Abstract
Background
Interleukin (IL)-17 is an important cytokine signature of a T helper differentiation pathway, Th17. This T cell subset is crucial in mediating autoimmune disease or antimicrobial immunity in animal models, but its presence and role in human disease remains to be completely characterized.
Objective
We set out to determine the frequency of Th17 cells in cystic fibrosis (CF), a disease in which there is recurrent infection with known pathogens.
Methods
Explanted lungs from patients undergoing transplant or organ donors (CF = 18, non-CF, non-bronchiectatic = 10) were collected. Hilar nodes and parenchymal lung tissue were processed. We examined them for Th17 signature by immunofluorescence and quantitative real time PCR. T cells were isolated and stimulated with antigens from Pseudomonas aeruginosa and Aspergillus. Cytokine profiles and staining by flow cytometry were used to assess the reactivity of these cells to antigen stimulation.
Results
We found a strong IL-17 phenotype in CF compared to non-CF controls. Within this tissue, we found pathogen-antigen-responsive CD4+IL17+ cells. There were double positive IL-17+IL-22+ cells and the IL-22+ population had higher proportions of memory characteristics. Antigen-specific Th17 responses were stronger in the draining lymph nodes compared to matched parenchymal lung.
Conclusion
Inducible proliferation of Th17(22) with memory cell characteristics is seen in CF lung. The function of these individual subpopulations will require further study regarding their development. T-cells are likely not the exclusive producers of IL-17 and IL-22 and this will require further characterization.
Keywords: Th17, Th22, IL-17, IL-22, cystic fibrosis, Pseudomonas, Aspergillus, chronic infectious disease, memory T cell response, lung transplant
Introduction
The discovery of the effector cytokine IL-17 has caused a major paradigm shift from our formerly dichotomous understanding of Th1/Th2 immune inflammation. It has emerged as a crucial regulator of mucosal immunity against infection in the lung, skin and gut. Moreover, the identification of subsets of memory T cells expressing IL-17 has led to the recognition of a distinct arm of immune response called Th17. However, as studies on the regulation of IL-17 demonstrate, the Th17 immune response walks a fine line between protective and destructive tissue inflammation.
At the height of the HIV epidemic, intense focus on CD4+ T cells led to the discovery of two classes of T helper cells, Th1 and Th2.1 Th1 cells are characterized by IFNγ production whereas Th2 cells produce IL-4, IL-5 and IL-13. This model of T helper cell development went far to explain immune defense against many intracellular pathogens (Th1) and parasites (Th2). However, mouse models lacking either class of T-helper response were still able to mount a protective response against Pneumocystis infection2. Therefore, the simple Th1/Th2 polarization model was insufficient to explain the rampant susceptibility to opportunistic bacterial and fungal infection associated with CD4+ deficiency.
Further studies on lymphotropic viruses found that they often orchestrate infection by producing homologues of host cytokines and IL-17 was identified in a T-cell hybridoma cDNA library in a search for viral protein homologues 3–6. Subsequent work has shown that IL-17 mediates neutrophil recruitment to sites of infection 6–8 and unequivocally established its protective host defense role in mucocutaneous bacterial and fungal infections 9–15.
On the flip side of the inflammatory coin, IL-17 pathways have been shown to exacerbate autoimmune disease. Mouse models of rheumatoid arthritis, systemic lupus erythematosis, inflammatory bowel disease and psoriasis have subsequently highlighted the importance of the Th17 subtype in maintenance of inflammation and this has been extensively reviewed elsewhere16. Whether it is infection or self-antigen that incites the initial inflammatory response, a sustained favorable cytokine milieu leads to the development of memory Th17 cells 13, 17–20. These cells mediate the crucial recall response to bacterial invaders but are disastrously destructive in autoimmunity. While this is well-understood in murine models of disease, the identification and characterization of Th17 cells in humans is still a developing frontier.
In human disease, the crucial role of IL-17 pathways in host defense is seen in patients with Job’s Syndrome, a disorder involving a STAT3 defect that results in impaired differentiation of Th17 cells. These patients present with hyper-IgE syndrome as well as recurrent bacterial and fungal infections of the skin and respiratory tract, particularly with S. aureus as well as oropharyngeal candidiasis. Furthermore, patients with autoimmune polyendrocrine syndrome type I who have chronic mucocutaneous candidiasis were found to have auto-antibodies against IL-17 and IL-22.21 Conversely, increased IL-17A and IL-17F levels are detected in asthmatics 22, 23, in bronchoalveolar lavage of cystic fibrosis (CF) patients undergoing exacerbation associated with Pseudomonal colonization24, and in patients with chronic obstructive pulmonary disease (COPD)25.
As important as Th17 responses are in host defense, it is conceivable that diseases involving anatomic or physiologic defects permissive of chronic infection could mimic the persistent Th17 inflammatory response seen in autoimmune models 26. While Th17 cells mediate memory adaptive immunity against infection, IL-17 also elicits robust innate inflammatory responses by recruiting neutrophils 7, 9, 27 and upregulating antimicrobial effectors15, 28–35. Thus, it presents a potential mechanistic target for remediation of the tissue destruction seen in chronic infection.
The human lung is a unique organ for examining Th17 development in this setting because it is the only organ with direct environmental contact but is not known to have a substantial normal flora. Investigation can be focused on the host memory response to a limited number of organisms in the lung. Indeed, there is evidence that lower respiratory tract colonization with select classes of organisms is associated with functional decline in COPD 36–40 as is extensively reviewed by A. Morris and colleagues 41. Moreover, mouse models of COPD demonstrate that matrix metalloproteinases, play a role in mediating tissue destruction 42, 43.
For the current study, our group has chosen to examine CF as the model of chronic infection and inflammation. We previously found increased levels of IL-17 in bronchoalveolar lavage fluid during exacerbations associated with positive Pseudomonas sputum cultures 24. Subsequently, we showed higher levels of IL-17 in the draining lymph nodes of CF patients undergoing transplant 44. In the current work, we now identify a source of IL-17 in these patients as CD4+ cells in the draining lymph node. Furthermore, we demonstrate that these cells have an antigen-specific response, producing a Th17 cytokine response to bacterial and fungal pathogens the patients were colonized with. This is a breakthrough in the isolation of Th17 cells that can be physiologically tested, and warrants prospective studies of its potential as a prognostic tool in transplant recipient outcome. Moreover, our finding that IL-17 levels can be augmented by simultaneous suppression of Th1 (IFNγ) and Th2 (IL-4 or IL-13) cytokines in humans, an effect mediated by many standard therapies for CF or airways hyperreactivity, has therapeutic implications that warrant further exploration.
Methods
Ethics Statement
All patient samples were collected after obtaining informed consent and were de-identified as approved by an Institutional Review Board at the University of Pittsburgh (IRB number REN10070105).
Collection of explanted lungs and tissue bank specimens
Explanted lungs were collected from patients undergoing lung transplant at the University of Pittsburgh following approval by the Institutional Review Board. Controls were lungs from non-CF patients that died of trauma and were not ultimately used for transplantation under the Center for Organ Recovery and Education (CORE), or patients with non-CF, non-bronchiectatic end-stage lung disease undergoing transplant. Eighteen CF patients and ten non-CF patients’ samples were used. Sample size was determined based on the volume of transplants done at the University of Pittsburgh and the availability of tissue for processing. For draining lymph node cells (DLN), hilar lymph nodes were dissected from the specimens and dispersed into single cell suspension per a protocol adapted from mouse mononuclear cell preparation45. For parenchymal leukocytes (PLC), peripheral lung tissue was processed as previously described46. Lung tissue was frozen in Tissue-Tek® OCT compound for immunofluorescence staining and RNA analysis.
