Abstract
The X-linked form of Charcot-Marie-Tooth disease (CMT1X) is the second most common form of hereditary motor and sensory neuropathy. The clinical phenotype is characterized by progressive weakness, atrophy, and sensory abnormalities that are most pronounced in the distal extremities. Some patients have CNS manifestations. Affected males have moderate to severe symptoms, whereas heterozygous females are usually less affected. Neurophysiology shows intermediate slowing of conduction and length-dependent axonal loss. Nerve biopsies show more prominent axonal degeneration than de/remyelination. Mutations in GJB1, the gene that encodes the gap junction (GJ) protein connexin32 (Cx32) cause CMT1X; more than 400 different mutations have been described. Many Cx32 mutants fail to form functional GJs, or form GJs with abnormal biophysical properties. Schwann cells and oligodendrocytes express Cx32, and the GJs formed by Cx32 play an important role in the homeostasis of myelinated axons. Animal models of CMT1X demonstrate that loss of Cx32 in myelinating Schwann cells causes a demyelinating neuropathy. Effective therapies remain to be developed.
Keywords: CMT, neuropathy, connexin32, gap junctions, Schwann cells, oligodendrocytes, myelin
Neuromsuscular manifestations of CMT1X
Shortly after Charcot, Marie, and Tooth published their descriptions of families with autosomal dominant inherited neuropathy that was later given their names (CMT), Herringham (Herringham, 1888) recognized a family in which males were selectively affected. This was well before Morgan’s demonstration of X-linked inheritance in 1910. Over the next 100 years, X-linked inherited neuropathy (CMT1X) was reported occasionally, and its existence was briefly questioned (Harding and Thomas, 1980), but CMT1X has emerged as the second most common form of CMT1 (Latour et al., 2006; Saporta et al., 2011).
Most males are clinically affected by 10 years of age (Birouk et al., 1998; Shy et al., 2007). The initial symptoms include difficulty running and frequent sprained ankles. The distal weakness progresses to involve the gastrocnemius and soleus muscles, eventually requiring assistive devices for ambulation. Weakness and atrophy also develop in the hands, particularly in thenar muscles. Thenar atrophy, positive sensory phenomena, and sensory loss may be more prominent in CMT1X than in CMT1A.
Men with CMT1X typically have “;intermediate” slowing of nerve conduction velocities (NCVs), as well as mildly prolonged distal motor and F-wave latencies. Forearm motor NCVs are typically 30-40 m/s in affected males, and 30-50 m/s in affected females (Birouk et al., 1998; Nicholson and Nash, 1993; Shy et al., 2007) -faster than what is typically seen in patients with the demyelinating forms of CMT (CMT1) and slower than what is typically seen in patients with the axonal/neuronal forms of CMT (CMT2). This intermediate slowing is characteristic of CMT1X and should raise the consideration of this diagnosis in an appropriate clinical setting. Compared to CMT1A (the most common form of CMT1), conduction slowing in CMT1X is less uniform among different nerves and dispersion is more pronounced (Gutierrez et al., 2000; Tabaraud et al., 1999). There is electrophysiologic evidence of distally accentuated axonal loss: the peroneal and tibial motor responses are frequently absent, while the median and ulnar motor amplitudes are reduced. Needle electromyography confirms the length-dependent loss of motor units as a result of axonal degeneration, which progresses with age (Birouk et al., 1998; Hahn et al., 1990; Hahn et al., 1999; Nicholson and Nash, 1993; Rouger et al., 1997; Rozear et al., 1987; Senderek et al., 1999). Even children have prominent axonal loss, while NCVs are normal or intermediate (Yiu et al., 2011).
Age-related loss of myelinated fibers, and an increasing numbers of regenerated axon clusters are the most prominent pathological finding in nerve biopsies (Hahn et al., 2001; Senderek et al., 1999). Many myelin sheaths are inappropriately thin for the axonal diameter (suggesting segmental demyelination and remyelination, or remyelination after axonal regeneration), although this is less prominent than in biopsies from other kinds of CMT1.
CMT1X is considered to be an X-linked dominant trait because it affects female carriers. Affected women usually have a later onset than men, and at every age the phenotype is milder. X-inactivation is the likely explanation for reduced severity in humans (Siskind et al., 2011) and has been documented in mice (Scherer et al., 1998). Women may be completely asymptomatic; thus a few kindreds have been reported to have “;recessive” CMT1X. Even in these kindreds, however, at least some obligate carriers have electrophysiological evidence of peripheral neuropathy. Furthermore, severe and early onset neuropathy may occur in some affected women (Dubourg et al., 2001; Karadima et al., 2004; Kuntzer et al., 2003; Liang et al., 2005; Wicklein et al., 1997), either because the random X-chromosome inactivation in each myelinating cell (Siskind et al., 2011) may be exceptionally skewed towards expression of the one carrying the mutation, or due to unusual gain-of-function mechanisms of certain mutants (Liang et al., 2005), discussed below.
GJB1 mutations cause CMT1X
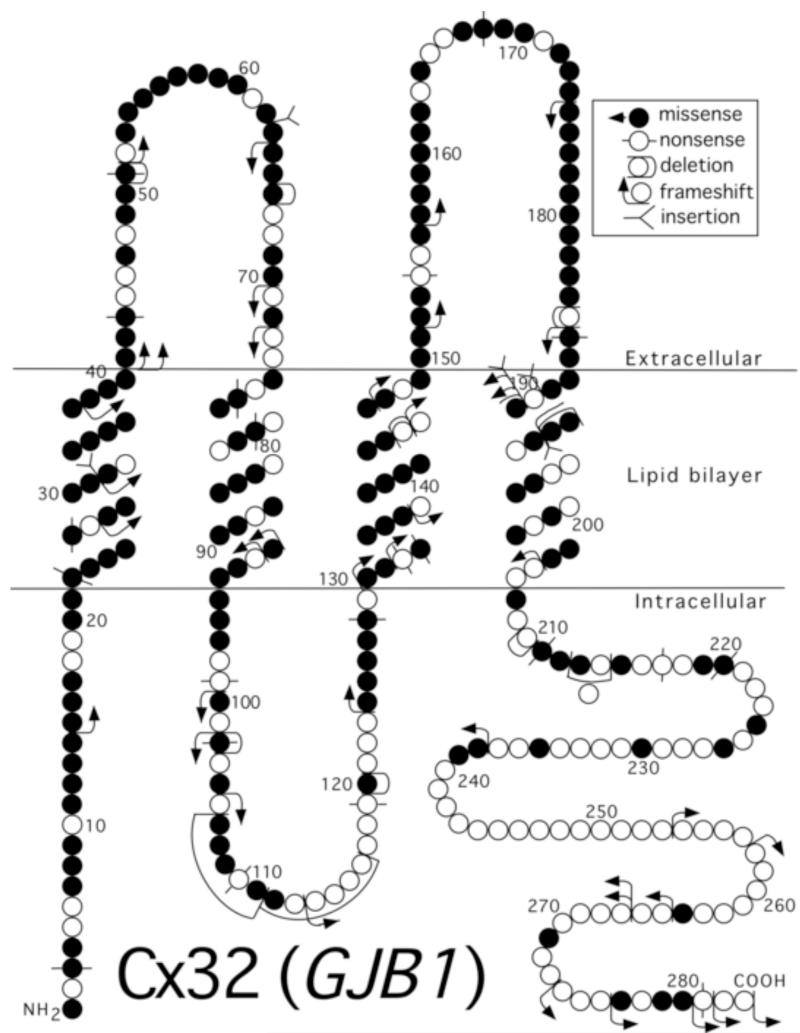
Connexins belong to a multigene family encoding ~20 highly homologous proteins (Willecke et al., 2002). Connexins are predicted to have the same overall topology (Fig. 1). Six connexins form a hemichannel (or connexon), arranged around a central pore (Nakagawa et al., 2010). Two apposed hemichannels form a functional channel that provides a contiguous pathway between the adjacent cells or cell compartments. The channel diameter is too small to allow transfer of proteins and nucleic acids, but large enough to allow the diffusion of ions and other small molecules (<1000 Da). Tens to thousands of channels connexin channels are localized to the cell membrane, in large aggregates called gap junction (GJ) plaques.
