) at 37° for 60 min or with 50 mmol H202 as a positive control. After centrifugation, cells were protoplasted by digesting with SCE-buffer (sorbitol/ sodium-citrat/ EDTA) containing 100-500 U/mg Lyticase from Arthrobacter luteus (Sigma Germany) and 2-mercaptoethanol (Roth Germany) at 37°C for 70 min. Samples were stained with FITC-labeled annexin-V (BD Pharmingen Germany) and propidium iodide (Molecular Probes Germany) to assess the externalization of phosphatidylserine and cell integrity. Cells were analyzed under a Leica TCS-SP/Leica DM RB confocal laser scanning microscope (Leica Microsystems, Wetzlar, Germany) with Leica Confocal Software LCS (version 2.61).
Cytokine analysis
GM-CSF, and IL-8 concentrations in the culture supernatants were determined using commercially available ELISA Kits (DuoSet, R&D Systems Minneapolis, MN, USA).
RNA isolation and quantitative RT-PCR
Total RNA was extracted with NucleoSpin RNAII (Macherey-Nagel, Düren, Germany) and cDNA was synthesized using 1μg total RNA with SuperScript III Reverse Transcriptase (Invitrogen, Karlsruhe, Germany). Amplification of cDNA was performed as described previously (Weindl et al., 2007). All samples were run in duplicates and the mean threshold cycle (Ct) reading was used. Fold difference in gene expression was normalized to the housekeeping gene (Weindl et al., 2007).
Models of oral candidiasis
For monolayer infection studies, the human buccal cell line TR146 was used. Cells were cultured in DMEM medium with 10% FCS and 0.1% gentamicin solution (50 mg/ml) at 37°C in 5% CO2. Infection studies (MOI 0.1) were performed in antibiotic and antimycotic free culture medium. Epithelial monolayers were infected with C. albicans SC5314 in the presence and absence of 5, 10, 50, 100 and 125 μg/ml hGAPDH (2-32) for 12 and 24 h. To evaluate tissue damage during infection, LDH analysis was performed (Weindl et al., 2007).
The in vitro model of oral candidiasis was based on multilayer reconstituted human epithelium (RHE). The mucosa equivalent and all culture media were prepared as described previously (Weindl et al., 2007). Epithelial cultures were infected with 2×106 C. albicans SC5314 cells in 50 μl medium (3 g/l TSB in 10 mM sodium phosphate buffer, pH 7.2) containing 5, 10, 50, 100, 125 μg/ml hGAPDH (2-32) or without peptide as a control. Infected cultures were incubated for 12 and 18 h at 37 °C in a 100 % humidified atmosphere containing 5 % CO2. To evaluate histological changes during infection, light microscopic studies were carried out as described previously (Weindl et al., 2007). Histological changes of mucosa were evaluated on the basis of 50 sections from five different sites for each infected epithelium.
For confocal microscopy, oral RHE was cryofixed in liquid nitrogen, and 5-μm sections were placed on Roti®-Bond adhesion slides (Roth GmbH). Sections were fixed in PLP (paraformaldehyde and lysine in PBS) for 2 min, followed by incubation with PBS for 5 min, PBS/BSA (0.1%) plus Tween 20 (0.1%) for 5 min, and PBS plus 10% donkey serum for 30 min at room temperature. Anti-hGAPDH (2-32) polyclonal rabbit antibodies (1:50) and human anti–C. albicans serum (1:60; Virion\Serion) were added for 60 min at room temperature. Sections were then incubated with donkey anti-rabbit–Dylight 549 (1:800; Dianova) and donkey anti-human–Cy5 (1:500; Dianova) for 60 min. All nuclei were stained with YOPRO (Invitrogen). All washing and antibody addition steps were performed with a combination of PBS, BSA, and Tween. The sections were analyzed with a confocal laser scanning microscope (Leica TCS SP; Leica Microsystems) at ×400 and ×250 magnification. Statistics. All experiments were performed at least 3 times if not otherwise indicated and revealed comparable results. Results are presented as mean ± SD. Statistical significance was determined using the 2-tailed paired Student’s t test. A P value of 0.05 or less was considered significant.