Antigen preparation and testing
Several antigens were tested for their ability to stimulate proliferation by BRDU incorporation in DLC cultures (FITC BRDU Flow Kit, BD Biosciences). Early log phase and late log phase Pseudomonas aeruginosa (PA01) cultures grown in Luria Broth were pelleted and subsequently either sonicated and sterile-filtered or heat-killed (HK). In addition, the supernatant from the pelleted culture was also sterile-filtered and tested. All samples were compared to fresh tryptic soy broth (TSB). From these studies, we found the early log phase [Pa(EL)] sonicated pellet and late log phase [Pa (LL)] culture filtrate had the highest activity and subsequently used these fractions. Aspergillus mitogilin (Asp, Indoor Biotechnologies) was used at 1 μg/ml as titrated previously47. Concanavalin A (Con A, Sigma) was used at 5 μg/ml, Candida antigen (Hollister-Stier) and tetanus toxoid (TT, adsorbed injectable solution, Aventis) both were tested at 1:10, 1:100 and 1:1000 dilution.
Culture conditions and antigen stimulation
DLN and PLC cells were resuspended at 5 × 106 cells/ml in RPMI 1640, 2 mM L-glutamine, 25 mM HEPES, 100 U/ml penicillin/streptomycin, 50 μM β-mercaptoethanol, 10% fetal calf serum, 100 U/ml IL-2 (Roche)] for stimulation. DLN and PLC cultures were stimulated in triplicate with Pa(EL) 1 μg/ml, Pa(LL) 10 μg/ml, Asp 1 μg/ml or Con A 5 μg/ml (Figure S1 in OR). For antibody neutralizations, anti-IL-4, anti-IFNγ, anti-IL-13 or appropriate isotype control (all R&D Systems) were added to wells at 10 μg/ml per antibody. Cultures were incubated for 5–7 days, collected and washed for flow cytometry staining and analysis, and supernatants were collected for cytokine analysis assays.
Immunofluorescence
Slides from the OCT-embedded tissue described above were fixed in 4% paraformaldehyde, washed with PBS and blocked with 5% secondary antibody source animal serum. They were stained with anti-CD4 (R&D AF-379-NA), anti-Zo-1 (Invitrogen 617300), anti-IL-17A (R&D MAB3171), anti-IL-17F (R&D MAB13351), anti-CD56 (BD Biosciences 559049) and/or anti-IL-22 (R&D AF782). They were counterstained with appropriate anti-isotype Alexa-fluor 488 or Alexa-fluor 594(Invitrogen). Isotype controls were also used to assess the level of non-specific binding (Figure S2 in OR). Confocal microscopy was done on an Olympus Fluoview1000 inverted laser scanning confocal microscope with a 20x oil objective (numerical aperture 0.85) and reviewed by an experienced microscopist (K. Lathrop) who was blinded to the identity of the slides and selected 6–8 representative fields on each slide for image quantitation. Images were acquired in 5 channels; four immunofluorescent and one DIC brightfield. For each fluorescent channel, images were thresholded to segment fluorescent signal above background levels and then binarized. Area measurements were read out into Excel (Microsoft) and the binary images were saved. Image arithmetic was used to determine total pixel area of CD4, Zo-1 and IL-17 individually as well as area of CD4 that was not coincident with ZO1 but was coincident with IL17. 3D image reconstructions of confocal stacks were also done (Video E1 and E2 in OR). All digital image processing was done using Metamorph 7.7.6 software and statistics were done in Graphpad Prism using two-way ANOVA with Bonferroni post test comparison.
Quantitative Real-Time PCR (qPCR) analysis
Serial sections from the OCT-embedded tissue blocks that were cut for immunofluorescence staining were placed in TRIzol® (Invitrogen). Total RNA was isolated per manufacturer’s protocol and re-purified using a cleanup procedure (RNeasy mini Kit, QIAGEN). Total RNA was reverse transcribed with Superscript II and random primers (Invitrogen Life Technologies). The cDNAs were amplified with specific primers and probes from Applied Biosystems, using custom-designed 384-well arrays (Micro Fluidic Card) and following the Applied Biosystems protocol. Level of mRNA expression was first normalized to human T-box transcription factor (TBX21) and fold change was calculated according to the ΔΔCt method and data was analyzed in Graphpad prism using two-way ANOVA with Bonferroni post test comparison.
Multiplex cytokine analysis and ELISA
Supernatants from the cultures were examined for multiple cytokine levels using a bead-based cytokine array. Specifically, DLN and PLC stimulated culture supernatants were processed using the Luminex (now Millipore) Human 30-plex cytokine kit. Results were read using a Bio-Rad Bio-Plex instrument. Data were then exported to Microsoft Excel and GraphPad Prism for analysis using two-way ANOVA with Bonferroni post test comparison.
Flow cytometry analysis of cell populations
Prior to harvest, DLN and PLC cultures intended for flow cytometry were re-stimulated with the antigen they were initially exposed to and monensin was added to 5 μM in the wells for 6 hr. Cells were blocked using Flow Cytometry Staining Buffer (eBioscience 00-4222) with 5% mouse IgG. Then, cells were surface labeled with conjugated antibodies directed against CD3, CD4, CD45RO and TCRαβ (all BDBiosciences). Intracellular cytokine expression for DLN and PLC cultures was also analyzed by flow cytometry. Intracellular staining for IL-17 (eBioscience 12-7179) and IL-22 (R&D Systems IC7821A) was carried out using reagents from a commercially available intracellular staining kit per their instructions (Ebioscience 00-5523). Samples were run on a BD FACSAria™ cell sorter and data was analyzed using FACSDiVa™ or FlowJo software. Acquisition gates were first set by size (FSC) and granularity (SSC) characteristics. Total live cells were further analyzed after doublet discrimination. Gate statistics for all samples were then exported to a spreadsheet and analyzed in Graphpad Prism using two-way ANOVA with Bonferroni post test comparison.
Results
Patient demographics
CF patients (Table 1) were evenly distributed by gender (9 males, 9 females). The average age at transplant among CF patients was 29 years (SD ± 6.9 years). Culture results for a majority of individuals revealed a history of polymicrobial colonization or infection prior to transplant. Ten of 18 individuals had positive cultures for Pseudomonas aeruginosa prior to transplant. Four patients were on oral prednisone at the time of transplant, while 9 patients were on an inhaled corticosteroid. Non-CF patients included in the comparison (Table 2) were a more heterogeneous group among the individuals for whom medical information was available. The average age of patients with available medical information was 48 years (SD ± 20 years). Unpaired, two-tailed t-test revealed a statistically significant difference in age of the populations for whom medical information was known. Patients that were transplanted for non-bronchiectatic or non-obstructive lung diseases (5 patients) were included, as well as lungs from CORE that were not used for transplant (5 patients). Medical information was available for one CORE patient due to that lung originating at our transplant center. Of the non-CF transplant recipients, 3 of 5 were on inhaled corticosteroids and oral prednisone at the time of transplant. Thus, the proportion of inhaled steroid usage was similar in recipient patients (CF 0.5, non-CF recipients 0.6), while the proportion of oral steroid use was similar among CF patients compared to all non-CF controls (CF 0.22, non-CF 0.30).
Table 1. Characteristics of CF patients.