Figure 1. CMT1X mutants.
This schematic shows the basic structure of Cx32, which has four transmembrane domains, one intracellular and two extracellular loops, as well as an amino- and a carboxy-terminal cytoplasmic tail. The GJB1 mutations of the coding region are indicated, more than 400 altogether (http://www.molgen.ua.ac.be/CMTMutations/Mutations/MutByGene.cfm).
Mutations in GJB1, the gene that encodes Cx32, cause CMT1X. Since the first report (Bergoffen et al., 1993), more than 400 GJB1 mutations, predicted to affect all regions of the Cx32 protein (Fig. 1), have been described .(www.molgen.ua.ac.be/CMTMutations/). Only one of the reported amino acid changes is a polymorphism, indicating that all of the other affected residues are required for the normal function of Cx32. Many of the mutations have been reported more than once; some probably represent founder effects, whereas others may represent mutational “;hot spots” in GJB1.
Many mutations would be predicted to cause loss-of-function. For example, nonsense or frameshift mutations that affect the N-terminus of Cx32 would not be expected to produce any functional channels. In addition, a few mutations likely abolish the expression of Cx32 by affecting the GJB1 promoter or the translation of Cx32 mRNA (Beauvais et al., 2006; Flagiello et al., 1998; Houlden et al., 2004; Ionasescu et al., 1996). Finally, the entire coding region of GJB1 is deleted in several CMT1X kindreds (Ainsworth et al., 1998; Gonzaga-Jauregui et al., 2010; Lin et al., 1999; Nakagawa et al., 2001). Because different GJB1 mutations, including deletions, appear to cause similar degree of neuropathy, most or all GJB1 mutations likely cause loss of function in myelinating Schwann cells (Shy et al., 2007).
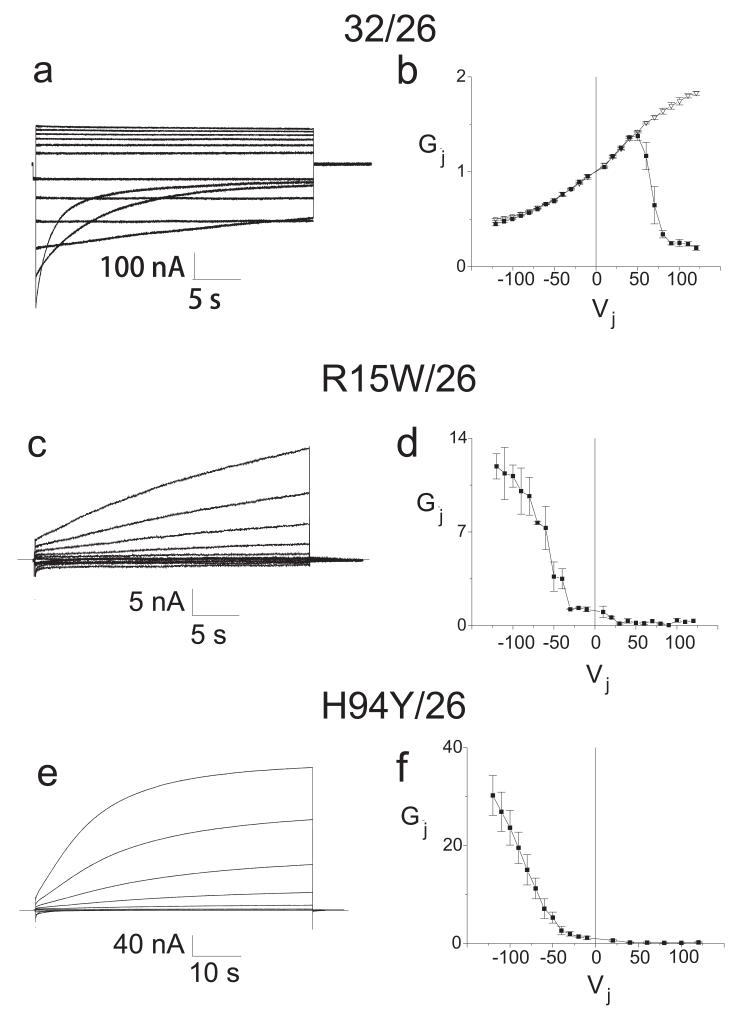
Several biophysical alterations lead to partial loss-of-function in model systems. For example, a mutant connexin may lead to disease through partial loss-of-function if the minimal luminal dimension is reduced, as has been inferred for the S26L (Bicego et al., 2006; Oh et al., 1997) and for the del 111-116 and R220stop (Bicego et al., 2006) mutants. Reduction in minimal luminal diameter may lead to reductions in biologically important small molecules such as glucose, lactate, cAMP, cGMP, IP3, and Ca2+. A second potential alteration is increased sensitivity to acidification-induced closure (Abrams et al., 2003; Ressot et al., 1998). Third, some mutations appear to stabilize the closed state of the channel (Abrams et al., 2001; Oh et al., 1997; Ri et al., 1999), leading to large shifts in the hemichannel conductance-voltage relation. In these cases, the very low junctional conductance for the some of the homotypically paired mutants can be explained on the basis of a reduced open probability (Po). Because of these shifts, Po may be not start to significantly increase until the voltage with respect to the apposed cell is +20 mV or more. If two such hemichannels are paired head to head, at least one hemichannel will be closed at all junctional voltages, leading to very low junctional conductances. Heterotypic pairing of Cx32 mutants with wild type Cx26 allows for the direct examination of the conductance voltage relation of the shifted mutant hemichannels, because the Cx26 hemichannel has a positive gating polarity and is fully open at voltages more negative than ~+60 mV with respect to the cell in which it is expressed (see Fig. 2 for examples). Such heterotypic pairings of the R15W and H94Y mutants with Cx26WT reveal markedly shifted conductance-voltage relations, so that the vast majority of homotypic channels formed by these mutants would be predicted to be fully closed at all transjunctional voltages. Fourth, some mutants, such as V181A (Abrams et al., 2003), may have reduced junctional coupling without alterations in channel properties, owing to reduced steady state levels of the protein.