ACKNOWLEDGEMENTS
We thank E. Januschke and J. Laude, University of Munich, and Birgit Fehrenbacher, Renate Nordin, Theresia Schneider, and Hannelore Bischof, University of Tübingen, for their excellent technical assistance. Gillian Teicke is acknowledged for help in revising the manuscript.
The work was supported by the Deutsche Forschungsgemeinschaft (Sch 897/1-3 to M.S., J.W., Graduate College 303, Infection and Immunity to J.S.) and by the National Institute of Dental and Craniofacial Research (R01 DE017514-01 to M.S.).
Footnotes
CONFLICT OF INTEREST W.G. Forssmann is CEO of IPF Pharmaceuticals GmbH and shareholder of Pharis Group. The other authors state no conflict of interest
REFERENCES
- Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics. 2005;21:389–95. doi: 10.1152/physiolgenomics.00025.2005. [DOI] [PubMed] [Google Scholar]
- Borelli C, Ruge E, Lee JH, Schaller M, Vogelsang A, Monod M, et al. X-ray structures of Sap1 and Sap5: structural comparison of the secreted aspartic proteinases from Candida albicans. Proteins. 2008;72:1308–19. doi: 10.1002/prot.22021. [DOI] [PubMed] [Google Scholar]
- Braga-Silva LA, Santos AL. Aspartic protease inhibitors as potential anti-Candida albicans drugs: impacts on fungal biology, virulence and pathogenesis. Curr Med Chem. 2011;18:2401–19. doi: 10.2174/092986711795843182. [DOI] [PubMed] [Google Scholar]
- Dalle F, Wachtler B, L’Ollivier C, Holland G, Bannert N, Wilson D, et al. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. 2010;12:248–71. doi: 10.1111/j.1462-5822.2009.01394.x. [DOI] [PubMed] [Google Scholar]
- Dumke R, Hausner M, Jacobs E. Role of Mycoplasma pneumoniae glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in mediating interactions with the human extracellular matrix. Microbiology. 2011;157:2328–38. doi: 10.1099/mic.0.048298-0. [DOI] [PubMed] [Google Scholar]
- Egea L, Aguilera L, Gimenez R, Sorolla MA, Aguilar J, Badia J, et al. Role of secreted glyceraldehyde-3-phosphate dehydrogenase in the infection mechanism of enterohemorrhagic and enteropathogenic Escherichia coli: interaction of the extracellular enzyme with human plasminogen and fibrinogen. Int J Biochem Cell Biol. 2007;39:1190–203. doi: 10.1016/j.biocel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Fera MT, La Camera E, De Sarro A. New triazoles and echinocandins: mode of action, in vitro activity and mechanisms of resistance. Expert Rev Anti Infect Ther. 2009;7:981–98. doi: 10.1586/eri.09.67. [DOI] [PubMed] [Google Scholar]
- Gauwerky K, Borelli C, Korting HC. Targeting virulence: a new paradigm for antifungals. Drug Discov Today. 2009;14:214–22. doi: 10.1016/j.drudis.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–82. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Korting HC, Schaller M, Eder G, Hamm G, Bohmer U, Hube B. Effects of the human immunodeficiency virus (HIV) proteinase inhibitors saquinavir and indinavir on in vitro activities of secreted aspartyl proteinases of Candida albicans isolates from HIV-infected patients. Antimicrob Agents Chemother. 1999;43:2038–42. doi: 10.1128/aac.43.8.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy O, Gangneux JP, Montravers P, Mira JP, Gouin F, Sollet JP, et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005-2006) Crit Care Med. 2009;37:1612–8. doi: 10.1097/CCM.0b013e31819efac0. [DOI] [PubMed] [Google Scholar]
- Liepke C, Baxmann S, Heine C, Breithaupt N, Standker L, Forssmann WG. Human hemoglobin-derived peptides exhibit antimicrobial activity: a class of host defense peptides. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791:345–56. doi: 10.1016/s1570-0232(03)00245-9. [DOI] [PubMed] [Google Scholar]
- Mookherjee N, Lippert DN, Hamill P, Falsafi R, Nijnik A, Kindrachuk J, et al. Intracellular receptor for human host defense peptide LL-37 in monocytes. J Immunol. 2009;183:2688–96. doi: 10.4049/jimmunol.0802586. [DOI] [PubMed] [Google Scholar]