Demographic information on CF patients from whom lungs were collected is shown along with a key describing the studies each sample underwent.
| Age | Gender | Immunomodulators | Pathogen status | Studies |
|---|---|---|---|---|
| 25 | M | None | Pseudomonas aeruginosa, Torulopsis glabrata | F, C |
| 36 | F | Fluticasone/salmeterol inhaled | Candida parapsilosis, Candida tropicalis, Candida albicans | F, C |
| 34 | M | Fluticasone/salmeterol inhaled Prednisone 15 mg daily | Stenotrophomonas maltophilia, Coagulase-negative Staphylococcus, Candida albicans | F, C |
| 26 | F | Prednisone 15 mg daily | Pseudomonas aeruginosa, Achromobacter xylosoxidans, Aspergillus fumigatus, Yeast, Staphylococcus aureus | F, C |
| 36 | M | Prednisone 20 mg daily | Pseudomonas aeruginosa, Staphylococcus aureus, Serratia marcescens | F, C, IF, R |
| 49 | M | None | Pseudomonas aeruginosa, Moraxella sp, Streptococcus viridans, Coagulase- negative Staphylococcus, Pseudomonas fluorescens putida, Neisseria sp, Candida albicans | F, C, IF, R |
| 25 | F | None | Candida albicans | IF, R |
| 25 | F | Fluticasone/salmeterol inhaled | Staphylococcus aureus, Stenotrophomonas maltophilia, Enterobacter aerogenes | F, C |
| 29 | M | Fluticasone/salmeterol inhaled Prednisone 10 mg daily | Pseudomonas aeruginosa | C |
| 26 | F | Fluticasone/salmeterol inhaled | Pseudomonas aeruginosa, Streptococcus viridans | F, C IF, R |
| 23 | F | None | Pseudomonas aeruginosa, Aspergillus fumigatus | F, C IF, R |
| 19 | M | Fluticasone/salmeterol inh | Burkholderia cepacia, Achromobacter xylosoxidans, Streptococcus viridans, Candida albicans | IF, R |
| 36 | F | Fluticasone/salmeterol inh | Pseudomonas aeruginosa, Streptococcus viridans, Neisseria sp | IF, R |
| 27 | M | Prednisone 10 mg daily | Burkholderia cepacia | IF, R |
| 29 | F | Mometasone inhaled | Staphylococcus aureus, Streptococcus viridans, Enterobacter cloacae, Neisseria sp, Coagulase-negative Staphylococcus, Yeast | IF, R |
| 29 | M | None | Burkholderia cepacia, Streptococcus viridans, Coagulase-negative Staphylococcus, Neisseria sp, Candida albicans | IF, R |
| 24 | F | Prednisone 10 mg daily | Pseudomonas aeruginosa, Staphylococcus aureus, Yeast | IF, R |
| 30 | M | Fluticasone/salmeterol inhaled Prednisone 5 mg daily | Pseudomonas aeruginosa, Streptococcus beta hemolytic Group C, Streptococcus viridans, Candida albicans, Exophiala jeanselmei | IF, R |
F = cell stimulation and flow cytometric analysis; C = cell stimulation, antibody neutralization and multiplex cytokine analysis; IF = immunofluorescence studies on OCT embedded tissue blocks; R = real time PCR on serial sections from OCT embedded blocks.
Table 2. Characteristics of non-CF patients.
Demographic information on non-CF control patients from whom lungs were collected are shown along with a key describing the studies each sample underwent.
| Age | Gender | Diagnosis | Immunomodulators | Pathogen status | Studies |
|---|---|---|---|---|---|
| 18 | M | CORE | None | None | F, C, IF, R |
| U | U | CORE | Unknown | Unknown | F, C |
| U | U | CORE | Unknown | Unknown | F, C, IF, R |
| U | U | CORE | Unknown | Unknown | F, C, IF, R |
| U | U | CORE | Unknown | Unknown | IF, R |
| 64 | M | IPF | Fluticasone inhaled Prednisone 12.5 mg daily | Streptococcus viridans, Neisseria sp | IF, R |
| 66 | M | IPF | Fluticasone/salmeterol inhaled Prednisone 20 mg daily | None | IF, R |
| 30 | M | PPH | None | Streptococcus viridans, Micrococcus sp, Neisseria sp | IF, R |
| 58 | F | PPH | None | Streptococcus viridans, Streptococcus Beta Hemolytic Group F, Staphylococcus aureus, Neisseria sp | IF, R |
| 54 | M | Sarcoidosis | Fluticasone/salmeterol inhaled Prednisone 30 mg taper | Candida albicans | IF, R |
U = unknown or unavailable information; F = cell stimulation and flow cytometric analysis; C = cell stimulation, antibody neutralization and multiplex cytokine analysis; IF = immunofluorescence studies on OCT embedded tissue blocks; R = real time PCR on serial sections from OCT embedded blocks.
Th17 cytokines levels are elevated in the CF lung
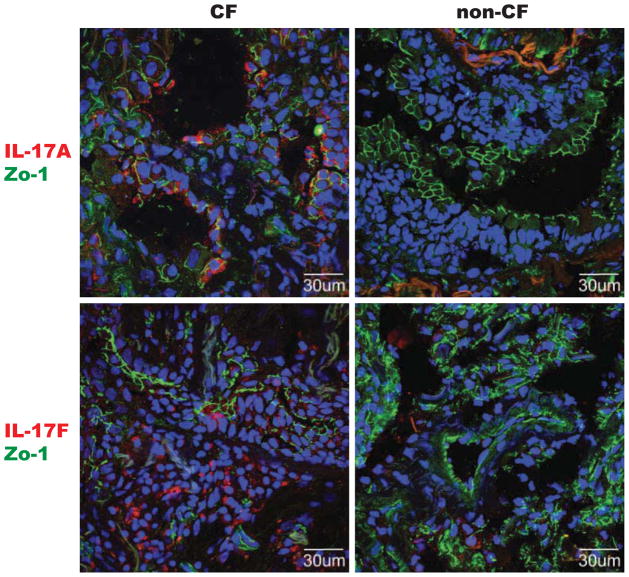
We examined the status of IL-17 in banked lung tissues from CF patients that had undergone transplant and compared them to CORE samples or patients undergoing transplant for non-bronchiectatic end-stage lung disease (primary pulmonary hypertension, idiopathic pulmonary fibrosis, sarcoidosis). CF tissues had many patches of dramatic staining for IL-17A and IL-17F (Figure 1). Examination of the airways that were seen revealed sheets of inflammatory cells in CF, while this was usually not present in non-CF genotypes. Quantitative analysis of IL-17F fluorescence area revealed that overall signal area of IL-17F trended higher for CF, and the signal area of co-localized CD4 and IL-17F was significantly higher in CF patients (Figure 2).
Figure 1. IL-17 positive cells are prominent in CF lung tissue.
Parenchymal lung tissue stained for IL-17A (red, top two) or IL-17F (red, bottom two) and co-stained with tight junction protein-1 (Zo-1, green, all) to delineate anatomic structure. DAPI counterstaining (blue) was done to highlight nuclei. Representative images from CF patients (left two) and non-CF controls (right two, patients transplanted for primary pulmonary hypertension and idiopathic pulmonary fibrosis) are shown.
Figure 2. CF lungs demonstrate higher numbers of dual positive CD4+IL-17+ cells and have stronger Th17 cytokine message profile.
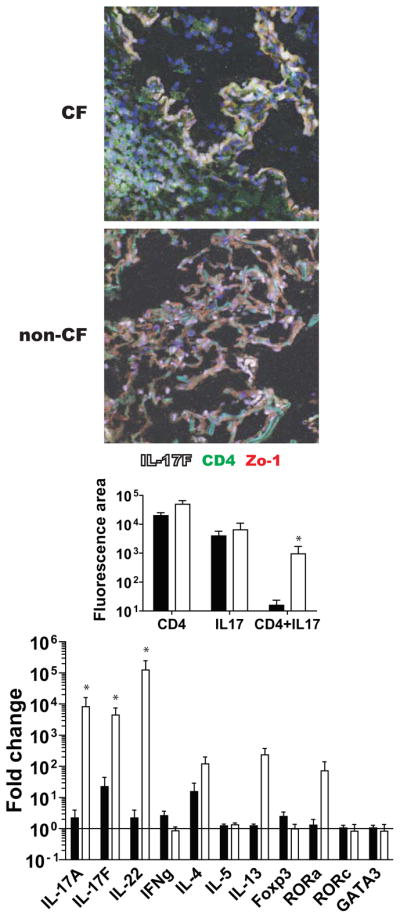
(Top photomicrographs) Representative images of parenchymal lung tissue stained for IL-17F (white) and co-stained with anti CD4 (green) and Zo-1 (red). (Middle graph) Quantitative confocal analysis of all image files obtained, compared by genotype control ■ (n = 9) vs CF □ (n = 12). (Bottom graph) qPCR reveals increased Th17 transcripts in CF lungs. ΔΔCt values for control ■ (n = 9) and CF □ (n = 12) are shown. IL-17A, IL-17F and IL-22 are upregulated in CF lung tissue while related transcript RORa also trends higher in CF. * = p < 0.05
Higher levels of Th17-related transcripts were also found in the CF samples compared to non-CF samples in serial sections taken from the same blocks used for immunofluorescent analysis (Figure 2). Notably, IL17A, IL17F and IL22 were significantly higher in CF tissues compared to non-CF controls. Consistent with these findings, RORA, a second Th17-specific transcription factor48, also trended higher in CF while RORC was not significantly different. IL-4 and IL-13 did trend higher in CF but this was not statistically significant. In contraposition, IFNg and FOXP3, a key regulatory T cell transcriptional activator and simultaneous inhibitor of Th17 trended lower in CF patients. This provided evidence that Th17 pathways were indeed significant in CF.