Figure 2. Representative current traces and average normalized junctional conductance-junctional voltage (Gj-Vj) relations.
Panels a, c, and e show the junctional current traces for wild type Cx32, R15W and H94Y paired heterotypically with wild type Cx26; all expressed in Xenopus oocytes. For clarity, only traces in 20 mV increments from +/−20 to +/−120 (or +/−10 to +/−110 for R15W) are shown. Panels b, d, and f show the average Gj-Vj relations for these pairs, averaged and plotted as mean +/− SEM as previously described (Abrams et al., 2001). The smooth curves approximating the steady state data correspond to the curves generated by the Boltzmann fits. Junctional voltages (Vj) were applied by varying the voltage applied to the cell expressing Cx26, with the cell expressing the wild type or mutant form of Cx32 held at V=0. Filled squares represent steady state conductances; hollow triangles represent instantaneous conductances. As show in 2b, the heterotypic Cx32/Cx26 conductance shows substantial instantaneous rectification between −120 and +120 mV, with instantaneous current increasing about 3 fold as the cell expressing Cx26 is made more positive with respect to the cell expressing Cx32; however, because the Cx26 hemichannel closes only at positive Vj and the Cx32 hemichannel closes on negative Vj, closure of these channels is seen only when the cell expressing Cx26 is made more positive than 50 mV with respect to Cx32 (and in turn the cell expressing Cx32 is 50 mV negative with respect to the cell expressing Cx26). In the case of the R15W and H94Y mutants paired with Cx26, channels remain predominantly closed until junctional voltage is more negative than +20 mV with respect to the cell expressing Cx26 (and more positive than −20 mV with respect to the cell expressing Cx32). Since the Cx26 hemichannel is predicted to be fully open at these voltages, the Gj-Vj curves in figures 2d and 2f reflect the relationships between open probability and junctional voltage for the mutant hemichannel. If two such hemichannels are placed head to head, as the case in a homotypic channel, the resulting open probability of that channel would be very low at all voltages.
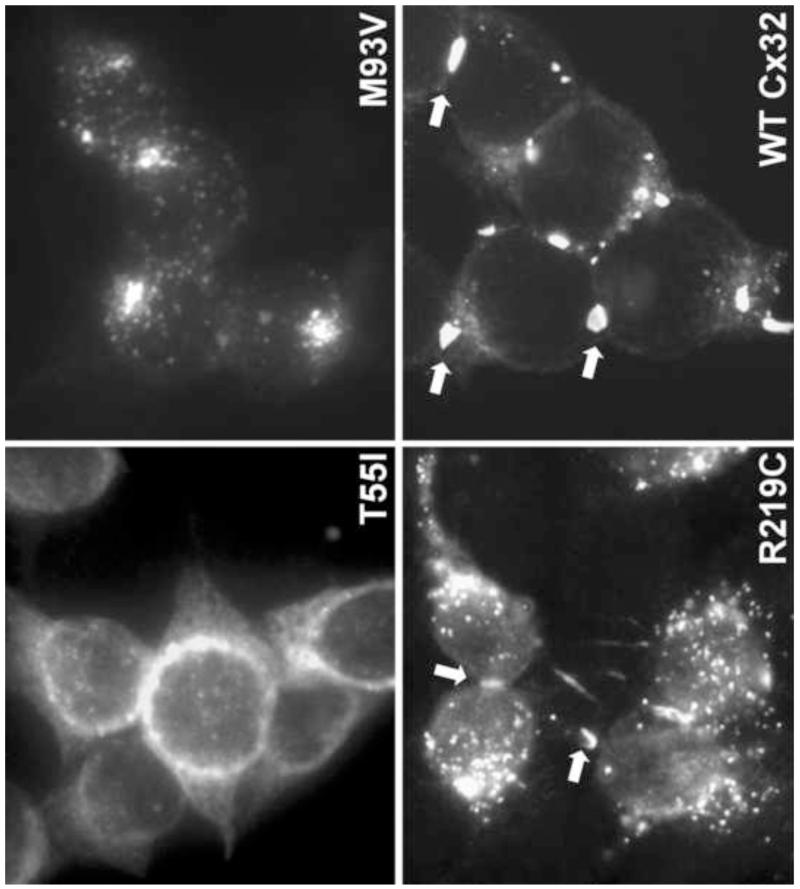
We (Abrams et al., 2003; Deschênes et al., 1997; Kleopa et al., 2002; Yum et al., 2002) and others (Martin et al., 2000; Matsuyama et al., 2001; Omori et al., 1996) have shown that many Cx32 mutants show abnormal trafficking when expressed by transfection in mammalian cell lines (Figure 3). Some mutants appear to be retained in the endoplasmic reticulum or Golgi, and some show a mixed picture of normally and abnormally distributed protein (Yum et al., 2002). Some missense mutations, however, particularly in the C-terminus, form GJ plaques that are indistinguishable from wild type Cx32 (Yum et al., 2002), and, furthermore, have electrophysiological features in Xenopus oocytes that are also indistinguishable from wild type Cx32 (Castro et al., 1999). Two of these C-terminal mutations, C280G and S281x, abolish the prenylation of Cx32 (Huang et al., 2005) – a lipid modification that is usually found in cytosolic proteins such as ras, and rarely in intrinsic membrane proteins. Prenylation would be expected to cause the C-terminus to be associated with the plasma membrane; Cx32 is the only connexin with this prenylation motif, which is conserved in Cx32 orthologs of some non-mammalian vertebrates (Huang et al., 2005). Thus, the relative contribution of abnormalities of trafficking and alterations of channel properties to the pathogenesis in patients carrying these mutations remains to be elucidated.
Figure 3. Different patterns of cellular expression characteristic of Cx32 mutants.
These are images of HeLa cells that have been transfected to express the indicated mutants. T55I is localized to the endoplasmic reticulum; M93V is localized to the Golgi; R219C forms GJ-like plaques (arrowheads), similar to wild type (WT) Cx32.
Similar studies have provided possible evidence that a gain-of-function of some Cx32 mutants contributes to their pathogenesis. Although the abnormal trafficking of mutants leads to accumulation of protein in the ER and Golgi (Deschênes et al., 1997; Kleopa et al., 2002), there is no evidence that this produces an abnormal effect (VanSlyke et al., 2000) including an unfolded protein response (Orthmann-Murphy et al., 2007), as has been documented for other myelin proteins (Scherer and Wrabetz, 2008). However, some Cx32 mutants can have dominant-negative effects on Cx32 or Cx26 (Bruzzone et al., 1994) and for at least two of them (R142W and R75W) a dominant effect on co-expressed wild type Cx32 in myelinating cells (Jeng et al., 2006; Sargiannidou et al., 2009) -discussed below. A third possibility is that some Cx32 mutants -S85C (Abrams et al., 2002) and F235C (Liang et al., 2005) -form functional hemichannels, which would likely have detrimental consequences in myelinating Schwann cells, such as collapse of ionic gradients, loss of metabolites, and influx of Ca2+. The F235C mutation was found in a girl with an unusually severe neuropathy suggesting that abnormal hemichannel activity may be a mechanism of gain of function in CMTX, accounting for the unusual severity of this girl’s illness (Liang et al., 2005).