- Morero RD, Vinals AL, Bloj B, Farias RN. Fusion of phospholipid vesicles induced by muscle glyceraldehyde-3-phosphate dehydrogenase in the absence of calcium. Biochemistry. 1985;24:1904–9. doi: 10.1021/bi00329a015. [DOI] [PubMed] [Google Scholar]
- Nuding S, Fellermann K, Wehkamp J, Mueller HA, Stange EF. A flow cytometric assay to monitor antimicrobial activity of defensins and cationic tissue extracts. J Microbiol Methods. 2006;65:335–45. doi: 10.1016/j.mimet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Peman J, Canton E, Espinel-Ingroff A. Antifungal drug resistance mechanisms. Expert Rev Anti Infect Ther. 2009;7:453–60. doi: 10.1586/eri.09.18. [DOI] [PubMed] [Google Scholar]
- Sakamoto N, Mukae H, Fujii T, Ishii H, Yoshioka S, Kakugawa T, et al. Differential effects of alpha- and beta-defensin on cytokine production by cultured human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L508–13. doi: 10.1152/ajplung.00076.2004. [DOI] [PubMed] [Google Scholar]
- Sanglard D, Coste A, Ferrari S. Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Res. 2009;9:1029–50. doi: 10.1111/j.1567-1364.2009.00578.x. [DOI] [PubMed] [Google Scholar]
- Schaller M, Borelli C, Korting HC, Hube B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses. 2005;48:365–77. doi: 10.1111/j.1439-0507.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- Schittek B, Paulmann M, Senyurek I, Steffen H. The role of antimicrobial peptides in human skin and in skin infectious diseases. Infect Disord Drug Targets. 2008;8:135–43. doi: 10.2174/1871526510808030135. [DOI] [PubMed] [Google Scholar]
- Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature. 2011;469:419–23. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- Singh R, Green MR. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science. 1993;259:365–8. doi: 10.1126/science.8420004. [DOI] [PubMed] [Google Scholar]
- Sirover MA. New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta. 1999;1432:159–84. doi: 10.1016/s0167-4838(99)00119-3. [DOI] [PubMed] [Google Scholar]
- Sirover MA. On the functional diversity of glyceraldehyde-3-phosphate dehydrogenase: Biochemical mechanisms and regulatory control. Biochim Biophys Acta. 2011;1810:741–51. doi: 10.1016/j.bbagen.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Tisdale EJ. Glyceraldehyde-3-phosphate dehydrogenase is required for vesicular transport in the early secretory pathway. J Biol Chem. 2001;276:2480–6. doi: 10.1074/jbc.M007567200. [DOI] [PubMed] [Google Scholar]
- van Wetering S, Mannesse-Lazeroms SP, van Sterkenburg MA, Hiemstra PS. Neutrophil defensins stimulate the release of cytokines by airway epithelial cells: modulation by dexamethasone. Inflamm Res. 2002;51:8–15. doi: 10.1007/pl00000282. [DOI] [PubMed] [Google Scholar]
- Weindl G, Naglik JR, Kaesler S, Biedermann T, Hube B, Korting HC, et al. Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling. J Clin Invest. 2007;117:3664–72. doi: 10.1172/JCI28115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Samaranayake LP, Leung WK, Sullivan PA. Inhibition of growth and secreted aspartyl proteinase production in Candida albicans by lysozyme. J Med Microbiol. 1999;48:721–30. doi: 10.1099/00222615-48-8-721. [DOI] [PubMed] [Google Scholar]
- Yamaji R, Chatani E, Harada N, Sugimoto K, Inui H, Nakano Y. Glyceraldehyde-3-phosphate dehydrogenase in the extracellular space inhibits cell spreading. Biochim Biophys Acta. 2005;1726:261–71. doi: 10.1016/j.bbagen.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Zaidi N, Herrmann T, Baechle D, Schleicher S, Gogel J, Driessen C, et al. A new approach for distinguishing cathepsin E and D activity in antigen-processing organelles. FEBS J. 2007;274:3138–49. doi: 10.1111/j.1742-4658.2007.05846.x. [DOI] [PubMed] [Google Scholar]
- Zhu W, Filler SG. Interactions of Candida albicans with epithelial cells. Cell Microbiol. 2010;12:273–82. doi: 10.1111/j.1462-5822.2009.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]