Antigen-specific IL-17 response is more dominant in CF compared to non-CF control draining lymph node
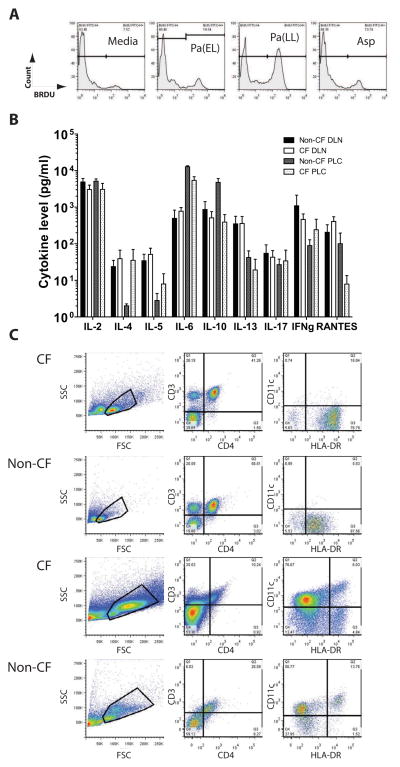
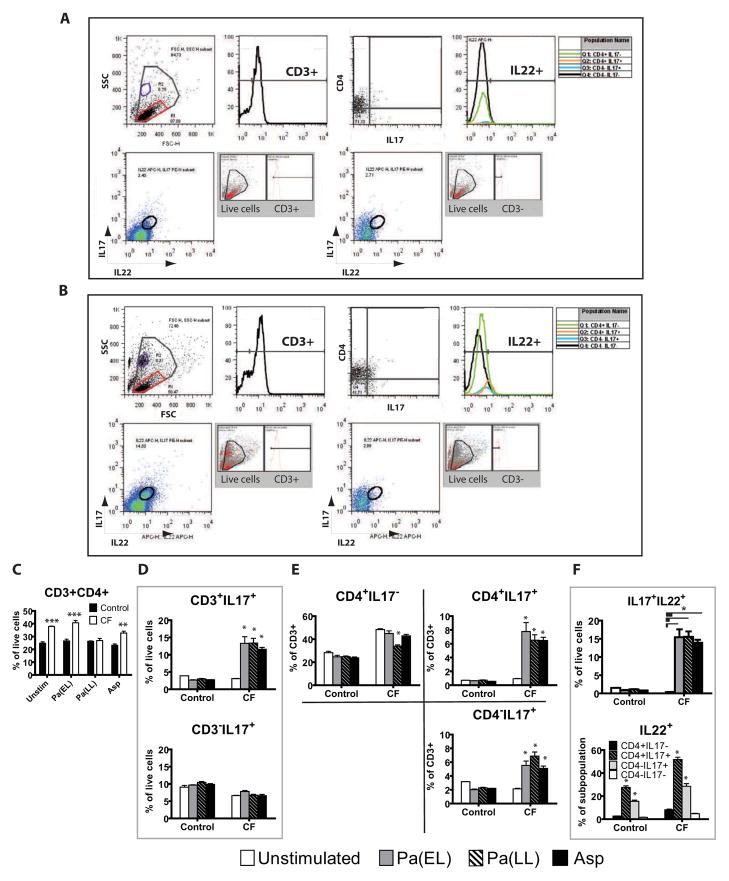
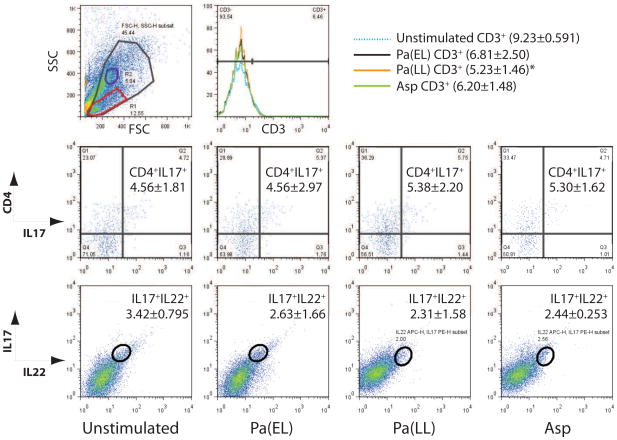
We next determined if there was a functional and physiologically relevant IL-17 response of live leukocytes derived from lung tissue. Most CF patients in this study had previously positive sputum cultures for Pseudomonas aeruginosa and some for Aspergillus. Therefore, we reasoned that there would likely be a memory Th17 response at least to these organisms. Pseudomonas antigens from different phases of culture were generated as described and tested against DLN and PLC cultures from patients early in the study. This was done in order to capture the diversity of antigens and virulence factors elaborated by Pseudomonas in its different phases of growth. The fractions eliciting the most robust proliferation measured by BRDU incorporation, Pa(EL) and Pa(LL), were used for all subsequent patients (Figure 3A). In addition, Aspergillus mitogilin (Asp) and a positive mitogenic control, Concanavalin A (Con A, not shown), were used in separate cell culture stimulations for comparison. Examination of the unstimulated culture supernatant results for selected cytokines reassuringly revealed that unstimulated IL-17 levels were not significantly different between cohorts for either the DLN or PLC cultures (Figure 3B). Also, pre-stimulated DLN and PLC population composition demonstrated the presence of potential antigen-presenting cells and T-cells (Figure 3C). Stimulated cells were first plotted by forward and side scatter, and gated live cells were further analyzed for CD3, CD4, IL-17, and IL-22 status. (Figure 4A and B). While CF patients started with higher baseline numbers of CD3+CD4+ cells which persisted after antigen stimulation (Figure 4C), the percentage of IL-17+ T cells clearly increased relative to non-CF samples after antigen stimulation (Figure 4D, top). Notably, there are CD3-IL-17+ cells in both non-CF and CF patients (Figure 4D, bottom) that are not inducible and could reflect a population of NK cells. Further subdivision by CD4 status reveals a strong T-cell IL-17 response in CF that may be equally divided among T-helper and other CD3+ T-cell subsets. Note the inducible fraction of CD4-IL-17+ cells which may reflect CD8+ or TCRγδ cells (Figure 4E). CF patients had more IL-17+IL-22+ double positive cells in the all live cell gate after stimulation (Figure 4F, top). Examination of the backgating and ancestry reveals that some of these cells appear in population R2 outside the lymphocyte gate R1 even though they are CD3+. IL-22+ cells made up a larger proportion of CD3+IL-17+ regardless of CD4 status in both CF and non-CF controls (Figure 4F, bottom).
Figure 3. Antigen stimulation preparations and baseline characteristics of CF and non-CF samples.