Myelinating Schwann cells express Cx32
Many cell types express Cx32, including hepatocytes, which express much higher levels of Cx32 than do oligodendrocytes and Schwann cells. Despite this broad expression pattern, peripheral neuropathy is usually the sole clinical manifestation of GJB1 mutations. The co-expression of other connexins may “protect” some tissues against the loss of Cx32. Oligodendrocytes, for example, also express Cx47, and the loss of both Cx32 and Cx47 is far more deleterious to CNS myelin in mice than the loss of either one alone (Menichella et al., 2003; Odermatt et al., 2003). Although myelinating Schwann cells in rodents express Cx29 (the human orthologue is Cx31.3), Cx29/Cx31.3 does not prevent the development of demyelinating neuropathy, perhaps because it does not form functional GJs (Ahn et al., 2008; Sargiannidou et al., 2008).
Cx32 is localized to non-compact myelin of incisures and paranodes (Bergoffen et al., 1993), where it likely forms these GJs between the layers of the Schwann cell myelin sheath (Balice-Gordon et al., 1998). A radial pathway formed by GJs at these locations would be up to a 300-fold shorter than the circumferential pathway within the Schwann cell cytoplasm. It remains to be shown that GJB1 mutations disrupt this shortcut, as well as the exact role this pathway plays in the homeostasis of myelinated axons.
Animal models of CMT1X
Several animal models have provided further insights into CMT1X pathogenesis. Mice with targeted deletion of the Gjb1 gene develop a progressive, predominantly motor demyelinating peripheral neuropathy beginning at about three months of age (Anzini et al., 1997; Scherer et al., 1998). Heterozygous females have fewer demyelinated and remyelinated axons than do age-matched Gjb1-null females or males (Scherer et al., 1998), in keeping with the clinical phenotype of affected women who are obligate carriers of CMT1X. Expression of wild type human Cx32 protein driven by the rat Mpz promoter (which is only expressed in myelinating Schwann cells) prevents demyelination in Gjb1-null mice (Scherer et al., 2005), confirming that the loss of Schwann cell autonomous expression of Cx32 is sufficient to account for CMT1X pathology. Gjb1-null mice show subtle CNS myelin defects, including diminished myelinated fiber and myelin volume density, particularly in white matter tracts with prominent Cx32 expression, such as the ventral and dorsal funiculus of the spinal cord (Sargiannidou et al., 2009), but also in the neocortex (Sutor et al., 2000).
Transgenic mice expressing the T55I, R75W, R142W, 175fs, C280G, and S281stop mutations have been generated. No Cx32 protein could be detected in 175frameshift transgenic mice despite expression of the transgenic mRNA, and there was no effect of this transgene on the histology of myelinated axons in transgenic mice (Abel et al., 1999). In contrast, R142W transgenic mice showed retention of the mutant protein in the Golgi and developed a mild demyelinating neuropathy (Jeng et al., 2006). Moreover, the presence of the mutant Cx32 reduced the level of the endogenous mouse Cx32, indicating that R142W has a dominant-negative effect on wild type Cx32. Such an effect is not clinically relevant in patients with CMT1X, however, as only one GJB1 allele is expressed in each cell, but demonstrates the possibility that some Cx32 mutants could have trans-dominant-negative effects on other connexins that are co-expressed in the same cell type, as previously found for some Cx26 mutants (Yum et al., 2010). The R142W mutant did not affect the localization of Cx29 in myelinating Schwann cells (Jeng et al., 2006), in keeping with the finding that Cx29 and Cx32 do not interact in transfected cells (Ahn et al., 2008). The C280G and S281stop mutants were properly localized to incisures and paranodes and appeared to prevent demyelination in Gjb1-null mice, indicating that they may form functional channels in the myelin sheath (Huang et al., 2005). How these mutants cause neuropathy in humans remains unclear.
Transgenic mice that express the T55I and R75W mutants in both Schwann cells and oligodendrocytes were generated in order to clarify whether Cx32 mutants associated with CNS phenotypes (Kleopa et al., 2002) could have gain-of-function effects in oligodendrocytes. In myelinating cells, as in cultured cells, these Cx32 mutants are retained intracellularly (T55I is localized in the ER and R75W is mostly found in the Golgi) and fail to reach the membrane and form GJ-like plaques. On a wild type background, the expression of endogenous Cx32 was partly impaired by R75W but not T55I. On a Gjb1-null background, neither T55I nor R75W had a detectable gain-of-function effect and do not appear to affect the localization of Cx29 in Schwann cells or Cx29 and Cx47 in oligodendrocytes. Thus, the loss of Cx32 function appears to be the main effect of the T55I and R75W mutants, in both the PNS and the CNS (Sargiannidou et al., 2009).
Taken together, these results show that all Cx32 mutants expressed in vivo have a comparable localization in myelinating cells and in transfected cell lines (Deschênes et al., 1997; Kleopa et al., 2002; Yum et al., 2002), and indicate that altered synthesis or trafficking and loss of GJ function is the fundamental alteration of most CMT1X mutants.
Axonal involvement in CMT1X
Axonal pathology in CMT patients is an important determinant of disability, and correlates with clinical progression not only in axonal forms but also in demyelinating types of CMT (Krajewski et al., 2000). The electrophysiological and pathological findings in nerves from people with CMT1X (Hattori et al., 2003; Yiu et al., 2011) suggest that axonal pathology is more prominent than in other forms of demyelinating CMT, in which axonal pathology is thought to be secondary to demyelination (Giese et al., 1992; Martini et al., 1995; Sancho et al., 1999). Axonal alterations have also been noted in a transplantation model: when nerve segments from humans are transplanted into sciatic nerves of nude mice, mouse axons regenerate into the transplanted segments and are ensheathed and remyelinated by human Schwann cells. As compared to axons that regenerate into grafts of normal human nerve, axons that regenerate into nerve-grafts from patients who have the E102G mutation have increased density of axonal neurofilaments, decreased microtubules, and increased density of vesicles and mitochondria (Sahenk and Chen, 1998). Nerve graft from humans with the V181A mutation showed delayed axonal regeneration (Abrams et al., 2003).
The initial characterization of Gjb1-null mice showed that demyelination preceded axonal loss (Anzini et al., 1997; Sargiannidou et al., 2009; Scherer et al., 1998). We have re-investigated this issue in Gjb1-null mice before the onset of demyelination, and found that the diameter of myelinated axons was progressively reduced in Gjb1-null mice, and that neurofilaments, the main component of axonal cytoskeleton, were increasingly dephosphorylated and more densely packed (Vavlitou et al., 2010). These cytoskeletal alterations were associated with slowing of axonal transport. Thus, impaired cytoskeletal organization and axonal transport defects appear to precede demyelination in this mouse model, providing clues to the early axonopathy in CMT1X. Disturbed axon-glial signalling and glial support of axon function (Nave and Trapp, 2008) is likely to account for this axonal pathology independent of myelination.