(A) CF DLN cells were stimulated as described and BRDU histograms from antigen concentrations that were chosen are shown. (B) Cultured DLN and PLC supernatants were assayed by multiplex cytokine bead array and IL-17 mean absolute values with SEM for each cohort and tissue sample are graphed. IL-17 levels are not remarkably different among all CF (n = 10) or control (n = 4) samples prior to stimulation. Culture media included IL-2. (C) DLN (top two rows of plots) and PLC (bottom two rows of plots) are assessed prior to culture with antigen stimulation. Representative dot plots are shown. Total events examined by size (FSC) vs granularity (SSC) were first plotted and a live cell gate was drawn. This live cell gate was subsequently analyzed for CD3 vs CD4 and CD11c vs HLA-DR.
Figure 4. IL-17+ and IL-22+ T cells are increased in CF.
Representative gating strategy for non-CF (A) and CF (B) is shown on a Pa(EL) stimulated sample. Total events were analyzed by size (FSC) vs granularity (SSC) and a live cell gate (largest, gray gate) was drawn. This gate was further examined by univariate histogram analysis of CD3 status (top row, second from left). Gated CD3+ cells were then analyzed by CD4 vs IL-17 (top row, third from left) and proportion of IL-22+ cells in each quadrant graphed in the second histogram. Second row in each lettered section shows all live cells that were CD3+ (first bivariate plot) and CD3− (second bivariate plot) analyzed for IL-17 vs IL-22. An IL-17+IL-22+ double positive gate was drawn and backgating to show ancestry is shown in the gray inset. (C) Non-CF ■ (n = 4) and CF □ (n = 7) population results of bivariate analysis of the live cell gate by CD3 vs CD4. (D) Cohort statistics by antigen stimulation of CD3+ and CD3− cells that are IL-17+. (E) Breakdown of quadrant statistics in the CD4 vs IL-17 plot by genotype and antigen stimulation. (F, top) Cohort statistics of the IL-17+IL-22+ double positive gate by genotype and antigen stimulation. (F, bottom) IL-22+ statistics for each quadrant in the CD4 vs IL-17 analysis for each genotype and antigen stimulation. Figure legend for D, E, F is shown on bottom unless otherwise noted in F, bottom graph. * p < 0.05; ** p < 0.001; *** p < 0.0001.
IL-22 expressing CD4+IL-17+ cells demonstrate higher proportions of memory cell markers
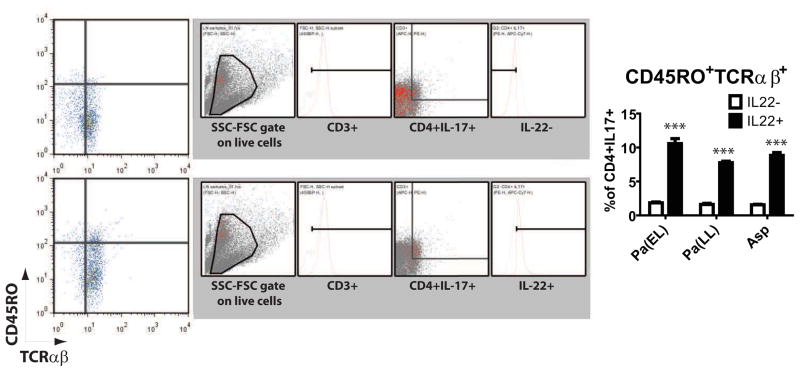
Further characterization of CF CD4+IL-17+ cells yielded intriguing results – when gated on their IL-22 status and then analyzed for CD45RO and TCRαβ expression, IL-22 positive cells had a higher proportion of CD45RO+ cells (Figure 5). IL-22+CD45RO+TCRαβ+ cells made up 7.770% (SEM 0.210%) — 10.58% (SEM 0.729%) in CF DLN across all pathogen stimuli, while IL-22− cells had only 1.553% (SEM 0.074%) — 1.840% (SEM 0.132%) CD45RO+TCRab+. This data endorses a potential role for IL-22 in maturation of the memory Th response to pathogen antigens.
Figure 5. IL-22+ status correlates with memory T cell characteristics.
IL-22+ and IL22− cells in the live cell gate were analyzed for CD45RO vs TCRαβ expression. Representative plots with backgating and ancestry shown in the gray inset next to each bivariate plot for Pa(EL) stimulated CF DLN. IL-22 positivity in CD4+ IL-17+ cells correlated to CD45RO+TCRab+ status (right graph) * p < 0.05; ** p < 0.001; *** p < 0.0001.
Alternative Th response may explain lower levels of Th17 cells in lung parenchyma
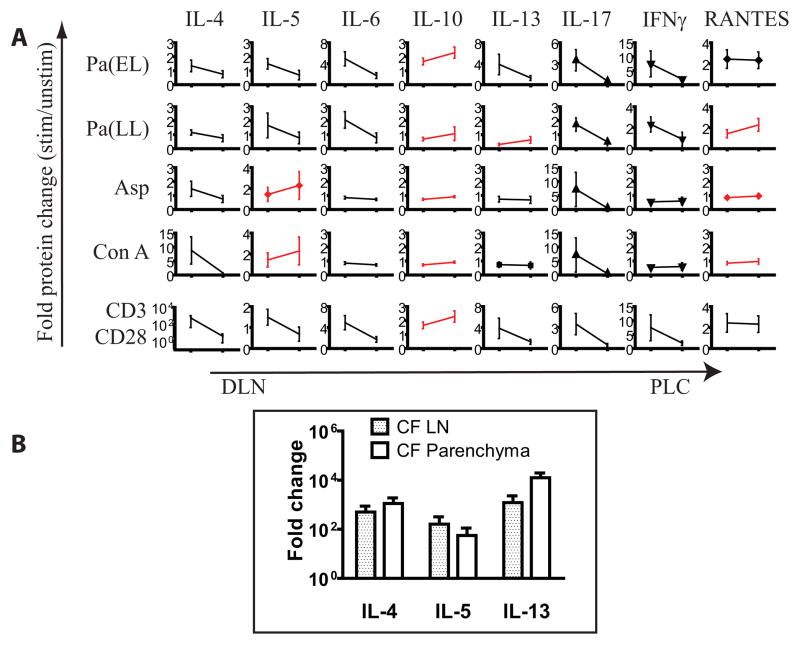
In contrast to these findings, we found a comparatively diminished Th17 response to antigen in PLC (Figure 6). Stimulated PLC CD3+ population was remarkably smaller than in DLN. The proportion of IL-17+IL-22+ in the live cell gate after stimulation was similar to that of unstimulated DLN. While there are many potential explanations for this, it is known that suppression of Th1 and Th2 pathways can foster the independent development of the Th17 lineage18, 19. Therefore, the lack of a PLC Th17 response could possibly be from strong peripheral Th1, Th2 or Treg signature. We examined these cytokine levels from the multiplex analysis in antigen-stimulated CF PLC cultures and plotted their average normalized values relative to the DLN result (Figure 7A). Here we note that IL-5, IL-10, IL-13 and RANTES were among cytokines that trended higher in PLC relative to DLN, suggesting that stronger presence of Treg or Th2 factors in the periphery could regulate the parenchymal Th17 response. Real time PCR of Th2 cytokines comparing the lymph node to parenchyma also showed a trend toward higher IL-4 and IL-13 levels in the parenchyma (Figure 7B).
Figure 6. PLC shows minimal to modest IL-17 and IL-22 response to antigen stimulation.
Representative plots on a CF PLC sample are shown. (Top row) Live cell gate in SSC vs FSC plot was examined for CD3 status (histogram). Statistics for each stimulation are shown in the histogram legend. One stimulation condition resulted in statistically significant reduction in the number of CD3+ cells relative to the unstimulated sample [Pa(LL)]. The live cell gate was then examined for CD4 vs IL-17 (second row of plots) with statistics for the CD4+IL-17+ quadrant shown for each antigen stimulation. Similarly the live cell gate was examined for IL-17 vs IL-22 in each antigen stimulation and statistics are shown for the IL-17+IL-22+ quadrant (bottom row of plots). * p < 0.05
Figure 7. Presence of Th2 or Treg cytokines in the parenchyma may blunt IL-17 response there.
(A) Average normalized antigen stimulated cytokine values for CF DLN and CF PLC were juxtaposed. Cytokines in which PLC > DLN are in red. Among cytokines exhibiting an increase in PLC relative to DLN are IL-10, IL-13 and RANTES for Pa stimulation; IL-5, IL-10 and IL-13 for Asp. (B) qPCR of Th2 cytokines in CF lymph node and parenchyma show a trend toward higher signals in the parenchyma.
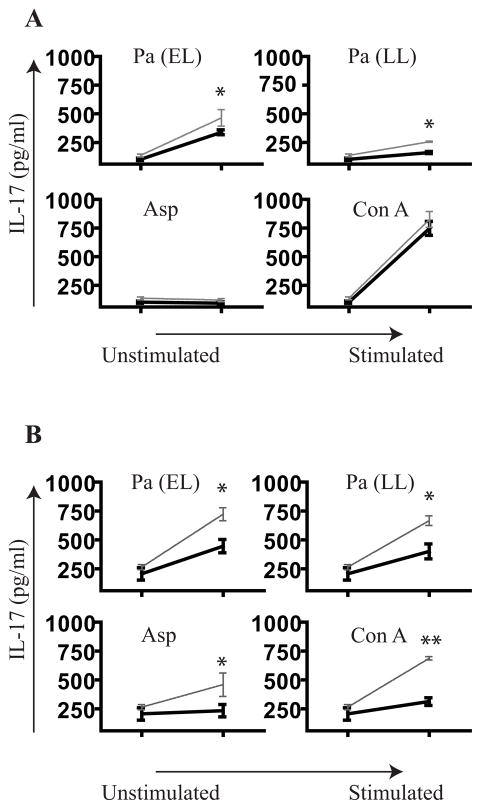
We reasoned that the Th17 response in human lung could be further amplified by suppression of Th1 and Th2 cytokines such as IL-4 and IFNγ, analogous to findings in the mouse model18, 49. Furthermore, recent evidence shows the presence of IL-13 receptors on Th17 cells which can attenuate IL-17A production50. Because of this precedent work that Th17 could be potently inhibited by IFNγ, IL-4, and IL-13 we proceeded to neutralize them in culture. Due to sparse recovery of T-cells in PLC, we stimulated DLN in the presence of neutralizing antibodies to IL-4 and IFNγ or IL-13 and IFNγ to see if IL-17A response in these cells could be amplified. After stimulation, supernatants were collected and analyzed for IL-17 cytokine levels. While the IL-17 gain was modest with IL-4 and IFNγ neutralization (Figure 8A), a more dramatic magnification of the IL-17 response was found with neutralization of IL-13 and IFNγ (Figure 8B). This effect was not observed with neutralization of each of the cytokines individually, except for Aspergillus stimulation where IL-17 responses were significantly unmasked with IL-13 neutralization alone (data not shown).
Figure 8. Neutralization of Th1 and Th2 cytokines augments the IL-17 response.
(A) CF DLN (n = 3) were cultured with indicated antigen along with neutralizing antibodies to IL-4 and IFNγ together (
 ) and compared to no neutralization (—) with 20 μg/ml of IgG. (B) IL-13 and IFNγ were also neutralized (n = 3).. * p < 0.05, ** p < 0.001
) and compared to no neutralization (—) with 20 μg/ml of IgG. (B) IL-13 and IFNγ were also neutralized (n = 3).. * p < 0.05, ** p < 0.001
Discussion
This work is an important advance in our understanding of IL-17 in human disease. First, we have shown that Th17 cells are isolable from draining lymph nodes of the lung in human CF. Moreover, these Th17 cells demonstrate an antigen-specific response rather than a merely non-specific mitogen-induced IL-17 release. We further find that the CD4+IL-17+ population expressing IL-22 has a higher proportion of CD45RO+TCRαβ+ compared to IL-22− cells, suggesting a role for IL-22 in maturation of the adaptive response to pathogens. However, there is sparse data in the literature to help judge if this is an adequate memory T cell fraction. Much of the current literature focuses on viral vaccination, which may elicit a different immune response. It would also be difficult to compare our findings since vaccination constitutes a discrete exposure in contrast to CF whereas bacterial colonization leads to chronic exposure. One study examining the human memory response to meningococcal vaccine suggests that our results are comparable to a one time administration of vaccine or persistence two years post-completion of a three dose prime and boost series.51 However, the dramatic shift of T cells into memory effector T cell populations in a mouse model of chronic antigen exposure suggests that the response we present in this work may be inadequate or below expected52, 53. This presents evidence that there may be acute generation of Th17 response to an antigen to which the host has had long term exposure. Given the high frequency of chronic colonization of late stage CF patients with Pseudomonas species, our findings may represent an ineffective or inefficient memory response in CF. This would also be consistent with recent findings that the antecedent and primary defect in CF is impaired host defense.54
Our findings help further characterize the nature of the host defense defect in CF. Although the IL-17 cytokine response is robust, it may be the balance of IL-17’s differing roles in early innate defense versus adaptive immune development that may ultimately determine effective bacterial eradication. Robust de novo Th17 responses may keep a pathogen at bay and prevent septic dissemination, but the memory response ultimately eliminates it. On the other hand, this Th17 response may represent unchecked tissue damaging inflammation in the setting of ineffective innate antimicrobial mechanisms, similar to what has been shown in a mouse model of Aspergillus infection in chronic granulomatous disease.55 In the case of CF, impaired epithelial bicarbonate secretion56 which is required for killing activity of antimicrobial peptides32, 57 leads to persistent IL-23/IL-17-mediated inflammatory response55, 58, 59. The interaction of IL-17 with IL-22 may be an important switchpoint in immune development from danger signal initiated tissue-damaging inflammation to a focused adaptive memory response. Further clinical studies characterizing the IL-17+ cell subsets identified in this study are certainly indicated. Additional work characterizing the proliferation and clonality of the Th17+22+ population as well as the status of other memory T cell markers CD62L and CCR7 would be fruitful future work on prospective transplant patients. If Th17 cells prove to be clonally expanded following stimulation, this would open the possibility of following the status of these cells through the natural course of disease at earlier stages prior to end-stage CF by bronchoscopic lavage or endobronchial ultrasound biopsy. If CD4+IL-17+IL-22− and CD4+IL-17+IL-22+ cells represent T helper cells along the same developmental pathway at different stages, then understanding their function or plasticity along this pathway may provide a key therapeutic target in remediating tissue inflammation in CF.
The striking difference between draining lymph nodes and the parenchymal lung also provides clues that Th17 response is defective or absent in areas of bacterial colonization. While the draining lymph nodes appear readily Th17 responsive to Pseudomonas and Aspergillus stimulation, parenchymal leukocytes were inert. This appears to be at odds with our findings that the parenchyma is simultaneously positive for IL-17 protein by immunofluorescence staining and Th17 pattern gene expression. A potential explanation for this is that IL-17 cytokine is likely elaborated by a variety of cell types in the parenchyma that were not purified in our preparation for flow cytometry and cytokine analysis. Because of findings by Cella et al that NK cells were a likely source of IL-22 in humans60 we also examined lung sections for IL-22+ NK cells by examining dual positivity for CD56 and IL-22 (Figure S3 in OR). While we did not replicate their findings as dramatically, this serves to illustrate the diversity of the innate immune response as it encounters infection from the upper airway to the lower respiratory tract. Alveolar epithelium has also been shown to be a significant source of IL-22 message61 and the lack of RORC upregulation (Figure 2) may point to a cell type other than T-cells producing IL-17 in the parenchyma. Finally, our own data showing inducible CD3+ but CD4− IL-17 producing cells speaks to the possibility of CD8 and TCR γδ IL-17 responders in various regions of the lung. The extent of parenchymal destruction in end-stage CF lung makes this difficult to discern and compare to others’ results in more readily accessed and architecturally-preserved tissue62 despite extensive 3D reconstruction of confocal images (Video E1 in OR). However, consistent with others’ results in COPD63, we found that IL-17 may be in the parenchymal subepithelial tissue when examining more architecturally preserved COPD lung (Figure S4 and Video E1 in OR). An alternative explanation to the paucity of IL-17+ cells in the parenchyma is that these cells are more apoptotic and sensitive to ischemic time following lung removal from the patient. Future studies devoted to characterizing apoptosis markers early on in this population would be important.