CNS manifestations of CMT1X
Many GJB1 mutations appear to be associated with electrophysiological, clinical, and/or MRI findings of CNS involvement (Nicholson and Corbett, 1996; Nicholson et al., 1998; Senderek et al., 1999). Furthermore, patients with R22Q, T55I, R75W, E102del, V139M, R142W, R164W, R164Q, C168Y, V177A, E186x mutations have developed the striking picture of an acute, transient encephalopathy associated with MRI changes in CNS myelin, often “triggered” by travel to high altitudes, intense physical activity, or acute infections. Signs of chronic corticospinal tract dysfunction such as spasticity, extensor plantar responses and hyperactive reflexes have also been reported in patients with the A39V (Marques et al., 1999), T55I (Panas et al., 1998), M93V (Bell et al., 1996), R164Q (Panas et al., 1998), R183H (Bort et al., 1997), T191 frameshift (Lee et al., 2002), and L143P (Kleopa et al., 2006) mutations.
CNS dysfunction caused by GJB1 mutations has even been reported in childhood as early as 5 years of age (Siskind et al., 2009), but does not appear to correlate with the stage and severity of the peripheral neuropathy; in some cases it is the first manifestation of CMT1X, while in others with exceptionally severe neuropathy no clinical CNS phenotypes are present. Because these electrophysiological findings have not been found in patients with a deleted GJB1 gene (Hahn et al., 2000), these mutants may cause an abnormal gain-of-function. However, two of these mutants (T55I and R75W) had no apparent trans-dominant effect on co-expressed Cx47 (Sargiannidou et al., 2009) when expressed in oligodendrocytes. Elucidating the consequences of GJB1 mutations both in PNS and CNS is an important requirement for developing effective CMT1X treatments in the future, such as gene replacement or gene deletion strategies.
Acknowledgements
We thank our collaborators for their contributions to the work summarized here. We thank the NIH, the National Multiple Sclerosis Society, the Muscular Dystrophy Association, the Charcot-Marie-Tooth Association, Cyprus Research Promotion Foundation and Telethon for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel A, Bone LJ, Messing A, Scherer SS, Fischbeck KH. Studies in transgenic mice indicate a loss of connexin32 function in X-linked Charcot-Marie-Tooth disease. J. Neuropathol. Exp. Neurol. 1999;58:702–710. doi: 10.1097/00005072-199907000-00004. [DOI] [PubMed] [Google Scholar]
- Abrams CK, Freidin MM, Verselis VK, Bennett MVL, Bargiello TA. Functional alterations in gap junction channels formed by mutant forms of connexin 32: evidence for loss of function as a pathogenic mechanism in the X-linked form of Charcot-Marie-Tooth disease. Brain Res. 2001;900:9–25. doi: 10.1016/s0006-8993(00)03327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams CK, Bennett MVL, Verselis VK, Bargiello TA. Voltage opens unopposed gap junction hemichannels formed by a connexin 32 mutant associated with X-linked Charcot-Marie-Tooth disease. Proc. Natl. Acad. Sci. USA. 2002;99:3980–3984. doi: 10.1073/pnas.261713499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams CK, Freidin M, Bukauskas F, Dobrenis K, Bargiello TA, Verselis VK, Bennett MVL, Chen L, Sahenk Z. Pathogenesis of X-linked Charcot-Marie-Tooth disease: Differential effects of two mutations in connexin 32. J. Neurosci. 2003;23:10548–10558. doi: 10.1523/JNEUROSCI.23-33-10548.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn M, Lee J, Gustafsson A, Enriquez A, Lancaster E, Sul J-Y, Haydon PG, Paul DL, Huang Y, Abrams CK, Scherer SS. Cx29 and Cx32, two connexins expressed by myelinating glia, do not interact and are functionally distinct. J. Neurosci. Res. 2008;86:992–1006. doi: 10.1002/jnr.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth PJ, Bolton CF, Murphy BC, Stuart JA, Hahn AF. Genotype/phenotype correlation in affected individuals of a family with a deletion of the entire coding sequence of the connexin 32 gene. Hum. Genet. 1998;103:242–244. doi: 10.1007/s004390050812. [DOI] [PubMed] [Google Scholar]
- Anzini P, Neuberg DH-H, Schachner M, Nelles E, Willecke K, Zielasek J, Toyka K, Suter U, Martini R. Structural abnormalities and deficient maintenance of peripheral nerve myelin in mice lacking the gap junction protein connexin32. J. Neurosci. 1997;17:4545–4561. doi: 10.1523/JNEUROSCI.17-12-04545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Bone LJ, Scherer SS. Functional gap junctions in the Schwann cell myelin sheath. J. Cell Biol. 1998;142:1095–1104. doi: 10.1083/jcb.142.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais K, Furby A, Latour P. Clinical, electrophysiological and molecular genetic studies in a family with X-linked dominant Charcot-Marie-Tooth neuropathy presenting a novel mutation in GJB1 promoter and a rare polymorphism in LITAF/SIMPLE. Neuromuscular Disord. 2006;16:14–18. doi: 10.1016/j.nmd.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Bell C, Willison H, Clark C, Haites N. CNS abnormalities in a family with a connexin32 mutation and peripheral neuropathy. Eur. J. Hum. Genet. 1996;4:S136. [Google Scholar]
- Bergoffen J, Scherer SS, Wang S, Oronzi-Scott M, Bone L, Paul DL, Chen K, Lensch MW, Chance P, Fischbeck K. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science. 1993;262:2039–2042. doi: 10.1126/science.8266101. [DOI] [PubMed] [Google Scholar]
- Bicego M, Morassutto S, Hemandez VH, Morgutti M, Mammano F, D’Andrea P, Bruzzone R. Selective defects in channel permeability associated with Cx32 mutations causing X-linked Charcot-Marie-Tooth disease. Neurobiol. Dis. 2006;21:607–617. doi: 10.1016/j.nbd.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Birouk N, Le Guern E, Maisonobe T, Rouger H, Gouider R, Gugenheim M, Tardieu S, Gugenheim M, Routon MC, Leger JM, Agid Y, Brice A, Bouche P. X-linked Charcot-Marie-Tooth disease with connexin 32 mutations - clinical and electrophysiological study. Neurology. 1998;50:1074–1082. doi: 10.1212/wnl.50.4.1074. [DOI] [PubMed] [Google Scholar]