In light of work that supports a more mutually exclusive developmental pathway for Th17 cells in the setting of Th1 and Th2 suppression18, 64, we report a similar result in humans. In contrast to mouse models however, we find that IL-13 plays a greater role than IL-4 and is consistent with work published by Newcomb et al50. This may explain the weak IL-17 signal in Pseudomonas-stimulated parenchymal leukocytes as these samples had higher normalized post-stimulation levels of IL-10, IL-13 and RANTES, a Th1 polarizing DC cytokine65. However, we found that concurrent neutralization of IFNγ was necessary for demonstrable IL-17 augmentation in Pseudomonas but not Aspergillus stimulation. The higher magnitude of IFNγ even prior to stimulation (Figure 3B) may be the reason for this, showing the powerful inhibition of Th17 that can be mediated by IFNγ. Conversely, IFNγ neutralization alone did not augment IL-17 in any cases. Further future studies examining the status of IL-13R on both DLN and PLC cells in this population of patients are indicated. This finding also advises greater scrutiny of the current state of medical management in patients with obstructive lung diseases, since it has been shown that combined therapy with prednisone and leukotriene inhibitors decrease IL-13 and IFNγ secretion.66 Potentially unopposed effects of IL-17 should be examined in this population. Issues that may confound this interpretation of the data are age differences and immunomodulating medication use in the two cohorts. Of the non-CF patients for whom demographic data was available, there is clearly a difference in average age. It is not known if IL-17 response changes with age independent of pathogen exposure. The best comparison population to test for age effects would be COPD patients with bronchiectasis who have also been colonized with Pseudomonas. The data in our supplemental results on COPD samples suggests that the findings may trend similarly with CF and this would need to be explored further. Presuming that CORE lungs came from patients that did not have a reason to be on long term systemic steroids, the rates of systemic steroid use rates were similar in both populations when comparing immunofluorescence and RNA data. However, our flow cytometry and cytokine comparisons were between CF and CORE lungs, presenting the possibility that systemic steroid use may have primed CF samples for a more robust IL-17 response. This again reinforces the need to evaluate the effects of routine clinical practice on lung immunologic response.
It would be interesting to further fractionate and explore the individual antigenic components of microbial extracts specifically leading to Th17 development as we arbitrarily chose Pseudomonas in early and late log phase of growth for our studies. It is possible that antigens created from Pseudomonas growing as a biofilm might more accurately reflect the true growth phase in live patients and that the antigens we used simulate CF “exacerbation” as the organism transforms from planktonic growth and disseminates. Further studies using clinical isolates of Pseudomonas and Aspergillus from the same patients may provide more information about how the immune system is primed against these organisms.
Proliferation assays were used as a starting point to determine antigen stimulation and it is recognized that measurement of proliferation and IL-17 release may only approximate Th17 physiologic effects. Furthermore, we separately measured IL-17F and IL-22, as these cytokines were not included in the 30-plex array. Again, CF DLN cultures trended toward a higher antigen-specific IL-17F and IL-22 response but the absolute magnitude of this response is comparatively larger than that for IL-17A (Figure S9 in OR). This suggests that assays for IL-17F and IL-22 may have greater sensitivity for detecting Th17 responses in CF.
Finally, this work establishes a streamlined protocol for isolating Th17 cells from a population of patients undergoing transplant. Th1/Th17 cells have been implicated in solid organ transplant rejection67–71 and these cells may have a role in predicting transplant outcome72. Thus, we present a possible tool for use and study in prognostication. Prospective studies examining the presence of these cells and ultimate transplant outcome are indicated.
Supplementary Material
Clinical Implications.
This work begins to elucidate the antimicrobial defect in CF. Examining lung Th17 cells is a potential tool for transplant follow-up, as Th17 has been implicated in transplant rejection.
Acknowledgments
Funding: This work is supported by research grants from the National Heart Lung and Blood Institute, NIH (Y. Chan K08 HL089189, J. Kolls and J. Pilewski P50 HL084932), Howard Hughes Medical Institute (Y. Chan, Early Career Physician Scientist Award), The CF Foundation (J. Pilewski) and Novartis (J. Kolls and J. Pilewski). It is also supported from the confocal imaging resources of the Core Grant for Vision Research at the University of Pittsburgh NIH P30-EY08098.
Abbreviations
- CF
Cystic Fibrosis
- IL-xx
Interleukin
- Th
T-helper
- DLN
draining lymph node cells
- PLC
parenchymal lung leukocytes
- Pa(EL)
Pseudomonas aeruginosa early log phase sonicate antigen
- Pa(LL)
Pseudomonas aeruginosa late log phase filtrate antigen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 2.Garvy BA, Wiley JA, Gigliotti F, Harmsen AG. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infect Immun. 1997;65:5052–6. doi: 10.1128/iai.65.12.5052-5056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–21. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 4.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–6. [PubMed] [Google Scholar]
- 5.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–56. [PubMed] [Google Scholar]
- 6.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–52. [PubMed] [Google Scholar]
- 8.Laan M, Prause O, Miyamoto M, Sjostrand M, Hytonen AM, Kaneko T, et al. A role of GM-CSF in the accumulation of neutrophils in the airways caused by IL-17 and TNF-alpha. Eur Respir J. 2003;21:387–93. doi: 10.1183/09031936.03.00303503. [DOI] [PubMed] [Google Scholar]
- 9.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, et al. Interleukin-17 and Lung Host Defense against Klebsiella pneumoniae Infection. Am J Respir Cell Mol Biol. 2001;25:335–40. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 11.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–9. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–8. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 14.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–19. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 129:311–21. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 18.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 19.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 207:291–7. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97:726–33. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 23.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111:1293–8. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 24.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, et al. Role of IL-17A, IL-17F, and the IL-17 Receptor in Regulating Growth-Related Oncogene-{alpha} and Granulocyte Colony-Stimulating Factor in Bronchial Epithelium: Implications for Airway Inflammation in Cystic Fibrosis. J Immunol. 2005;175:404–12. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. 2009;157:316–24. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sethi S. Bacterial infection and the pathogenesis of COPD. Chest. 2000;117:286S–91S. doi: 10.1378/chest.117.5_suppl_1.286s. [DOI] [PubMed] [Google Scholar]
- 27.Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, et al. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109:3794–802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan YR, Liu JS, Pociask DA, Zheng M, Mietzner TA, Berger T, et al. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol. 2009;182:4947–56. doi: 10.4049/jimmunol.0803282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao C-Y, Chen Y, Thai P, Wachi S, Huang F, Kim C, et al. IL-17 Markedly Up-Regulates {beta}-Defensin-2 Expression in Human Airway Epithelium via JAK and NF-{kappa}B Signaling Pathways. J Immunol. 2004;173:3482–91. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 30.Karlsen JR, Borregaard N, Cowland JB. Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with interleukin-17 and tumor necrosis factor-alpha is controlled by IkappaB-zeta but neither by C/EBP-beta nor C/EBP-delta. J Biol Chem. 2010;285:14088–100. doi: 10.1074/jbc.M109.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolls JK, McCray PB, Jr, Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol. 2008;8:829–35. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreindler JL, Bertrand CA, Lee RJ, Karasic T, Aujla S, Pilewski JM, et al. Interleukin-17A induces bicarbonate secretion in normal human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L257–66. doi: 10.1152/ajplung.00344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peric M, Koglin S, Kim SM, Morizane S, Besch R, Prinz JC, et al. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol. 2008;181:8504–12. doi: 10.4049/jimmunol.181.12.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-{alpha}-induced genes in bone cells. J Leukoc Biol. 2005;77:388–99. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 36.Meshi B, Vitalis TZ, Ionescu D, Elliott WM, Liu C, Wang XD, et al. Emphysematous lung destruction by cigarette smoke. The effects of latent adenoviral infection on the lung inflammatory response. Am J Respir Cell Mol Biol. 2002;26:52–7. doi: 10.1165/ajrcmb.26.1.4253. [DOI] [PubMed] [Google Scholar]
- 37.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–21. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 38.Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;57:759–64. doi: 10.1136/thorax.57.9.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1090–5. doi: 10.1164/rccm.200210-1179OC. [DOI] [PubMed] [Google Scholar]
- 40.Morris A, Sciurba FC, Lebedeva IP, Githaiga A, Elliott WM, Hogg JC, et al. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am J Respir Crit Care Med. 2004;170:408–13. doi: 10.1164/rccm.200401-094OC. [DOI] [PubMed] [Google Scholar]
- 41.Morris A, Sciurba FC, Norris KA. Pneumocystis: A Novel Pathogen in Chronic Obstructive Pulmonary Disease? COPD: Journal of Chronic Obstructive Pulmonary Disease. 2008;5:43–51. doi: 10.1080/1541255070181756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61:259–66. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–29. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 44.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008 doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kruisbeek A. Current protocols in immunology. New York: Wiley; 2001. Isolation of Mouse Mononuclear Cells; pp. 3.1–3.1.5. [DOI] [PubMed] [Google Scholar]
- 46.Lefrançois L, Lycke N. Current Protocols in Immunology. New York: Wiley; 2001. Isolation of Mouse Small Intestinal Intraepithelial Lymphocytes, Peyer’s Patch, and Lamina Propria Cells; pp. 3.19.1–3..6. [DOI] [PubMed] [Google Scholar]
- 47.Kreindler JL, Steele C, Nguyen N, Chan YR, Pilewski JM, Alcorn JF, et al. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J Clin Invest. 120:3242–54. doi: 10.1172/JCI42388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newcomb DC, Zhou W, Moore ML, Goleniewska K, Hershey GK, Kolls JK, et al. A functional IL-13 receptor is expressed on polarized murine CD4+ Th17 cells and IL-13 signaling attenuates Th17 cytokine production. Journal of immunology. 2009;182:5317–21. doi: 10.4049/jimmunol.0803868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newcomb DC, Boswell MG, Zhou W, Huckabee MM, Goleniewska K, Sevin CM, et al. Human TH17 cells express a functional IL-13 receptor and IL-13 attenuates IL-17A production. The Journal of allergy and clinical immunology. 2011;127:1006–13. e1–4. doi: 10.1016/j.jaci.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naess LM, Oftung F, Aase A, Wetzler LM, Sandin R, Michaelsen TE. Human T-cell responses after vaccination with the Norwegian group B meningococcal outer membrane vesicle vaccine. Infect Immun. 1998;66:959–65. doi: 10.1128/iai.66.3.959-965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arce F, Rowe HM, Chain B, Lopes L, Collins MK. Lentiviral vectors transduce proliferating dendritic cell precursors leading to persistent antigen presentation and immunization. Mol Ther. 2009;17:1643–50. doi: 10.1038/mt.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rowe HM, Lopes L, Ikeda Y, Bailey R, Barde I, Zenke M, et al. Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene. Mol Ther. 2006;13:310–9. doi: 10.1016/j.ymthe.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 54.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romani L, Fallarino F, De Luca A, Montagnoli C, D’Angelo C, Zelante T, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–5. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 56.Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci U S A. 2003;100:16083–8. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dorschner RA, Lopez-Garcia B, Peschel A, Kraus D, Morikawa K, Nizet V, et al. The mammalian ionic environment dictates microbial susceptibility to antimicrobial defense peptides. FASEB J. 2006;20:35–42. doi: 10.1096/fj.05-4406com. [DOI] [PubMed] [Google Scholar]
- 58.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 59.Dubin PJ, Kolls JK. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol. 2007;292:L519–28. doi: 10.1152/ajplung.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–5. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whittington HA, Armstrong L, Uppington KM, Millar AB. Interleukin-22: a potential immunomodulatory molecule in the lung. Am J Respir Cell Mol Biol. 2004;31:220–6. doi: 10.1165/rcmb.2003-0285OC. [DOI] [PubMed] [Google Scholar]
- 62.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–85. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shan M, Cheng HF, Song LZ, Roberts L, Green L, Hacken-Bitar J, et al. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci Transl Med. 2009;1:4ra10. doi: 10.1126/scitranlsmed.3000154. [DOI] [PubMed] [Google Scholar]
- 64.Walsh KP, Brady MT, Finlay CM, Boon L, Mills KH. Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. J Immunol. 2009;183:1577–86. doi: 10.4049/jimmunol.0803803. [DOI] [PubMed] [Google Scholar]
- 65.Lebre MC, Burwell T, Vieira PL, Lora J, Coyle AJ, Kapsenberg ML, et al. Differential expression of inflammatory chemokines by Th1- and Th2-cell promoting dendritic cells: a role for different mature dendritic cell populations in attracting appropriate effector cells to peripheral sites of inflammation. Immunol Cell Biol. 2005;83:525–35. doi: 10.1111/j.1440-1711.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 66.Gibbons FK, Israel E, Deykin A, Schaub B, He HZ, Perkins DL, et al. The combined effects of zafirlukast, prednisone, and inhaled budesonide on IL-13 and IFN-gamma secretion. J Clin Immunol. 2005;25:437–44. doi: 10.1007/s10875-005-5625-6. [DOI] [PubMed] [Google Scholar]
- 67.Braun RK, Molitor-Dart M, Wigfield C, Xiang Z, Fain SB, Jankowska-Gan E, et al. Transfer of tolerance to collagen type V suppresses T-helper-cell-17 lymphocyte-mediated acute lung transplant rejection. Transplantation. 2009;88:1341–8. doi: 10.1097/TP.0b013e3181bcde7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deteix C, Attuil-Audenis V, Duthey A, Patey N, McGregor B, Dubois V, et al. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol. 184:5344–51. doi: 10.4049/jimmunol.0902999. [DOI] [PubMed] [Google Scholar]
- 69.Vokaer B, Van Rompaey N, Lemaitre PH, Lhomme F, Kubjak C, Benghiat FS, et al. Critical role of regulatory T cells in Th17-mediated minor antigen-disparate rejection. J Immunol. 185:3417–25. doi: 10.4049/jimmunol.0903961. [DOI] [PubMed] [Google Scholar]
- 70.Wang S, Li J, Xie A, Wang G, Xia N, Ye P, et al. Dynamic changes in Th1, Th17, and FoxP3(+) T cells in patients with acute cellular rejection after cardiac transplantation. Clin Transplant. doi: 10.1111/j.1399-0012.2010.01362.x. [DOI] [PubMed] [Google Scholar]
- 71.Xie A, Wang S, Zhang K, Wang G, Ye P, Li J, et al. Treatment With Interleukin-12/23p40 Antibody Attenuates Acute Cardiac Allograft Rejection. Transplantation. doi: 10.1097/tp.0b013e3181fdd948. [DOI] [PubMed] [Google Scholar]
- 72.Carvalho A, Cunha C, Di Ianni M, Pitzurra L, Aloisi T, Falzetti F, et al. Prognostic significance of genetic variants in the IL-23/Th17 pathway for the outcome of T cell-depleted allogeneic stem cell transplantation. Bone Marrow Transplant. 45:1645–52. doi: 10.1038/bmt.2010.28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.