- Bort S, Nelis E, Timmerman V, Sevilla T, Cruz-Martinez A, Martinez F, Millan JM, Arpa J, Vilchez JJ, Prieto F, Van Broeckhoven C, Palau F. Mutational analysis of the MPZ, PMP22 and Cx32 genes in patients of Spanish ancestry with Charcot-Marie-Tooth disease and hereditary neuropathy with liability to pressure palsies. Hum. Genet. 1997;99:746–754. doi: 10.1007/s004390050442. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Scherer SS, Fischbeck KH, Paul DL. Null mutations of connexin32 in patients with X-linked Charcot-Marie-Tooth disease. Neuron. 1994;13:1253–1260. doi: 10.1016/0896-6273(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Castro C, Gomez-Hernandez JM, Silander K, Barrio LC. Altered formation of hemichannels and gap junction channels caused by C-terminal connexin-32 mutations. J. Neurosci. 1999;19:3752–3760. doi: 10.1523/JNEUROSCI.19-10-03752.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes SM, Walcott JL, Wexler TL, Scherer SS, Fischbeck KH. Altered trafficking of mutant connexin32. J. Neurosci. 1997;17:9077–9084. doi: 10.1523/JNEUROSCI.17-23-09077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubourg O, Tardieu S, Birouk N, Gouider R, Leger JM, Maisonobe T, Brice A, Bouche P, Le Guern E. Clinical, electrophysiological and molecular genetic characteristics of 93 patients with X-linked Charcot-Marie-Tooth disease. Brain. 2001;124:1958–1967. doi: 10.1093/brain/124.10.1958. [DOI] [PubMed] [Google Scholar]
- Flagiello L, Cirigliano V, Strazzullo M, Cappa V, Ciccodicola A, D’Esposito M, Torrente I, Werner R, De Lorio G, Rinaldi M, Dallapiccola B, Forabosco A, Venruto V, D’Urso M. Mutation in the nerve-specific 5′ non-coding region of Cx32 gene and absence of specific mRNA in a CMTX1 Italian family. Hum. Mutat. 1998;12:361–363. [PubMed] [Google Scholar]
- Giese KP, Martini R, Lemke G, Soriano P, Schachner M. Mouse P0 gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell. 1992;71:565–576. doi: 10.1016/0092-8674(92)90591-y. [DOI] [PubMed] [Google Scholar]
- Gonzaga-Jauregui C, Zhang F, Towne CF, Batish SD, Lupski JR. GJB1/Connexin 32 whole gene deletions in patients with X-linked Charcot-Marie-Tooth disease. Neurogenetics. 2010;11:465–470. doi: 10.1007/s10048-010-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A, England JD, Sumner AJ, Ferer S, Warner LE, Lupski JR, Garcia CA. Unusual electrophysiological findings in X-linked dominant Charcot-Marie-Tooth disease. Muscle Nerve. 2000;23:182–188. doi: 10.1002/(sici)1097-4598(200002)23:2<182::aid-mus6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hahn A, Ainsworth PJ, Bolton CF, Bilbao JM, Vallat J-M. Pathological findings in the X-linked form of Charcot-Marie-Tooth disease: a morphometric and ultrastructural analysis. Acta Neuropathol. 2001;101:129–139. doi: 10.1007/s004010000275. [DOI] [PubMed] [Google Scholar]
- Hahn AF, Brown WF, Koopman WJ, Feasby TE. X-linked dominant hereditary motor and sensory neuropathy. Brain. 1990;113:1511–1525. doi: 10.1093/brain/113.5.1511. [DOI] [PubMed] [Google Scholar]
- Hahn AF, Bolton CF, Whiate CM, Brown WF, Tuuga SE, Tan CC, Ainsworth PJ. Genotype/phenotype correlations in X-linked Charcot-Marie-Tooth disease. Ann. N.Y. Acad. Sci. 1999;883:366–382. [PubMed] [Google Scholar]
- Hahn AF, Ainsworth PJ, Naus CCG, Mao J, Bolton CF. Clinical and pathological observations in men lacking the gap junction protein connexin 32. Muscle Nerve. 2000:S39–S48. [PubMed] [Google Scholar]
- Harding AE, Thomas PK. Genetic aspects of hereditary motor and sensory neuropathy (types I and II) J. Med. Genet. 1980;176:329–336. doi: 10.1136/jmg.17.5.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Yamamoto M, Yoshihara T, Koike H, Nakagawa M, Yoshikawa H, Ohnishi A, Hayasaka K, Onodera O, Baba M, Yasuda H, Saito T, Nakashima K, Kira J, Kaji R, Oka N, Sobue G. Demyelinating and axonal features of Charcot-Marie-Tooth disease with mutations of myelin-related proteins (PMP22, MPZ and Cx32): a clinicopathological study of 205 Japanese patients. Brain. 2003;126:134–151. doi: 10.1093/brain/awg012. [DOI] [PubMed] [Google Scholar]
- Herringham WP. Muscular atrophy of the peroneal type affecting many members of a family. Brain. 1888;11:230–236. [Google Scholar]
- Houlden H, Girard M, Cockerell C, Ingram D, Wood NW, Goossens M, Walker RWH, Reilly MM. Connexin 32 promoter P2 mutations: A mechanism of peripheral nerve dysfunction. Ann. Neurol. 2004;56:730–734. doi: 10.1002/ana.20267. [DOI] [PubMed] [Google Scholar]
- Huang Y, Sirkowski EE, Stickney JT, Scherer SS. Prenylation-defective human connexin32 mutants are normally localized and function equivalently to wild type connexin32 in myelinating Schwann cells. J. Neurosci. 2005;25:7111–7120. doi: 10.1523/JNEUROSCI.1319-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionasescu VV, Searby C, Ionasescu R, Neuhaus IM, Werner R. Mutations of noncoding region of the connexin32 gene in X-linked dominant Charcot-Marie-Tooth neuropathy. Neurology. 1996;47:541–544. doi: 10.1212/wnl.47.2.541. [DOI] [PubMed] [Google Scholar]
- Jeng LJB, Messing A, Balice-Gordon R, Fischbeck KH, Scherer SS. The effects of a dominant connexin32 mutant in myelinating Schwann cells. Mol. Cell. Neurosci. 2006;32:283–298. doi: 10.1016/j.mcn.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Karadima G, Panas M, Floroskufi P, Kalfakis N, Vassilopoulos D. A V38A mutation in X-linked Charcot-Marie-Tooth neuropathy with unusual clinical features. J. Neurol. 2004;251:222–223. doi: 10.1007/s00415-004-0284-8. [DOI] [PubMed] [Google Scholar]
- Kleopa KA, Yum SW, Scherer SS. Cellular mechanisms of connexin32 mutations associated with CNS manifestations. J. Neurosci. Res. 2002;68:522–534. doi: 10.1002/jnr.10255. [DOI] [PubMed] [Google Scholar]
- Kleopa KA, Zamba-Papanicolaou E, Alevra X, Nicolaou P, Georgiou D-M, Hadjisavvas A, Kyriakides T, Christodoulou K. Phenotypic and cellular expression of two novel connexin32 mutations causing CMT1X. Neurology. 2006;66:396–402. doi: 10.1212/01.wnl.0000196479.93722.59. [DOI] [PubMed] [Google Scholar]
- Krajewski KM, Lewis RA, Fuerst DR, Turansky C, Hinderer SR, Garbern J, Kamholz J, Shy ME. Neurological dysfunction and axonal degeneration in Charcot-Marie-Tooth disease. Brain. 2000 doi: 10.1093/brain/123.7.1516. [DOI] [PubMed] [Google Scholar]
- Kuntzer T, Dunarnd M, Schorderet DF, Vallat J-M, Hahn AF, Bogousslavsky J. Phenotypic expresssion of a Pro 87 to Leu mutatin in the connexin 32 gene in a large Swiss family with Charcot-Marie-Tooth neuropathy. J. Neurol. Sci. 2003;87:77–86. doi: 10.1016/s0022-510x(02)00394-5. [DOI] [PubMed] [Google Scholar]
- Latour P, Gonnaud PM, Ollagnon E, Chan V, Perelman S, Stojkovic T, Stoll C, Vial C, Ziegler F, Vandenberghe A, Maire I. SIMPLE mutation analysis in dominant demyelinating Charcot-Marie-Tooth disease: three novel mutations. J. Peripher. Nerv. Syst. 2006;11148:155. doi: 10.1111/j.1085-9489.2006.00080.x. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Nelson I, Houlden H, Sweeney MG, Hilton-Jones D, Blake J, Wood NW, Reilly MM. Six novel connexin32 (GJB1) mutations in X-linked Charcot-Marie-Tooth disease. J. Neurol. Neurosurg. Psychiat. 2002;73:304–306. doi: 10.1136/jnnp.73.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang GSL, de Miguel M, Gomez-Hernandez JM, Glass JD, Scherer SS, Mintz M, Barrio LC, Fischbeck KH. Severe neuropathy with leaky connexin32 hemichannels. Ann. Neurol. 2005;57:749–754. doi: 10.1002/ana.20459. [DOI] [PubMed] [Google Scholar]
- Lin CQ, Numakura C, Ikegami T, Shizuka M, Shoji M, Nicholson G, Hayasaka K. Deletion and nonsense mutations of the connexin 32 gene associated with Charcot-Marie-Tooth disease. Tohoku J. Exp. Med. 1999;188:239–244. doi: 10.1620/tjem.188.239. [DOI] [PubMed] [Google Scholar]
- Marques W, Sweeney MG, Wood NW, Wroe SJ. Central nervous system involvement in a novel connexin 32 mutation affecting identical twins. J. Neurol. Neurosurg. Psychiat. 1999;66:803–804. doi: 10.1136/jnnp.66.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PEM, Mambetisaeva ET, Archer DA, George CH, Evans WH. Analysis of gap junctions assembly using mutated connexins detected in Charcot-Marie-Tooth X-linked disease. J. Neurochem. 2000;74:711–720. doi: 10.1046/j.1471-4159.2000.740711.x. [DOI] [PubMed] [Google Scholar]
- Martini R, Zielasek J, Toyka KV, Giese KP, Schachner M. Protein zero (P0)-deficient mice show myelin degeneration in peripheral nerves characteristic of inherited human neuropathies. Nat. Genet. 1995;11:281–285. doi: 10.1038/ng1195-281. [DOI] [PubMed] [Google Scholar]
- Matsuyama W, Nakagawa M, Moritoyo T, Takashima H, Umehara F, Hirata K, Suehara M, Osame M. Phenotypes of X-linked Charcot-Marie-Tooth disease and altered trafficking of mutant Connexin 32 (GJB1) J. Hum. Genet. 2001;46:307–313. doi: 10.1007/s100380170064. [DOI] [PubMed] [Google Scholar]
- Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Connexins are critical for normal myelination in the central nervous system. J. Neurosci. 2003;23:5963–5973. doi: 10.1523/JNEUROSCI.23-13-05963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Takashima H, Umehara F, Arimura K, Miyashita F, Takenouchi N, Matsuyama W, Osame M. Clinical phentoype in X-linked Charcot-Marie-Tooth disease with an entire deletion of the connexin 32 coding sequence. J. Neurol. Sci. 2001;185:31–36. doi: 10.1016/s0022-510x(01)00454-3. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Maeda S, Tsukihara T. Structural and functional studies of gap junction channels. Curr. Opin. Struct. Biol. 2010;20:423–430. doi: 10.1016/j.sbi.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Nave K-A, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu. Rev. Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- Nicholson G, Nash J. Intermediate nerve conduction velocities define X-linked Charcot-Marie-Tooth neuropathy families. Neurology. 1993;43:2558–2564. doi: 10.1212/wnl.43.12.2558. [DOI] [PubMed] [Google Scholar]
- Nicholson G, Corbett A. Slowing of central conduction in X-linked Charcot-Marie-Tooth neuropathy shown by brain auditory evoked responses. J. Neurol. Neurosurg. Psychiatry. 1996;61:43–46. doi: 10.1136/jnnp.61.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson GA, Yeung L, Corbett A. Efficient neurophysiological selection of X-linked Charcot-Marie-Tooth families. Neurology. 1998;51:1412–1416. doi: 10.1212/wnl.51.5.1412. [DOI] [PubMed] [Google Scholar]
- Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen G, Euwens C, Fuss B, Bussow H, Schilling K, Stenhauser C, Willecke K. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J. Neurosci. 2003;23:4549–4559. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Ri Y, Bennett MVL, Trexler EB, Verselis VK, Bargiello TA. Changes in permeability caused by connexin 32 mutations underlie X-linked Charcot-Marie-Tooth disease. Neuron. 1997;19:927–938. doi: 10.1016/s0896-6273(00)80973-3. [DOI] [PubMed] [Google Scholar]
- Omori Y, Mesnil M, Yamasaki H. Connexin 32 mutations from X-linked Charcot-Marie-Tooth disease patients: functional defects and dominant negative effects. Mol. Biol. Cell. 1996;7:907–916. doi: 10.1091/mbc.7.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Enriquez AD, Abrams CK, Scherer SS. Loss-of-function GJA12/Connexin47 mutations cause Pelizaeus-Merzbacher-like disease. Mol. Cell. Neurosci. 2007;34:629–641. doi: 10.1016/j.mcn.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas M, Karadimas C, Avramopoulos D, Vassilopoulos D. Central nervous system involvement in four patients with Charcot-Marie-Tooth disease with connexin 32 extracellular mutations. J. Neurol. Neurosurg. Psychiat. 1998;65:947–948. doi: 10.1136/jnnp.65.6.947a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressot C, Gomes D, Dautigny A, Pham-Dinh D, Bruzzone R. Connexin32 mutations associated with X-linked Charcot-Marie-Tooth disease show two distinct behaviors: Loss of function and altered gating properties. J. Neurosci. 1998;18:4063–4075. doi: 10.1523/JNEUROSCI.18-11-04063.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ri Y, Ballesteros JA, Abrams CK, Oh S, Verselis VK, Weinstein H, Bargiello TA. The role of a conserved proline residue in mediating conformational changes associated with voltage gating of Cx32 gap junctions. Biophy. J. 1999;76:2887–2898. doi: 10.1016/S0006-3495(99)77444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouger H, Le Guern E, Birouk N, Gouider R, Tardieu S, Plassart E, Gugenheim M, Vallat JM, Louboutin JP, Bouche P, Agid E, Brice A. Charcot-Marie-Tooth disease with intermediate motor nerve conduction velocities: Characterization of 14 Cx32 mutations in 35 families. Hum. Mutat. 1997;10:443–450. doi: 10.1002/(SICI)1098-1004(1997)10:6<443::AID-HUMU5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Rozear MP, Pericak-Vance MA, Fischbeck K, Stajich JM, Gaskell PC, Jr., Krendel DA, Graham DG, Dawson DV, Roses AD. Hereditary motor and sensory neuropathy, X-linked: a half century follow-up. Neurology. 1987;37:1460–1465. doi: 10.1212/wnl.37.9.1460. [DOI] [PubMed] [Google Scholar]
- Sahenk Z, Chen L. Abnormalities in the axonal cytoskeleton induced by a connexin32 mutation in nerve xenografts. J. Neurosci. Res. 1998;51:174–184. doi: 10.1002/(SICI)1097-4547(19980115)51:2<174::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Sancho S, Magyar JP, Aguzzi A, Suter U. Distal axonopathy in peripheral nerves of PMP22 mutant mice. Brain. 1999;122:1563–1577. doi: 10.1093/brain/122.8.1563. [DOI] [PubMed] [Google Scholar]
- Saporta ASD, Sottile SL, Miller LJ, Feely SME, Siskind CE, Shy ME. Charcot-Marie-Tooth disease subtypes and genetic testing strategies. Ann. Neurol. 2011;69:22–33. doi: 10.1002/ana.22166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiannidou I, Ahn M, Enriquez AD, Peinado A, Reynolds R, Abrams C, Scherer SS, Kleopa KA. Human oligodendrocytes express Cx31.3: function and interactions with Cx32 mutants. Neurobiol. Dis. 2008;30:221–233. doi: 10.1016/j.nbd.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiannidou I, Vavlitou N, Aristodemou S, Hadjisavvas A, Kyriacou K, Scherer SS, Kleopa KA. Connexin32 mutations cause loss of function in Schwann cells and oligodendrocytes leading to PNS and CNS myelination defects. J. Neurosci. 2009;29:4736–4749. doi: 10.1523/JNEUROSCI.0325-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Xu Y-T, Nelles E, Fischbeck K, Willecke K, Bone LJ. Connexin32-null mice develop a demyelinating peripheral neuropathy. Glia. 1998;24:8–20. doi: 10.1002/(sici)1098-1136(199809)24:1<8::aid-glia2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Xu Y-T, Messing A, Willecke K, Fischbeck KH, Jeng LJB. Transgenic expression of human Connexin32 in myelinating Schwann cells prevents demyelination in connexin32-null mice. J. Neurosci. 2005;25:1550–1559. doi: 10.1523/JNEUROSCI.3082-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Wrabetz L. Molecular mechanisms of inherited demyelinating neuropathies. Glia. 2008;56:1578–1589. doi: 10.1002/glia.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderek J, Hermanns B, Bergmann C, Boroojerdi B, Bajbouj M, Hungs M, Ramaekers VT, Quasthoff S, Karch D, Schröder JM. X-linked dominant Charcot-Marie-Tooth neuropathy: clinical, electrophysiological, and morphological phenotype in four families with different connexin32 mutations. J. Neurol. Sci. 1999;167:90–101. doi: 10.1016/s0022-510x(99)00146-x. [DOI] [PubMed] [Google Scholar]
- Shy ME, Siskind C, Swan ER, Krajewski KM, Doherty T, Fuerst DR, Ainsworth PJ, Lewis RA, Scherer SS, Hahn A. CMT1X phenotypes represent loss of GJB1 gene function. Neurology. 2007;68:849–855. doi: 10.1212/01.wnl.0000256709.08271.4d. [DOI] [PubMed] [Google Scholar]
- Siskind C, Feely SME, Bernes S, Shy ME, Garbern JY. Persistent CNS dysfunction in a boy with CMT1X. J. Neurol. Sci. 2009;279:109–113. doi: 10.1016/j.jns.2008.12.031. [DOI] [PubMed] [Google Scholar]
- Siskind C, Murphy S, Ovens R, Polke J, Reilly M, Shy M. Phenotype expression in women with CMT1X. J. Perpheral Nerv. Syst. 2011;16:102–107. doi: 10.1111/j.1529-8027.2011.00332.x. [DOI] [PubMed] [Google Scholar]
- Sutor B, Schmolke C, Teubner B, Schirmer C, Willecke K. Myelination defects and neuronal hyperexcitability in the neocortex of connexin 32-deficient mice. Cereb Cortex. 2000;10:684–697. doi: 10.1093/cercor/10.7.684. [DOI] [PubMed] [Google Scholar]
- Tabaraud F, Lagrange E, Sindou P, Vandenberghe A, Levy N, Vallat JM. Demyelinating X-linked Charcot-Marie-Tooth disease: Unusual electrophysiological findings. Muscle Nerve. 1999;22:1442–1447. doi: 10.1002/(sici)1097-4598(199910)22:10<1442::aid-mus16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- VanSlyke JK, Deschênes SM, Musil LS. Intracellular transport, assembly, and degradation of wild-type and disease-linked mutant gap junction proteins. Mol. Biol. Cell. 2000;11:1933–1946. doi: 10.1091/mbc.11.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavlitou N, Sargiannidou I, Markoullis K, Kyriacou K, Scherer SS, Kleopa KA. Axonal pathology precedes demyelination in a mouse model of X-linked demyelinating/type I Charcot-Marie Tooth neuropathy. J. Neuropathol. Exp. Neurol. 2010;69:945–958. doi: 10.1097/NEN.0b013e3181efa658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklein EM, Orth U, Gal A, Kunze K. Missense mutation (R15W) of the connexin32 gene in a family with X chromosomal Charcot-Marie-Tooth neuropathy with only female family members affected. J. Neurol. Neurosurg. Psychiatry. 1997;63:379–381. doi: 10.1136/jnnp.63.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Söhl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- Yiu E, Geevasinga N, Nicholson G, Fagan E, Ryan M, Ouvrier R. A retrospective review of X-linked Charcot-Marie-Tooth disease in childhood. Neurology. 2011;76:461–466. doi: 10.1212/WNL.0b013e31820a0ceb. [DOI] [PubMed] [Google Scholar]
- Yum SW, Kleopa KA, Shumas S, Scherer SS. Diverse trafficking abnormalities for connexin32 mutants causing CMTX. Neurobiol. Dis. 2002;11:43–52. doi: 10.1006/nbdi.2002.0545. [DOI] [PubMed] [Google Scholar]
- Yum SW, Zhang J.-x., Scherer SS. Dominant connexin26 mutants associated with human hearing loss have trans-dominant effects on connexin30. Neurobiol Dis. 2010;38:226–236. doi: 10.1016/j.nbd.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]